Changes in Woody Vegetation over 31 Years in Farmed Parkland of the Central Plateau, Burkina Faso
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Site
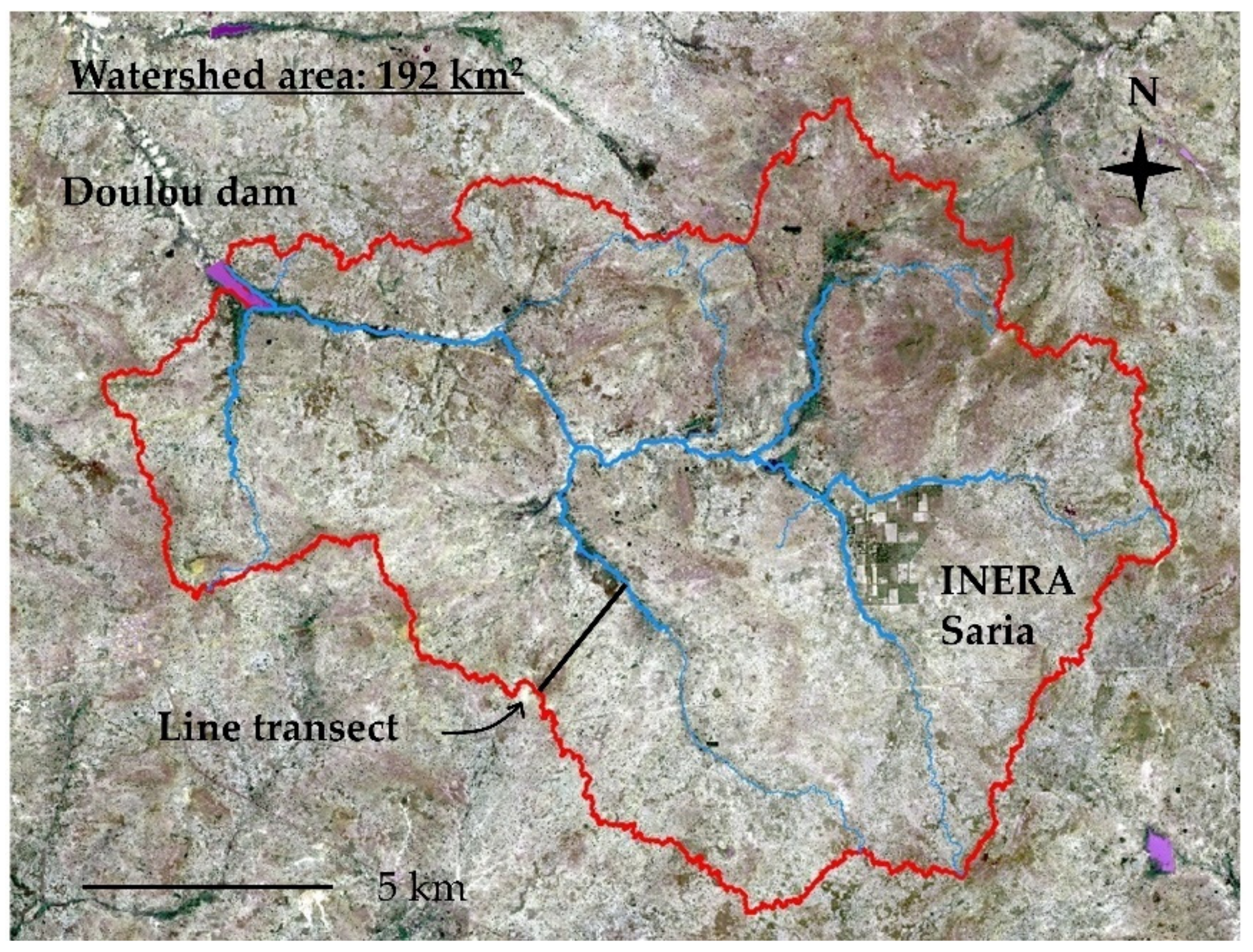
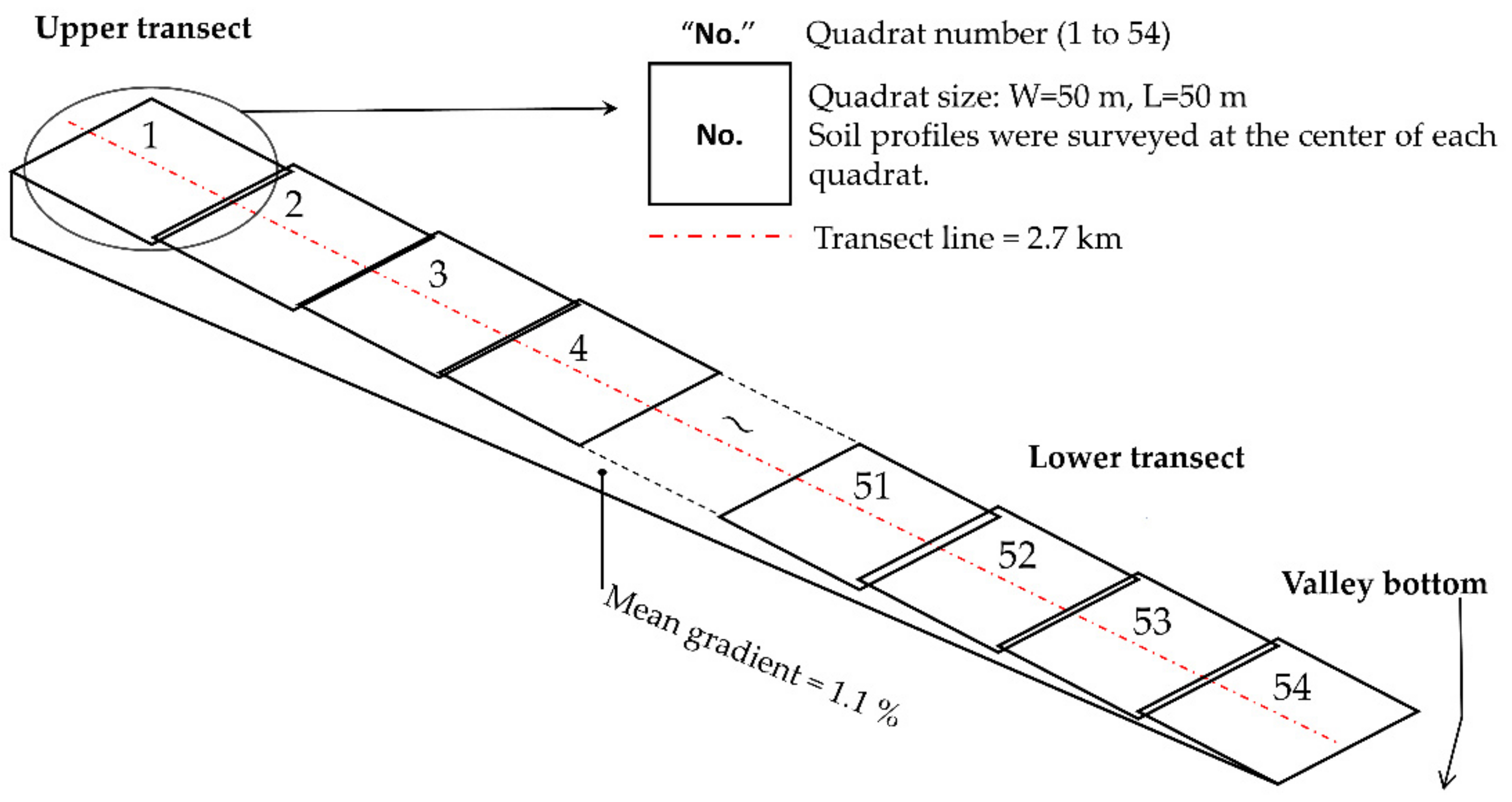
2.2. Soil Survey
2.3. Woody Vegetation Survey
2.4. Vegetation Parameters
2.5. Survey of Residents on Preferred Useful Trees
2.6. Comparison with Previous Research
3. Results
3.1. Composition of the Woody Vegetation
3.2. Soil Distribution and Properties
3.3. Relationships between Topographic Position, Soil Type, and Woody Vegetation
3.4. Preferred Useful Trees
4. Discussion
4.1. Composition of Wood Vegetation
4.2. Relationships between Topographic Position, Soil Type, and Woody Vegetation
4.3. Changes in Woody Vegetation over the Last 31 Years
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pullan, R.A. Farmed parkland in West Africa. Savanna 1974, 3, 119–131. [Google Scholar]
- Cole, M.M. The Savannas: Biogeography and Geobotany; Academic Press: London, UK, 1986. [Google Scholar]
- Boffa, J.-M. Agroforestry Parklands in Sub-Saharan Africa; FAO Conservation Guide 34; Forestry Department, Food and Agriculture Organization of the United Nations: Rome, Italy, 1999. [Google Scholar]
- Tomomatsu, Y. Parkia biglobosa-dominated cultural landscape: An ethnohistory of the Dagomba political institution in farmed parkland of northern Ghana. J. Ethnobiol. 2014, 34, 153–174. [Google Scholar] [CrossRef]
- Pélissier, P. L’Arbre dans les paysages agraires de l’Afrique Noire. Cah. ORSTOM (Sci. Hum.) 1980, 17, 131–136. (In French) [Google Scholar]
- Seignobos, C. Vegetations anthropiques dans la zone soudano-sahélienne: La problématique des Parcs. Rev. Geog. Cameroun 1982, 3, 1–23. (In French) [Google Scholar]
- Pouliot, M.; Treue, T.; Obiri, B.D.; Ouedraogo, B. Deforestation and the limited contribution of forests to rural livelihoods in West Africa: Evidence from Burkina Faso and Ghana. Ambio 2012, 41, 738–750. [Google Scholar] [CrossRef]
- Middleton, N.J.; Thomas, D.S.G. World Atlas of Desertification, 2nd ed.; Arnold: London, UK, 1997. [Google Scholar]
- Angeluccetti, I.; Coviello, V.; Grimaldi, S.; Vezza, P.; Koussubé, A. Soil conservation in Burkina Faso: Is international cooperation effective? In Proceedings of the 19th European Geosciences Union General Assembly (EGU 2017), Vienna, Austria, 23–28 April 2017; p. 1541. [Google Scholar]
- FAO. Global Guidelines for the Restoration of Degraded Forests and Landscapes in Drylands: Building Resilience and Benefiting Livelihoods; FAO Forestry Paper 175; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015. [Google Scholar]
- Slingerland, M.; Masdewel, M. Mulching on the Central Plateau of Burkina Faso. In Sustaining the Soil: Indigenous Soil and Water Conservation in Africa; Reij, C., Scoones, I., Toulmin, C., Eds.; Earthscan: London, UK, 1996; pp. 85–89. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015. [Google Scholar]
- Jones, A.; Breuning-Madsen, H.; Brossard, M.; Dampha, A.; Deckers, J.; Dewitte, O.; Gallali, T.; Hallett, S.; Jones, R.; Kilasara, M.; et al. (Eds.) Soil Atlas of Africa; European Commission, Publications Office of the European Union: Luxembourg, 2013. [Google Scholar]
- Hounkpatin, K.O.L.; Welp, G.; Akponikpè, P.B.I.; Rosendahl, I.; Amelung, W. Carbon losses from prolonged arable cropping of Plinthosols in southwest Burkina Faso. Soil Tillage Res. 2018, 175, 51–61. [Google Scholar] [CrossRef]
- Ikazaki, K.; Nagumo, F.; Simporé, S.; Barro, A. Soil toposequence, productivity, and a simple technique to detect petroplinthites using ground-penetrating radar in the Sudan Savanna. Soil Sci. Plant Nutr. 2018, 64, 623–631. [Google Scholar] [CrossRef]
- Archer, S.R.; Andersen, E.M.; Predick, K.I.; Schwinning, S.; Steidl, R.J.; Woods, S.R. Woody Plant Encroachment: Causes and Consequences. In Rangeland Systems: Processes, Management and Challenges; Briske, D., Ed.; Springer: Cham, Switzerland, 2017; pp. 25–84. [Google Scholar] [CrossRef]
- Hänke, H.; Börjeson, L.; Hylander, K.; Enfors-Krautsky, E. Drought tolerant species dominate as rainfall and tree cover returns in the West African Sahel. Land Use Policy 2016, 59, 111–120. [Google Scholar] [CrossRef]
- Zida, W.A.; Bationo, B.A.; Waaub, J.-P. Regreening of agrosystems in the Burkina Faso Sahel: Greater drought resilience but falling woody plant diversity. Environ. Conserv. 2020, 47, 174–181. [Google Scholar] [CrossRef]
- Brandt, M.; Tappan, G.; Diouf, A.A.; Beye, G.; Mbow, C.; Fensholt, R. Woody Vegetation Die off and Regeneration in Response to Rainfall Variability in the West African Sahel. Remote Sens. 2017, 9, 39. [Google Scholar] [CrossRef]
- Guinko, S. Végétation de la Haute-Volta. Ph.D. Thesis, University of Bordeaux III, Bordeaux, France, 1984. (In French). [Google Scholar]
- Zerbo, L. Caracterisation des Sols des Stations de Recherches Agricoles d l’INERA -Kamboinse, Farako-ba, Saria, Niangoloko; Institut d’Etudes et de Recherches Agricoles: Ouagadougou, Burkina Faso, 1995. (In French) [Google Scholar]
- FAO. Global Forest Resources Assessment 2015: Desk Reference; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015. [Google Scholar]
- Climate-data.org. Koudougou Climate (Burkina Faso). Available online: https://en.climate-data.org/location/3744/ (accessed on 20 April 2018).
- D-Maps. Maps Burkina Faso. Available online: http://d-maps.com/pays.php?num_pay=13&lang=en (accessed on 15 September 2017).
- FAO. Guidelines for Soil Description, 4th ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006. [Google Scholar]
- The Plant List. Available online: http://www.theplantlist.org/ (accessed on 21 September 2017).
- Arbonnier, M. Trees, Shrubs and Lianas of West African Dry Zones; Margraf Publishers: Weikersheim, Germany, 2004. [Google Scholar]
- Sidiyasa, K.; Zakaria, R.I. The Forests of Setulang and Sengayan in Malinau, East Kalimantan: Their Potential and the Identification of Steps for Their Protection and Sustainable Management; Center for International Forestry Research: Bogor, Indonesia, 2006. [Google Scholar]
- Weigel, J. Agroforesterie Pratique: À L’usage des Agents de Terrain en Afrique Tropicale Sèche; Institut de Recherches et d’Applications de Méthodes de Développement: Paris, France, 1994. [Google Scholar]
- Nikiema, A. Agroforestry Parkland Species Diversity: Uses and Management in Semi-Arid West Africa (Burkina Faso). Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2005. [Google Scholar]
- Ifo, S.A.; Moutsambote, J.-M.; Koubouana, F.; Yoka, J.; Ndzai, S.F.; Orcellie Bouetou-Kadilamio, L.N.; Mampouya, H.; Jourdain, C.; Bocko, Y.; Mantota, A.B.; et al. Tree species diversity, richness, and similarity in intact and degraded forest in the tropical rainforest of the Congo Basin: Case of the forest of Likouala in the Republic of Congo. Int. J. For. Res. 2016, 2016, 7593681. [Google Scholar] [CrossRef]
- Ouedraogo, I.; Savadogo, P.; Tigabu, M.; Cole, R.; Odén, P.C.; Ouadba, J.-M. Is rural migration a threat to environmental sustainability in southern Burkina Faso? Land Degrad. Dev. 2009, 20, 217–230. [Google Scholar] [CrossRef]
- Kessler, J.J. The influence of karité (Vitellaria paradoxa) and néré (Parkia biglobosa) trees on sorghum production in Burkina Faso. Agrofor. Syst. 1992, 17, 97–118. [Google Scholar] [CrossRef]
- Sinare, H.; Gordon, L.J. Ecosystem services from woody vegetation on agricultural lands in Sudano-Sahelian West Africa. Agric. Ecosyst. Environ. 2015, 200, 186–199. [Google Scholar] [CrossRef]
- Zougmoré, R. Integrated Water and Nutrient Management for Sorghum Production in Semi-Arid Burkina Faso; Tropical Resource Management Resource Papers No. 45; Wageningen University and Research Center: Wageningen, The Netherlands, 2003. [Google Scholar]
- Ikazaki, K.; Nagumo, F.; Simporé, S.; Barro, A. Are all three components of conservation agriculture necessary for soil conservation in the Sudan Savanna? Soil Sci. Plant Nutr. 2017, 64, 230–237. [Google Scholar] [CrossRef]
- Lahmar, R.; Bationo, B.A.; Dan Lamso, N.; Guéro, Y.; Tittonell, P. Tailoring conservation agriculture technologies to West Africa semi-arid zones: Building on traditional local practices for soil restoration. Field Crops Res. 2012, 132, 158–167. [Google Scholar] [CrossRef]
- Singh, K.P.; Kushwaha, C.P. Deciduousness in tropical trees and its potential as indicator of climate change: A review. Ecol. Indic. 2016, 69, 699–706. [Google Scholar] [CrossRef]
- Etongo Bau, D. Deforestation and Forest Degradation in Southern Burkina Faso: Understanding the Drivers of Change and Options for Revegetation. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2016. [Google Scholar]
- Fischer, D.; Kleinn, C.; Fehrmann, L.; Fuchs, H.; Panferov, O. A national level forest resource assessment for Burkina Faso—A field based forest inventory in a semiarid environment combining small sample size with large observation plots. For. Ecol. Manag. 2011, 262, 1532–1540. [Google Scholar] [CrossRef]
- FAO. Global Forest Resources Assessment 2010; FAO Forestry Paper 163; Food and Agriculture Organization of the United Nations: Rome, Italy, 2010. [Google Scholar]
- Kindt, R.; Kalinganire, A.; Larwanou, M.; Belem, M.; Dakouo, J.M.; Bayala, J.; Kairé, M. Species accumulation within land use and tree diameter categories in Burkina Faso, Mali, Niger and Senegal. Biodivers. Conserv. 2008, 17, 1883–1905. [Google Scholar] [CrossRef]
- Ræbild, A.; Hansen, U.B.; Kambou, S. Regeneration of Vitellaria paradoxa and Parkia biglobosa in a parkland in southern Burkina Faso. Agrofor. Syst. 2012, 85, 443–453. [Google Scholar] [CrossRef]
- Yélémou, B.; Zoungrana, I.; Hien, F. Distribution spatiale des principales especes agroforestieres, Butyrospermum paradoxum, Parkia biglobosa, Acacia albida et Azadirachta indica dans les systemes agraires de la province du Bulkiemde (Burkina Faso). In Proceedings of the International Symposium on Agroforestry Parklands in the Semi-Arid Lands of West Africa ICRAF/IRBET/CILSS/LTC, Ouagadougou, Burkina Faso, 25–27 October 1993. [Google Scholar]
- Louppe, D.; Depommier, D. Expansion, research and development of the eucalyptus in Africa. Wood production, livelihoods and environmental issues: An unlikely reconciliation? In Proceedings of the FAO/MEEATU Workshop “Eucalyptus in East Africa, the Socio-Economic and Environmental Issues”, Bujumbura, Burundi, 1 March–1 April 2010. [Google Scholar]
- Mialhe, P.; Piot, J. Haute-Volta, essais de préparation et de plantation d’Eucalyptus camaldulensis à racines nues. Revue Bois Forêts Trop. 1979, 187, 31–51. [Google Scholar]


| Taxon | Family | Plant Form | Total No. of Individuals | ||
|---|---|---|---|---|---|
| DBH ≥ 5 cm | DBH < 5 cm | Total | |||
| Azadirachta indica | MELIACEAE | Tree | 8 | 84 | 92 |
| Cochlospermum sp. | COCHLOSPERMACEAE | Tree/Shrub | 0 | 4 | 4 |
| Combretum glutinosum | COMBRETACEAE | Tree/Shrub | 0 | 8 | 8 |
| Combretum micranthum | COMBRETACEAE | Tree/Shrub | 0 | 217 | 217 |
| Daniellia oliveri | LEGUMINOSAE | Tree | 0 | 34 | 34 |
| Detarium microcarpum | LEGUMINOSAE | Tree | 0 | 3 | 3 |
| Diospyros mespiliformis | EBENACEAE | Tree | 3 | 169 | 172 |
| Eucalyptus camaldulensis | MYRTACEAE | Tree | 0 | 182 | 182 |
| Feretia apodanthera | RUBIACEAE | Shrub | 0 | 26 | 26 |
| Ficus sp. | MORACEAE | Tree | 0 | 1 | 1 |
| Guiera senegalensis | COMBRETACEAE | Shrub | 0 | 3426 | 3426 |
| Khaya senegalensis | MELIACEAE | Tree | 2 | 0 | 2 |
| Lannea microcarpa | ANACARDIACEAE | Tree | 36 | 72 | 108 |
| Maytenus senegalensis | CELASTRACEAE | Tree/Shrub | 0 | 1 | 1 |
| Parkia biglobosa | LEGUMINOSAE | Tree | 14 | 0 | 14 |
| Piliostigma reticulatum | LEGUMINOSAE | Tree/Shrub | 6 | 433 | 439 |
| Saba senegalensis | APOCYNACEAE | Shrub/Liana | 0 | 2 | 2 |
| Sclerocarya birrea | ANACARDIACEAE | Tree | 2 | 3 | 5 |
| Senegalia pennata | LEGUMINOSAE | Shrub | 0 | 46 | 46 |
| Sterculia sp. | STERCULIACEAE | Tree | 2 | 0 | 2 |
| Tamarindus indica | LEGUMINOSAE | Tree | 1 | 0 | 1 |
| Terminalia sp. | COMBRETACEAE | Tree | 2 | 87 | 89 |
| Vachellia seyal | LEGUMINOSAE | Tree | 0 | 24 | 24 |
| Vitellaria paradoxa | SAPOTACEAE | Tree | 47 | 0 | 47 |
| Waltheria indica | STERCULIACEAE | Shrub | 0 | 1 | 1 |
| Ximenia americana | OLACACEAE | Tree/Shrub | 0 | 53 | 53 |
| Total (26 spp.) | 123 | 4876 | 4999 | ||
| Number of species (spp.) † | 11 | 21 | 26 (6) | ||
| Density of woody plants (individuals ha−1) ‡ | 9.1 | 361.2 | 370.3 | ||
| Basal area at breast height (m2 ha−1) | 1.6 | N/A | N/A | ||
| Canopy coverage (m2 ha−1) | 421.2 | 266.1 | 687.3 | ||
| Shannon diversity index (H′) | 1.36 | - | - | - | |
| Pielou’s evenness (E) | 0.41 | - | - | - | |
| Taxon | Total No. of Individuals | DBH (cm) *1, † | Height (m) † | Canopy Coverage † (m2 Individual−1) | Canopy Coverage (m2 ha−1) | Basal Area (m2 ha−1) | RD *2 | RF *3 | Rdom *4 | IVI *5 |
|---|---|---|---|---|---|---|---|---|---|---|
| Azadirachta indica | 8 | 24.7 ± 3.1 | 6.3 ± 0.8 | 19.8 ± 6.1 | 11.7 | 0.03 | 6.5 | 9.2 | 2.0 | 17.7 |
| Diospyros mespiliformis | 3 | 22.5 ± 9.5 | 4.4 ± 0.6 | 0.3 ± 0.0 | 0.1 | 0.01 | 2.4 | 3.1 | 0.7 | 6.3 |
| Khaya senegalensis | 2 | 66.0 ± 15.0 | 8.0 ± 0.8 | 15.3 ± 2.8 | 2.3 | 0.05 | 1.6 | 1.5 | 3.3 | 6.5 |
| Lannea microcarpa | 36 | 25.2 ± 3.1 | 6.1 ± 0.4 | 23.1 ± 4.2 | 61.5 | 0.20 | 29.3 | 24.6 | 12.7 | 66.5 |
| Parkia biglobosa | 14 | 70.3 ± 6.2 | 12.2 ± 0.7 | 141.0 ± 17.6 | 146.2 | 0.44 | 11.4 | 15.4 | 27.4 | 54.2 |
| Piliostigma reticulatum | 6 | 43.3 ± 14.3 | 7.7 ± 1.7 | 79.9 ± 33.7 | 35.5 | 0.10 | 4.9 | 4.6 | 6.2 | 15.7 |
| Sclerocarya birrea | 2 | 38.3 ± 7.3 | 5.6 ± 0.3 | 31.3 ± 8.7 | 4.6 | 0.02 | 1.6 | 3.1 | 1.1 | 5.8 |
| Sterculia sp. | 2 | 49.5 ± 12.5 | 7.1 ± 1.0 | 32.9 ± 15.8 | 4.9 | 0.03 | 1.6 | 3.1 | 1.9 | 6.6 |
| Tamarindus indica | 1 | 45.0 | 9.5 | 60.3 | 4.5 | 0.01 | 0.8 | 1.5 | 0.7 | 3.1 |
| Terminalia sp. | 2 | 16.8 ± 0.3 | 3.8 ± 0.6 | 6.0 ± 0.4 | 0.9 | < 0.01 | 1.6 | 1.5 | 0.2 | 3.4 |
| Vitellaria paradoxa | 47 | 48.2 ± 2.4 | 8.7 ± 0.4 | 42.8 ± 3.5 | 149.1 | 0.71 | 38.2 | 32.3 | 43.8 | 114.3 |
| Total (11 spp.) | 123 | 41.2 ± 2.2 | 7.9 ± 0.3 | 46.2 ±4.6 | 421.2 | 1.60 | 100.0 | 100.0 | 100.0 | 300.0 |
| Position | Soil Type | Effective Soil Depth *1, *2 | Mean Number of Individuals *2 | Mean Number of Species *2 | Dominant Species in 1984 | Dominant Species in 2015 *3 |
|---|---|---|---|---|---|---|
| (cm) | (Plants Quadrat−1) | (Number Quadrat−1) | [20,21] *4 | (This Study) | ||
| Valley bottom | Gleysols (2) | >100 | 175.0 ± 121.0 ab | 8.0 ± 2.0 ns | Anogeissus leiocarpus Butyrospermum paradoxum subsp. parkii *5 Sclerocarya birrea Lannea microcarpa Diospyros mespiliformis | Diospyros mespiliformis Guiera senegalensis Piliostigma reticulatum Vachellia seyal Azadirachta indica |
| Lower transect | Lixisols (8) | >100 | 390.5 ± 172.7 b | 5.8 ± 0.6 ns | Guiera senegalensis Piliostigma reticulatum Combretum micranthum Terminalia sp. Ximenia americana | |
| Pisolithic Plinthosols (10) | 16.5 ± 4.2 | 20.7 ± 6.5 a | 3.9 ± 0.7 ns | Guiera senegalensis Piliostigma reticulatum Lannea microcarpa Azadirachta indica Combretum micranthum | ||
| Middle transect | Petric Plinthosols (21) | 45.3 ± 3.7 | 45.6 ± 13.2 a | 4.6 ± 0.4 ns | Butyrospermum paradoxum subsp. parkii *5 Guiera senegalensis Gardenia erubescens Senegalia macrostachya Piliostigma reticulatum Combretum glutinosum Adansonia digitata Lannea microcarpa Combretum micranthum Ximenia americana | Guiera senegalensis Eucalyptus camaldulensis Piliostigma reticulatum Diospyros mespiliformis Lannea microcarpa |
| Upper transect | Pisolithic Petric Plinthosols (13) | 4.3 ± 2.4 | 27.8 ± 8.4 a | 3.8 ± 0.8 ns | Senegalia macrostachya Guiera senegalensis Piliostigma reticulatum Gardenia erubescens | Guiera senegalensis Piliostigma reticulatum Combretum micranthum Senegalia pennata Lannea microcarpa |
| Taxon | Total Abundance of Woody Plants | Petric Pisolithic Plinthosols | Petric Plinthosol | Lixisol | Pisolithic Plinthosol | Gleysols |
|---|---|---|---|---|---|---|
| N = 13 | N = 21 | N = 8 | N = 10 | N = 2 | ||
| Azadirachta indica | 92 | 0.15 a | 0.90 a | 4.38 a | 2.00 a | 8.00 a |
| Cochlospermum sp. | 4 | 0.00 a | 0.19 a | 0.00 a | 0.00 a | 0.00 a |
| Combretum glutinosum | 8 | 0.54 a | 0.05 a | 0.00 a | 0.00 a | 0.00 a |
| Combretum micranthum | 217 | 1.69 a | 0.10 a | 20.88 b | 1.10 a | 7.50 a |
| Daniellia oliveri | 34 | 0.23 a | 1.48 a | 0.00 a | 0.00 a | 0.00 a |
| Detarium microcarpum | 3 | 0.23 a | 0.00 a | 0.00 a | 0.00 a | 0.00 a |
| Diospyros mespiliformis | 172 | 0.00 a | 2.14 a | 0.88 a | 1.00 a | 55.00 b |
| Eucalyptus camaldulensis | 182 | 0.00 a | 8.48 a | 0.00 a | 0.40 a | 0.00 a |
| Feretia apodanthera | 26 | 0.31 a | 0.05 a | 1.88 b | 0.60 a | 0.00 a |
| Ficus sp. | 1 | 0.00 a | 0.05 a | 0.00 a | 0.00 a | 0.00 a |
| Guiera senegalensis | 3426 | 17.00 a | 25.10 a | 313.63 b | 6.10 a | 54.00 a |
| Khaya senegalensis | 2 | 0.00 a | 0.10 a | 0.00 a | 0.00 a | 0.00 a |
| Lannea microcarpa | 108 | 1.38 a | 1.62 a | 1.25 a | 3.10 a | 7.50 a |
| Maytenus senegalensis | 1 | 0.00 a | 0.00 a | 0.00 a | 0.00 a | 0.50 b |
| Parkia biglobosa | 14 | 0.31 a | 0.19 a | 0.00 a | 0.20 a | 2.00 b |
| Piliostigma reticulatum | 439 | 3.69 a | 3.43 a | 26.13 b | 5.70 a | 26.50 a |
| Saba senegalensis | 2 | 0.00 a | 0.05 a | 0.13 a | 0.00 a | 0.00 a |
| Sclerocarya birrea | 5 | 0.00 a | 0.14 a | 0.13 a | 0.10 a | 0.00 a |
| Senegalia pennata | 46 | 1.46 a | 0.00 a | 3.38 b | 0.00 a | 0.00 a |
| Sterculia sp. | 2 | 0.08 a | 0.05 a | 0.00 a | 0.00 a | 0.00 a |
| Tamarindus indica | 1 | 0.00 a | 0.00 a | 0.13 a | 0.00 a | 0.00 a |
| Terminalia sp. | 89 | 0.00 a | 0.00 a | 10.75 b | 0.00 a | 1.50 a |
| Vachellia seyal | 24 | 0.00 a | 0.00 a | 0.00 a | 0.00 a | 12.00 b |
| Vitellaria paradoxa | 47 | 0.46 a | 1.48 a | 0.75 a | 0.30 a | 0.50 a |
| Waltheria indica | 1 | 0.08 a | 0.00 a | 0.00 a | 0.00 a | 0.00 a |
| Ximenia americana | 53 | 0.15 a | 0.00 a | 6.25 b | 0.10 a | 0.00 a |
| Total 26 spp. | 4999 | 27.77 a | 45.57 a | 390.50 b | 20.70 a | 175.00 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takenaka, K.; Ikazaki, K.; Simporé, S.; Kaboré, F.; Thiombiano, N.; Koala, J. Changes in Woody Vegetation over 31 Years in Farmed Parkland of the Central Plateau, Burkina Faso. Land 2021, 10, 470. https://doi.org/10.3390/land10050470
Takenaka K, Ikazaki K, Simporé S, Kaboré F, Thiombiano N, Koala J. Changes in Woody Vegetation over 31 Years in Farmed Parkland of the Central Plateau, Burkina Faso. Land. 2021; 10(5):470. https://doi.org/10.3390/land10050470
Chicago/Turabian StyleTakenaka, Koichi, Kenta Ikazaki, Saïdou Simporé, François Kaboré, Natacha Thiombiano, and Jonas Koala. 2021. "Changes in Woody Vegetation over 31 Years in Farmed Parkland of the Central Plateau, Burkina Faso" Land 10, no. 5: 470. https://doi.org/10.3390/land10050470
APA StyleTakenaka, K., Ikazaki, K., Simporé, S., Kaboré, F., Thiombiano, N., & Koala, J. (2021). Changes in Woody Vegetation over 31 Years in Farmed Parkland of the Central Plateau, Burkina Faso. Land, 10(5), 470. https://doi.org/10.3390/land10050470






