Abstract
The application of superabsorbents to soils and seed coatings is a pre-sowing seed treatment method that is commonly used to improve early vigor and establish stability and uniformity under water deficit conditions. To evaluate the interaction of seed coating and superabsorbent on Calotropisprocera L. (milkweed) under water deficit conditions, a greenhouse experiment was conducted. The experiment was conducted with four coating material levels (non-coated seeds and seeds coated with peat moss, vermicompost, and canola residue), four growth medium levels (soil, sand + soil, soil + 2 g superabsorbent, and soil + 4 g superabsorbent), and three field capacity regimes (25, 50%, and 100%) in a completely randomized design factorial arrangement with four replications. Reducing the field capacity from 100 to 25% led to decreased growth (shoot and root dry weights and leaf area) and chlorophyll content. The activities of SOD, CAT, APX antioxidant enzymes, and proline increased under drought stress. The use of superabsorbent polymers in growth media enhanced growth indices and chlorophyll content and decreased the activity of antioxidant enzymes and proline under water deficit conditions. The highest chlorophyll and growth indices were observed when 4 g of superabsorbent was added to the growth medium under drought stress. The application of 4 g of superabsorbent to the growth medium reduced the activity of antioxidant enzymes and proline. The use of seed coatings improved the growth indices, antioxidant enzyme activity, and chlorophyll content under drought stress. The most adaptive morphological and physiological responses to water stress were observed in the vermicompost-coated seeds. The vermicompost coating containing a superabsorbent polymer (4 g/kg soil) proved to be the best for establishing milkweed under mild (50% FC) and severe water deficits (25% FC).
1. Introduction
The significance of the rehabilitation and development of rangelands has recently been highlighted because of the increasing trend of their degradation under the effects of human factors and climate change, such as reduced rainfall [1,2,3,4]. In this regard, seeding projects and the application of superabsorbent polymers are of paramount importance for regenerating rangelands. Therefore, a great deal of time and money are spent on rangeland seeding projects [5,6]. Nevertheless, various factors, such as soil erosion and climate change, including increased average temperature and environmental stresses, particularly drought, can decrease seed emergence percentage in rangelands [7,8]. Iran is located in the Northern Hemisphere, between 46° and 64° E [2]. The geographical location, rainfall regime, and temperature conditions, as well as the annual rainfall of approximately 250 to 300 mm [1], make our country (Iran) one of the arid and semi-arid regions in the world.
Drought and water deficit are among the most significant factors limiting crop production worldwide. The most sensitive stages of plant development to drought stress are the germination and seedling stages [9,10,11,12,13,14]. In this context, decreased seed emergence percentage and seedling establishment in lentils (Lens culinaris Medik.), walnut (Juglansregia L.), and maize (Zeamays L.) under drought stress conditions have been reported [15,16,17]. Dryland soils are characterized by low organic matter content, low water-holding capacity, low infiltration, and low fertility, which lowers the percentage of seedling establishment due to low water maintenance. One way to improve seedling emergence and early seedling development is to use seed coating fillers [18] to increase the water stored in the soil for a longer period [19]. Organic materials, such as peat moss and vermicompost, can be used as soil water stores to increase the moisture around planted seeds.
Hence, it is of paramount importance to apply methods such as seed coating with organic material to reduce the effects of water stress and enhance the seed emergence percentage [20]. Some studies have reported increased seed germination uniformity and speed, as well as seedling establishment, under salinity and drought stress conditions using the seed coating method in rice, barley, rye, and wheat [21,22].
In addition, the use of superabsorbent polymers for soil amendment may improve moisture availability for germinating seeds and during later growth stages under water deficit conditions. Superabsorbent polymers are hydrophilic polymer gels that can absorb an amount of water many times their size [23]. After absorption, owing to the drying environment, water inside the polymer is gradually released, and the rhizosphere soil is kept wet for a long time without re-watering [24,25]. In this context, the use of superabsorbent polymers in soil significantly improved the germination of corn (Zea mays L.), pearl millet (Pennisetum glaucum), and oat (Avena sativa L.) seeds under drought stress [26,27,28].
Calotropisprocera L. (milkweed), which belongs to the family Apocynaceae, is an evergreen and perennial plant. This species is mostly distributed from the hot and desert areas of Southwest Asia and the Mediterranean region to the shores of Africa and southern Iran [29]. Milkweed is a unique species that plays an important role in the restoration of degraded land in arid and desert regions. Milkweed also has industrial and medicinal properties and is used as an anti-rheumatic, anti-diarrheal, and anti-fungal anticancer agent [30]. Milkweed plants are very important in revitalizing pastures and the pharmaceutical industry. This plant is propagated only by seeds. Milkweed produces many seeds, but its density is very low in arid regions. Its seeds are exposed to drought stress during the germination stage in spring, leading to a very low percentage of germination and seedling establishment because its seeds are sensitive to water stress during germination [31]. Little information is available regarding the use of a growing medium with superabsorbents or the use of organic fillers to reduce water stress and increase the percentage of plant establishment in arid lands. Our hypothesis is that the decrease in green percentage in the pasture is due to the type of growing medium or soil; that is, the percentage of silt is high, and the percentage of organic matter is very low, which cannot absorb a significant amount of water from accidental spring rain. Furthermore, the use of superabsorbent and organic matter can increase the absorption of spring rain; that is, the application of superabsorbent material can help the soil absorb a significant amount of rainwater, thus allowing it to provide water to the plant for a longer period of time, thereby reducing water stress during the emergence stage.
On the other hand, the use of organic material as a filler or seed cover can improve the amount of water absorption and retention time around the seed and increase the percentage of this plant’s establishment in early spring. Hence, the aim of this study was to investigate the effects of the addition of superabsorbent polymers to growth media and the use of different types of organic matter seed coatings to help improve the early growth and establishment of milkweed under water deficit conditions.
2. Materials and Methods
2.1. Collection and Preparation of Seeds Coat
Five hundred fresh capsules of Calotropis procera L. were collected in August 2022 from one of its natural habitats in the rangelands of Sarbaz (61°25′ E and 26°63′ N, 892 m asl), Sistan, Iran. The environment consists of a degraded pasture with scattered cover of Calotropis procera L. trees and silty clay loam soils. One hundred milkweed fruits were dried, and the seeds were removed from the capsules and cleaned. Dried, uniform seeds were selected and disinfected in a carboxin–thiram solution (2 g L−1) for 2 min.
After preparing the seeds, three organic compounds of peat moss, vermicompost, and canola residue were dried and sterilized at 100 °C for 30 min in an oven and then ground by a mill passed through a sieve with a 1 mm mesh (in this experiment, vermicompost and peat moss were prepared by Kian Pars Company, Teheran, Iran, and Miracle-Gro Company, Marysville, Ohio, USA, respectively, and canola residue was prepared by Shiraz University, Iran).
Subsequently, 2 g of each seed coating material was injected into a plastic half-circle (with a diameter of 5 cm), and the molds were dried at room temperature. They were maintained in a sterile environment until the beginning of the experiment (Figure 1). It should be noted that in the middle of one of the molds, there was a 4 mm deep hole for placing seeds. At the time of planting, two seeds were placed between the two halves of the molds and were planted in 5 L volume pots filled with growth medium “based on experimental treatments” (with one hole drilled in the bottom of each pot for drainage) (Figure 1), and one of them was removed. To apply the water stress, the field capacity of the soil was determined.
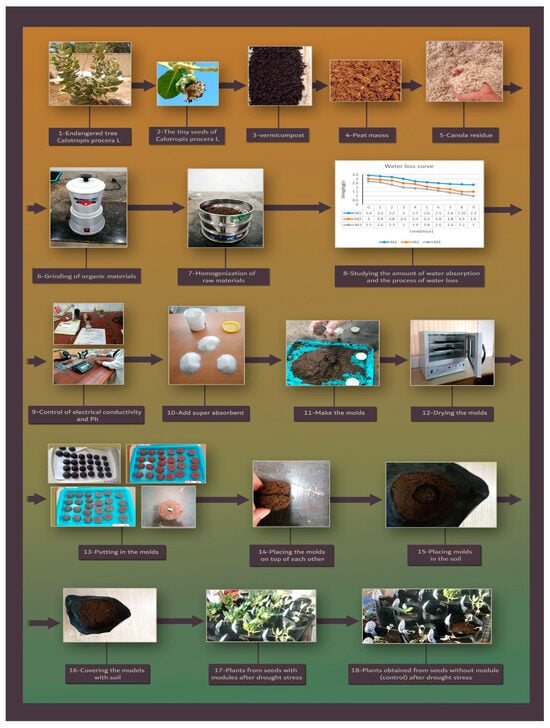
Figure 1.
The steps of preparing seed coating.
The physicochemical features of the soils used in this study are listed in Table 1.

Table 1.
Some of the physical and chemical properties of soil used in experiment.
After planting the seeds, the pots were placed in a greenhouse with 16/8 h day/night length at 25/15 °C day/night temperatures for a period of 20 days before the beginning of treatments.
2.2. Experimental Detail
This experiment was performed at the Research Greenhouse of the Department of Crop Production and Plant Breeding, School of Agriculture, Shiraz University, Shiraz, Iran (52°32′ E and 29°36′ N, 1810 m asl) in a completely randomized factorial design including four coating material levels (control, peat moss, vermicompost, and canola residue), four growth media levels (soil (S1), sand + soil 1:1 ratio (S2), 2 g superabsorbent per kg soil (S3), 4 g superabsorbent per kg soil (S4)), and irrigation at three levels (25, 50, and 100% field capacity) with four replicates. F1 superabsorbent polymer (a cationic amphoteric surfactant, as white crystalline fine powder (potassium polyacrylate), produced by the German Stockosorb® company, Essen, Germany) ISO 14001:2005 [32] was used in this experiment. The chemical characteristics of vermicompost, peat moss, and canola residues are listed in Table 2. Seeds coated with organic matter (peat moss, vermicompost, and canola residue) were sown in culture media. After 15 days of sowing, water deficit treatments (25, 50, and 100% of the field capacity) were carried out for three months and then the plants were harvested to determine indices. Every alternate day, to apply the field capacity, the pots were weighed, water treatments were applied to the weight base, and water was added to achieve the target soil moisture. The plants were harvested three months after the imposition of water deficit to record morphological and physiological parameters.

Table 2.
Some chemical characteristics of vermicompost, peat moss, and canola residue used.
2.3. Measurements
2.3.1. Plant Morphology
At the end of the experiment, shoots and roots of the harvested plants were separated and placed in an oven (model no. ODWF24-SD) at 70 °C until a constant weight was recorded for shoot and dry weights. Leaves were separated from the harvested plants, and leaf area was measured using a leaf area meter (LI-3000C, LI-COR, Lincoln, Nebraska).
2.3.2. Leaf Free Proline Content
The leaf free proline content was determined as described by Bates et al. (1973) [33]. Fresh leaf samples (0.5 g) were mixed with 10 mL of a 3% sulfosalicylic acid solution. Two milliliters of the extract was mixed with 2 mL ninhydrin acid reagent (2, 2- dihydroxyindane-1,3-dione) and 2 mL acetic acid, and the mixture was incubated at 100 °C for 60 min. After incubation, the mixture was placed in a water bath, 4 mL of toluene was added, and the absorbance was read at 520 nm using a spectrophotometer (Biowave II model, UK). The leaf free proline content was determined from the standard curve.
2.3.3. Leaf Chlorophyll Content
Fresh leaf samples (1 g) were crushed with 15 mL 80% acetone, and the supernatant was collected after centrifugation at 3000 rpm for 15 min and diluted to 25 mL using 80% acetone. The absorption was measured using a spectrophotometer (20–120-UV model, Japan) at 645 and 663 nm. The chlorophyll content was calculated using the following equation:
where A = absorption at 645 nm, B = absorption at 663 nm, V = final volume of the extract and acetone, and W = fresh leaf weight.
Chlorophyll content (mg g−1 FW) = [20.2 (A) + 8.02 (B) × V/(W × 1000)]
2.3.4. Activity of Enzymatic Antioxidant
Briefly, fresh leaf (0.5 g) samples were ground with liquid nitrogen in a Chinese mortar; 1 mL of 50 mM phosphate buffer containing 0.5 M EDTA polyvinyl and 2% polypyrrolidone was added to the extracted tissue and centrifuged at 14,000× g for 20 min at 4 °C [34].
The supernatant was used to measure ascorbate peroxidase (APX), catalase (CAT), and superoxide dismutase (SOD). APX activity was estimated as described by Nakano and Asada (1981) [35], CAT activity was determined following Beauchamp and Fridovich (1971) [36], and SOD activity was estimated following McCord and Fridovitch (1969) [37].
2.4. Statistical Analysis
Prior to analysis, data population normality was verified using the Kolmogorov–Smirnov and Shapiro–Wilk tests in SPSS (10.0) software (Table 3). For the kind of organic mater and superabsorbent amount experiment, the collected data were analyzed by analysis of variance using ‘SAS’ v. 9.4. The least significant difference (LSD) test was used for mean separation at p ≤ 0.05.

Table 3.
Data normality is determined by Kolmogorov–Smirnov and Shapiro–Wilk tests.
3. Results and Discussion
The ANOVA results (see table below) showed that the main effects of seed coating, growth media, and irrigation significantly influenced the measured traits. In addition, the interactions of seed coating × irrigation and growth media × irrigation had a significant effect on the measured traits at the 1% probability level. The interactions of seed coating × growth medium and seed coating × growth medium × irrigation had no significant effect on the measured traits (Table 4).

Table 4.
Analysis of variance of the effect of seed coating, growth media, and irrigation and their interaction on the measured traits in milkweed.
3.1. Emergence Percentage, Leaf Area, Specific Leaf Weight, Shoot and Root Dry Weights, and Root/Shoot Dry Weights
Drought is one of the most important growth-limiting factors that reduces plant growth. Plant injury occurs under water shortages during any vegetative growth stage [38]. Impaired mitotic division due to reduced turgor pressure has been reported to reduce growth indices under drought stress. When there is a shortage of water in the soil, the water flow from the roots to the cells declines, leading to reduced cell division and elongation, resulting in decreased growth traits in the plant [39]. Another reason for the decreased growth indices in plants is the closed stomata, which reduce water loss under drought stress. Under these conditions, CO2 uptake by stomata and dry matter production are reduced [40,41,42].
Our findings showed that reducing the field capacity from 100 to 25% led to decreased shoot and root dry weight, leaf area, specific leaf weight, and root/shoot dry weight in milkweed compared to 100% FC, leaf area, root dry weight, and shoot dry weight, which, respectively, decreased by 2.82-, 1.99-, and 2.27-fold at 25% of field capacity (Table 5). Similarly, the effects of drought on morphological traits have been reported in many experiments on crops such as apples and sour cherries [43,44].

Table 5.
Effect of interaction of coat and media, coat and irrigation, media and irrigation on leaf area and shoot and root dry weights of milkweed under drought stress. S1 = soil, S2 = sand + soil 1:1 ratio, S3 = 2 g superabsorbent per kg soil, S4 = 4 g superabsorbent per kg soil (S3), and FC = field capacity.
According to our results, the highest emergence percentage, leaf area, specific leaf weight, shoot and root dry weights, and root/shoot dry weights were observed in S4 media, indicating that, in this study, the use of superabsorbent in growth medium enhanced growth indices under drought stress (Table 5). In the S4 growth medium, leaf area and shoot and root dry weights increased by 1.09-, 1.02-, and 1.04-fold compared to the control (Table 5).
It has been reported that superabsorbents may absorb water several times their own weight, providing water to plants under drought stress [45]. The consequences of soil moisture variability during plant establishment might be reduced by increasing the soil water-holding capacity (Patra et al., 2019). The use of hydrogel “superabsorbent polymers” (SAPs) to increase topsoil water-holding capacity (WHC) is a suitable management tool that absorbs a significant amount of water and improves the ability of the topsoil to store water. Therefore, plants exhibit a better growth performance under drought stress conditions. Patra et al. (2022) stated that superabsorbents reduced drought stress and enhanced dry matter production in bean [46].
Our findings showed that using a seed coat (peat moss, vermicompost, and canola residue) improved leaf area and shoot and root dry weights under drought stress, and the highest leaf area and shoot and root dry weights were achieved in the seeds coated with vermicompost, peat moss, and canola residue (Table 5). Compared to uncoated seeds, leaf area and shoot and root dry weights increased by 1.05-, 1.03-, and 1.04-fold in the vermicompost seed coating (Table 5).
Organic materials such as vermicompost have a strong water-holding capacity and ensure long-term plant water availability, in addition to increasing nutrient absorption by plants. Therefore, the use of organic matter as a seed coat helps plants to access water and nutrients under drought stress [47]. In agreement with our results, using seeds coated with organic matter enhanced the germination percentage and growth indices of wheat seedlings under drought stress [48].
3.2. Free Leaf Proline Content
One of the common responses of plants to mitigating drought stress is the production and accumulation of organic solutes, known as compatible solutes, such as proline [49]. Proline is believed to be one of the most important compatible solutes that plays a vital role in osmotic adjustment in plants subjected to drought stress [50]. Proline protects plants from drought by contributing to cellular osmotic adjustment, protection of membrane integrity, enzyme or protein stabilization, and reactive oxygen species [51].
Our results showed that reduced field capacity led to the accumulation of proline content in plants, with the highest proline content observed in 25% FC, i.e., 4.36-fold higher than that in the control plants (Table 6). Proline accumulation under drought stress has been reported in rose and coffee plants, consistent with our results. Previous studies have shown that proline accumulation is necessary to reduce the destructive effects of drought stress [50,51].

Table 6.
Effect of different seed coating materials and growth media and their interaction on chlorophyll and proline contents of milkweed under drought stress.
The results showed that the highest proline accumulation was observed in the S2, S1, S3, and S4 growth media (Table 6). Proline accumulation in S2 media increased by 1.16-fold compared to that in the control (Table 6). Compared to other media, the S2 growth medium had a lighter texture owing to its sand. Thus, this condition had a lower ability to maintain water, and its plants experienced drought stress earlier than those in other growth media [52].
Accordingly, in this media (S2), proline accumulation in plants was higher. Proline accumulation in Hammada salicornica in sandy soils has been reported [53,54]. Our results showed the highest proline content in the control and in seeds coated with canola residue, peat moss, and vermicompost (Table 6). Hence, in the present study, seeds coated with three types of organic matter showed the lowest proline accumulation. It seems that, because of their ability to maintain water, coating seeds with organic matter causes them to experience less drought stress; hence, they have less proline accumulation [55].
3.3. Leaf Chlorophyll Content
Chlorophyll is one of the major chloroplast components for photosynthesis, and the relative chlorophyll content has a positive relationship with the photosynthetic rate. The decline in chlorophyll content under drought stress is considered a typical symptom of oxidative stress and may result from pigment photooxidation and chlorophyll degradation [39].
Our results showed that the reduction in field capacity from 100% to 25% resulted in decreased chlorophyll content in the milkweed, such that the lowest chlorophyll content was observed at 25% FC, i.e., a 2.65-fold decrease compared to the control (Table 6). In this regard, the chlorophyll content decreased significantly at higher water deficits in Catharanthusroseus L. [56], Helianthusannuus L. [57], and Vacciniummyrtillus L. [58].
According to the results, the highest chlorophyll content was obtained in S4, S3, S1, and S2 growth media, indicating an increase of 1.02-fold in chlorophyll content in S4 growth media compared to the control (Table 6). Furthermore, the S2 growth medium showed the lowest chlorophyll content, a 1.07-fold decline compared to the control (Table 6).
Our results showed that the application of a superabsorbent could help preserve chlorophyll under drought stress. It has been reported that because of its water retention, adding a superabsorbent to soil may prevent chlorophyll degradation under drought stress [23]. In media containing superabsorbents, increased chlorophyll accumulation has been reported in Acaciavictoriae L. and Cynodondactylon L. plants under drought stress [23,59,60]. In addition, the lowest chlorophyll content was observed in S2 growth media (Table 6). The amount of sand in this medium is the reason for this phenomenon, as the plant is exposed to less stress than in other growth media [52].
Our results showed the highest chlorophyll content in seeds coated with vermicompost, peat moss, and canola residue and the control (Table 6). The chlorophyll content in seeds coated with vermicompost, peat moss, and canola residue increased by 1.02-, 1.01-, and 1.006-fold, respectively, compared with the control plants (Table 6). In the present study, coating seeds with the three types of organic matter appeared to prevent chlorophyll degradation under drought stress. Enhanced chlorophyll content under drought stress has been reported in indica rice and bread wheat plants [21,61].
3.4. Activity of Enzymatic Antioxidant
Exposure of plants to water deficit conditions increases the production of reactive oxygen species (ROS), such as hydroxyl radicals and superoxide [62]. These ROS may initiate destructive oxidative processes such as lipid peroxidation, chlorophyll bleaching, protein oxidation, and damage to nucleic acids. Therefore, according to [61], ROS scavenging is the most important defense mechanism for plants to cope with stressful conditions. However, antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX) play key roles in scavenging activated species [63]. Modulation of the activity of these enzymes may be an important factor in the tolerance of various plants to drought stress [64].
The results indicated that reducing the field capacity from 100% to 25% enhanced the activity of SOD, CAT, and APX enzymes in milkweed; in 25% FC, the activity of SOD, CAT, and APX enzymes increased 4.55-, 2.99-, and 1.57-fold compared to the control (Table 7). Increased activity of antioxidant enzymes in different plants under drought stress has been reported in many studies. Sales et al. (2013) reported increased activity of APX and SOD enzymes in sugarcane under water-shortage conditions [65]. Increased activities of SOD, CAT, and APX enzymes under drought stress have been shown in Amaranthustricolor L. and Pyruscommunis L. plants [66,67].

Table 7.
Effect of different seed coating materials and growth media and their interaction on activity of superoxide dismutase, catalase, and ascorbate peroxidase enzymes of milkweed under drought stress.
In our study, the accumulation of antioxidant enzymes appeared to reduce damage caused by ROS production under drought stress [65]. The highest activities of SOD, CAT, and APX enzymes were observed in S2, S1, S3, and S4 growth media (Table 7). Compared with the control, the highest activities of SOD, CAT, and APX enzymes in the S2 growth media were 1.11-, 1.27-, and 1.01-fold greater, respectively (Table 7). Based on these results, the higher ratio of sand in S2 growth media was the reason for the increased activity of enzymes in this media; thus, in this media, the plants experienced drought stress earlier than in other media employed in the experiment [68].
The results showed that, compared to the control, the application of superabsorbent in soil reduced the activity of SOD, CAT, and APX enzymes (Table 7). This phenomenon is due to the ability of a superabsorbent to maintain water in the environment, providing more water to plants and reducing the severity of drought stress [69]. In line with our results, reduced activity of POD and CAT enzymes in Acaciavictoriae L. and SOD, CAT, POD, and APX enzymes in Avenasativa L. under the application of superabsorbents in soil has been reported under drought stress [28,59,60].
Our findings indicate that, compared to the control, the highest activities of SOD, CAT, and APX enzymes were observed in seeds coated with vermicompost, peat moss, and canola residue, respectively (Table 7). In addition, compared with the control, the activities of SOD, CAT, and APX enzymes in seeds coated with vermicompost increased 1.05-, 1.23-, and 1.01-fold, respectively (Table 7).
In this study, seed coating enhanced enzyme activity. Seed coating increases plant access to nutrients and water, contributing to increased enzyme activity to eliminate free radicals under drought stress. Enhanced antioxidant enzyme activity in rice and wheat plants has been reported under stress conditions [61,70].
4. Conclusions
Our findings showed that drought led to reduced growth indices in milkweed. During the emergence stage, the plant faces a lack of moisture because the soil in which it grows cannot absorb a significant amount of spring rain and store it for a long time. The use of superabsorbents increases soil moisture and improves seedling growth in the emergence stage. In contrast, proline and antioxidant enzyme activities increase as defence mechanisms to reduce the destructive effects of drought stress. The application of organic seed carriers improved the growth indices under drought stress, especially leaf surface, leaf specific weight, and root-to-shoot ratio. The application of superabsorbents with coatings made with organic seed carriers decreased proline accumulation and enzyme activity under drought stress owing to improved plant access to water. Among the growth media treatments, the application of 4 g of superabsorbent to coatings made with vermicompost exhibited the best physiological and morphological performance under drought conditions. Seed coating with organic carriers improved growth indices and enhanced enzyme activity and chlorophyll content under drought stress. Among the seed coatings employed in the experiment, the vermicompost coating was superior to other coatings under drought conditions. Generally, seed coating with vermicompost, together with the use of a superabsorbent polymer (4 g per kg soil), was the best to establish milkweed under mild (50% FC) and severe water deficits (25% FC). Therefore, to increase the success rate in the establishment of this plant, instead of directly planting the seeds, the farmer can put the seeds of this plant inside seed balls made of vermicompost and superabsorbent and then plant them in the soil.
Author Contributions
Conceptualization, M.T. and M.D.; methodology, M.T. and M.D.; formal analysis, M.T. and M.D.; data curation, M.T., M.D., and A.M.; writing—original draft preparation, M.T.; writing—review and editing, M.T. and A.M.; supervision, M.T.; project administration, M.T.; funding acquisition, A.M. All authors read and agreed to the published version of the manuscript.
Funding
This study did not receive any external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the corresponding author upon request.
Acknowledgments
We would like to express our special thanks to the Department of Plant Production and Genetics, School of Agriculture, Shiraz University, and the University of Brescia for the initial financial support provided to conduct this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aghajanlou, F.; Mirdavoudi, H.; Shojaee, M.; Mac Sweeney, E.; Mastinu, A.; Moradi, P. Rangeland Management and Ecological Adaptation Analysis Model for Astragalus curvirostris Boiss. Horticulturae 2021, 7, 67. [Google Scholar] [CrossRef]
- Mirdavoudi, H.; Ghorbanian, D.; Zarekia, S.; Soleiman, J.M.; Ghonchepur, M.; Sweeney, E.M.; Mastinu, A. Ecological Niche Modelling and Potential Distribution of Artemisia sieberi in the Iranian Steppe Vegetation. Land 2022, 11, 2315. [Google Scholar] [CrossRef]
- Moradi, P.; Aghajanloo, F.; Moosavi, A.; Monfared, H.H.; Khalafi, J.; Taghiloo, M.; Khoshzaman, T.; Shojaee, M.; Mastinu, A. Anthropic Effects on the Biodiversity of the Habitats of Ferula gummosa. Sustainability 2021, 13, 7874. [Google Scholar] [CrossRef]
- Yousefi, A.R.; Ahmadikhah, A.; Fotovat, R.; Rohani, L.; Soheily, F.; Uberti, D.L.; Mastinu, A. Molecular Characterization of a New Ecotype of Holoparasitic Plant Orobanche L. on Host Weed Xanthium spinosum L. Plants 2022, 11, 1406. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.D.; Kostka, S.J.; Inouye, A.L.; Zvirzdin, D.L. Postfire Restoration of Soil Hydrology and Wildland Vegetation Using Surfactant Seed Coating Technology. Rangel. Ecol. Manag. 2012, 65, 253–259. [Google Scholar] [CrossRef]
- Williams, M.I.; Dumroese, R.K.; Page-Dumroese, D.S.; Hardegree, S.P. Can biochar be used as a seed coating to improve native plant germination and growth in arid conditions? J. Arid. Environ. 2016, 125, 8–15. [Google Scholar] [CrossRef]
- Lipiec, J.; Doussan, C.; Nosalewicz, A.; Kondracka, K. Effect of drought and heat stresses on plant growth and yield: A review. Int. Agrophysics 2013, 27, 463–477. [Google Scholar] [CrossRef]
- Ravi, S.; Breshears, D.D.; Huxman, T.E.; D’Odorico, P. Land degradation in drylands: Interactions among hydrologic–aeolian erosion and vegetation dynamics. Geomorphology 2010, 116, 236–245. [Google Scholar] [CrossRef]
- Toscano, S.; Romano, D.; Tribulato, A.; Patanè, C. Effects of drought stress on seed germination of ornamental sunflowers. Acta Physiol. Plant 2017, 39, 184. [Google Scholar] [CrossRef]
- Bayati, P.; Karimmojeni, H.; Razmjoo, J.; Pucci, M.; Abate, G.; Baldwin, T.C.; Mastinu, A. Physiological, Biochemical, and Agronomic Trait Responses of Nigella sativa Genotypes to Water Stress. Horticulturae 2022, 8, 193. [Google Scholar] [CrossRef]
- Chaichi, M.; Nemati, A.; Dadrasi, A.; Heydari, M.; Hassanisaadi, M.; Yousefi, A.R.; Baldwin, T.C.; Mastinu, A. Germination of Triticum aestivum L.: Effects of Soil-Seed Interaction on the Growth of Seedlings. Soil Syst. 2022, 6, 37. [Google Scholar] [CrossRef]
- Jam, B.J.; Shekari, F.; Andalibi, B.; Fotovat, R.; Jafarian, V.; Najafi, J.; Uberti, D.; Mastinu, A. Impact of Silicon Foliar Application on the Growth and Physiological Traits of Carthamus tinctorius L. Exposed to Salt Stress. Silicon 2022, 15, 1235–1245. [Google Scholar] [CrossRef]
- Kamali, N.; Sadeghipour, A.; Souri, M.; Mastinu, A. Variations in Soil Biological and Biochemical Indicators under Different Grazing Intensities and Seasonal Changes. Land 2022, 11, 1537. [Google Scholar] [CrossRef]
- Taghvaei, M.; Nasrolahizadehi, A.; Mastinu, A. Effect of Light, Temperature, Salinity, and Halopriming on Seed Germination and Seedling Growth of Hibiscus sabdariffa under Salinity Stress. Agronomy 2022, 12, 2491. [Google Scholar] [CrossRef]
- Muscolo, A.; Sidari, M.; Anastasi, U.; Santonoceto, C.; Maggio, A. Effect of PEG-induced drought stress on seed germination of four lentil genotypes. J. Plant Interact. 2013, 9, 354–363. [Google Scholar] [CrossRef]
- Lotfi, N.; Soleimani, A.; Vahdati, K.; Çakmakçı, R. Comprehensive biochemical insights into the seed germination of walnut under drought stress. Sci. Hortic. 2019, 250, 329–343. [Google Scholar] [CrossRef]
- Partheeban, C.; Chandrasekhar, C.N.; Jeyakumar, P.; Ravikesavan, R.; Gnanam, R. Effect of PEG Induced Drought Stress on Seed Germination and Seedling Characters of Maize (Zea mays L.) Genotypes. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1095–1104. [Google Scholar] [CrossRef]
- Amirkhani, M.; Mayton, H.; Loos, M.; Taylor, A. Development of Superabsorbent Polymer (SAP) Seed Coating Technology to Enhance Germination and Stand Establishment in Red Clover Cover Crop. Agronomy 2023, 13, 438. [Google Scholar] [CrossRef]
- Beigi, S.; Azizi, M.; Iriti, M. Application of Super Absorbent Polymer and Plant Mucilage Improved Essential Oil Quantity and Quality of Ocimum basilicum var. Keshkeni Luvelou. Molecules 2020, 25, 2503. [Google Scholar] [CrossRef]
- Johnson, E.N.; Miller, P.R.; Blackshaw, R.E.; Gan, Y.; Harker, K.N.; Clayton, G.W.; Kephart, K.D.; Wichman, D.M.; Topinka, K.; Kirkland, K.J. Seeding date and polymer seed coating effects on plant establishment and yield of fall-seeded canola in the Northern Great Plains. Can. J. Plant Sci. 2004, 84, 955–963. [Google Scholar] [CrossRef]
- Rehman, A.; Farooq, M. Zinc seed coating improves the growth, grain yield and grain biofortification of bread wheat. Acta Physiol. Plant 2016, 38, 238. [Google Scholar] [CrossRef]
- Gorim, L.; Asch, F. Seed coating reduces respiration losses and affects sugar metabolism during germination and early seedling growth in cereals. Funct. Plant Biol. 2015, 42, 209. [Google Scholar] [CrossRef] [PubMed]
- Cerasola, V.A.; Perlotti, L.; Pennisi, G.; Orsini, F.; Gianquinto, G. Potential Use of Superabsorbent Polymer on Drought-Stressed Processing Tomato (Solanum lycopersicum L.) in a Mediterranean Climate. Horticulturae 2022, 8, 718. [Google Scholar] [CrossRef]
- Milani, P.; França, D.; Balieiro, A.G.; Faez, R. Polymers and its applications in agriculture. Polímeros 2017, 27, 256–266. [Google Scholar] [CrossRef]
- Motamedi, M.; Zahedi, M.; Karimmojeni, H.; Motamedi, H.; Mastinu, A. Effect of rhizosphere bacteria on antioxidant enzymes and some biochemical characteristics of Medicago sativa L. subjected to herbicide stress. Acta Physiol. Plant 2022, 44, 84. [Google Scholar] [CrossRef]
- AbdAllah, A.M.; Mashaheet, A.M.; Burkey, K.O. Super absorbent polymers mitigate drought stress in corn (Zea mays L.) grown under rainfed conditions. Agric. Water Manag. 2021, 254, 106946. [Google Scholar] [CrossRef]
- Bagherifard, A.; Hamidoghli, Y.; Biglouei, M.H.; Ghaedi, M. Effects of drought stress and superabsorbent polymer on morpho-physiological and biochemical traits of Caper (Capparis spinosa L.). Aust. J. Crop Sci. 2020, 14, 13–20. [Google Scholar] [CrossRef]
- Islam, M.R.; Xue, X.; Mao, S.; Ren, C.; Eneji, A.E.; Hu, Y. Effects of water-saving superabsorbent polymer on antioxidant enzyme activities and lipid peroxidation in oat (Avena sativa L.) under drought stress. J. Sci. Food Agric. 2011, 91, 680–686. [Google Scholar] [CrossRef]
- Taghvaei, M.; Sadeghi, H.; Khaef, N. Cardinal Temperatures for Germination of the Medicinal Anddesert Plant, Calotropis procera. Planta Daninha 2015, 33, 671–678. [Google Scholar] [CrossRef]
- Khanzada, A.K.; Shaikh, W.; Kazi, T.G.; Kabir, S.; Soofia, S. Antifungal activity, elemental analysis and determination of total protein of seaweed, Solieria robusta (Greville) Kylin from the coast of Karachi. Pak. J. Bot. 2007, 39, 931–937. [Google Scholar]
- Nouman, W.; Aziz, U. Seed priming improves salinity tolerance in Calotropis procera (Aiton) by increasing photosynthetic pigments, antioxidant activities, and phenolic acids. Biologia 2022, 77, 609–626. [Google Scholar] [CrossRef]
- Taghvaei, M.; Kordestani, M.D.; Saleh, M.; Mastinu, A. The Reinforcement of Early Growth, Extract, and Oil of Silybum marianum L. by Polymer Organic Cover and Bacteria Inoculation under Water Deficit. Soil Syst. 2023, 7, 61. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Gong, Y.P.; Toivonen, P.M.A.; Lau, O.L.; Wiersma, P.A. Antioxidant system level in ‘Braeburn’ apple is related to its browning disorder. Bot. Bull. Acad. Sin. 2001, 42, 259–264. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen-Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach-Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide Dismutase. J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Tátrai, Z.A.; Sanoubar, R.; Pluhár, Z.; Mancarella, S.; Orsini, F.; Gianquinto, G. Morphological and Physiological Plant Responses to Drought Stress in Thymus citriodorus. Int. J. Agron. 2016, 2016, 4165750. [Google Scholar] [CrossRef]
- Anjum, S.A.; Xie, X.Y.; Wang, L.C.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Zargar, S.M.; Gupta, N.; Nazir, M.; Mahajan, R.; Malik, F.A.; Sofi, N.R.; Shikari, A.B.; Salgotra, R.K. Impact of drought on photosynthesis: Molecular perspective. Plant Gene 2017, 11, 154–159. [Google Scholar] [CrossRef]
- Chaves, M.M.; Costa, J.M.; Saibo, N.J.M. Recent Advances in Photosynthesis Under Drought and Salinity. Adv. Bot. Res. 2011, 57, 49–104. [Google Scholar] [CrossRef]
- Zangani, E.; Afsahi, K.; Shekari, F.; Mac Sweeney, E.; Mastinu, A. Nitrogen and Phosphorus Addition to Soil Improves Seed Yield, Foliar Stomatal Conductance, and the Photosynthetic Response of Rapeseed (Brassica napus L.). Agriculture 2021, 11, 483. [Google Scholar] [CrossRef]
- Sivritepe, N.; Erturk, U.; Yerlikaya, C.; Turkan, I.; Bor, M.; Ozdemir, F. Response of the cherry rootstock to water stress induced in vitro. Biol. Plant. 2008, 52, 573–576. [Google Scholar] [CrossRef]
- Nemeskeri, E.; Kovacs-Nagy, E.; Nyeki, J.; Sardi, E. Responses of apple tree cultivars to drought: Carbohydrate composition in the leaves. Turk. J. Agric. For. 2015, 39, 949–957. [Google Scholar] [CrossRef]
- Nge, T.T.; Hori, N.; Takemura, A.; Ono, H. Swelling behavior of chitosan/poly(acrylic acid) complex. J. Appl. Polym. Sci. 2004, 92, 2930–2940. [Google Scholar] [CrossRef]
- Patra, S.K.; Poddar, R.; Brestic, M.; Acharjee, P.U.; Bhattacharya, P.; Sengupta, S.; Pal, P.; Bam, N.; Biswas, B.; Barek, V.; et al. Prospects of Hydrogels in Agriculture for Enhancing Crop and Water Productivity under Water Deficit Condition. Int. J. Polym. Sci. 2022, 2022, 4914836. [Google Scholar] [CrossRef]
- Yazdani, F.; Allahdadi, I.; Akbari, G.A. Impact of Superabsorbent Polymer on Yield and Growth Analysis of Soybean (Glycine max L.) Under Drought Stress Condition. Pak. J. Biol. Sci. 2007, 10, 4190–4196. [Google Scholar] [CrossRef]
- Ahmadi, A.; Mardeh, A.S.-S.; Poustini, K.; Jahromi, M.E. Influence of Osmo and Hydropriming on Seed Germination and Seedling Growth in Wheat (Triticum aestivum L.) Cultivars under Different Moisture and Temperature Conditions. Pak. J. Biol. Sci. 2007, 10, 4043–4049. [Google Scholar] [CrossRef] [PubMed]
- Per, T.S.; Khan, N.A.; Reddy, P.S.; Masood, A.; Hasanuzzaman, M.; Khan, M.I.R.; Anjum, N.A. Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: Phytohormones, mineral nutrients and transgenics. Plant Physiol. Biochem. 2017, 115, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Hosseinifard, M.; Stefaniak, S.; Ghorbani Javid, M.; Soltani, E.; Wojtyla, L.; Garnczarska, M. Contribution of Exogenous Proline to Abiotic Stresses Tolerance in Plants: A Review. Int. J. Mol. Sci. 2022, 23, 5186. [Google Scholar] [CrossRef]
- Adamipour, N.; Khosh-Khui, M.; Salehi, H.; Razi, H.; Karami, A.; Moghadam, A. Metabolic and genes expression analyses involved in proline metabolism of two rose species under drought stress. Plant Physiol. Biochem. 2020, 155, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Ben Ahmed, C.; Magdich, S.; Ben Rouina, B.; Boukhris, M.; Ben Abdullah, F. Saline water irrigation effects on soil salinity distribution and some physiological responses of field grown Chemlali olive. J. Environ. Manag. 2012, 113, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Towhidi, A.; Saberifar, T.; Dirandeh, E. Nutritive value of some herbage for dromedary camels in the central arid zone of Iran. Trop. Anim. Health Prod. 2011, 43, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Bahrani, M.J.; Niknejad-Kazempour, H. Effect of dormancy breaking treatments and salinity on seed germination of two desert shrubs. Arid. Land. Res. Manag. 2007, 21, 107–118. [Google Scholar] [CrossRef]
- Su, L.-Q.; Li, J.-G.; Xue, H.; Wang, X.-F. Super absorbent polymer seed coatings promote seed germination and seedling growth of Caragana korshinskii in drought. J. Zhejiang Univ.-Sci. B 2017, 18, 696–706. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Sankar, B.; Murali, P.V.; Gomathinayagam, M.; Lakshmanan, G.M.A.; Panneerselvam, R. Water deficit stress effects on reactive oxygen metabolism in Catharanthus roseus; impacts on ajmalicine accumulation. Colloids Surf. B Biointerfaces 2008, 62, 105–111. [Google Scholar] [CrossRef]
- Poormohammad Kiani, S.; Maury, P.; Sarrafi, A.; Grieu, P. QTL analysis of chlorophyll fluorescence parameters in sunflower (Helianthus annuus L.) under well-watered and water-stressed conditions. Plant Sci. 2008, 175, 565–573. [Google Scholar] [CrossRef]
- Tahkokorpi, M.; Taulavuori, K.; Laine, K.; Taulavuori, E. After-effects of drought-related winter stress in previous and current year stems of Vaccinium myrtillus L. Environ. Exp. Bot. 2007, 61, 85–93. [Google Scholar] [CrossRef]
- Tongo, A.; Jalilvand, H.; Hosseininasr, M.; Naji, H.R. Leaf morphological and physiological variations in response to canopy dieback of Persian Oak (Quercus brantii Lindl.). Forest Pathol. 2021, 51, e12671. [Google Scholar] [CrossRef]
- Mahdavi, A.; Moradi, P.; Mastinu, A. Variation in Terpene Profiles of Thymus vulgaris in Water Deficit Stress Response. Molecules 2020, 25, 1091. [Google Scholar] [CrossRef]
- Zhang, H.-Q.; Zou, Y.-B.; Xiao, G.-C.; Xiong, Y.-F. Effect and Mechanism of Cold Tolerant Seed-Coating Agents on the Cold Tolerance of Early Indica Rice Seedlings. Agric. Sci. China 2007, 6, 792–801. [Google Scholar] [CrossRef]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Khan, A.L.; Waqas, M.; Lee, I.-J. Silicon Regulates Antioxidant Activities of Crop Plants under Abiotic-Induced Oxidative Stress: A Review. Front. Plant Sci. 2017, 8, 00510. [Google Scholar] [CrossRef]
- Terzi, R.; Kadioglu, A. Drought stress tolerance and the antioxidant enzyme system in Ctenanthe setosa. Acta Biol. Cracov. Bot. 2006, 48, 89–96. [Google Scholar]
- Sales, C.R.G.; Ribeiro, R.V.; Silveira, J.A.G.; Machado, E.C.; Martins, M.O.; Lagôa, A.M.M.A. Superoxide dismutase and ascorbate peroxidase improve the recovery of photosynthesis in sugarcane plants subjected to water deficit and low substrate temperature. Plant Physiol. Biochem. 2013, 73, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci. Rep. 2018, 8, 16496. [Google Scholar] [CrossRef]
- Azarabadi, S.; Abdollahi, H.; Torabi, M.; Salehi, Z.; Nasiri, J. ROS generation, oxidative burst and dynamic expression profiles of ROS-scavenging enzymes of superoxide dismutase (SOD), catalase (CAT) and ascorbate peroxidase (APX) in response to Erwinia amylovora in pear (Pyrus communis L). Eur. J. Plant Pathol. 2016, 147, 279–294. [Google Scholar] [CrossRef]
- Lai, K.M.; Ye, D.Y.; Wong, J.W.C. Enzyme activities in a sandy soil amended with sewage sludge and coal fly ash. Water Air Soil Poll. 1999, 113, 261–272. [Google Scholar] [CrossRef]
- Zhong, Y.; Yang, S.; Hu, X.; Cai, J.; Tang, Z.; Xu, Q. Whole Process Inhibition of a Composite Superabsorbent Polymer-Based Antioxidant on Coal Spontaneous Combustion. Arab. J. Sci. Eng. 2018, 43, 5999–6009. [Google Scholar] [CrossRef]
- Zeng, D.; Zhao, H. Activity test and mechanism study of an environmentally friendly wheat seed coating agent. Agric. Sci. 2013, 4, 334–339. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).