Influence of Forest Conditions on the Spread of Scots Pine Blister Rust and Red Ring Rot in the Priangarye Pine Stands
Abstract
1. Introduction
2. Materials and Methods
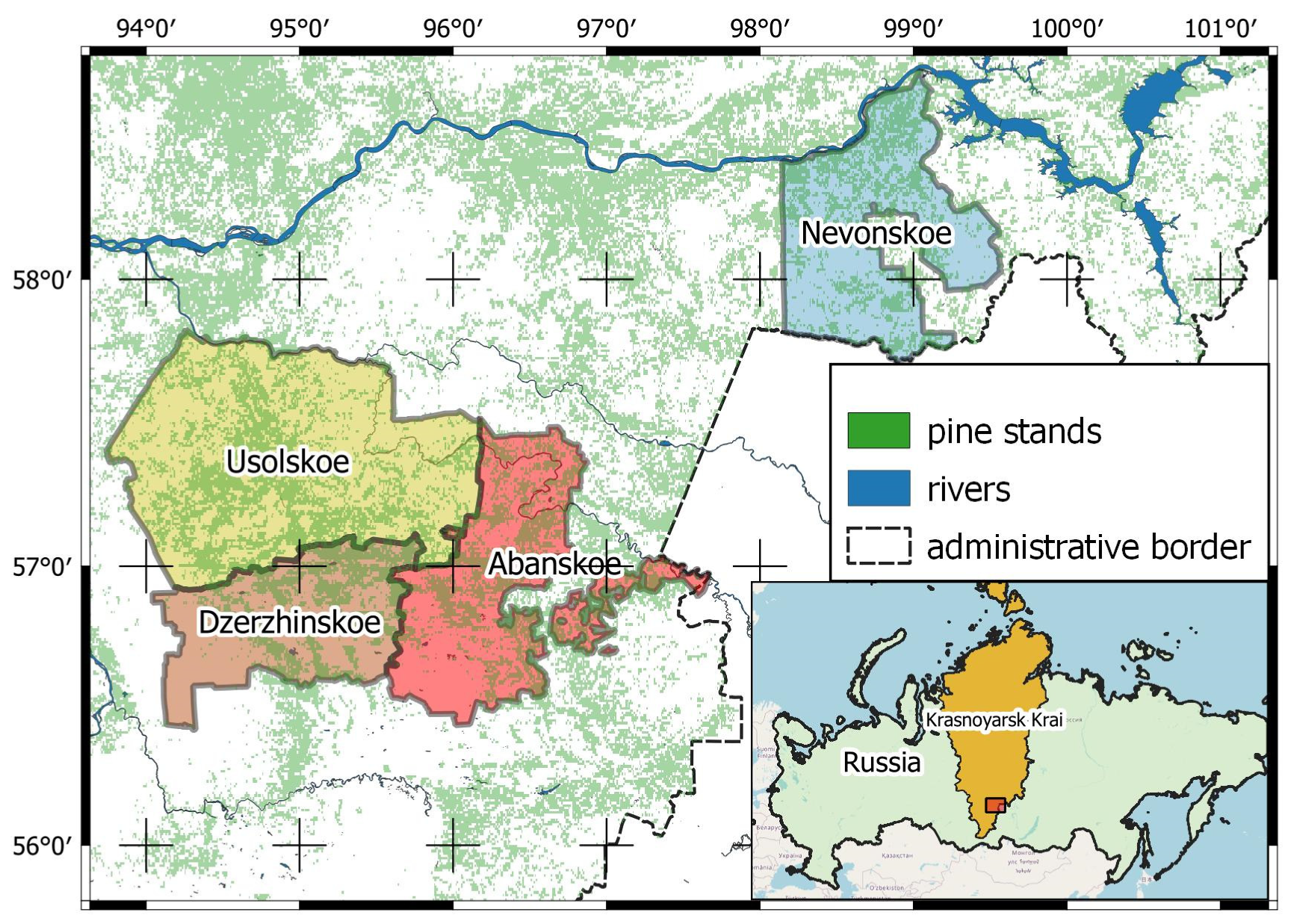
2.1. Study Area and Objects
2.2. Fieldwork Methods
2.3. Materials Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giertych, M.; Mátyás, C. Genetics of Scots Pine. In Developments in Plant Genetics and Breeding; Elsevier Science Publisher: Amsterdam, The Netherland, 1991; Volume 3, p. 280. [Google Scholar]
- Buzykin, A.I. The resource and ecological potential of forests of the Krasnoyarsk region. Conifers Boreal Zone 2008, XXV, 327–332. [Google Scholar]
- Onuchin, A.; Burenina, T.; Ziryukina, N.; Gaparov, K. Land use impacts on river hydrological regimes in Northern Asia. Iahs-Aish Publ. 2009, 331, 163–170. [Google Scholar]
- Onuchin, A.; Burenina, T.; Ziryukina, N.; Farber, S. Impact of forest harvesting and forest regeneration on runoff dynamics at watersheds of Central Siberia. Sib. J. For. Sci. 2014, 1, 110–118. [Google Scholar]
- Government Report on Condition of the Environment and Protective Measures in the Krasnoyarsk Territory in 2018. In Ministry of Natural Resources and Ecology for Krasnoyarsk Krai; Regional State Budgetary Institution Center for the Implementation of Measures for the Use of Natural Resources and Environmental Protection of the Krasnoyarsk Krai: Krasnoyarsk, Russia, 2019; p. 298.
- Chakraborty, S.; Tiedemann, A.V.; Teng, P.S. Climate change: Potential impact on plant diseases. Environ. Pollut. 2000, 108, 317–326. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Trumbore, S.; Brando, P.; Hartmann, H. Forest health and global change. Science 2015, 349, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Shirk, A.J.; Cushman, S.A.; Waring, K.M.; Wehenkel, C.A.; Leal-Sáenz, A.; Toney, C.; Lopez-Sanchez, C.A. Southwestern white pine (Pinus strobiformis) species distribution models project a large range shift and contraction due to regional climatic changes. For. Ecol. Manag. 2018, 411, 176–186. [Google Scholar] [CrossRef]
- Haagsma, M.; Page, G.F.M.; Johnson, J.S.; Still, C.; Waring, K.M.; Sniezko, R.A.; Selker, J.S. Using Hyperspectral Imagery to Detect an Invasive Fungal Pathogen and Symptom Severity in Pinus strobiformis Seedlings of Different Genotypes. Remote Sens. 2020, 12, 4041. [Google Scholar] [CrossRef]
- Moriondo, F. Caratteristiche epidemiche della ruggine vescicolosa del Pino: Cronartium flaccidum (Alb. et Schw.) Wint in Italia. Ann. Accad. Ital. Sci. For. 1975, 24, 331–406. [Google Scholar]
- Diamandis, S.; De Kam, M. A severe attack of Scots pine blister rust in N. Greece. Eur. J. Plant Pathol. 1986, 16, 247–249. [Google Scholar]
- Greig, B.J.W. History of Peridermium stem rust of Scots pine (Pinus sylvestris L.) in Thetford Forest, East Anglia. Forestry 1987, 60, 193–202. [Google Scholar] [CrossRef]
- Kaitera, J. Analysis of Cronartium flaccidum lesion development on pole-stage Scots pines. Silva Fenn. 2000, 34, 21–27. [Google Scholar] [CrossRef]
- Davis, C.; Meyer, T. Field Guide to Tree Diseases of Ontario; Natural Resources Canada, Canadian Forest Service: Sault Ste. Maire, ON, Canada, 1997.
- Fedorov, N.I.; Yarmolovich, V.A. Scots pine blister rust in the forests of Belarus. Mushroom Communities For. Ecosyst. 2004, 2, 239–254. [Google Scholar]
- Ozkazanc, N.; Maden, S. Some important shoot and stem fungi in pine (Pinus spp.) and firs (Abies sp.) in western Blacksea region, Turkey. Bartın Orman Fakültesi Derg. 2013, 15, 32–38. [Google Scholar]
- Szewczyk, W.; Kwaśna, H.; Behnke-Borowczyk, J.; Baranowska-Wasilewska, M. Phylogenetic relationships among Porodaedalea pini from Poland and related Porodaedalea species. Cent. Eur. J. Biol. 2014, 9, 614–627. [Google Scholar] [CrossRef]
- Kaitera, J.; Hiltunen, R.; Kauppila, T.; Hantula, J. Five plant families support natural sporulation of Cronartium ribicola and C. flaccidum in Finland. Eur. J. Plant Pathol. 2017, 149, 367–383. [Google Scholar] [CrossRef]
- Ezhov, O.N. Distribution of the Pine Sponge (Phellinus pini [Thore ex. Fr.] Pil.) in the Middle Subzone of the Taiga and the Limitation of its Harmfulness: Extended Abstract of Dissertation; St. Petersburg State Forestry Academy: St. Petersburg, Russia, 1998; 18p. [Google Scholar]
- Gninenko, Y.I. The main diseases of the Russian forests and their economic significance, Problems of forest phytopathology and mycology. In Proceedings of the 5th International Conference “Problems of Forest Phytopathology and Mycology”, Moskva, Russia, 7–10 October 2002; All-Russian Research Institute for Silviculture and Mechanization of Forestry: Moscow, Russia, 2002; pp. 63–66. [Google Scholar]
- Maslov, A.A. Scots pine blister rust as a factor of natural mortality in boreal pine forests: 20 years of monitoring in forest reserves. In Proceedings of the 5th International Conference “Problems of Forest Phytopathology and Mycology”, Moskva, Russia, 7–10 October 2002; All-Russian Research Institute for Silviculture and Mechanization of Forestry: Moscow, Russia, 2002; pp. 155–158. [Google Scholar]
- Storozhenko, V.G. Rotten lesion of the primary Forests of spruce and pine formations on the Russian plain. For. Bull. 2003, 2, 77–81. [Google Scholar]
- Churakov, B.P.; Kandrashkin, A.I. Infection of pine forest stands with a pine sponge in various types of forests and its impact on the output of business wood. For. J. 2009, 3, 37–41. [Google Scholar]
- Vlasenko, V.A. Ecological characteristics of bracket fungi in the forest steppe of Western Siberia. Contemp. Probl. Ecol. 2013, 6, 390–395. [Google Scholar] [CrossRef]
- Bubnova, M.A.; Leontiev, D.F. Tree wart in the forests of the Shelekhovskoye forestry in 2016–2019 (southern Baikalia). Biosph. Econ. Theory Pract. 2020, 5, 96–103. [Google Scholar]
- Samils, B.; Ihrmark, K.; Kaitera, J.; Hansson, P.; Barklund, P. Genetic structure of scots pine blister rust (Cronartium flaccidum and Peridermium pini). Phytopathol. Mediterr. 2010, 49, 428. [Google Scholar]
- Wulff, S.; Liendelow, A.; Lundin, L.; Hansson, P.; Axelsson, A.-L.; Barklund, P.; Wijk, S.; Stahl, G. Adapting forest health assessments to changing perspectives on threats—A case example from Sweden. Environ. Monit. Assess. 2012, 184, 2453–2464. [Google Scholar] [CrossRef]
- Skyttä, V. Tervasroso piinaa pohjoisessa. Metsälehti 2017, 6, 2. [Google Scholar]
- Nevalainen, S.; Nuorteva, H.; Pouttu, A. Metsätuhot Vuonna 2017; Luonnonvarakeskus: Helsinki, Finland, 2018; Available online: https://jukuri.luke.fi/bitstream/handle/10024/542725/luke-luobio_44_2018.pdf (accessed on 5 April 2020).
- Normark, E. Multiskadad Ungskog I Västerbottens—Och Norrbottens Län. Möjliga Åtgärder För Att Mildra Problemen; Skogsstyrelsen: Mars, Sweden, 2019; Volume 10, p. 24.
- Samils, B.; Kaitera, J.; Persson, T.; Stenlid, J.; Barklund, P. Relationship and genetic structure among autoecious and heteroecious populations of Cronartium pini in northern Fennoscandia. Fungal Ecol. 2021, 50, 101032. [Google Scholar] [CrossRef]
- Tatarintsev, A.I.; Aminev, P.I. Infestation of pine forests with Scots pine blister rust in the Krasnoyarsk Priangarye: Eco-coenotic features of the prevalence of the disease. Conifers Boreal Zone 2014, XXXII, 58–65. [Google Scholar]
- Tatarintsev, A.I. Stem rot in anthropogenically disturbed pine forests of the Krasnoyarsk Priangarye. Conifers Boreal Zone 2016, XXXIV, 259–265. [Google Scholar]
- Konev, G.I. Central rot of pine in the Angara region. Forestry 1982, 8, 68–69. [Google Scholar]
- Tatarintsev, A.I. To the question of the regularities of the development of stem rot in the pine forests of the Krasnoyarsk Priangarye. For. Inventory For. Manag. 1996, 32–36. [Google Scholar]
- Fedorov, N.I. On the issue of contamination of plantations in Belovezhskaya Pushcha with a pine sponge. Forest J. 1963, 5, 45–47. [Google Scholar]
- Drachkov, V.N. Trunk cancer in the plantations of the European North. Forestry 1972, 10, 64–65. [Google Scholar]
- Churakov, B.P. Fungi and Fungal Diseases of Scotch Pine in the Band Pine Forests of Altai Krai; Irkutsk. Gos. Univ.: Irkutsk, Russia, 1983; p. 151. [Google Scholar]
- Vaskov, S.P.; Alekseev, I.A. Pine resin cancer in the Mari ASSR. Forestry 1991, 9, 52–53. [Google Scholar]
- Mertvishchev, L.V. The occurrence of pine sponges in the pine forests of the Angara region. For. Inf. Cent. Bur. Sci. Tech. Inf. 1979, I, 19–20. [Google Scholar]
- Ivanchikov, A.A. Qualitative characteristics of pine forest stands. Pine For. Karelia Elev. Prod. 1974, 72–84. [Google Scholar]
- Kartavenko, N.T. Fungus Disease of Pine in the Island Pine Wood of the Trans-Urals Forest-Steppe; Institute of Biology Uralsk. Branch of the USSR Academy of Sciences: Sverdlovsk, Russia, 1960; Volume 15, pp. 107–130. [Google Scholar]
- Lyubarsky, L.V. Wood-destroying mushrooms of the Soviet Far East, their study, significance and control measures. Collect. Far East For. Res. Inst. 1963, 5, 132–164. [Google Scholar]
- Ventsenostsev, M.A. Distribution patterns of wood defects in pine forests. For. J. 1967, 3, 156–157. [Google Scholar]
- Zhukov, A.M. Pathogenic Fungi of the Forests of the Southern Ob Region; Lomonosov Moscow State University: Moscow, Russia, 1982; p. 397. [Google Scholar]
- Kaitera, J.; Jalkanen, R. Distribution of Endocronartium pini in northern Finland. In Proceedings of the 4th IUFRO Rusts of Pines Working Party Conference, Tsukuba, Japan, 2–7 October 1995; pp. 115–118. [Google Scholar]
- Smirnova, V.K. Infection of Pine Forests with Pine Sponge—Phellinus Pini—In Various Groups of Forest Types (Green Moss and Dense-Pine Forests) and Its Influence on the Output of Commercial Wood. Ph.D. Thesis, Institute of Biology Uralsk. Branch of the USSR, Sverdlovsk, Russia, 1968. [Google Scholar]
- Korotkov, I.A. Forest growing zoning of Russia and the republics of the former USSR. In Carbon in the Ecosystems of Forests and Swamps of Russia; Alekseev, V.A., Birdsey, R.A., Eds.; Forest Institute of the Siberian Branch of the Russian Academy of Sciences: Krasnoyarsk, Russia, 1994; pp. 29–47. [Google Scholar]
- Mozolevskaya, E.G.; Kataev, O.A.; Sokolova, E.S. Methods of Forest Pathological Examination of Foci of Stem Pests and Forest Diseases; Lesnaya Promyshlennost: Moscow, Russia, 1984; p. 152. [Google Scholar]
- Tuzova, V.K. (Ed.) Methods of Monitoring Pests and Forest Disease; All-Russian Research Institute for Silviculture and Mechanization of Forestry: Tuzov, Russia, 2004; 200p. [Google Scholar]
- Guidelines for Planning, Organizing and Conducting Forest Pathological Examinations; Appendix 3 to the Order of Federal Forestry Agency Dated December 29; Federal Forestry Agency: Moscow, Russia, 2007; Volume 523, p. 74.
- Cheremisinov, N.A.; Negrutsky, S.F.; Leshkovtseva, I.I. Mushrooms and Fungal Diseases of Trees and Shrubs; Forest Industry: Moscow, Russia, 1970; p. 392. [Google Scholar]
- Zhuravlev, I.I.; Selivanova, T.N.; Cheremisinov, N.A. Keys to Fungal Diseases of Trees and Shrubs; Forest Industry: Moscow, Russia, 1979; p. 246. [Google Scholar]
- Kuzmichev, E.P.; Sokolova, E.S.; Mozolevskaya, E.G. Diseases of Woody Plants: Handbook. In Diseases and Insect Pests in Forests of Russia; All-Russian Research Institute for Silviculture and Mechanization of Forestry: Moscow, Russia, 2004; Volume I. [Google Scholar]
- Zhuravlyov, I.I. The Diagnostics of Forest Diseases; Publishing House of Agricultural Literature, Magazines and Posters: Moscow, Russia, 1962; p. 192. [Google Scholar]
- Sukachev, V.N. Selected Works; Nauka: Moscow, Russia, 1972; p. 345. [Google Scholar]
- Melehov, I.S. Lesnaya Tipologiya [Forest Typology]; Moscow Forestry Institute: Moscow, Russia, 1976; p. 72. [Google Scholar]
- Churakov, B.P. Scots pine blister rust in the Barnaul ribbon pine forest. Mycol. Phytopathol. 1986, 20, 317–321. [Google Scholar]
- Minkevich, I.I.; Dorofeeva, T.B.; Kovyazin, V.F. Plant Pathology. Diseases of Tree and Shrub Species; Publishing House “Lan”: St. Petersburg, Russia, 2011; p. 160. [Google Scholar]
- Guseva, A.N. Blister rust in pine forests of Southern Yakutia. Forestry 1957, 3, 39–41. [Google Scholar]
- Belov, D.A.; Belova, N.K. Distribution of Scots Pine Blister Rust in Urban Forests of the Korolev City. Materials of the XV International Scientific-Practical Conference “Problems of Greening of Large Cities”; All-Russian Exhibition Center: Moscow, Russia, 2012; pp. 26–29. [Google Scholar]
- Romanovsky, V.P.; Kochanovsky, S.V.; Mikhalevich, P.K. Some patterns of distribution of a pine sponge rot. Belovezhskaya Pushcha 1973, 7, 12–24. [Google Scholar]
- Arefiev, S.P. Rotting Diseases of Siberian Pine in the Forests of the Priirtysh Region Middle Taiga: Extended Abstract. Ph.D. Thesis, Ural Forest Engineering Institute, Sverdlovsk, Russia, 1990; p. 22. [Google Scholar]
- Tatarintsev, A.I. Dynamics of the prevalence of pine stem rot depending on the inventory indicators of plantations in the conditions of the Krasnoyarsk Priangarye. For. Inventory For. Manag. 1995, 132–138. [Google Scholar]
| Studied Diseases | Total | Including Distribution by Pine Forest Types | ||
|---|---|---|---|---|
| Lichen | Mossy | Herb-Rich | ||
| Blister rust | 63 | 13 | 24 | 26 |
| Red ring rot | 75 | 22 | 27 | 26 |
| Studied Diseases | Including Distribution by Pine Forest Types | ||
|---|---|---|---|
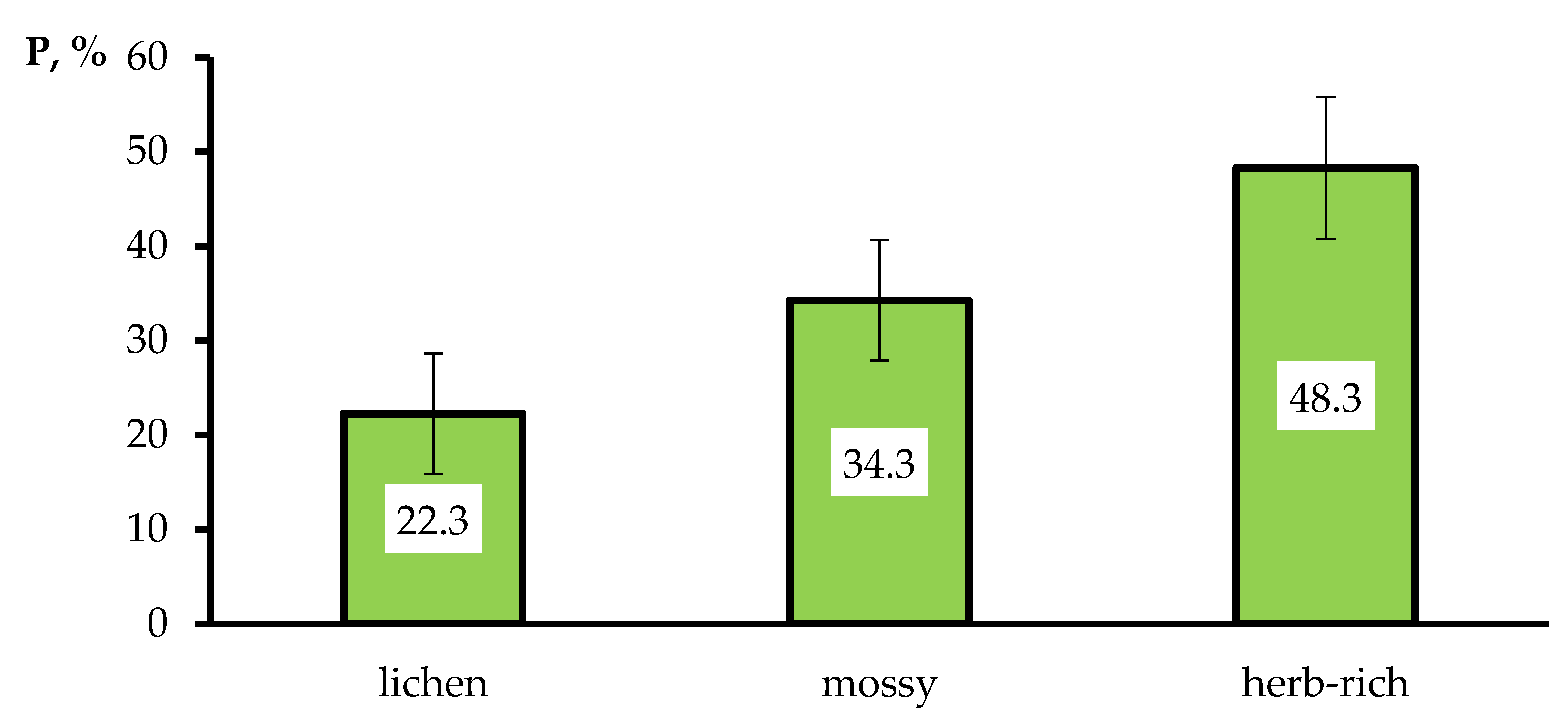
| Lichen | Mossy | Herb-Rich | |
| Blister rust | 11.4 ± 2.0 | 4.2 ± 0.6 | 4.8 ± 0.9 |
| 1.9–28.2 | 0.4–12.7 | 0–16.5 | |
| Red ring rot | 31.1 ± 3.3 | 37.6 ± 3.4 | 44.2 ± 3.6 |
| 0–51.4 | 4.4–70.0 | 14.9–75.0 | |
| Compared Samples Distributed by Forest Types | Studied Diseases | |
|---|---|---|
| Blister Rust | Red Ring Rot | |
| lichen—mossy | 46 (<0.05) | 10 (>0.05) |
| lichen—herb-rich | 63 (<0.05) | 5 (<0.05) |
| mossy—herb-rich | 290 (>0.05) | 11 (>0.05) |
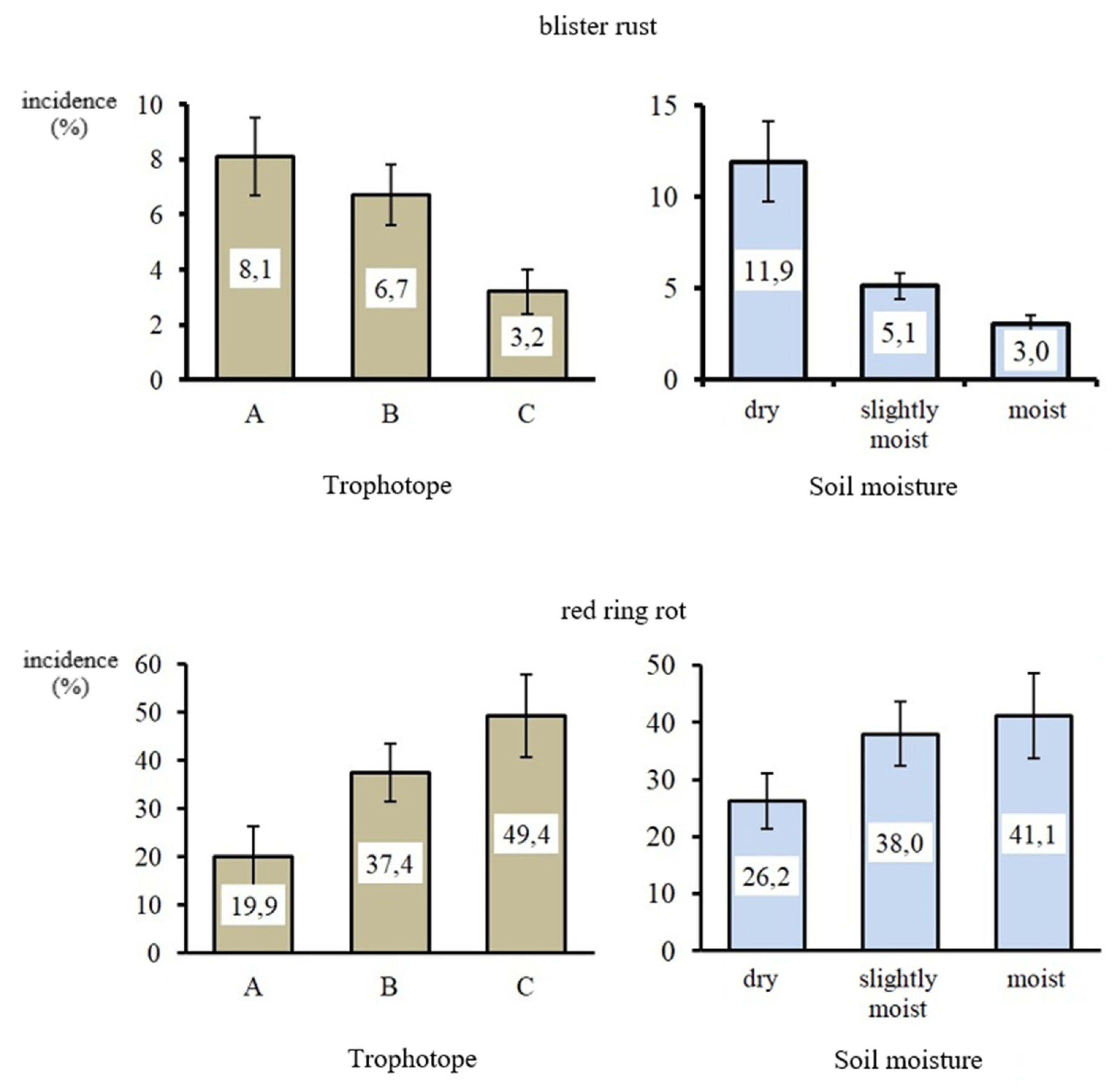
| Compared Samples in the Gradations of Edaphotope | Studied Diseases | |
|---|---|---|
| Blister Rust | Red Ring Rot | |
| trophotope | ||
| A–B | 130 (>0.05) | 8 (>0.05) |
| A–C | 29 (<0.05) | 4 (<0.05) |
| B–C | 171 (<0.05) | 10 (>0.05) |
| soil moistening | ||
| dry—slightly moist | 86 (<0.05) | 2 (<0.05) |
| dry—moist | 14 (<0.05) | 4 (>0.05) |
| slightly moist—moist | 180 (>0.05) | 11 (>0.05) |
| Studied Diseases | Checking Samples (Incidence) for Normal Distribution: dK-S (p-Value) | Correlation Coefficient (p-Value) |
|---|---|---|
| Blister rust | dK-S = 0.195 (p < 0.05) | 0.345 (p < 0.05) 1 |
| Red ring rot | dK-S = 0.047 (p > 0.05) | −0.047 (p > 0.05) 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatarintsev, A.I.; Aminev, P.I.; Mikhaylov, P.V.; Goroshko, A.A. Influence of Forest Conditions on the Spread of Scots Pine Blister Rust and Red Ring Rot in the Priangarye Pine Stands. Land 2021, 10, 617. https://doi.org/10.3390/land10060617
Tatarintsev AI, Aminev PI, Mikhaylov PV, Goroshko AA. Influence of Forest Conditions on the Spread of Scots Pine Blister Rust and Red Ring Rot in the Priangarye Pine Stands. Land. 2021; 10(6):617. https://doi.org/10.3390/land10060617
Chicago/Turabian StyleTatarintsev, Andrey I., Pavel I. Aminev, Pavel V. Mikhaylov, and Andrey A. Goroshko. 2021. "Influence of Forest Conditions on the Spread of Scots Pine Blister Rust and Red Ring Rot in the Priangarye Pine Stands" Land 10, no. 6: 617. https://doi.org/10.3390/land10060617
APA StyleTatarintsev, A. I., Aminev, P. I., Mikhaylov, P. V., & Goroshko, A. A. (2021). Influence of Forest Conditions on the Spread of Scots Pine Blister Rust and Red Ring Rot in the Priangarye Pine Stands. Land, 10(6), 617. https://doi.org/10.3390/land10060617






