Rooting Volume Impacts Growth, Coverage and Thermal Tolerance of Green Façade Climbing Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growing Conditions
2.2. Plant Growth and Canopy Cover Measurements
2.3. Chlorophyll Fluorescence
2.4. Statistical Analysis
3. Results
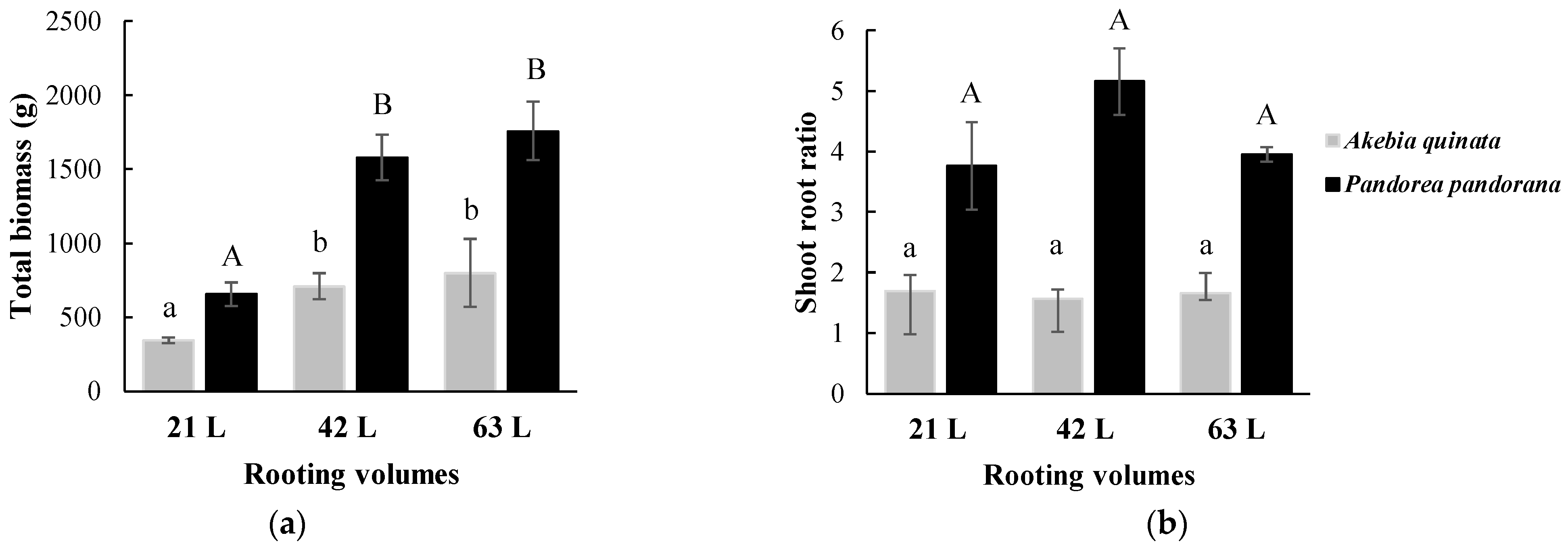
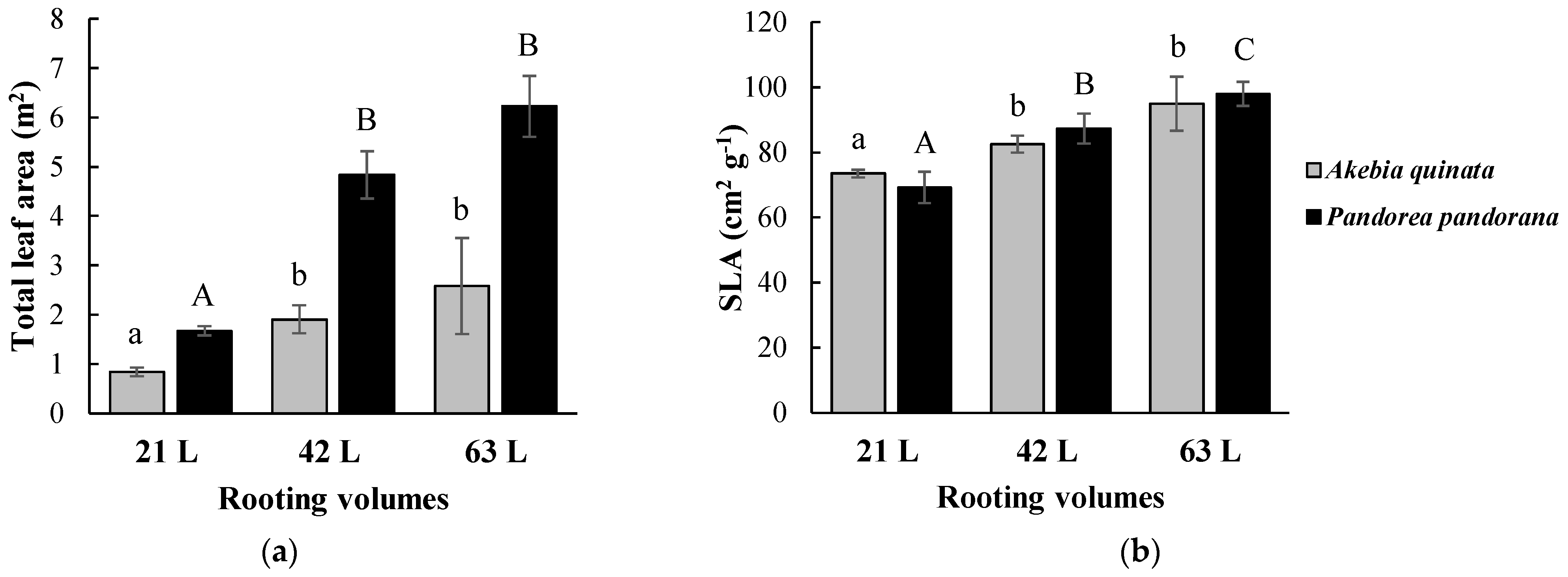
3.1. Climbing Plant Coverage and Growth Response to Different Rooting Volumes
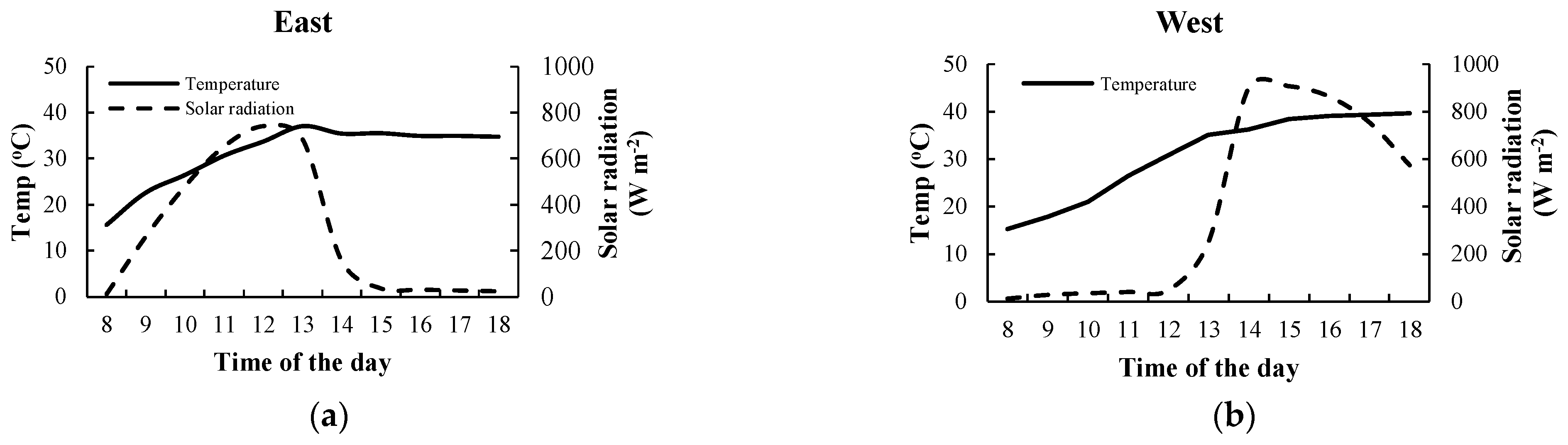
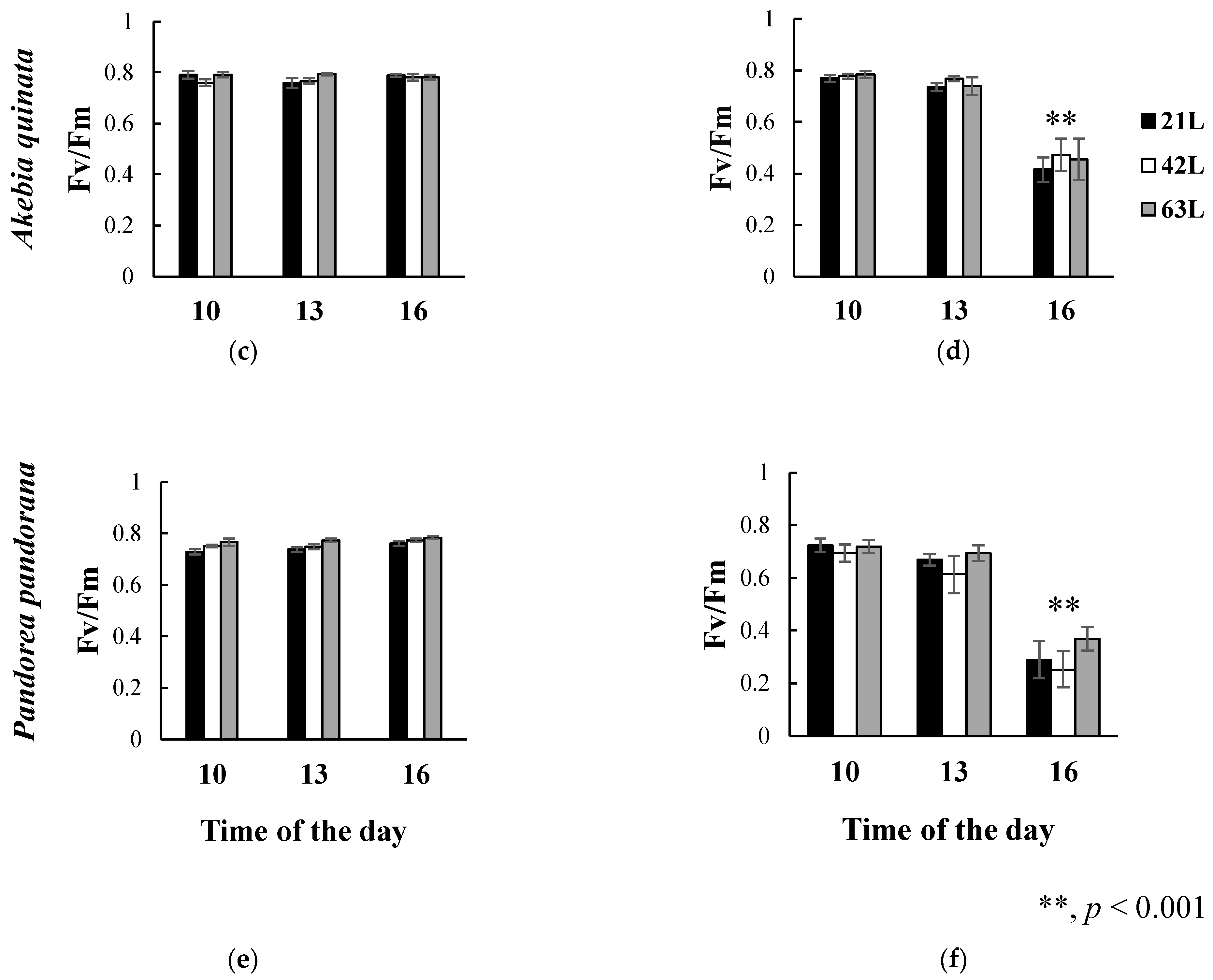
3.2. Effects of Air Temperature and Solar Radiation on the Fv/Fm Ratio of Climbing Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bambrick, H.J.; Capon, A.G.; Barnett, G.B.; Beaty, R.M.; Burton, A.J. Climate change and health in the urban environment: Adaptation opportunities in Australian cities. Asia Pac. J. Public Health 2011, 23, 67S–79S. [Google Scholar] [CrossRef] [PubMed]
- Oleson, K.; Monaghan, A.; Wilhelmi, O.; Barlage, M.; Brunsell, N.; Feddema, J.; Hu, L.; Steinhoff, D. Interactions between urbanization, heat stress, and climate change. Clim. Chang. 2015, 129, 525–541. [Google Scholar] [CrossRef]
- Manso, M.; Teotónio, I.; Silva, C.M.; Cruz, C.O. Green roof and green wall benefits and costs: A review of the quantitative evidence. Renew. Sustain. Energy Rev. 2021, 135, 110111. [Google Scholar] [CrossRef]
- Pérez-Urrestarazu, L.; Fernández-Cañero, R.; Franco-Salas, A.; Egea, G. Vertical greening systems and sustainable cities. J. Urban Technol. 2015, 22, 65–85. [Google Scholar] [CrossRef]
- Haaland, C.; van Den Bosch, C.K. Challenges and strategies for urban green-space planning in cities undergoing densification: A review. Urban For. Urban Green. 2015, 14, 760–771. [Google Scholar] [CrossRef]
- Hunter, A.M.; Williams, N.S.; Rayner, J.P.; Aye, L.; Hes, D.; Livesley, S.J. Quantifying the thermal performance of green façades: A critical review. Ecol. Eng. 2014, 63, 102–113. [Google Scholar] [CrossRef]
- Shamanga, K.J.; Idris, H.A.; Babangida, A. Integrating Green Wall in Healthcare Buildings to Curb Climate Change. J. Eng. Comput. Appl. Sci. (JECAS) 2021, 5. Available online: https://journal.gjbeacademia.com/index.php/jecas/article/view/8 (accessed on 26 October 2021).
- Medl, A.; Stangl, R.; Florineth, F. Vertical greening systems–a review on recent technologies and research advancement. Build. Environ. 2017, 125, 227–239. [Google Scholar] [CrossRef]
- Tudiwer, D.; Korjenic, A. The effect of living wall systems on the thermal resistance of the façade. Energy Build. 2017, 135, 10–19. [Google Scholar] [CrossRef]
- Cameron, R.W.; Taylor, J.E.; Emmett, M.R. What’s ‘cool’ in the world of green façades? How plant choice influences the cooling properties of green walls. Build. Environ. 2014, 73, 198–207. [Google Scholar] [CrossRef]
- Bustami, R.A.; Belusko, M.; Ward, J.; Beecham, S. Vertical greenery systems: A systematic review of research trends. Build. Environ. 2018, 146, 226–237. [Google Scholar] [CrossRef]
- Hoelscher, M.-T.; Nehls, T.; Jänicke, B.; Wessolek, G. Quantifying cooling effects of facade greening: Shading, transpiration and insulation. Energy Build. 2016, 114, 283–290. [Google Scholar] [CrossRef]
- Koyama, T.; Yoshinaga, M.; Hayashi, H.; Maeda, K.-i.; Yamauchi, A. Identification of key plant traits contributing to the cooling effects of green façades using freestanding walls. Build. Environ. 2013, 66, 96–103. [Google Scholar] [CrossRef]
- Vox, G.; Blanco, I.; Schettini, E. Green façades to control wall surface temperature in buildings. Build. Environ. 2018, 129, 154–166. [Google Scholar] [CrossRef]
- Besir, A.B.; Cuce, E. Green roofs and facades: A comprehensive review. Renew. Sustain. Energy Rev. 2018, 82, 915–939. [Google Scholar] [CrossRef]
- Fernández-Cañero, R.; Urrestarazu, L.P.; Perini, K. Vertical Greening Systems: Classifications, Plant Species, Substrates. In Nature Based Strategies for Urban and Building Sustainability; Elsevier: Oxford, UK, 2018; pp. 45–54. [Google Scholar]
- Manso, M.; Castro-Gomes, J. Green wall systems: A review of their characteristics. Renew. Sustain. Energy Rev. 2015, 41, 863–871. [Google Scholar] [CrossRef]
- Jim, C.Y. Greenwall classification and critical design-management assessments. Ecol. Eng. 2015, 77, 348–362. [Google Scholar] [CrossRef]
- Teotónio, I.; Silva, C.M.; Cruz, C.O. Economics of green roofs and green walls: A literature review. Sustain. Cities Soc. 2021, 69, 102781. [Google Scholar] [CrossRef]
- Thottathi, V.; Balamuralikrishna, C.; Ghosh, S. Use of Green Facades In Sustainable Building Environments: Quantifying the Uptake Rates of Air Pollutants by Facades Draped with Tropical Creepers. In Proceedings of the International Conference on Sustainable Built Environment (ICSBE-2010), Kandy, Sri Lanka, 13–14 December 2010; pp. 363–373. [Google Scholar]
- Köhler, M. Green facades—a view back and some visions. Urban Ecosyst. 2008, 11, 423–436. [Google Scholar] [CrossRef]
- Ascione, F.; De Masi, R.F.; Mastellone, M.; Ruggiero, S.; Vanoli, G.P. Green walls, a critical review: Knowledge gaps, design parameters, thermal performances and multi-criteria design approaches. Energies 2020, 13, 2296. [Google Scholar] [CrossRef]
- Dunnett, N.; Kingsbury, N. Planting Green Roofs and Living Walls; Timber Press: Portland, OR, USA, 2008. [Google Scholar]
- Growing Green Guide: A Guide to Green Roofs, Walls and Facades in Melbourne and Victoria, Australia; Department of Environment and Primary Industries: Melbourne, Victoria, Australia, 2014.
- Hsu, Y.; Tseng, M.; Lin, C. Container volume affects growth and development of wax-apple. HortScience 1996, 31, 1139–1142. [Google Scholar] [CrossRef]
- Dubik, S.P.; Krizek, D.T.; Stimart, D.P.; McIntosh, M.S. Growth analysis of spreading euonymus subjected to root restriction. J. Plant Nutr. 1992, 15, 469–486. [Google Scholar] [CrossRef]
- Andrade, J.L.; Meinzer, F.C.; Goldstein, G.; Schnitzer, S.A. Water uptake and transport in lianas and co-occurring trees of a seasonally dry tropical forest. Trees 2005, 19, 282–289. [Google Scholar] [CrossRef]
- Schnitzer, S.A. A mechanistic explanation for global patterns of liana abundance and distribution. Am. Nat. 2005, 166, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Haege, E.; Leake, S. Soils for Landscape Development: Selection, Specification and Validation; Csiro Publishing: Melbourne, Australia, 2014. [Google Scholar]
- Handreck, K.A.; Black, N.D. Growing Media for Ornamental Plants and Turf, 4th ed.; UNSW Press: Sydney, Australia, 2010. [Google Scholar]
- NeSmith, D.S.; Duval, J.R. The effect of container size. HortTechnology 1998, 8, 495–498. [Google Scholar] [CrossRef]
- Lambers, H.; Poorter, H. Inherent Variation in Growth Rate Between Higher Plants: A Search for Physiological Causes and Ecological Consequences. In Advances in Ecological Research; Elsevier: Cambridge, MA, USA, 1992; Volume 23, pp. 187–261. [Google Scholar]
- Wang, S.; Okamoto, G.; Hirano, K.; Lu, J.; Zhang, C. Effects of restricted rooting volume on vine growth and berry development of Kyoho grapevines. Am. J. Enol. Vitic. 2001, 52, 248–253. [Google Scholar]
- Aghai, M.M.; Pinto, J.R.; Davis, A.S. Container volume and growing density influence western larch (Larix occidentalis Nutt.) seedling development during nursery culture and establishment. New For. 2014, 45, 199–213. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Puértolas, J.; Cuesta, B.; Penuelas, J.L.; Uscola, M.; Heredia-Guerrero, N.; Benayas, J.M.R. Increase in size and nitrogen concentration enhances seedling survival in Mediterranean plantations. Insights from an ecophysiological conceptual model of plant survival. New For. 2012, 43, 755–770. [Google Scholar] [CrossRef]
- Darbah, J.N.; Sharkey, T.D.; Calfapietra, C.; Karnosky, D.F. Differential response of aspen and birch trees to heat stress under elevated carbon dioxide. Environ. Pollut. 2010, 158, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Brunel, S.; Schrader, G.; Brundu, G.; Fried, G. Emerging invasive alien plants for the Mediterranean Basin. EPPO Bull. 2010, 40, 219–238. [Google Scholar] [CrossRef]
- Yen, N.T.; Thu, N.V.; Zhao, B.T.; Lee, J.H.; Kim, J.A.; Son, J.K.; Choi, J.S.; Woo, E.R.; Woo, M.H.; Min, B.S. Quantitative determination of compounds from Akebia quinata by high-performance liquid chromatography. Bull. Korean Chem. Soc. 2014, 35, 1956–1964. [Google Scholar] [CrossRef]
- Andresen, L. High impact bush regeneration: Is there a role for heavy machinery? Australas. Plant Conserv. J. Aust. Netw. Plant Conserv. 2012, 21, 9–11. [Google Scholar]
- James, E.; Knox, R. Reproductive-biology of the Australian species of the genus Pandorea (Bignoniaceae). Aust. J. Bot. 1993, 41, 611–626. [Google Scholar] [CrossRef]
- Farrell, C.; Mitchell, R.; Szota, C.; Rayner, J.; Williams, N. Green roofs for hot and dry climates: Interacting effects of plant water use, succulence and substrate. Ecol. Eng. 2012, 49, 270–276. [Google Scholar] [CrossRef]
- Wilkinson, K.M.; Landis, T.D.; Haase, D.L.; Daley, B.F.; Dumroese, R.K. Tropical Nursery Manual: A Guide to Starting and Operating a Nursery for Native and Traditional Plants. In Agriculture Handbook 732; US Department of Agriculture, Forest Service: Washington, DC, USA, 2014. [Google Scholar]
- Chung, P.-W. The Effects of Rooting Volume, Greywater Irrigation and Reduced Sunlight on Climbing Plants for Indirect Green Façades in Urban Environments. Ph.D. Thesis, The University of Melbourne, Melbourne, Australia, 31 January 2021. [Google Scholar]
- Dias, D.P.; Marenco, R.A. Fluorescence characteristics and photoinhibition in saplings of manwood on clear days and under overcast conditions. Sci. Agric. 2007, 64, 595–600. [Google Scholar] [CrossRef]
- Yamane, Y.; Kashino, Y.; Koike, H.; Satoh, K. Increases in the fluorescence Fo level and reversible inhibition of Photosystem II reaction center by high-temperature treatments in higher plants. Photosynth. Res. 1997, 52, 57–64. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—a practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- McConnaughay, K.; Bazzaz, F. Is physical space a soil resource? Ecology 1991, 72, 94–103. [Google Scholar] [CrossRef]
- Kostopoulou, P.; Radoglou, K.; Dini-Papanastasi, O.; Adamidou, C. Effect of mini-plug container depth on root and shoot growth of four forest tree species during early developmental stages. Turk. J. Agric. For. 2011, 35, 379–390. [Google Scholar]
- Fransen, B.; de Kroon, H.; De Kovel, C.; van den Bosch, F. Disentangling the effects of root foraging and inherent growth rate on plant biomass accumulation in heterogeneous environments: A modelling study. Ann. Bot. 1999, 84, 305–311. [Google Scholar] [CrossRef][Green Version]
- Perez, G.; Rincon, L.; Vila, A.; Gonzalez, J.M.; Cabeza, L.F. Green vertical systems for buildings as passive systems for energy savings. Appl. Energy 2011, 88, 4854–4859. [Google Scholar] [CrossRef]
- Kent, D.; Shultz, S.; Wyatt, T.; Halcrow, D. Soil volume and tree condition in Walt Disney World parking lots. Landsc. J. 2006, 25, 94–107. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M. Strategy shifts in leaf physiology, structure and nutrient content between species of high-and low-rainfall and high-and low-nutrient habitats. Funct. Ecol. 2001, 15, 423–434. [Google Scholar] [CrossRef]
- Antúnez, I.; Retamosa, E.C.; Villar, R. Relative growth rate in phylogenetically related deciduous and evergreen woody species. Oecologia 2001, 128, 172–180. [Google Scholar] [CrossRef]
- Keever, G.; Cobb, G.; Reed, R. Effects of container dimension and volume on growth of three woody ornamentals. HortScience 1985, 20, 89–93. [Google Scholar]
- Rieger, M.; Marra, F. Responses of young peach trees to root confinement. J. Am. Soc. Hortic. Sci. 1994, 119, 223–228. [Google Scholar] [CrossRef]
- Robbins, N.S.; Pharr, D.M. Effect of restricted root growth on carbohydrate metabolism and whole plant growth of Cucumis sativus L. Plant Physiol. 1988, 87, 409–413. [Google Scholar] [CrossRef]
- Krizek, D.T.; Carmi, A.; Mirecki, R.M.; Snyder, F.W.; Bunce, J.A. Comparative effects of soil moisture stress and restricted root zone volume on morphogenetic and physiological responses of soybean [Glycine max (L.) Merr.]. J. Exp. Bot. 1985, 36, 25–38. [Google Scholar] [CrossRef]
- Van Iersel, M. Root restriction effects on growth and development of salvia (Salvia splendens). HortScience 1997, 32, 1186–1190. [Google Scholar] [CrossRef]
- Kramer, P.J.; Kozlowski, T.T. Physiology of Woody Plants; Academic Press: New York, NY, USA, 1979. [Google Scholar]
- Camejo, D.; Rodríguez, P.; Morales, M.A.; Dell’Amico, J.M.; Torrecillas, A.; Alarcón, J.J. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant Physiol. 2005, 162, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Haldimann, P.; Feller, U. Inhibition of photosynthesis by high temperature in oak (Quercus pubescens L.) leaves grown under natural conditions closely correlates with a reversible heat-dependent reduction of the activation state of ribulose-1, 5-bisphosphate carboxylase/oxygenase. Plant Cell Environ. 2004, 27, 1169–1183. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, P.-W.; Livesley, S.J.; Rayner, J.P.; Farrell, C. Rooting Volume Impacts Growth, Coverage and Thermal Tolerance of Green Façade Climbing Plants. Land 2021, 10, 1281. https://doi.org/10.3390/land10121281
Chung P-W, Livesley SJ, Rayner JP, Farrell C. Rooting Volume Impacts Growth, Coverage and Thermal Tolerance of Green Façade Climbing Plants. Land. 2021; 10(12):1281. https://doi.org/10.3390/land10121281
Chicago/Turabian StyleChung, Pei-Wen, Stephen J. Livesley, John P. Rayner, and Claire Farrell. 2021. "Rooting Volume Impacts Growth, Coverage and Thermal Tolerance of Green Façade Climbing Plants" Land 10, no. 12: 1281. https://doi.org/10.3390/land10121281
APA StyleChung, P.-W., Livesley, S. J., Rayner, J. P., & Farrell, C. (2021). Rooting Volume Impacts Growth, Coverage and Thermal Tolerance of Green Façade Climbing Plants. Land, 10(12), 1281. https://doi.org/10.3390/land10121281





