Comparing Two Saline-Gypseous Wetland Soils in NE Spain
Abstract
:1. Introduction
2. Materials and Methods
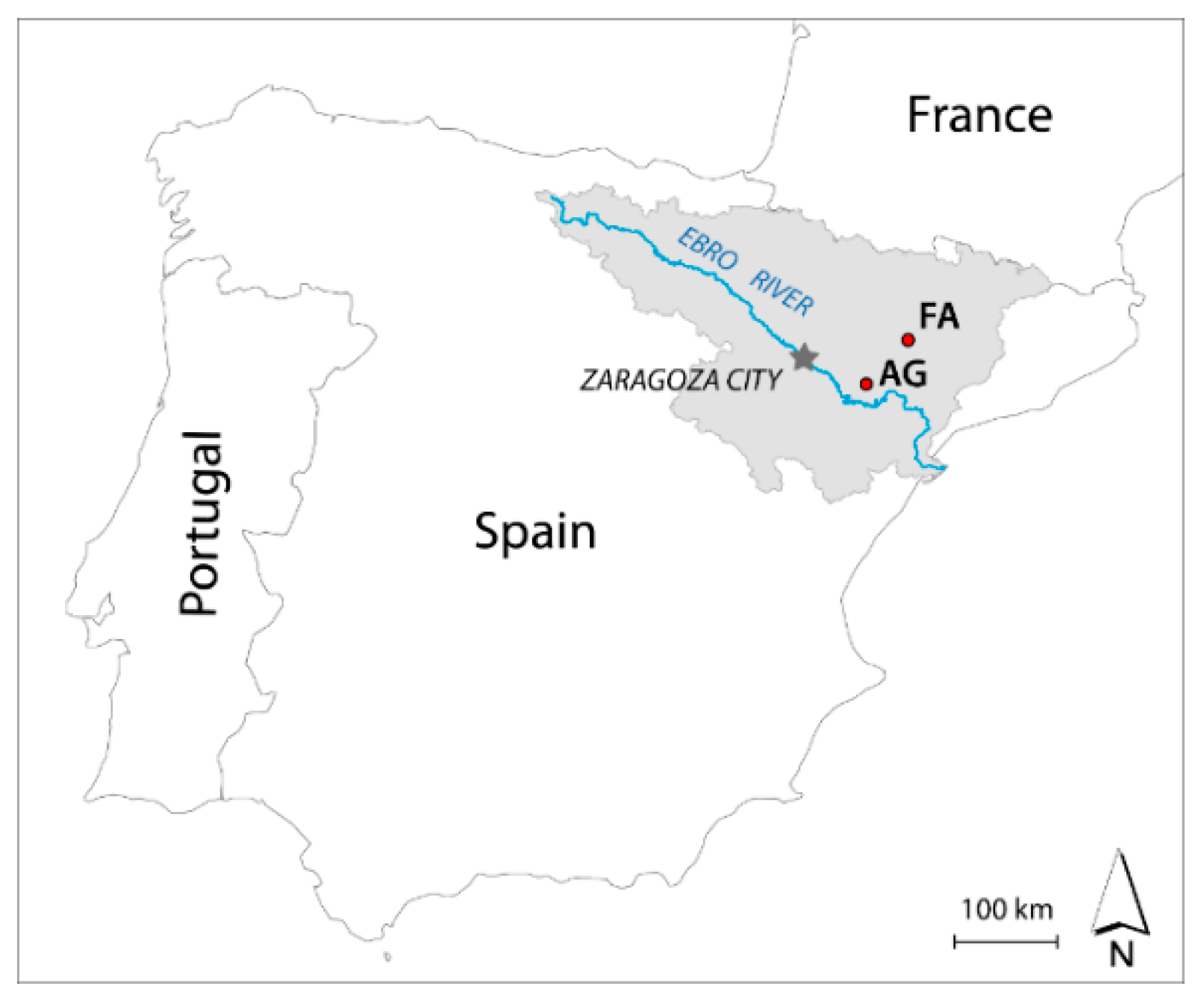
2.1. The Context of the Wetlands Studied
2.2. Climate
2.3. Sampling
2.4. Analyses
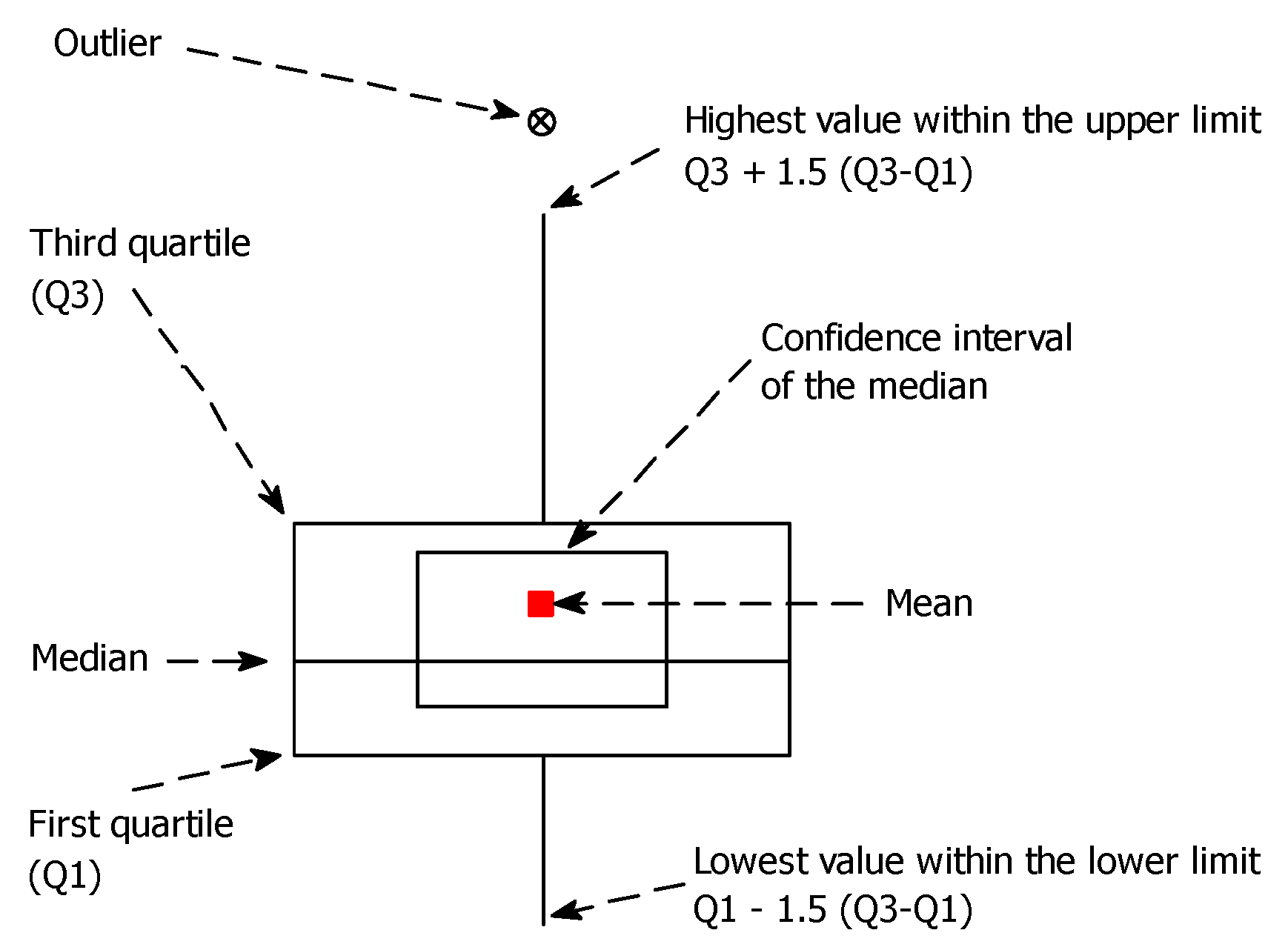
2.5. Statistical Procedures
3. Results and Discussion
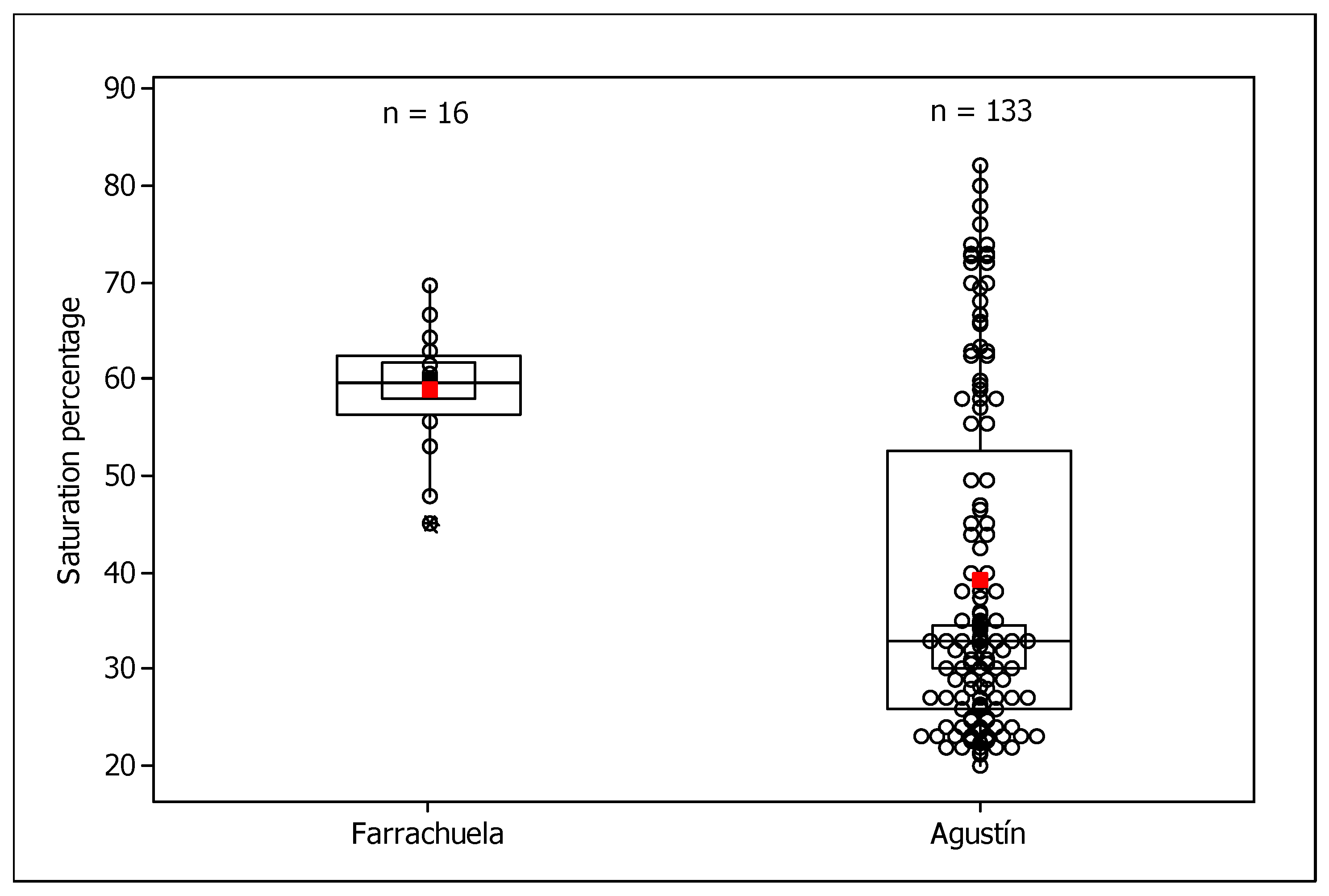
3.1. Saturation Percentages
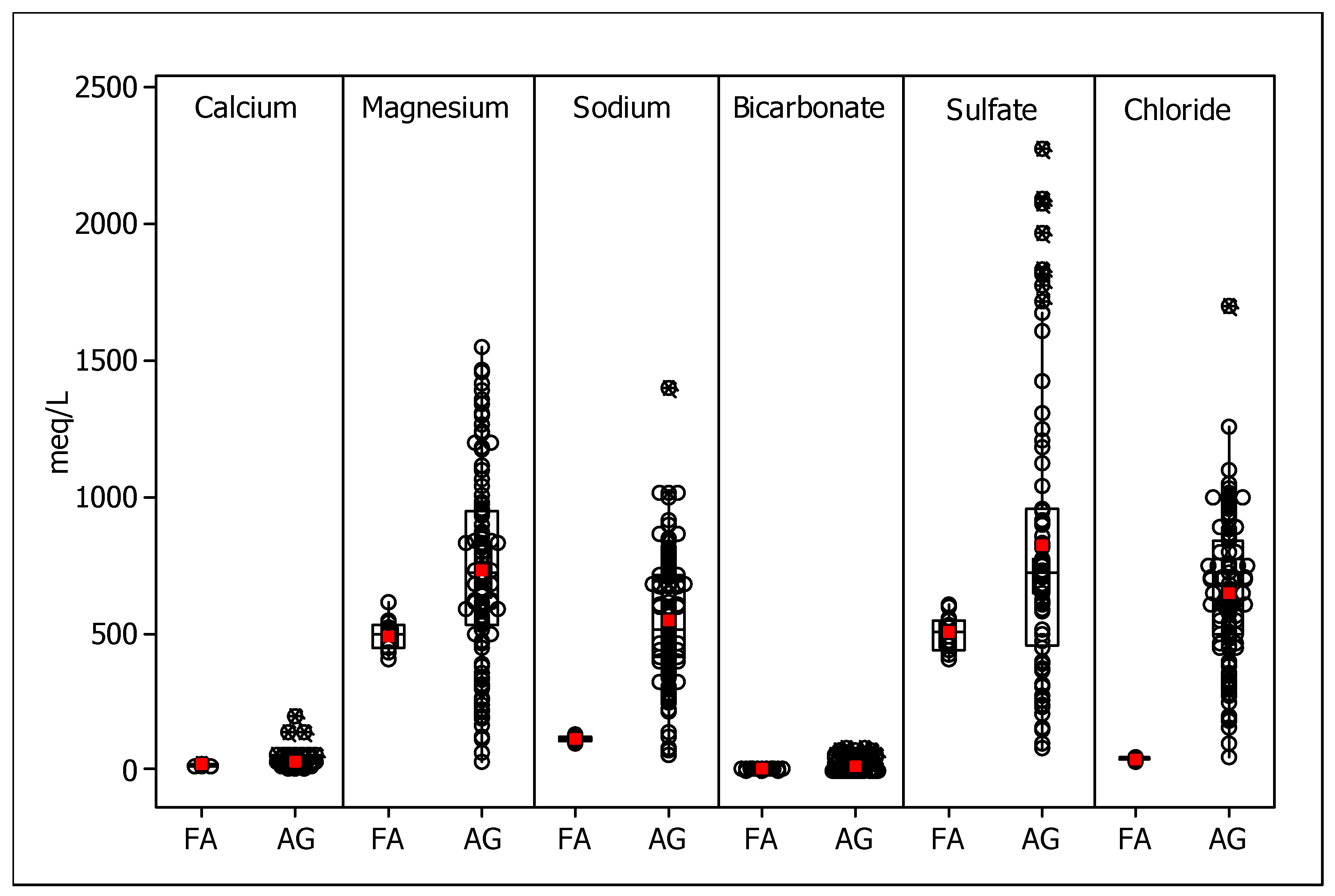
3.2. Major Mineral Components
3.3. Soil Salinity
3.4. Soil Classification
3.5. Ionic Contents of the Saturation Extracts
3.6. SAR and pH
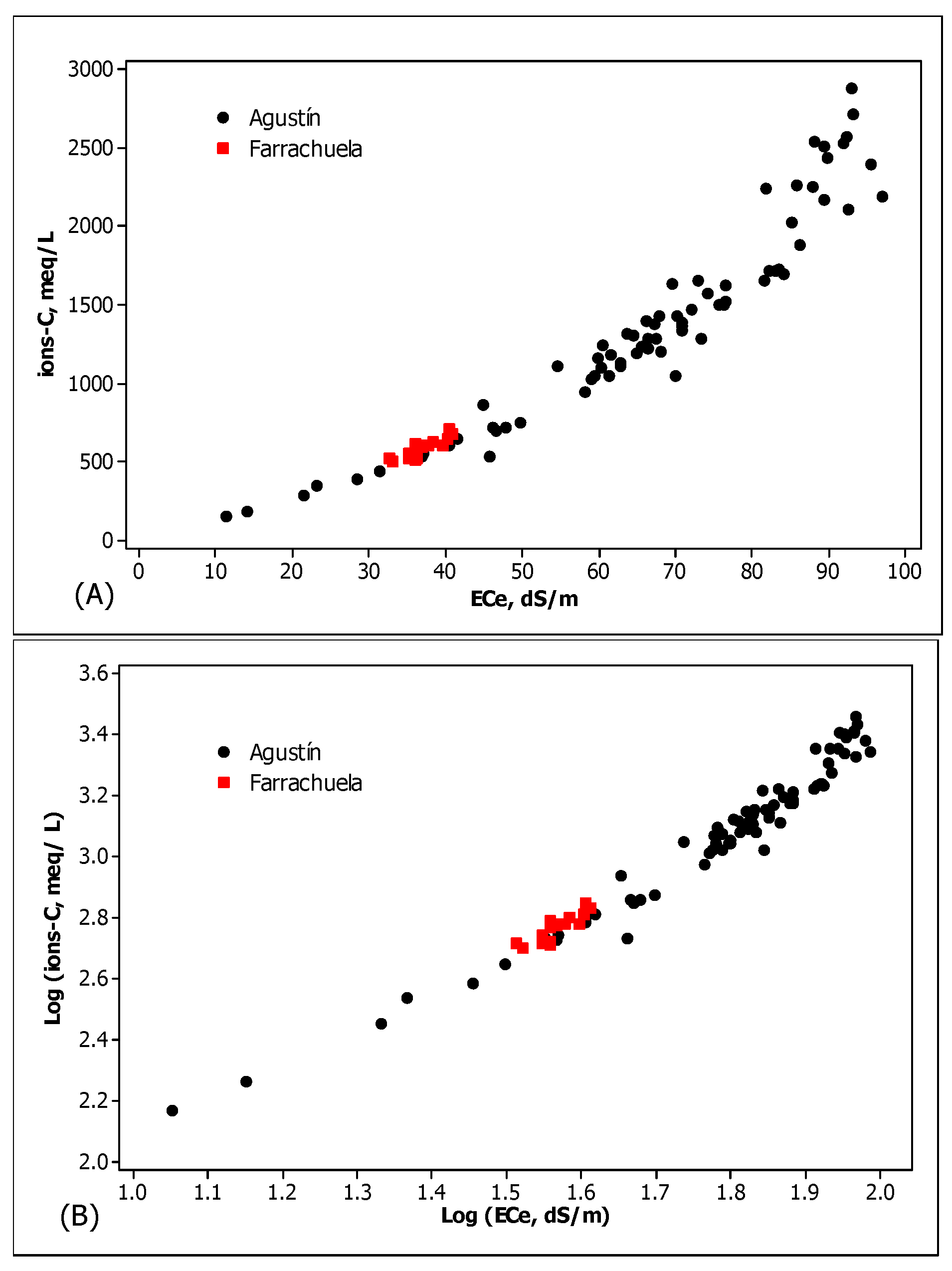
3.7. Relationship between Ionic Concentration and ECe
3.8. Relationship between the Concentration of Individual Ions and ECe
3.9. The Specificity in the Analytics of Gypseous Materials
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


| Farrachuela | Agustín | |
|---|---|---|
| q | R | |
| 0 | 0.291 | 0.360 |
| 0.50 | 0.862 | 0.887 |
| 0.55 | 0.863 | 0.902 |
| 0.60 | 0.857 | 0.913 |
| 0.65 | 0.848 | 0.920 |
| 0.70 | 0.837 | 0.925 |
| 0.80 | 0.812 | 0.928 |
| 0.90 | 0.787 | 0.925 |
| 1 | 0.763 | 0.919 |
| Salada | Method | a | b | R2, % |
|---|---|---|---|---|
| Farrachuela | OLS | 0.659 | 1.34 | 75.4 |
| Theil | 0.701 | 1.32 | 75.4 | |
| Agustín | OLS | 0.541 | 1.42 | 95.5 |
| Theil | 0.302 | 1.54 | 95.5 |
References
- Cížková, E.; Navrátilová, J.; Martinát, S.; Navrátil, J.; Frazier, R.J. Impact of water level on species quantity and composition grown from the soil seed bank of the inland salt marsh: An ex-situ experiment. Land 2020, 9, 533. [Google Scholar] [CrossRef]
- Domínguez-Beisiegel, M.; Herrero, J.; Castañeda, C. Saline wetlands’ fate in inland deserts: An example of 80 years’ decline in Monegros, Spain. Land Degrad. Dev. 2013, 24, 250–265. [Google Scholar] [CrossRef]
- Köninger, J.; Panagos, P.; Jones, A.; Briones, M.J.I.; Orgiazzi, A. In defence of soil biodiversity: Towards an inclusive protection in the European Union. Biol. Conserv. 2022, 268, 109475. [Google Scholar] [CrossRef]
- Casby-Horton, S.; Herrero, J.; Rolong, N.A. Gypsum soils—Their morphology, classification, function, and landscapes. Adv. Agron. 2015, 130, 231–290. [Google Scholar] [CrossRef]
- Goryachkin, S.V.; Spiridonova, I.A.; Sedov, S.N.; Targulian, V.O. Boreal soils on hard gypsum rocks: Morphology, properties, and genesis. Eurasian Soil Sci. 2003, 36, 691–703. [Google Scholar]
- Tuyukina, T.Y. Geochemical studies of northern taiga (gypsum) karst ecosystems and their high vulnerability to natural and anthropogenic hazards. Environ. Geol. 2009, 58, 269–274. [Google Scholar] [CrossRef]
- Palacio, S.; Cera, A.; Escudero, A.; Luzuriaga, A.L.; Sánchez, A.M.; Mota, J.F.; Pérez-Serrano, M.; Merlo, M.E.; Martínez-Hernández, F.; Salmerón-Sánchez, E.; et al. Recent and ancient evolutionary events shaped plant elemental composition of edaphic endemics: A phylogeny-wide analysis of Iberian gypsum plants. New Phytol. 2022, 235, 2406–2423. [Google Scholar] [CrossRef]
- Pearson, M.J.; Monteith, S.E.; Ferguson, R.R.; Hallmark, C.T.; Hudnall, W.H.; Monger, H.C.; Reinsch, T.G.; West, L.T. A method to determine particle size distribution in soils with gypsum. Geoderma 2015, 237–238, 318–324. [Google Scholar] [CrossRef]
- Heino, J.; Alahuhta, J.; Bini, L.M.; Cai, Y.; Heiskanen, A.S.; Hellsten, S.; Kortelainen, P.; Kotamaki, N.; Tolonen, K.T.; Vihervaara, P.; et al. Lakes in the era of global change: Moving beyond single-lake thinking in maintaining biodiversity and ecosystem services. Biol. Rev. 2021, 96, 89–106. [Google Scholar] [CrossRef]
- Lehner, B.; Doll, P. Development and validation of a global database of lakes, reservoirs and wetlands. J. Hydrol. 2004, 296, 1–22. [Google Scholar] [CrossRef]
- Prăvălie, R.; Sîrodoev, I.; Ruiz-Arias, J.; Dumitraşcu, M. Using renewable (solar) energy as a sustainable management pathway of lands highly sensitive to degradation in Romania. A countrywide analysis based on ex-ploring the geographical and technical solar potentials. Renew. Energy 2022, 193, 976–990. [Google Scholar] [CrossRef]
- Wang, G.; Li, G.; Liu, Z. Wind farms dry surface soil in temporal and spatial variation. Sci. Total Environ. 2023, 857, 159293. [Google Scholar] [CrossRef]
- Alberto, F.; Navas, A. La participación de los yesos en la salinización de las aguas superficiales de la cuenca del Ebro. I. Cartografía de síntesis de las formaciones con yesos. An. Aula Dei 1986, 18, 7–18. Available online: http://hdl.handle.net/10261/13516 (accessed on 3 September 2023).
- Herrero, J.; Castañeda, C. A Dataset of Scientific References on Saline Wetlands of Monegros, NE Spain. Mendeley Data. 2023. Available online: https://data.mendeley.com/datasets/kxbgpc8y5r/3 (accessed on 20 September 2023).
- Reid, R.P.; Oehlert, A.M.; Suosaari, E.P.; Demergasso, C.; Chong, G.; Escudero, L.V.; Piggot, A.M.; Lascu, I.; Palma, A.T. Electrical conductivity as a driver of biological and geological spatial heterogeneity in the Pu-quios, Salar de Llamara, Atacama Desert, Chile. Sci. Rep. 2021, 11, 12769. [Google Scholar] [CrossRef]
- Pardo, G.; Villena, J. Aportación a la geología de la región de Barbastro. Acta Geol. Hisp. 1979, 14, 289–292. [Google Scholar]
- Castañeda, C.; Herrero, J.; Conesa, J.A. Distribution, morphology and habitats of saline wetlands: A case study from Monegros. Geol. Acta 2013, 11, 371–388. [Google Scholar] [CrossRef]
- Salvany, J.M.; García-Vera, M.A.; Samper, J. Geología e hidrogeología de la zona endorreica de Bujaraloz-Sástago (Los Monegros, provincias de Zaragoza y Huesca). Acta Geol. Hisp. 1995, 30, 31–51. [Google Scholar]
- Samper-Calvete, F.J.; García-Vera, M.A. Inverse modeling of groundwater flow in the semiarid evaporitic closed basin of Los Monegros, Spain. Hydrogeol. J. 1998, 6, 33–49. [Google Scholar] [CrossRef]
- Herrero, J.; Castañeda, C.; Velayos, M. Salada Farrachuela, a saline wetland in Tamarite de Litera, Spain. Bol. Real Soc. Esp. Hist. Nat. 2020, 114, 67–80. [Google Scholar] [CrossRef]
- Conesa, J.A.; Castañeda, C.; Pedrol, J. Las saladas de Monegros y su entorno. In Hábitats y Paisaje Vegetal; Consejo de Protección de la Naturaleza de Aragón: Zaragoza, Spain, 2011; 540p, ISBN 978-84-89862-76-0. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 13th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2022. [Google Scholar]
- M.A.P.A. Métodos Oficiales de Análisis; Tomo III; Ministerio de Agricultura, Pesca y Alimentación, Secretaría General Técnica: Madrid, Spain, 1994; 662p. [Google Scholar]
- Artieda, O.; Herrero, J.; Drohan, P.J. A refinement of the differential water loss method for gypsum deter-mination in soils. Soil Sci. Soc. Am. J. 2006, 70, 1932–1935. [Google Scholar] [CrossRef]
- USDA; United States Salinity Laboratory Staff. Diagnosis and Improvement of Saline and Alkali Soils. Agriculture Handbook no. 60. Reprint 1969. 1954. Available online: https://www.ars.usda.gov/ARSUserFiles/20360500/hb60_pdf/hb60complete.pdf (accessed on 24 September 2023).
- Tukey, J.W. Exploratory Data Analysis; Addison-Wesley: Reading, MA, USA, 1977. [Google Scholar]
- Chambers, J.M.; Cleveland, W.S.; Kleiner, B.; Tukey, P.A. Graphical Methods for Data Analysis; Reissued in 2018 by CRC Press; Chapman and Hall: New York, NY, USA, 1983. [Google Scholar]
- Hettmansperger, T.P.; Sheather, S.J. Confidence intervals based on interpolated order statistics. Stat. Probab. Lett. 1986, 4, 75–79. [Google Scholar] [CrossRef]
- Daniel, W.W. Applied Nonparametric Statistics, 2nd ed.; Pws-Kent Publishing Company: Boston, MA, USA, 1990. [Google Scholar]
- Glaister, P. A comparison of best fit lines for data with outliers. Int. J. Math. Educ. Sci. Technol. 2005, 36, 110–117. [Google Scholar] [CrossRef]
- Bower, C.A.; Wilcox, L.V. Soluble salts. In Methods of Soil Analysis, Part 2 Chemical and Microbiological Properties; Black, C.A., Ed.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 933–951. [Google Scholar]
- Hanson, B.R.; Grattan, S.R.; Fulton, A. Agricultural Salinity and Drainage; Water Management Series, Publication 113375; University of California Davis: Davis, CA, USA, 2006. [Google Scholar]
- Stiven, G.A.; Khan, M.A. Saturation percentage as a measure of soil texture in the Lower Indus Basin. J. Soil Sci. 1966, 17, 255–263. [Google Scholar] [CrossRef]
- Minasny, B.; McBratney, A.B.; Field, D.J.; Tranter, T.; McKenzie, N.J.; Brough, D.M. Relationships between field texture and particle-size distribution in Australia and their implications. Aust. J. Soil Res. 2007, 45, 428–437. [Google Scholar] [CrossRef]
- Briggs, L.J.; McLane, J.W. Moisture equivalent determinations and their application. Agron. J. 1910, 2, 138–147. [Google Scholar] [CrossRef]
- Bodman, G.B.; Mahmud, A.J. The use of the moisture equivalent in the textural classification of soils. Soil Sci. 1932, 33, 363–374. [Google Scholar] [CrossRef]
- Gharaibeh, M.A.; Albalasmeh, A.A.; Hanandeh, A.E. Estimation of saturated paste electrical conductivity using three modelling approaches: Traditional dilution extracts; saturation percentage and artificial neural networks. Catena 2021, 200, 105141. [Google Scholar] [CrossRef]
- Elsharkawy, M.M.; Sheta, A.S.; D’Antonio, P.; Abdelwahed, M.S.; Scopa, A. Tool for the establishment of agro-management zones using GIS techniques for precision farming in Egypt. Sustainability 2020, 14, 5437. [Google Scholar] [CrossRef]
- Herrero, J.; Castañeda, C. Changes in soil salinity in the habitats of five halophytes after 20 years. Catena 2013, 109, 58–71. [Google Scholar] [CrossRef]
- Herrero, J.; Mandado, J. Clarifications on statements in Badía et al. (2013), Geoderma 193–194, 13–21. Geoderma 2016, 275, 82–83. [Google Scholar] [CrossRef]
- Al-Kayssi, A.W. Quantifying soil physical quality by using indicators and pore volume-function characteris-tics of the gypsiferous soils in Iraq. Geoderma Reg. 2022, 30, e00556. [Google Scholar] [CrossRef]
- Moret-Fernández, D.; Herrero, J. Effect of gypsum content on soil water retention. J. Hydrol. 2015, 528, 122–126. [Google Scholar] [CrossRef]
- Herrero, J.; Weindorf, D.C.; Castañeda, C. Two fixed ratio dilutions for soil salinity monitoring in hypersaline wetlands. PLoS ONE 2015, 10, e0126493. [Google Scholar] [CrossRef]
- Carpena, O.; Guillén, M.G.; Fernández, F.G.; Caro, M.G. Saline soil classification using the 5:1 aqueous extract. In Transactions of the 9th International Congress of Soil Science; American Elsevier Publishing Company: Adelaide, Australia, 1968; Volume I, pp. 483–490. [Google Scholar]
- Sumner, M.E.; Rengasamy, P.; Naidu, R. Sodic Soils: A Reappraisal. In Sodic Soils: Distribution, Properties, Management, and Environmental Consequences; Sumner, M.E., Naidu, R., Eds.; Oxford University Press: New York, NY, USA, 1998; pp. 3–17. [Google Scholar]
- Quirk, J.P.; Schofield, R.K. Landmark Papers: No. 2. The effect of electrolyte concentration on soil permeability. Eur. J. Soil Sci. 2013, 64, 8–15. [Google Scholar] [CrossRef]
- Darab, K.; Csillag, J.; Painter, I. Studies on the ion composition of salt solutions and of saturation extracts of salt-affected soils. Geoderma 1980, 23, 95–111. [Google Scholar] [CrossRef]
- Marion, G.M.; Babcock, K.L. Predicting specific conductance and salt concentration in dilute aqueous solutions. Soil Sci. 1976, 122, 181–187. [Google Scholar] [CrossRef]
- Schoeneberger, P.J.; Wysocki, D.A.; Benham, E.C.; Soil Survey Staff. Field Book for Describing and Sampling Soils; Version 3.0., Reprint 2021; National Soil Survey Center: Lincoln, NE, USA, 2012. Available online: https://www.nrcs.usda.gov/sites/default/files/2022-09/field-book.pdf (accessed on 29 August 2023).
- Zhu, Y.; Weindorf, D.C.; Zhang, W. Characterizing soils using a portable X-ray fluorescence spectrometer: 1. Soil texture. Geoderma 2011, 167–168, 167–177. [Google Scholar] [CrossRef]
- Wang, S.Q.; Li, W.D.; Li, J.; Liu, X.S. Prediction of soil texture using FT-NIR spectroscopy and PXRF spectrometry with data fusion. Soil Sci. 2013, 178, 626–638. [Google Scholar] [CrossRef]
- Gozukara, G.; Akça, E.; Dengiz, O.; Kapur, S.; Alper, A. Soil particle size prediction using Vis-NIR and pXRF spectra in a semiarid agricultural ecosystem in Central Anatolia of Türkiye. Catena 2022, 217, 106514. [Google Scholar] [CrossRef]



| Farrachuela, 1981 | Agustín, 2004 to 2007 |
| Hornungia procumbens (L.) Hayek | Aeluropus littoralis (Gouan) Parl. |
| Helianthemum salicifolium (L.) Mill. | Artemisia herba-alba Asso |
| Frankenia pulverulenta L. | Arthrocnemum macrostachyum (Moric.) Moris in Moris and Delponte |
| Lepidium subulatum L. | Atriplex halimus L. |
| Phragmites australis (Cav.) Trin. ex Steud. | Frankenia pulverulenta L. |
| Gypsophila struthium subsp. hispánica (Willk.) G. López | |
| Farrachuela, 2013 | Hordeum marinum Huds. |
| Suaeda spicata (Willd.) Moq. | Kochia prostrata = Bassia prostrata (L.) Beck in Rchb. |
| Salsola soda L. | Limonium sp. pl. |
| Puccinellia festuciformis (Host) Parl. | Puccinellia fasciculata (Torrey) E.P. Bicknell |
| Frankenia pulverulenta L. | Salsola vermiculata L. |
| Suaeda vera subsp. braun-blanquetii Castrov. and Pedrol |
| Name of the Salada | Farrachuela | Agustín |
|---|---|---|
| Sheet name of the National Topographic Map of Spain at 1:25 000 scale | Tamarite | Bujaraloz |
| Geographical coordinates (N, E) | 41.8845, 0.3742 | 41.4311, −0.1091 |
| Surface area, ha | 3.4 | 68.1 |
| Elevation, meters above sea level | 400 | 329 |
| Number of augerings | 4 | 24 |
| Augering mean deep, cm | 200 | 63 |
| Number of soil samples for chemical analyses | 32 | 133 |
| Ca2+ | Mg2+ | Na+ | Cl− | SO42− | HCO3− | |
|---|---|---|---|---|---|---|
| mmolc L−1 | ||||||
| (a) Farrachuela | 18.9 | 492.3 | 113.0 | 40.7 | 503.1 | 2.2 |
| (b) all determinations from Agustín * | 29.0 | 734.0 | 546.4 | 646.4 | 820.6 | 16.7 |
| (c) differences between saladas, (b) minus (a) | 10.1 | 241.7 | 433.4 | 605.7 | 317.5 | 14.5 |
| (d) Agustín, only the 76 samples with the six ions titrated | 27.5 | 716.7 | 523.4 | 634.5 | 820.6 | 3.0 |
| (e) differences, b minus d | 1.5 | 17.3 | 23.0 | 11.9 | --- | 13.7 |
| Number of Analyzed Samples | Minimum | Maximum | Median | 95% Confidence Interval for The Median | ||
|---|---|---|---|---|---|---|
| SAR (mmolc/L)0.5 | ||||||
| FA | 16 | 6.70 | 7.80 | 7.00 | 6.80 | 7.30 |
| AG | 124 | 10.41 | 45.08 | 27.96 | 26.80 | 28.94 |
| pH | ||||||
| FA | 16 | 7.92 | 8.44 | 8.26 | 8.17 | 8.36 |
| AG | 52 | 7.28 | 8.33 | 7.92 | 7.71 | 8.00 |
| ECe | Mg2+ | Na+ | SO42− | |
|---|---|---|---|---|
| Farrachuela | ||||
| Mg2+ | 0.822 | |||
| Na+ | 0.792 | 0.815 | ||
| SO42− | 0.815 | 0.840 | n.s. | |
| Cl− | n.s. | n.s. | n.s. | n.s. |
| Agustín | ||||
| Mg2+ | 0.913 | |||
| Na+ | 0.936 | 0.901 | ||
| SO42− | 0.794 | 0.907 | 0.792 | |
| Cl− | 0.945 | 0.888 | 0.957 | 0.723 |
| Method | a | b | R2 | S | Range of Ionic-C | |
|---|---|---|---|---|---|---|
| % | mmolc L−1 | |||||
| Farrachuela | OLS | −213.1 | 21.59 | 76.2 | 29.60 | 502.4 to 705.2 |
| Theil | −200.4 | 21.33 | 76.2 | 32.71 | ||
| Agustín | OLS | −620.6 | 30.30 | 88.8 | 218.8 | 147.8 to 2876.0 |
| Theil | −629.6 | 29.50 | 88.8 | 281.1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrero, J.; Castañeda, C. Comparing Two Saline-Gypseous Wetland Soils in NE Spain. Land 2023, 12, 1990. https://doi.org/10.3390/land12111990
Herrero J, Castañeda C. Comparing Two Saline-Gypseous Wetland Soils in NE Spain. Land. 2023; 12(11):1990. https://doi.org/10.3390/land12111990
Chicago/Turabian StyleHerrero, Juan, and Carmen Castañeda. 2023. "Comparing Two Saline-Gypseous Wetland Soils in NE Spain" Land 12, no. 11: 1990. https://doi.org/10.3390/land12111990
APA StyleHerrero, J., & Castañeda, C. (2023). Comparing Two Saline-Gypseous Wetland Soils in NE Spain. Land, 12(11), 1990. https://doi.org/10.3390/land12111990








