Farmland Carbon and Water Exchange and Its Response to Environmental Factors in Arid Northwest China
Abstract
:1. Introduction
2. Materials and Methods
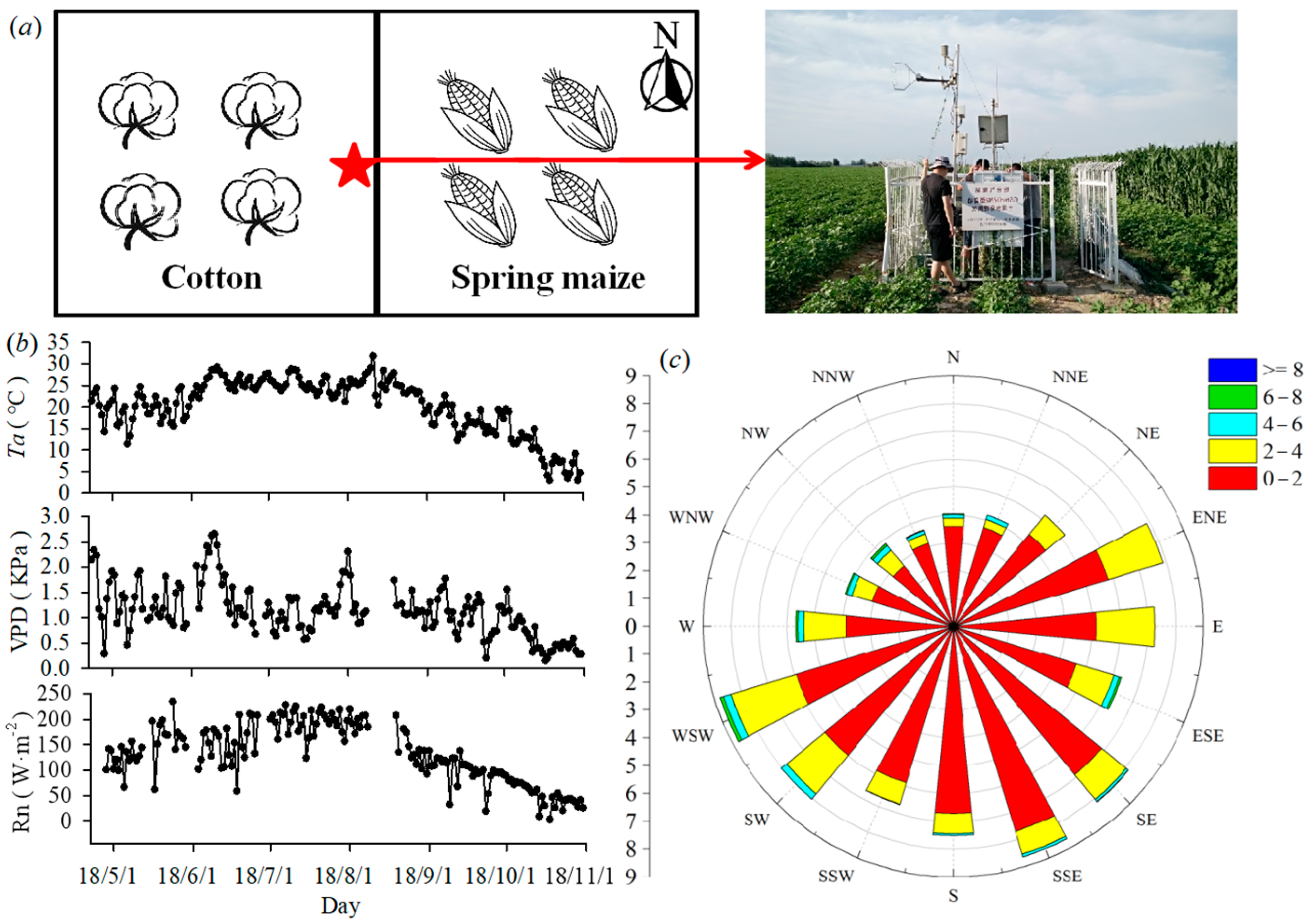
2.1. Site Description
2.2. Experimental Design
2.3. Data Processing
Conventional Meteorological Data
2.4. Statistical Analyses
3. Results
3.1. Monthly Mean Diurnal Variation of Sensible and Latent Heat Fluxes
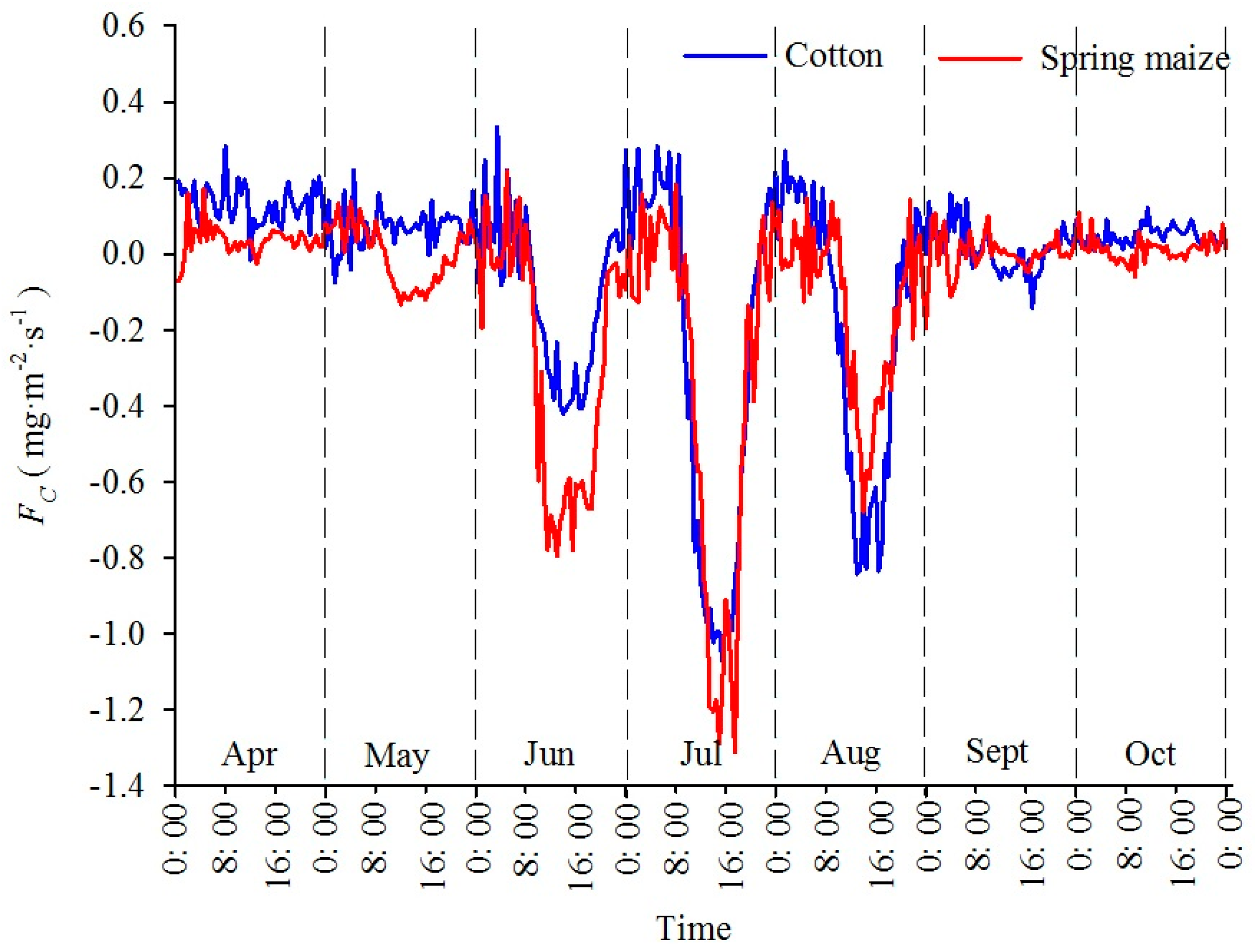
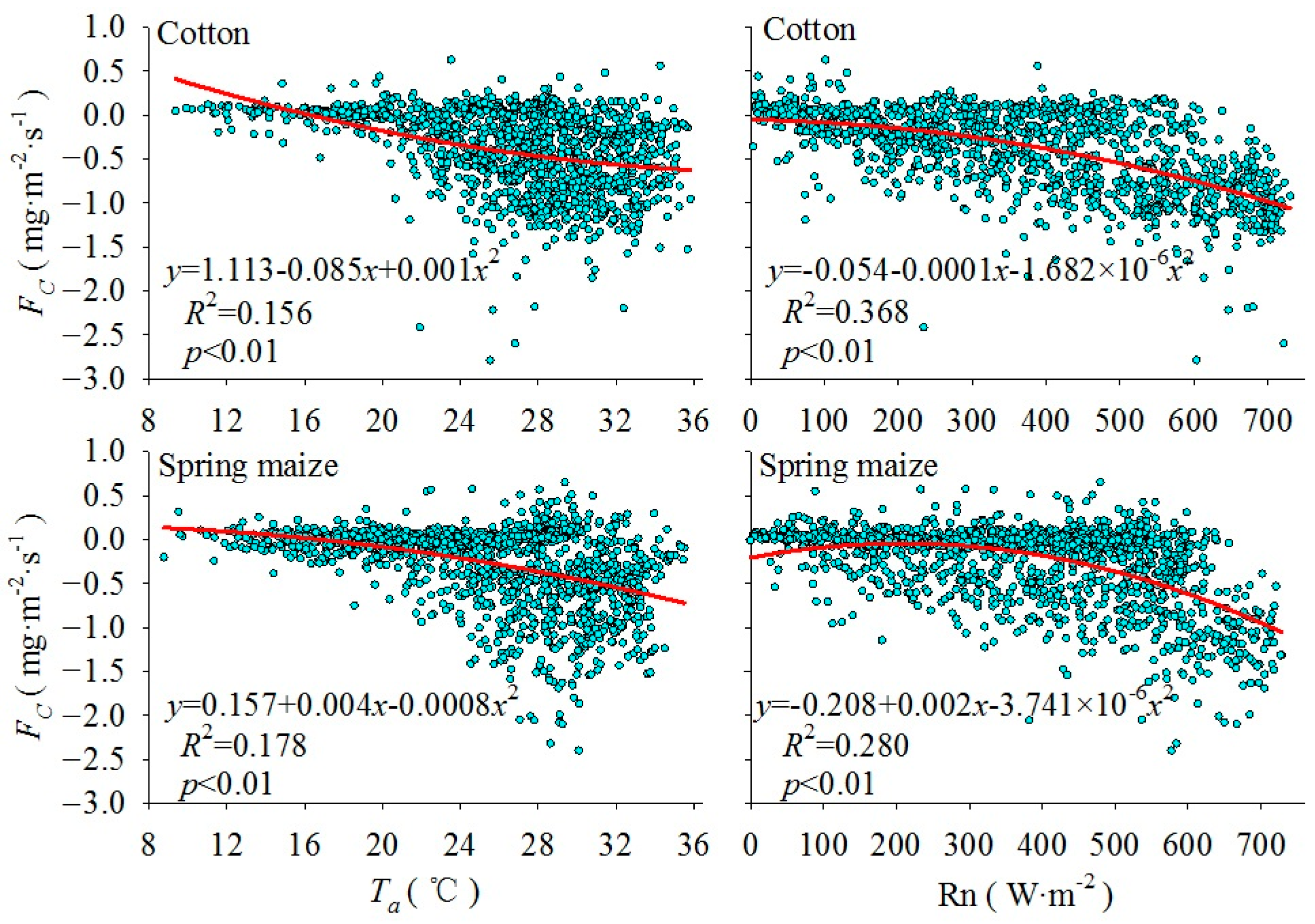
3.2. Monthly Mean Diurnal Variation of CO2 Flux and Its Correlation with Meteorological Factors
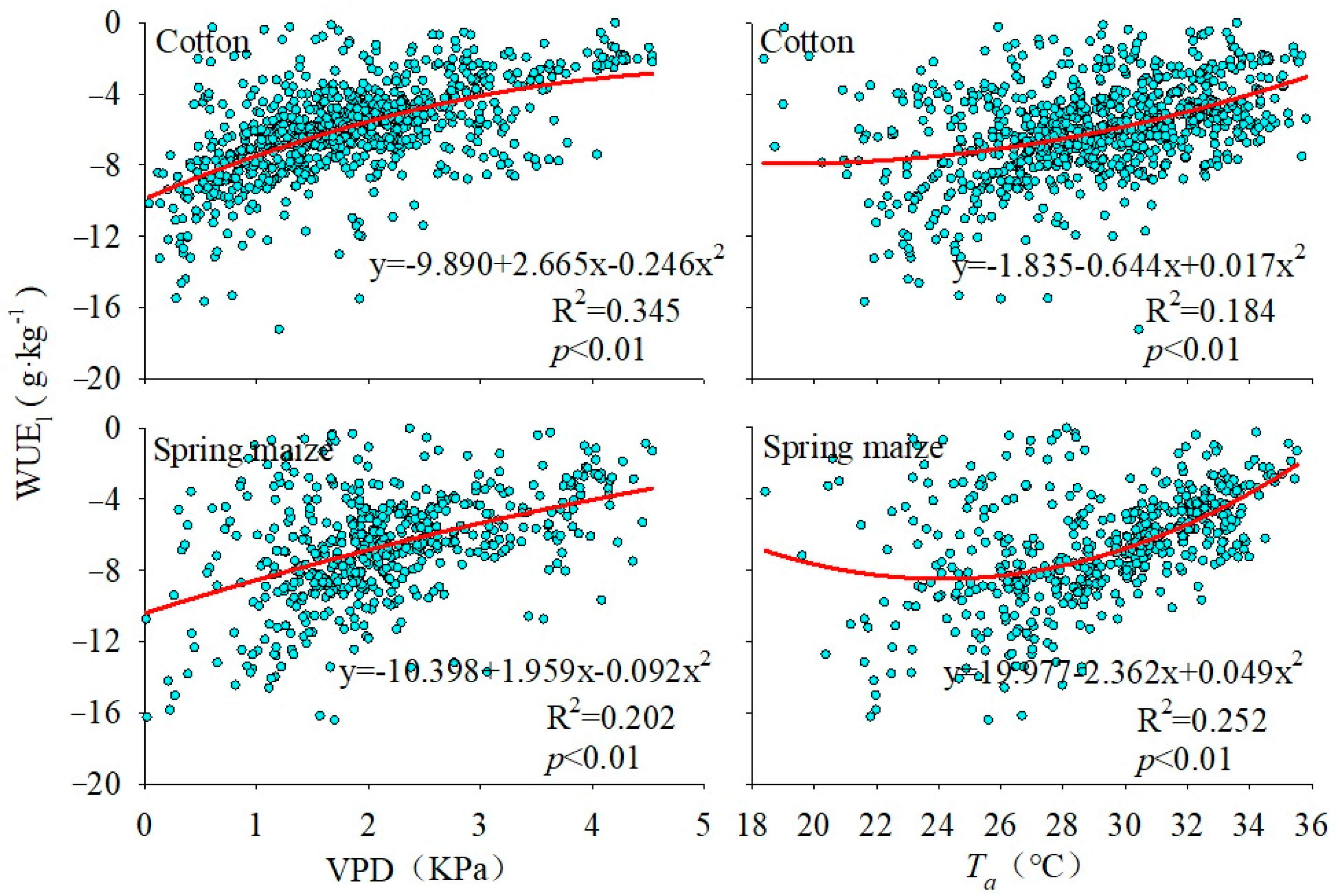
3.3. Monthly Average Daily Variation of Water Vapor Flux and Its Correlation with Meteorological Factors
4. Discussion
| Site | Ecosystem Type | NEE/ (g C·m−2) | Research Period | Source |
|---|---|---|---|---|
| Ulan Wusu, China | Farmland (cotton) | −478.6 | April–October 2009–2010 | [49] |
| Washington, USA | Farmland (wheat) | −261 | 2012–2013 | [50] |
| Nebraska, USA | Farmland (maize) | −590.0 | May–October 2002 | [51] |
| Yingke, China | Farmland (maize) | −626.0 | 2008–2009 | [52] |
| Weishan, China | Farmland (wheat, maize) | −533 ~ −585 | 2006–2008 | [24] |
| Central Oregon, USA | Forest | −534 ~ −415 | 2004–2008 | [40] |
| Northeast China | Grassland | −89.57 | 2014 | [47] |
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Houghton, R.A.; Davidson, E.A.; Woodwell, G.M. Missing sinks, feedbacks, and understanding the role of terrestrial ecosystems in the global carbon balance. Glob. Biogeochem. Cycles 1998, 12, 25–34. [Google Scholar] [CrossRef]
- Rustad, L.E.; Huntington, T.G.; Boone, R.D. Controls on soil respiration: Implications for climate change. Biogeochemistry 2000, 48, 1–6. [Google Scholar] [CrossRef]
- Friedlingstein, P.; Bopp, L.; Ciais, P.; Dufresne, J.-L.; Fairhead, L.; LeTreut, H.; Monfray, P.; Orr, J. Positive feedback between future climate change and the carbon cycle. Geophys. Res. Lett. 2001, 28, 1543–1546. [Google Scholar] [CrossRef]
- Li, C.; Han, Q.; Luo, G.; Zhao, C.; Li, S.; Wang, Y.; Yu, D. Effects of Cropland Conversion and Climate Change on Agrosystem Carbon Balance of China’s Dryland: A Typical Watershed Study. Sustainability 2018, 10, 4508. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, Y.; Sun, W.; Chang, J.; Zhu, J.; Chen, L.; Wang, X.; Guo, Y.; Zhang, H.; Yu, L.; et al. Terrestrial carbon sinks in China and around the world and their contribution to carbon neutrality. Sci. China Life Sci. 2022, 65, 861–895. [Google Scholar] [CrossRef]
- FAO. Land Statistics and Indicators Global, Regional and Country Trends, 2000–2020. FAOSTAT Analytical Brief, 48. 2022. Available online: https://www.fao.org/food-agriculture-statistics/data-release/data-release-detail/zh/c/1599856/ (accessed on 15 July 2022).
- Wang, B.; Li, R.; Wan, Y.; Li, Y.; Cai, W.; Guo, C.; Qin, X.; Song, C.; Wilkes, A. Air warming and CO2 enrichment cause more ammonia volatilization from rice paddies: An OTC field study. Sci. Total Environ. 2020, 752, 142071. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Kang, S.; Li, F.; Zhang, L. Comparison of three evapotranspiration models to Bowen ratio-energy balance method for a vineyard in an arid desert region of northwest China. Agric. For. Meteorol. 2008, 148, 1629–1640. [Google Scholar] [CrossRef]
- Yu, G.R.; Sun, X.M. Principles of Flux Measurement in Terrestrial Ecosystems; Higher Education Press: Beijing, China, 2017; 243p, ISBN 7-04-018401-X. [Google Scholar]
- Li, G.; Zhang, F.; Jing, Y.; Liu, Y.; Sun, G. Response of evapotranspiration to changes in land use and land cover and climate in China during 2001–2013. Sci. Total. Environ. 2017, 596-597, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Baldocchi, D.D. Assessing the eddy covariance technique for evaluating carbon dioxide exchange rates of ecosystems: Past, present and future. Glob. Chang. Biol. 2003, 9, 479–492. [Google Scholar] [CrossRef]
- Yang, F.; Huang, J.; Zheng, X.; Huo, W.; Zhou, C.; Wang, Y.; Han, D.; Gao, J.; Mamtimin, A.; Yang, X.; et al. Evaluation of carbon sink in the Taklimakan Desert based on correction of abnormal negative CO2 flux of IRGASON. Sci. Total Environ. 2022, 838, 155988. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, J.Z.; Li, J.Z.; Chen, F.L.; Feng, P. Review on research and development of carbon and water fluxes charac-teristics in terrestrial ecosystem. J. Earth Environ. 2022, 13, 645–666. [Google Scholar]
- Yan, X.-D.; Li, H.-Y.; Liu, F.; Gao, Z.-Q.; Liu, H.-Z. Modeling of surface flux in Tongyu using the Simple Biosphere Model 2 (SiB2). J. For. Res. 2010, 21, 183–188. [Google Scholar] [CrossRef]
- Jing, Z.; Jing, Y.; Zhang, F.; Qiu, R.; Wido, H. Application of the Simple Biosphere Model 2 (SiB2) with Irrigation Module to a Typical Low-Hilly Red Soil Farmland and the Sensitivity Analysis of Modeled Energy Fluxes in Southern China. Water 2019, 11, 1128. [Google Scholar] [CrossRef]
- Cao, M.; Woodward, F.I. Net primary and ecosystem production and carbon stocks of terrestrial ecosystems and their responses to climate change. Glob. Chang. Biol. 1998, 4, 185–198. [Google Scholar] [CrossRef]
- Wang, Q.; Watanabe, M.; Ouyang, Z. Simulation of water and carbon fluxes using BIOME-BGC model over crops in China. Agric. For. Meteorol. 2005, 131, 209–224. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, Y.; Zhang, W.; Sun, W.; Liu, S.; Jiang, J.; Wu, J.; Yu, W.; Wang, Y.; Yang, Z. Agro-C: A biogeophysical model for simulating the carbon budget of agroecosystems. Agric. For. Meteorol. 2009, 149, 106–129. [Google Scholar] [CrossRef]
- Cui, F.; Zheng, X.; Liu, C.; Wang, K.; Zhou, Z.; Deng, J. Assessing biogeochemical effects and best management practice for a wheat–maize cropping system using the DNDC model. Biogeosciences 2014, 11, 91–107. [Google Scholar] [CrossRef]
- Ouyang, W.; Shan, Y.; Hao, F.; Lin, C. Differences in soil organic carbon dynamics in paddy fields and drylands in northeast China using the CENTURY model. Agric. Ecosyst. Environ. 2014, 194, 38–47. [Google Scholar] [CrossRef]
- Vuichard, N.; Ciais, P.; Viovy, N.; Li, L.; Ceschia, E.; Wattenbach, M.; Bernhofer, C.; Emmel, C.; Grünwald, T.; Jans, W.; et al. Simulating the net ecosystem CO2 exchange and its components over winter wheat cultivation sites across a large climate gradient in Europe using the ORCHIDEE-STICS generic model. Agric. Ecosyst. Environ. 2016, 226, 1–17. [Google Scholar] [CrossRef]
- Osborne, T.; Gornall, J.; Hooker, J.; Williams, K.; Wiltshire, A.; Betts, R.; Wheeler, T. JULES-crop: A parametrisation of crops in the Joint UK Land Environment Simulator. Geosci. Model Dev. 2015, 8, 1139–1155. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, M.P.; Chen, J.N. Progress and perspectives in studies on agro-ecosystem carbon cycle model. Trans. Chin. Soc. Agric. Eng. 2015, 31, 1–9. [Google Scholar] [CrossRef]
- Lei, H.-M.; Yang, D.-W. Seasonal and interannual variations in carbon dioxide exchange over a cropland in the North China Plain. Glob. Chang. Biol. 2009, 16, 2944–2957. [Google Scholar] [CrossRef]
- Yu, G.; Song, X.; Wang, Q.; Liu, Y.; Guan, D.; Yan, J.; Sun, X.; Zhang, L.; Wen, X. Water-use efficiency of forest ecosystems in eastern China and its relations to climatic variables. New Phytol. 2007, 177, 927–937. [Google Scholar] [CrossRef]
- Dufranne, D.; Moureaux, C.; Vancutsem, F.; Bodson, B.; Aubinet, M. Comparison of carbon fluxes, growth and productivity of a winter wheat crop in three contrasting growing seasons. Agric. Ecosyst. Environ. 2011, 141, 133–142. [Google Scholar] [CrossRef]
- Jans, W.W.; Jacobs, C.M.; Kruijt, B.; Elbers, J.A.; Barendse, S.; Moors, E.J. Carbon exchange of a maize (Zea mays L.) crop: Influence of phenology. Agric. Ecosyst. Environ. 2010, 139, 316–324. [Google Scholar] [CrossRef]
- Cao, Y.Y.; Wang, J.; Wang, J.L. Diurnal change characteristics of H2O and CO2 flux of corn farmland ecosystem. Chin. Agric. Sci. Bull. 2016, 32, 137–141. [Google Scholar]
- Cai, X.; Zhang, F.H.; Yang, H.C. Net ecosystem exchange changes of agricultural ecosystem and its influencing factors in Xinjiang. J. Arid. Land Resour. Environ. 2016, 30, 59–64. [Google Scholar]
- Chen, Y. Study on Variation and Estimation Model of Maize Carbon Flux Based on Eddy Covariance in South of Anhui Province. Master’s Thesis, Hefei University of Technology, Hefei, China, 2019. [Google Scholar]
- Li, X.; Liu, L.; Yang, H.; Li, Y. Relationships between carbon fluxes and environmental factors in a drip-irrigated, film-mulched cotton field in arid region. PLoS ONE 2018, 13, e0192467. [Google Scholar] [CrossRef] [PubMed]
- Machado, C.B.; Lima, J.R.d.S.; Antonino, A.C.D.; de Souza, E.S.; Souza, R.M.S.; Alves, E.M. Daily and seasonal patterns of CO2 fluxes and evapotranspiration in maize-grass intercropping. Rev. Bras. De Eng. Agrícola E Ambient. 2016, 20, 777–782. [Google Scholar] [CrossRef]
- Li, C.; Li, Z.; Zhang, F.; Lu, Y.; Duan, C.; Xu, Y. Seasonal dynamics of carbon dioxide and water fluxes in a rice-wheat rotation system in the Yangtze-Huaihe region of China. Agric. Water Manag. 2023, 275, 107992. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, C.; Dong, W.; Li, X.; Zhang, Y.; Qin, S.; Oenema, O. Carbon budget of a winter-wheat and summer-maize rotation cropland in the North China Plain. Agric. Ecosyst. Environ. 2015, 206, 33–45. [Google Scholar] [CrossRef]
- Sun, X.; Chang, Z.; Yang, G.; Xu, X. Characteristics of net ecosystem exchange and environmental factors of rice-wheat rotation system in the Yangtze River delta of China. Chin. J. Eco-Agric. 2015, 23, 803–811. [Google Scholar]
- Sharma, S.; Rajan, N.; Cui, S.; Casey, K.; Ale, S.; Jessup, R.; Maas, S. Seasonal variability of evapotranspiration and carbon exchanges over a biomass sorghum field in the Southern U.S. Great Plains. Biomass Bioenergy 2017, 105, 392–401. [Google Scholar] [CrossRef]
- Chi, J.; Waldo, S.; Pressley, S.; O’keeffe, P.; Huggins, D.; Stöckle, C.; Pan, W.L.; Brooks, E.; Lamb, B. Assessing carbon and water dynamics of no-till and conventional tillage cropping systems in the inland Pacific Northwest US using the eddy covariance method. Agric. For. Meteorol. 2016, 218-219, 37–49. [Google Scholar] [CrossRef]
- Zhan, L.; Yang, H.B.; Lei, H.M. Analysis of corn water consumption, carbon assimilation and ecosystem water use efficiency based on flux observations. Trans. Chin. Soc. Agric. Eng. 2016, 32, 88–93. [Google Scholar]
- Xinjiang Bureau of Statistics 2021. Available online: http://tjj.xinjiang.gov.cn/ (accessed on 1 March 2022).
- Vickers, D.; Thomas, C.K.; Pettijohn, C.; Martin, J.G.; Law, B.E. Five years of carbon fluxes and inherent water-use efficiency at two semi-arid pine forests with different disturbance histories. Tellus B Chem. Phys. Meteorol. 2012, 64, 17159. [Google Scholar] [CrossRef]
- Schotanus, P.; Nieuwstadt, F.T.M.; De Bruin, H.R. Temperature measurement with a sonic anemometer and its application to heat and moisture fluxes. Boundary-Layer Meteorol. 1983, 26, 81–93. [Google Scholar] [CrossRef]
- Wilczak, J.M.; Oncley, S.P.; Stage, S.A. Sonic Anemometer Tilt Correction Algorithms. Boundary-Layer Meteorol. 2001, 99, 127–150. [Google Scholar] [CrossRef]
- Moore, C.J. Frequency response corrections for eddy correlation systems. Boundary-Layer Meteorol. 1986, 37, 17–35. [Google Scholar] [CrossRef]
- Webb, E.K.; Pearman, G.I.; Leuning, R. Correction of flux measurements for density effects due to heat and water vapour transfer. Q. J. R. Meteorol. Soc. 1980, 106, 85–100. [Google Scholar] [CrossRef]
- Hibberd, J.M.; Sheehy, J.E.; Langdale, J.A. Using C4 photosynthesis to increase the yield of rice—Rationale and feasibility. Curr. Opin. Plant Biol. 2008, 11, 228–231. [Google Scholar] [CrossRef]
- Li, J.; Yu, Q.; Sun, X.; Tong, X.; Ren, C.; Wang, J.; Liu, E.; Zhu, Z.; Yu, G. Carbon dioxide exchange and the mechanism of environmental control in a farmland ecosystem in North China Plain. Sci. China Ser. D Earth Sci. 2006, 49, 226–240. [Google Scholar] [CrossRef]
- Sun, B.; Zhao, H.; Lu, F.; Wang, X. Spatial and temporal patterns of carbon sequestration in the Northeastern Forest Regions and its impact factors analysis. Acta Ecol. Sin. 2018, 38, 4975–4983. [Google Scholar] [CrossRef]
- Qin, W.; Hu, C.; Oenema, O. Soil mulching significantly enhances yields and water and nitrogen use efficiencies of maize and wheat: A meta-analysis. Sci. Rep. 2015, 5, 16210. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bai, J.; Chen, X.; Luo, G.; Wang, S. Carbon fluxes in cotton field with plastic mulched drip irrigation in Xinjiang Oasis. Trans. Chin. Soc. Agric. Mach. 2015, 46, 70–78+136. [Google Scholar] [CrossRef]
- Waldo, S.; Chi, J.; Pressley, S.N.; O’keeffe, P.; Pan, W.L.; Brooks, E.S.; Huggins, D.R.; Stöckle, C.O.; Lamb, B.K. Assessing carbon dynamics at high and low rainfall agricultural sites in the inland Pacific Northwest US using the eddy covariance method. Agric. For. Meteorol. 2016, 218-219, 25–36. [Google Scholar] [CrossRef]
- Aubinet, M.; Moureaux, C.; Bodson, B.; Dufranne, D.; Heinesch, B.; Suleau, M.; Vancutsem, F.; Vilret, A. Carbon sequestration by a crop over a 4-year sugar beet/winter wheat/seed potato/winter wheat rotation cycle. Agric. For. Meteorol. 2009, 149, 407–418. [Google Scholar] [CrossRef]
- Wang, X.; Ma, M.; Huang, G.; Veroustraete, F.; Zhang, Z.; Song, Y.; Tan, J. Vegetation primary production estimation at maize and alpine meadow over the Heihe River Basin, China. Int. J. Appl. Earth Obs. Geoinf. 2012, 17, 94–101. [Google Scholar] [CrossRef]


| Cotton | Spring Maize | ||||||
|---|---|---|---|---|---|---|---|
| Growth Period | Main Period | Plant Height (cm) | Irrigation Volume (m3·hm−2) | Growth Period | Main Period | Plant Height (cm) | Irrigation Volume (m3·hm−2) |
| Sowing | Mid-April ~ Late April | - | 675 | Sowing | Early April ~ Mid-April | - | 600 |
| Seedling | Early May ~ Early June | 28 | - | Seedling | Late April ~ Early May | 36 | - |
| Budding | Mid-June ~ Late June | 50 | 1350 | Jointing | Late May ~ Mid-June | 106 | 1550 |
| Flowering and boll | Early July ~ Late Aug | 63 | - | Tasseing stage | Late June ~ Mid-July | 221 | 1700 |
| Boll opening | Early Sept ~ Late Sept | 66 | 2100 | Milky maturation stage | Late July ~ Early Aug | 230 | 1850 |
| Maturity | Mid-Aug ~ Late Aug | 230 | - | ||||
| Observation Systems | Sensors | Observation Variables | Height/Depth (m) | Acquisition Frequency |
|---|---|---|---|---|
| The eddy covariance system | Three–dimensional ultrasonic anemometer (CSAT3, Campbell Scientific Inc., Logan, UT, USA) | Three-dimensional wind speed and direction, Ta, RH, CO2/H2O concentration, H, LE, Fc, FH | 3.0 | 10 Hz |
| Open–path infrared gas analyzer (LI-7500A, LI-COR Inc., Lincoln, NE, USA) | ||||
| The environmental element observation system | Four-component radiometer (CNR4, Kipp and Zonen B. V., Delft, The Netherlands) | DR, UR, DLR, ULR, Rn | 1.5 | 1 Hz |
| Soil temperature sensors (109, Campbell Scientific Inc., UT, USA) | Soil temperature | 0, −0.05 | ||
| Soil humidity sensor (Hydra 93640, Stevens Inc., Portland, OR, USA) | Soil moisture | −0.05 | ||
| Soil heat flux plate (HFP01SC, Hukseflux, Inc., Delft, The Netherlands) | Soil heat flux | −0.05 |
| Ta | VPD | Rn | |
|---|---|---|---|
| FC of Cotton | −0.387 * | 0.083 | −0.592 ** |
| FC of Spring maize | −0.417 ** | −0.127 | −0.456 ** |
| Ta | VPD | Rn | |
|---|---|---|---|
| WUEl of Cotton | 0.418 * | 0.577 ** | −0.039 |
| WUEl of Spring maize | 0.456 ** | 0.449 * | 0.077 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Yang, F.; Mamtimin, A.; Huo, X.; Gao, J.; Ji, C.; Abudukade, S.; Li, C.; Sun, Y.; Wang, W.; et al. Farmland Carbon and Water Exchange and Its Response to Environmental Factors in Arid Northwest China. Land 2023, 12, 1988. https://doi.org/10.3390/land12111988
Zheng X, Yang F, Mamtimin A, Huo X, Gao J, Ji C, Abudukade S, Li C, Sun Y, Wang W, et al. Farmland Carbon and Water Exchange and Its Response to Environmental Factors in Arid Northwest China. Land. 2023; 12(11):1988. https://doi.org/10.3390/land12111988
Chicago/Turabian StyleZheng, Xinqian, Fan Yang, Ali Mamtimin, Xunguo Huo, Jiacheng Gao, Chunrong Ji, Silalan Abudukade, Chaofan Li, Yingwei Sun, Wenbiao Wang, and et al. 2023. "Farmland Carbon and Water Exchange and Its Response to Environmental Factors in Arid Northwest China" Land 12, no. 11: 1988. https://doi.org/10.3390/land12111988
APA StyleZheng, X., Yang, F., Mamtimin, A., Huo, X., Gao, J., Ji, C., Abudukade, S., Li, C., Sun, Y., Wang, W., Cui, Z., Wang, Y., Ma, M., Huo, W., Zhou, C., & Yang, X. (2023). Farmland Carbon and Water Exchange and Its Response to Environmental Factors in Arid Northwest China. Land, 12(11), 1988. https://doi.org/10.3390/land12111988








