Abstract
Natural resource extraction has been linked to habitat loss and declines in biodiversity. Insects, the most diverse and abundant animals on Earth, provide a wide array of critical ecosystem services, but are typically understudied in terrestrial restoration projects. Here, we examine how insects and other arthropods respond to reclamation efforts in the Pinedale Anticline natural gas field in semi-arid Wyoming, USA. Vegetation on two-year-old well pads seeded with native grass or one-year-old well pads seeded with a native annual forb, Rocky Mountain bee plant (Cleome serrulata), was measured and compared to reference areas adjacent to the well pads with a free software program called SamplePoint. Reference areas in the Pinedale Anticline natural gas field consist primarily of decadent sagebrush stands with low floral diversity. Insect and arthropod communities were also collected and assessed for family richness and abundance on these well pads and reference areas over two years. Based on the mass flowering hypothesis, we expected higher insect abundance and diversity on well pads seeded with the Rocky Mountain bee plant compared to adjacent reference areas. Based on the plant vigor hypothesis, we expected higher insect abundance and diversity on well pads seeded with native grass than reference communities. In year one, 893 insects from 30 insect families with an additional 12 arthropods from 4 families were captured. In year two, 685 insects from 17 families were collected. Reclaimed well pads had significantly higher abundance in both years and vegetation types. In year one, we did not detect a significant difference in richness on native-grass-treated well pads vs. the reference site. We found a significant difference in richness on bee-plant-treated well pads vs. the reference in both years, as well as native-grass-treated well pads vs. the reference in year two. Implications of these findings are discussed in the manuscript.
1. Introduction
Natural resource extraction is required to provide anthropogenic amenities on a global scale. In Western North America, the extraction of oil and natural gas has been linked to habitat loss, biodiversity decline and changes in vegetation communities [1]. Ecological restoration, defined as the ‘process of assisting the recovery of an ecosystem that has been degraded, damaged, or destroyed’ [2], may assist in reversing negative impacts of land surface disturbance associated with resource extraction and is essential to regain biodiversity and ecosystem services of degraded lands [3]. In Wyoming, natural gas well pads undergoing ecological restoration are typically judged by comparing recently disturbed sites which have been seeded with an approved seed mix to adjacent reference sites and by determining how these sites are progressing based upon existing regulatory frameworks [4,5]. These frameworks are often focused on land reclamation, a component of ecological restoration which emphasizes revegetation, regrading topography and erosion control [6,7,8]. In this sense, human-mediated reclamation efforts are often viewed as ‘assisted succession’ and focus on structural rather than functional endpoints [9]. Insects, the most diverse group of animals on Earth, have been used as indicators to gauge restoration success [7], but few studies have examined how insects respond to the restoration of vegetation communities at different successional stages [10,11] and most studies involving insects and restoration are focused on crop systems [12].
While biodiversity is an ecosystem service in itself, it is also used as a measure for ecosystem services [13]. Aside from providing biodiversity, insects play critical roles in ecosystem services such as nutrient cycling, pollination, pest control and nutrition of higher trophic levels [14,15]. Maintaining and restoring ecosystem services is a goal of ecological restoration and several studies suggest certain services provided by insects are critical in achieving restoration success [14,16]. The main focus of restoring insects and their services has been towards pollinators in crop agriculture systems [12], and recent studies have suggested that ecosystem restoration efforts often positively benefit pollinators [17]. Few studies have examined how insects respond to restoration efforts in arid and semi-arid rangeland systems, none are associated with natural gas development nor its reclamation and little is known about native rangeland pollinators in general [18].
In Wyoming, natural gas well pads undergo an interim reclamation process which involves spreading stockpiled topsoil to the non-active portion of a well pad (typically ~75% of initial disturbance) and applying seed mixes containing early seral plant species [5,19]. A final reclamation process is implemented after the life of the well is complete, which includes plugging the well, removing equipment and seeding the remaining area of initial disturbance [19]. While policies in Wyoming associated with natural gas restoration often focus on erosion control, site stability, control of noxious and invasive weeds and vegetation comparisons to an adjacent reference site [6], selecting reference sites as measures of success has been debated by restoration ecologists [20,21]. In general, well pads undergoing reclamation can meet regulatory success criteria by establishing native plant species similar in composition to a reference site and controlling undesirable species (e.g., noxious weeds) [5,19]. However, in arid sagebrush steppe ecosystems, reclamation efforts and establishment of old sagebrush stands may take decades [22]. Therefore, young well pads undergoing reclamation are expected to be in earlier seral stages than reference communities.
In areas where reference or pre-disturbance vegetation communities are culturally influenced ecosystems which have been under various human management prior to a new disturbance [2], identifying an appropriate reference system upon which to gauge restoration success is particularly difficult. The Pinedale Anticline natural gas field (Pinedale, WY, USA) is considered to lie within a cultural, or semi-natural, ecosystem subjected to domestic livestock grazing since the early 1870s [23]. More recently (ca. 2000), natural gas development began occurring in the area. Natural gas development in this area has resulted in 2140 hectares of surface disturbance in the principal forms of pipelines, roads and well pads. While roads are the most persistent form of disturbance, pipelines and well pads are required to be reclaimed after construction [18] and 1,485 hectares (69.3%) of the initial surface disturbance is currently in the reclamation process (Pinedale Anticline Data Management System Version 3.3.6). One of the primary goals of reclamation in the Pinedale Anticline is to establish vegetation similar in composition to adjacent reference sites which have not been directly disturbed by natural gas development. However, data from the Pinedale Anticline Data Management System and the Wyoming Reclamation and Restoration Center Oil and Gas Reclamation Database [4] suggest these disturbed sites undergoing reclamation are generally in earlier successional states and contain higher species diversity than the decadent, old growth sagebrush communities common at many reference sites. While reclamation efforts in the Pinedale Anticline natural gas field were not developed with the intent of studying insects, differences between vegetation communities on reclaimed well pads compared to reference site vegetation communities provided an opportunity to do so in this ecosystem.
Whereas pollinating insects have been studied extensively in crop systems, often focusing on bees (Apoidea spp.), few studies have been conducted on bees and non-bee pollinators in non-crop systems or in rangelands in general [12,18]. However, studies on rangelands are becoming more popular [24,25]. Mass flowering plants introduced to crop monocultures have proven to enhance all pollinator abundance [26,27,28,29]. It has been documented that vegetation structure, especially height and color, has a positive influence on flying insects [30]. Additionally, previous research has shown that most herbivorous insects avoid terpenoids produced by sagebrush (Artemisia spp.) [31] and prefer young, vigorous plants compared to older, decadent plants [32,33] (plant vigor hypothesis). Relatively few studies have been conducted to determine how domestic livestock grazing influences insect communities, with most suggesting negative consequences for insects [34,35]. Additionally, domestic livestock grazing has proven to diminish vegetation in the understory of sagebrush steppe ecosystems [36]. While overgrazing typically has negative consequences on insects, other studies suggest moderate grazing by domestic livestock may allow for the coexistence of livestock and insects [37].
The objective of this study is to examine insect community response to ecosystem disturbance associated with drilling for natural gas and subsequent reclamation efforts. We compared two types of early successional plant communities on reclaimed well pads with adjacent reference communities over the course of two years. A total of eight one-year-old reclaimed well pads dominated by the native annual Rocky Mountain bee plant (Cleome serrulata) and eight 2–3-year-old well pads primarily dominated by native grasses were compared to adjacent reference system locations which consisted primarily of Wyoming big sagebrush (Artemisia tridentata ssp. wyomingensis. Based on previous ‘mass flowering’ literature, coupled with the knowledge of vegetation structure and color influencing flying insects [26,27,28,29,30], we hypothesized that insect abundance and family richness would be greater on reclaimed well pads with the Rocky Mountain bee plant than in the adjacent reference system. Based on the ‘plant vigor hypothesis’ [32] and due to a lack of understory in the reference vegetation communities, we hypothesized that insect abundance and family richness would be greater on reclaimed well pads with native grass than in the adjacent reference system. Additionally, we examined if insect abundance and family richness varied between native grass reclaimed sites and those with bee plant. Finally, we sought to determine if different insect families were equally distributed among vegetation types on reclaimed sites and their adjacent reference locations. Due to a short growing season in the Pinedale Anticline natural gas field, we conducted our study late in the growing season when we were sure bee plant flowers would be in bloom.
2. Methods
2.1. Study Site
The Pinedale Anticline natural gas field is located in a semi-arid and semi-natural sagebrush steppe ecosystem in Sublette County, WY, USA (Figure 1). A total of 16 well pads and 16 adjacent reference sites (i.e., 8 one-year-old reclaimed well pads which were seeded with the Rocky Mountain bee plant and their adjacent 8 reference locations which were seeded with grasses as well as 8 two-year-old reclaimed well pads and their adjacent reference locations) were sampled in our study, all of which were located in Ecological Site Description (ESD) R034AY222WY, receiving 254–356 mm annual precipitation evenly spread throughout the year. The elevation is greater than 2100 m throughout the study area. Soils in the area are loamy and a historic climax plant community is characterized as mixed grass and Wyoming big sagebrush. The climate has a wide range in variability of seasonal and daily temperature and experiences an average frost-free period of 42 days. Daily minimum temperatures during the growing season range between −1 and 6 °C, while daily maximum temperatures range between 19 and 26 °C. More information about the area may be found through the United States Department of Agriculture-Natural Resources Conservation Service’s Ecological Site Information System.
Figure 1.
A location map showing where the Pinedale Anticline natural gas field lies within Wyoming, USA. A detailed map of the gas field can be found using the Pinedale Anticline Data Management System found at https://my.usgs.gov/papo/home (accessed on 30 March 2022).
All sites were selected within the same ESD in an effort to reduce effects of different reference communities on reclaimed sites. Additionally, all sites were at least 0.8 km to the next closest location in this study, with some locations being up to 10 km apart. In both 2015 and 2016, 8 well pads were selected with 4 consisting of mass flowering Rocky Mountain bee plant and 4 consisting predominantly of native, perennial grasses (Figure 2 is an example of the site types). Sites were inconsistent across years, as vegetation communities on reclaimed sites did not remain entirely consistent.

Figure 2.
The left image shows the edge of a reclaimed well pad with mass flowering Rocky Mountain bee plant near the reference site, consisting primarily of Wyoming big sagebrush. The right image shows the edge of a reclaimed well pad consisting primarily of native, perennial grasses with the Wyoming big sagebrush reference system in the backdrop.
Sites were selected when Rocky Mountain bee plant flowering was prevalent. All sampling was conducted when winds were less than 8 kph. Daily temperature fluctuations were as follows: 6 September 2015 ranged from −4 °C–17 °C; 7 September 2015 from 0 °C–20 °C; 4 September 2016 ranged from 7 °C–20 °C; and 5 September 2016 ranged from 1 °C–17 °C. All sampling was conducted in sunshine conditions, although a rain event occurred the evening of 4 September 2016.
2.2. Vegetation Sampling and Analysis
On 6–7 September 2015 and 4–5 September 2016, two 40 m transects were set up on well pads at 5 m and 10 m from the reference community as well as at 5 m and 10 m away from the well pad in the reference community. All transects were placed perpendicular to the edge of the well pad. A 0.5 m2 image was taken every 5 m along all transects using methods described in [38], starting at the 0 m point on the transect tape, resulting in nine images per transect. A 12.1-megapixel Ricoh G700SE digital camera was held 1.3 m above ground level to acquire a ~0.4 mm ground sample distance (GSD) image at each location which is consistent with previous literature [39,40]. Each photo (in JPG format) was quantified for vegetation, litter and ground surface cover by having 40 pixels randomly distributed throughout each image (i.e., 360 total pixels per transect) in a free software program, ‘SamplePoint’ [41]. The categories, or buttons, used in our SamplePoint analysis included bare ground, herbaceous litter, woody litter, dung, rock and vegetation by species.
2.3. Insect Sampling
In each year, two runs of forty sweeps were taken on each reclaimed well pad and adjacent reference location transect. Sweep net samples were conducted on 6–7 September 2015 and 4–5 September 2016 using a 38 cm diameter net with a 50 cm handle. All sampling was conducted between the hours of 0930 and 1300. Sampled insects were transferred from the sweep net to a Zip-Lock® bag and placed in a cooler immediately after collection. Upon return to the University of Wyoming (Laramie, WY, USA) from the Pinedale Anticline natural gas field, samples were placed in a freezer for identification in a laboratory at a later date.
All insects were identified to family by a PhD Entomologist (author DIS) using a dichotomous key and with assistance from the National Audubon Society field guide [42]. Adult insects were also classified into functional groups based on potential ecological roles which may be performed by individuals from various families by DIS. These functional groups were detritivore, herbivore, parasitoid, pollinator and predator.
2.4. Statistical Analysis
SamplePoint reports, as .csv files, were used for vegetation analysis. The total number of pixel counts per transect was lumped together based on site type. We separated sites into the following groups before comparing insect communities: (1) mass flowering Rocky Mountain bee plant reclaimed sites (beeplant treatment), (2) reference sites adjacent to mass flowering Rocky Mountain bee plant reclaimed sites (beeplant reference), (3) native grass reclaimed sites (grass treatment) and (4) reference sites adjacent to native grass reclaimed sites (grass reference). The total number of pixel counts per cover type (a total of 2880 pixels) was used for each site type. Chi-square tests for equal distribution were conducted separately for each year by comparing the total number of pixel counts per vegetation classification group between reclaimed sites and their reference pairs in accordance with [6].
As vegetation data and insect sampling were conducted at distinctly different times throughout each day, and because time of day and weather conditions may have influenced insect sampling results [43,44], we did not attempt to correlate insect abundance and family richness to vegetation cover or vegetation richness. Instead, we grouped insects based on the four vegetation site types and conducted Welch’s t-tests. The design is a randomized block design with fixed blocks rather than a randomized block design with random blocks—the sites are treated as blocks and a portion of each block received the treatment and the other portion of the block was left as control/reference. As raw responses from insect data were non-normal, the non-normality was addressed by using the square-root transformation and then back-transforming once the model was built. Normal probability plots were used to check that the transformed data were normally distributed. The analysis of the transformed data was carried out separately for each year, each site type and for abundance and family richness tests. Non-insect arthropods (i.e., spiders and mites), since they made up only ~0.01% of the abundance in 2015, were not included in insect abundance or family richness tests, though all other insects were included in these tests.
As this is the first study to examine insect response to natural gas well pad reclamation in a sagebrush steppe ecosystem, we used stacked bar charts as a visual plotting technique to determine the best way to analyze how insect and arthropod families’ distribution differed by site type in this study. Upon creating these stacked box charts and looking at raw insect count data, all insect families which made up greater than 5% of the total amount of collected insects were analyzed using chi-square tests to test for equal distribution of insects among vegetation types. Additionally, both insects and arthropods were categorized into functional groups based on potential ecological role (detritivore, herbivore, parasitoid, pollinator or predator) and chi-square tests were used to test for distribution across site types. All statistical analyses were conducted in Program R [45].
3. Results
3.1. Vegetation Sampling
In both years, vegetation communities on reclaimed well pads differed significantly from adjacent reference sites (Table 1 and Table 2). Overall vegetation cover was significantly greater on the reclaimed well pads with Rocky Mountain bee plant than adjacent reference sites over both years (p < 0.001), while overall vegetation cover was significantly less on reclaimed sites with native grasses than their adjacent reference sites (Table 1 and Table 2). Russian thistle (Salsola tragus) was the only non-native plant recorded and occurred on all Rocky Mountain bee plant reclaimed sites (Table 1 and Table 2). In both years, no forbs were recorded on reference sites and only one individual forb, Western yarrow (Achilleum millefolium), was recorded on native grass reclaimed sites (Table 1 and Table 2). Grass cover was higher on reference sites adjacent to native grass reclaimed sites compared to reference sites adjacent to Rocky Mountain bee plant reclaimed sites, whereas sagebrush cover was higher on reference sites adjacent to Rocky Mountain bee plant reclaimed sites (Table 1 and Table 2).

Table 1.
Relative mean percent of sample point analysis in 2015. All site types had a total of 2880 secondary sampling units (pixels) analyzed, which are converted to percentages in this table. Reclaimed sites were compared directly to their paired adjacent reference sites. All sites were significantly different (p < 0.001). Columns with * indicate those which were responsible for significant differences. Bolded and underlined numbers suggest higher proportion of that cover type within that site type compared to the paired reclamation or reference site.

Table 2.
Relative mean percent cover of sample point analysis in 2016. All sites had a total of 2880 secondary sampling units (pixels) analyzed, which are converted to percentages in this table. Reclaimed sites were compared directly to their paired adjacent reference sites. All sites were significantly different (p < 0.001). Columns with * indicate those which were responsible for significant differences. Bolded and underlined numbers suggest higher proportion of that cover type within that site type compared to the paired reclamation or reference site.
3.2. Insect Sampling
In 2015, 893 individual insects representing 30 families were collected along with 12 individuals from 4 non-insect arthropod families; in 2016, 685 individual insects representing 17 families were collected. In 2015 and 2016, insect abundance and richness were significantly greater on reclaimed well pads with the Rocky Mountain bee plant than their reference sites (Figure 3a,c, 2015 abundance p < 0.001, 2016 abundance p < 0.001; 2015 richness p = 0.07, 2016 richness p = 0.031). Mean insect abundance was also significantly greater on reclaimed well pads with native grasses than their adjacent reference sites in 2015 and 2016, while richness was only significantly greater on native grass reclamation sites in 2016 (Figure 3b,d, 2015 abundance p < 0.001, 2016 abundance p < 0.001; 2015 richness p = 0.07, 2016 richness p = 0.014). Mean insect abundance differed significantly between Rocky Mountain bee plant reclaimed well pads and native grass reclaimed well pads in both 2015 and 2016, with the Rocky Mountain bee plant having a higher average number of insects in both years of our survey (2015 abundance p < 0.001, 2016 abundance p < 0.001; 2015 richness p = 0.002, 2016 richness p = 0.014).
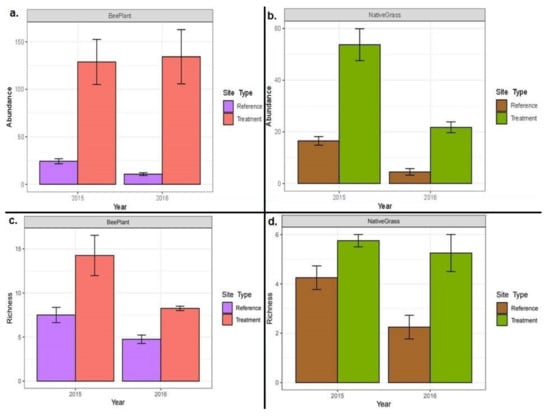
Figure 3.
Bar plot showing mean insect abundance (a,b) and richness (c,d) on reclaimed well pads that were predominantly Rocky Mountain bee plant or native grass, compared to their reference sites. Error bars are standard error. Note, the range of values on the y-axis varies among each plot.
Of the 34 families of insects which were collected in 2015, only 7 families made up greater than 5% of the total count; therefore, these were the focus of our chi-square tests for equal distributions. Results from our chi-square tests found unequal distributions (p < 0.001) for each of the seven families, with Anthomyiidae, Chrysomelidae, Halictidae, Lygaeidae and Miridae being concentrated most heavily on reclaimed sites with the Rocky Mountain bee plant and Cercopidae and Cicadellidae being concentrated most heavily on sites reclaimed with native perennial grasses (Figure 4). In 2016, individuals from only three families made up >5% of the total collection. Our chi-square tests revealed unequal distribution for each family (p < 0.001) with Chrysomelidae and Lygaeidae being more heavily concentrated on Rocky Mountain bee plant reclaimed sites compared to other vegetation types and Cicadellidae being most heavily concentrated on sites reclaimed with native perennial grasses (Figure 5).
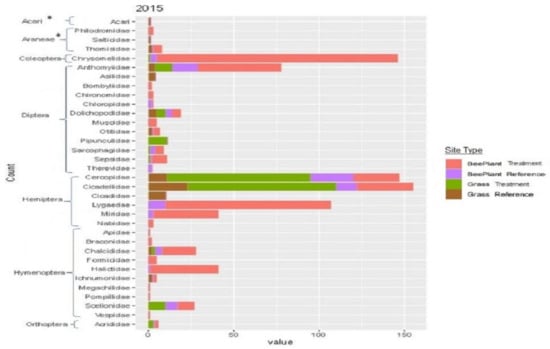
Figure 4.
A stacked bar chart of insect abundance by family found on each site type in 2015. The * denotes arthropod orders and families within them which are not considered insects, but rather mite and spiders.
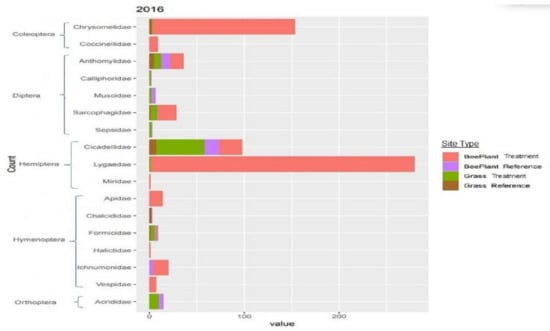
Figure 5.
A stacked bar chart of insect abundance by family found on each site type in 2016.
In both years, insects were also categorized by functional role (Supplementary Table S1). In instances where certain families have the potential to serve multiple roles, they were counted multiple times. Due to the sampling method (sweep netting), it was not possible to be certain which role(s) each insect was serving at the time of capture. Ecological roles included detritovore, herbivore, parasitoid, pollinator and predator. In 2015, all functional roles were unequally distributed (p < 0.001) with reclaimed sites containing the Rocky Mountain bee plant having the highest concentrations for each group (Supplementary Figure S1). In 2016, significant distribution differences (p < 0.001) of predators were found between Rocky Mountain bee plant reclaimed sites and their reference sites (no predators were found on grass reclaimed sites or their reference sites), and all other groups were most heavily concentrated on reclaimed sites with Rocky Mountain bee plant (p < 0.001; Supplementary Figure S2).
4. Discussion
Our results suggest well pads undergoing interim reclamation support more insect abundance and diversity in the form of family richness than adjacent reference areas. Our findings suggest that the mass flowering Rocky Mountain bee plant supports more insect families, insect functional groups and insect abundance than a reference system which is nearly a monoculture of decadent sagebrush, corroborating previous mass flowering literature, though not focused on bees in a crop agriculture environment [26,27,28,29]. Additionally, our results support the plant vigor hypothesis [32] with significantly more insect abundance and family richness being found on young reclaimed well pads with native grasses compared to decadent sagebrush reference systems. Finally, it is worth noting that well pads which contained flowering Rocky Mountain bee plant and their reference sites had higher insect abundance and family richness than well pads which were reclaimed with native grasses and their reference sites, respectively.
Previous research has suggested islands of mass flowering plants may have positive benefits in terms of increasing the number of insects to surrounding areas [26,27,28]. While the reference sites adjacent to Rocky Mountain bee plant reclaimed well pads differed from reference sites adjacent to native grass reclaimed well pads, both groups of reference sites occur in the same Ecological Site Description and consist of older sagebrush/mixed grass plant communities than the reclaimed well pads. Our findings also suggest that insect abundance and family richness are greater on reference sites adjacent to Rocky Mountain bee plant well pads than reference sites adjacent to native grass reclaimed well pads are consistent with aforementioned studies [26,27,28,29], though it is not conclusive these higher insect counts were solely due to flowering bee plant or in combination with different vegetation, rock and bare ground components in the reference systems. While birds and other wildlife were not accounted for in this study, previous studies have suggested forbs which attract insects with food web benefits to higher trophic levels may be especially important for future restoration efforts in sagebrush steppe ecosystems [46,47].
While insects in this particular ecosystem are not well studied, our findings may help serve as a road map for future research in the area and surrounding areas. Although we only identified insects and other arthropods to the family level, to our knowledge this is the most comprehensive study related to insects in Sublette County, WY to date. While Halictidae (i.e., sweat bees) was the only family of bees to be significant enough to report statistics on (i.e., they made up >5% of total abundance), it should also be noted that all individuals from other bee families were found on sites with the Rocky Mountain bee plant. This information may help inform future insect studies in this ecosystem as the majority of pollinators which were found in this study were non-bee species, which is not atypical in other rangeland settings [18]. Additionally, when combined into ecological roles, insects and other arthropods from each functional group were found in higher abundance on reclaimed well pads with the Rocky Mountain bee plant compared to other vegetation types. While insect sampling techniques can bias results due to a variety of factors [48], the fact we used a sweep net technique undoubtedly led to us capturing fewer ground dwelling insects than truly existed in our study system. Even so, more detritivores and predatory insects were found on bee plant sites than in other communities. Although we did not quantify ecosystem services other than biodiversity (in the form of family richness), the high abundance of insect functional groups on sites with a native flowering plant may suggest higher ecosystem functionality which would require future studies to verify. While it is more common for reclamation practitioners to utilize seed mixes containing primarily perennial species [49], there may be benefits to using native annual plants in reclamation mixes other than those associated with increased insect diversity and abundance [50]. For example, the Rocky Mountain bee plant performs favorably on disturbed sites in arid environments [51,52], and along with several other native annual forb species has been shown to compete well with non-desirable invasive species in these systems [49]. Rocky Mountain bee plant is typically not a persistent plant in reclamation seed mixes and interseeding with perennial forbs, shrubs and grasses or seeding those later seral species after the bee plant becomes established has shown to be effective at establishing native plant diversity as reclaimed well pads advance in age [52]. This is important as reclamation and restoration projects in arid environments have high potential for failure [53]. Another reason as to why the Rocky Mountain bee plant is commonly used in reclamation seed mixes in the Western US is because it is commercially available, while many other forbs are currently not [46]. While Russian thistle (Salsola kali) existed on well pads with the bee plant, it was not in the seed mix and we do not recommend the use of non-native plants in reclamation practices. We do not believe S. kali to have attracted many insects to the site as it was not flowering and was mostly senesced, and it should be noted that it is a non-native which often disappears rapidly after reclamation processes begin [54].
Our study was limited to one portion of the growing season in both years, which is common among other mass flowering studies [26,27,28]. While our results clearly demonstrate an increase in insect abundance and diversity on reclaimed sites during this portion of the growing season, future research comparing reclaimed well pads at different seral stages in earlier portions of the growing season would be beneficial in creating a temporal and spatial mosaic of diverse flowering plants which have different blooming cycles; which is likely to be beneficial to large numbers of insects over the course of their life cycle [28]. Additionally, further studies to determine the extent to which insects move among reclaimed sites within natural gas fields and the extent of which plants and other wildlife benefit from these insects will shed light on overall ecosystem functionality. Previous research has shown restoration efforts which increase insect abundance (as prey) have positive bottom-up impacts on food webs, benefiting predators (e.g., insectivorous birds) at higher trophic levels [55]. In terms of insect abundance and diversity, reclaimed well pads in this system show significantly higher numbers than adjacent reference sites.
5. Conclusions
In this study, we found significantly higher insect abundance and family richness on natural gas well pads undergoing interim reclamation when compared to reference areas within the Pinedale Anticline natural gas field. We found insect abundance on well pads reclaimed with the native annual forb, Rocky Mountain bee plant, to be 6–12× higher than reference areas and insect abundance on well pads reclaimed with native grasses to be 3–4× higher than reference areas. Overall, reclaimed well pads with the native forb had greater insect abundance and diversity than reclaimed well pads with native grasses. Pollinator abundance was 8× higher on reclaimed well pads with the Rocky Mountain bee plant than reference areas in 2015 and nearly 12× higher on reclaimed well pads with the Rocky Mountain bee plant than reference areas in 2016. Future research is needed to determine if the significant increase in insect abundance on reclaimed areas within the Pinedale Anticline natural gas field has positive impacts on food webs within the area as well as to determine if increased pollinators in the reclaimed areas have benefits to surrounding areas. Overall, this research suggests reclamation practices in the Pinedale Anticline natural gas field result in an improved insect habitat when compared to reference areas, both in terms of abundance and diversity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/land11040527/s1, Figure S1: A stacked bar chart of insect abundance by ecological role on each site type in 2015; Figure S2: A stacked bar chart of insect abundance by ecological role on each site type in 2016; Table S1: A table depicting potential ecological roles/functional groups of each insect family.
Author Contributions
Conceptualization, M.F.C.; Formal Analysis, M.F.C., T.J.R., T.M.C. and D.I.S.; Investigation, M.F.C. and P.G.; Methodology, M.F.C.; Project Administration, M.F.C., P.G. and P.D.S.; Resources, P.G., J.S., M.F.C. and P.D.S.; Supervision, P.D.S.; Validation, M.F.C.; Visualization, T.J.R., T.M.C. and M.F.C.; Writing—Original Draft, M.F.C.; Writing—Review and Editing, T.J.R., P.G., J.S., T.M.C., D.I.S. and P.D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This project received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the study being conducted on QEP Resources’ land.
Acknowledgments
For general advice, we thank David Legg at the University of Wyoming. For access to field sites, we are grateful for QEP Resources. We are thankful to Zoe Craft for helping organize insect spreadsheet data. Funding was provided by the Wyoming Reclamation and Restoration Center at University of Wyoming and Jonah Energy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Finn, S.P.; Knick, S.T. Changes in the Wyoming basins landscape from oil and natural gas development. In Sagebrush Ecosystem Conservation and Management: Ecoregional Assessment Tools and Models of the Wyoming Basins; Allen Press: Lawrence, KS, USA, 2011; pp. 46–68. [Google Scholar]
- Society for Ecological International Science and Policy Working Group. The SER International Primer on Restoration Ecology; Society for Ecological Restoration International: Tucson, AZ, USA, 2004. [Google Scholar]
- Wortley, L.; Hero, J.M.; Howes, M. Evaluating restoration success: A review of the literature. Restor. Ecol. 2013, 21, 537–543. [Google Scholar] [CrossRef]
- Curran, M.F.; Wolff, B.J.; Stahl, P.D. Approaching oil and gas pad reclamation with data management: A framework for the future. J. Am. Soc. Min. Reclam. 2013, 2, 195–204. [Google Scholar]
- Curran, M.F.; Stahl, P.D. Database management for large scale reclamation projects in Wyoming: Developing better data acquisition, monitoring, and models for applications to future projects. J. Environ. Solut. Oil Gas Min. 2015, 1, 31–43. [Google Scholar] [CrossRef]
- Curran, M.F.; Cox, S.E.; Robinson, T.J.; Robertson, B.L.; Rogers, K.A.; Sherman, Z.A.; Adams, T.A.; Strom, C.F.; Stahl, P.D. Spatially balanced sampling and ground-level imagery for revegetation monitoring on reclaimed well pads. Restor. Ecol. 2019, 27, 947–980. [Google Scholar] [CrossRef]
- Longcore, T. Terrestrial arthropods as indicators of ecological restoration success in coastal sage scrub (California, USA). Restor. Ecol. 2003, 11, 397–409. [Google Scholar] [CrossRef]
- Ruiz-Jaen, M.C.; Aide, T.M. Restoration success: How is it being measured? Restor. Ecol. 2005, 13, 569–577. [Google Scholar] [CrossRef]
- Walker, L.R.R.; Walker, J.; del Moral, R. Forging a new alliance between succession and restoration. In Linking Restoration and Ecological Succession; Walker, L.R.R., Walker, J., Hobbs, R.H., Eds.; Springer: New York, NY, USA, 2007; pp. 1–18. [Google Scholar]
- Albrecht, M.; Riesen, M.; Schmid, B. Plant-pollinator network assembly along the chronosequence of a glacier foreland. Oikos 2010, 119, 1610–1624. [Google Scholar] [CrossRef]
- Devoto, M.; Bailey, S.; Craze, P.; Memmott, J. Understanding and planning ecological restoration of plant-pollinator networks. Ecol. Lett. 2012, 15, 319–328. [Google Scholar] [CrossRef]
- Menz, M.M.H.; Phillips, R.D.; Winfree, R.; Kremen, C.; Aizen, M.A.; Johnson, S.D.; Dixon, K.W. Reconnecting plants and pollinators: Challenges in the restoration of pollination mutualisms. Trends Plant Sci. 2011, 16, 4–12. [Google Scholar] [CrossRef]
- Brophy, C.; Dooley, A.; Kirwan, L.; Finn, J.A.; McDonnell, J.; Bell, T.; Cadotte, M.W.; Connolly, J. Biodiversity and ecosystem function: Making sense of numerous species interactions in multi-species communities. Ecology 2017, 98, 1771–1778. [Google Scholar] [CrossRef]
- Losey, J.E.; Vaughan, M. The economic value of ecological services provided by insects. Bioscience 2006, 56, 311–323. [Google Scholar] [CrossRef]
- Dangles, O.; Casas, J. Ecosystem services provided by insects for achieving sustainable development goals. Ecosyst. Serv. 2019, 35, 109–115. [Google Scholar] [CrossRef]
- Cusser, S.; Goodell, K. Diversity and distribution of floral resources influence the restoration of plant-pollinator networks on a reclaimed strip mine. Restor. Ecol. 2013, 26, 713–721. [Google Scholar] [CrossRef]
- Tonietto, R.K.; Larkin, D.J. Habitat restoration benefits wild bees: A meta-analysis. J. Appl. Ecol. 2018, 55, 582–590. [Google Scholar] [CrossRef]
- Harmon, J.P.; Ganguli, A.C.; Solga, M.A. An overview of pollination in rangelands: Who, why, and how. Rangelands 2011, 33, 4–9. [Google Scholar] [CrossRef][Green Version]
- United States Department of the Interior; United States Department of Agriculture. The Gold Book: Surface Operating Standards and Guidelines for Oil and Gas Exploration and Development, 4th ed.; BLM/WO/ST-06/021+3071/REV 07; Bureau of Land Management: Denver, CO, USA, 2007.
- Pickett, S.T.A.; Parker, V.T. Avoiding the old pitfalls: Opportunities in a new discipline. Restor. Ecol. 1994, 2, 75–79. [Google Scholar] [CrossRef]
- Aronson, J.; Dhillion, S.; le Floc’h, E. On the need to select a reference site, however imperfect: A reply to Pickett and Parker. Restor. Ecol. 1995, 3, 1–3. [Google Scholar] [CrossRef]
- Monroe, A.P.; Aldridge, C.L.; O’Donnell, M.S.; Manier, D.J.; Homer, C.G.; Anderson, P.J. Using remote sensing products to predict recovery of vegetation across space and time following energy development. Ecol. Indic. 2020, 110, 105872. [Google Scholar] [CrossRef]
- Sommers, J. Green River Drift: A History of the Upper Green River Cattle Association; Falcon Press Publishing Co., Inc.: Helena, MT, USA, 1994. [Google Scholar]
- Harmon-Threatt, A.N.; Chin, J. Common methods for tallgrass prairie restoration and their potential effects on bee diversity. Nat. Areas J. 2016, 36, 400–411. [Google Scholar] [CrossRef]
- Buckles, B.J.; Harmon-Threatt, A.N. Bee diversity in tallgrass prairies affected by management and its effect on above-and-below-ground resources. J. Appl. Ecol. 2019, 56, 2443–2453. [Google Scholar] [CrossRef]
- Westphal, C.; Steffan-Dewenter, I.; Tscharntke, T. Mass flowering crops enhance pollinator densities at landscape scales. Ecol. Lett. 2003, 6, 961–965. [Google Scholar] [CrossRef]
- Holzschuh, A.; Dormann, C.F.; Tscharntke, T.; Steffan-Dewenter, I. Mass-flowering crops enhance wild bee abundance. Oecologia 2013, 172, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Todd, K.J.; Gardiner, M.M.; Lindquist, E.D. Mass flowering crops as a conservation resource for wild pollinators (Hymenoptera: Apoidea). J. Kans. Entomol. Soc. 2016, 89, 158–167. [Google Scholar] [CrossRef]
- Rundlöf, M.; Persson, A.S.; Smith, H.G.; Bommarco, R. Late-season mass-flowering red clover increases bumble bee queen and male densities. Biol. Conserv. 2014, 172, 138–145. [Google Scholar] [CrossRef]
- Wenninger, E.J.; Inouye, R.S. Insect community response to plant diversity and productivity in a sagebrush-steppe ecosystem. J. Arid Environ. 2008, 72, 24–33. [Google Scholar] [CrossRef]
- Martin, C.A.; Guldan, S.J. Sagebrush as a short-term arthropod repellent. J. Sustain. Agric. 1998, 11, 77–85. [Google Scholar] [CrossRef]
- Price, P.W. The plant vigor hypothesis and herbivore attack. Oikos 1991, 62, 244–251. [Google Scholar] [CrossRef]
- Pan, V.S.; Pepi, A.; Goidell, J.; Karban, R. Retention of green leaves not brown leaves increases spring cynipid diversity on large valley oaks. Arthropod-Plant Interact. 2021, 15, 353–362. [Google Scholar] [CrossRef]
- Flieschner, T.L. Ecological costs of livestock grazing in western North America. Conserv. Biol. 1994, 8, 629–644. [Google Scholar] [CrossRef]
- Debano, S.J. Effects of livestock grazing on aboveground insect communities in semi-arid grasslands of southeastern Arizona. Biodivers. Conserv. 2006, 15, 2547–2564. [Google Scholar] [CrossRef]
- Holechek, J.L.; de Souza Gomes, H.; Molinar, F.; Galt, D. Grazing intensity: Critique and approach. Rangel. Arch. 1998, 20, 15–18. [Google Scholar]
- Shapira, T.; Henkin, Z.; Dag, A.; Mandelik, Y. Rangeland sharing by cattle and bees: Moderate grazing does not impair bee communities and resource availability. Ecol. Appl. 2020, 30, e02066. [Google Scholar] [CrossRef]
- Cagney, J.; Cox, S.E.; Booth, D.T. Comparison of point intercept and image analysis for monitoring rangeland composition and trend. Rangel. Ecol. Manag. 2011, 64, 309–315. [Google Scholar] [CrossRef]
- Curran, M.F.; Cox, S.E.; Robinson, T.J.; Strom, C.F.; Stahl, P.D. Combining spatially balanced sampling, route optimization and remote sensing to assess biodiversity response to reclamation practices on semi-arid well pads. Biodiversity 2020, 21, 1–11. [Google Scholar] [CrossRef]
- Curran, M.F.; Hodza, P.; Cox, S.E.; Lanning, S.G.; Robertson, B.L.; Robinson, T.J.; Stahl, P.D. Ground-level unmanned aerial system imagery coupled with spatially balanced sampling and route optimization to monitor rangeland vegetation. J. Vis. Exp. 2020, 160, e61052. [Google Scholar] [CrossRef]
- Booth, D.T.; Cox, S.E.; Berryman, R.D. Sampling digital imagery with ‘SamplePoint’. Environ. Monit. Assess. 2006, 123, 97–108. [Google Scholar] [CrossRef]
- Milne, L.; Milne, M. National Audubon Society: Field Guide to North American Insects and Spiders; No. 595.7 M659; Toppan Printing Co., Ltd.: Tokyo, Japan, 2011. [Google Scholar]
- McCall, C.; Primack, R.B. Influence of flower characteristics, weather, time of day, and season on insect visitation rates in three plant communities. Am. J. Bot. 1992, 79, 434–442. [Google Scholar] [CrossRef]
- Solga, M.J.; Harmon, J.P.; Ganguli, A.C. Timing is everything: An overview of phenological changes to plants and their pollinators. Nat. Areas J. 2014, 34, 227–235. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: https://www.Rproject.org/ (accessed on 1 March 2022).
- Curran, M.F.; Crow, T.M.; Hufford, K.M.; Stahl, P.D. Forbs and greater sage-grouse habitat restoration efforts: Suggestions for improving commercial seed availability and restoration practices. Rangelands 2015, 37, 211–216. [Google Scholar] [CrossRef]
- Dumroese, R.K.; Luna, T.; Pinito, J.R.; Landis, T.D. Forbs: Foundation for restoration of monarch butterflies, other pollinators, and greater sage-grouse in Western United States. Nat. Areas J. 2016, 36, 499–511. [Google Scholar] [CrossRef]
- Curran, M.F.; Summerfield, K.; Alexander, E.-J.; Lanning, S.G.; Schwyter, A.R.; Torres, M.L.; Schell, S.; Vaughan, K.; Robinson, T.J.; Smith, D.I. Use of 3-dimensional videography as a non-lethal way to improve visual insect sampling. Land 2020, 9, 340. [Google Scholar] [CrossRef]
- Prasser, N.K.; Hilld, A.L. Competitive interactions between an exotic annual, Halogeton glomeratus, and 10 North American native species. Nativ. Plants J. 2016, 17, 244–255. [Google Scholar] [CrossRef]
- Leger, E.A.; Goergen, E.M.; de Queiroz, T.F. Can native annual forbs reduce Bromus tectorum biomass and indirectly facilitate establishment of a native perennial grass? J. Arid Environ. 2014, 102, 9–16. [Google Scholar] [CrossRef]
- Cane, J.H. Breeding biologies, seed production and species-rich bee guilds of Cleome lutea and Cleome serrulata (Cleomaceae). Plant Species Biol. 2008, 23, 152–158. [Google Scholar] [CrossRef]
- Curran, M.F.; Sorenson, J.; Stahl, P.D. Rocky mountain beeplant aids revegetation in an arid natural gas field. Environ. Connect. 2019, 14, 27–29. [Google Scholar]
- Shackelford, N.; Paterno, G.B.; Winkler, D.E.; Erickson, T.E.; Leger, E.A.; Svejcar, L.N.; Breed, M.F.; Faist, A.M.; Harrison, P.A.; Curran, M.F.; et al. Drivers of seedling establishment success in dryland restoration efforts. Nat. Ecol. Evol. 2021, 5, 1283–1290. [Google Scholar] [CrossRef]
- Lodhi, M.A.K. Allelopathic potential of Salsola kali L. and its possible role in rapid disappearance of weedy stage during revegetation. J. Chem. Ecol. 1979, 5, 429–437. [Google Scholar] [CrossRef]
- Loch, J.M.; Walters, L.J.; Cook, G.S. Recovering trophic structure through habitat restoration: A review. Food Webs 2020, 25, e00162. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).