Abstract
Mining activities generate residues during the ore concentration process. These wastes are placed into large tailing storage facilities, and upon mine closure, these tailings must be reclaimed. This study aimed to determine how different reclamation methods, involving combinations of planted boreal woody species and organic amendments application (paper mill sludge biosolids, chicken manure, and topsoil) affected plant community diversity at two tailing storage facilities in Québec, Canada. We recorded the composition of the plant communities using the percent cover of plant species within 1 m × 1 m quadrats. At the Niobec mine site, paper mill sludge mixed with topsoil enhanced total plant cover was compared with the use of topsoil only; the former amendment, however, reduced evenness (J′) and diversity (1−D) due to the increased growth of grasses and invasive forbs. At the Mont-Wright site, plots having received paper mill sludge mixed with a “Norco” treatment (a mixture of chicken manure, hay, and grass seeds) produced the highest total plant cover. The Norco treatment mixed with topsoil and the single application of topsoil and biosolids produced the highest evenness (J′) and diversity (1−D). Overall, organic amendment applications promoted vegetation cover on tailings and contributed to the colonization of diverse plant communities.
Keywords:
biodiversity; vegetation; reclamation; mine; tailings; organic amendment; plantation; paper mill sludges; chicken manure; topsoil 1. Introduction
Mining activities produce mining rock wastes (tailings) that can cover vast areas. In Québec (Canada), these tailing storage facilities cover over 13,000 ha [1]. Tailing impoundments are among the most damaging and longest-lasting environmental liabilities of the mining industry [2]. Their creation converts productive ecosystems into degraded landscapes, representing a loss of ecological services, such as wildlife habitat, nutrient cycling, and carbon sequestration [3,4,5,6].
Reclamation efforts by the mining industry aim to mitigate some of these environmental impacts by accelerating plant colonization on tailing storage facilities. Re-establishing ecological functions is challenging because multiple factors limit colonization, including soil compaction, low soil organic matter content, soil erosion as well as the poor nutrient availability and low water-holding capacity of the wastes [7,8,9,10]. Standard reclamation methods include the use of amendments and the seeding of herbaceous plants to favor colonization [11,12,13,14,15,16]. This method allows the establishment of a rapid protective vegetation cover that helps limit erosion, as requested by the guidelines for preparing mine closure plans in Québec [17]. Although this business-as-usual (BAU) minimal requirement can initiate the reclamation process, the planting of trees combined with the application of organic amendments and the seeding of herbaceous plants could benefit even more the reclamation of tailings.
Plantations on mining sites may initiate natural succession, help integrate former mining sites into natural forested landscapes, and increase carbon sequestration [18,19]. Plantations also modify the microclimate in the understorey, which influences the colonising plant community [20,21,22,23]. For instance, a tree canopy decreases light availability. This shading reduces the growth of grasses, which compete with tree seedlings [8,24]. Few studies have examined the role of plantations in enhancing understorey biodiversity on mine sites; however, the limited available data suggest that planting native trees on degraded landscapes fosters more diverse plant communities [23,25].
Organic amendments can enhance soil fertility and increase plant success on reclaimed sites [5,11,26]. Their application on tailing storage facilities can increase tree survival [2], biomass, and coverage of understorey vegetation [26,27,28,29,30]. When applied at high rates, however, amendments can negatively influence plant communities by, for example, decreasing richness and diversity [31,32,33]. In Québec, Canada, various organic amendments are used to reclaim mining sites. Topsoil, as an amendment, is often available on site, as it is collected when the tailing storage facility is created or enlarged. Topsoil can enhance soil conditions on tailings in the short term by increasing C and N concentrations and enhancing microbial activity [34]. However, this amendment is not always available in sufficient quantities and is often stockpiled before being applied, which can lead to its compaction and decreased seed viability [29,35,36,37]. As an alternative to topsoil, paper mill sludge biosolid (PMS), a by-product of the pulp and paper industry, represents a potential source of organic amendments for tailing reclamation, in particular because PMSs are presently landfilled and, therefore, lost to other uses [38,39]. Animal manure is another amendment used to increase crop productivity in agriculture; it could also benefit plant growth on reclaimed tailing storage facilities [27,29].
The major goal of mine site reclamation is to enhance site conditions and establish an ecosystem to a near-predisturbance state [37]. Evaluating progress towards this goal requires comparing the reclaimed plant community to a “natural” state at a similar successional stage. In the boreal forest, the greatest plant diversity is found in the forest understorey, a layer dominated by vascular plants, mosses, and lichens [40,41,42]. Nonetheless, cyclical and frequent natural disturbances, such as wildfires and insect outbreaks, continually modify the composition of these communities [43]. These frequent disturbances allow reclaimed mine site communities to be compared with naturally disturbed understorey communities; for example, Errington and Pinno [44] used post-fire forests as natural references to capture the first years of plant community succession following the removal of the forest canopy and understorey vegetation. Post-fire reference plots located close to tailing storage facilities can serve as valid points of comparison for recovering plant communities.
Although organic amendments and plantations are expected to benefit multiple aspects of tailing storage facility reclamation in the boreal region, little is known about their effect on plant community diversity in these settings. We hypothesised that (1) reclamation of mine tailing storage facilities through the planting of woody species, the seeding of herbaceous plants, and the use of soil organic amendments produces a greater plant diversity relative to the business-as-usual minimal requirements, which rely on seeding herbaceous plants and applying amendments; (2) reclamation methods using tree planting, herbaceous plants, and amendments establish plant communities more similar to those found on recently naturally disturbed reference sites (i.e., 11 years post-fire) than the business-as-usual method. We compared the effect of various organic amendment applications (topsoil, PMS, and chicken manure) on the response of the understorey plant community (total percent cover, richness, evenness, diversity, and functional-group abundance) at two mine tailing storage facilities in Québec, Canada, and compare these reclaimed plant communities with natural reference sites.
2. Materials and Methods
2.1. Site Description
We collected field data in 2018 at two tailings storage facilities in Québec, Canada. The first site is located at a niobium mine (Niobec, Inc., Saint-Honoré, QC, Canada) in St-Honoré, Saguenay (48°32′ N, 71°08′ W). This site lies within the balsam fir–yellow birch bioclimatic domain of the boreal zone [45]. The region receives 934.5 mm of precipitation annually (of which 223 mm is snow) and has a mean annual temperature of 2.8 °C [46]. The second site is situated at an iron mine tailings facility operated by ArcelorMittal Mining Canada at Mont-Wright, Fermont (52°46′ N, 67°20′ W). This mine lies within the spruce–lichen bioclimatic domain [45]. The region receives 839.5 mm of precipitation annually (of which 428.7 mm falls as snow) and has a mean daily temperature of −3.1 °C [47]. At both sites, the tailings are non-acidic (pH 7–8) with a relatively coarse texture (150–180 μm). Our experimental plots were established on slopes of 15% at the Niobec mine and 10% at Mont-Wright.
We compared our reclaimed sites with recent post-fire natural reference sites, which were previously forested stands. We selected the reference sites, one per mining site, according to their proximity to the respective mine sites and the time since the last fire disturbance. Given the very limited number of reference sites fitting our criteria, our selected natural reference sites did not burn in the same years as the reclamation (2012 at Niobec and 2015 at Mont-Wright) within our experimental sites. Near the Niobec mine, our reference site was a mixed forest stand that burned in 2007 (48°29′30.4″ N, 71°00′53.3″ W), and the Mont-Wright reference stand was a young black spruce forest that burned in 2007 (52°15′11.0″ N, 67°41′27.5″ W).
2.2. Experimental Design
At the Niobec tailing storage facilities site, we established, in 2012, a complete randomized block and factorial design (split-split-plot) with four replicates. We tested ten reclamation treatments involving combinations of organic amendments (topsoil or topsoil paper mill sludges (PMS) mixture) and revegetation with trees (larch (Larix laricina (Du Roi) K. Koch), paper birch (Betula papyrifera Marshall), red pine (Pinus resinosa Aiton), a treatment with the three mixed tree species (larch, paper birch, and red pine), and a control without trees).
At the Niobec site, a 10 cm layer of topsoil was spread onto the tailings from an all-terrain vehicle (Caterpillar D10) and evened out over the ground. The vehicle operator also removed any large rocks found within the topsoil. The topsoil had been excavated from another area of the tailing facility and stockpiled for two years prior to its use. We split each block (25 m × 600 m) into two main plots. Each plot was randomly attributed to one of two organic amendment treatments: (1) no application of amendment or (2) the application (and rototilling) of PMS (obtained from Resolute Forest Products, Jonquière, Québec; see Table 1 for its chemical characteristics) at a rate of 35 Mg(dry)·ha−1. The PMS used contained a very low concentration of heavy metals (analyses not shown), according to Canadian standards [48]. We, then, divided each main plot into five equal subplots (25 m × 60 m) in which we planted 40 to 60 cm-tall trees at a density of 2250 trees·ha−1 (2 × 2 m spacing). We selected the tree species based on 11 criteria, including tolerance to drought, nutrient requirements, and cost (unpublished data), which had been developed by Niobec, Inc. We randomly attributed one of five selected tree covers to each subplot. The tree cover was either (1) no trees planted; (2) Larix laricina; (3) Pinus resinosa (specimens of both Larix laricina and Pinus resinosa were obtained from the Normandin nursery of the Québec Ministry of Forests, Wildlife and Parks); (4) Betula papyrifera (obtained from the Boucher nursery, St-Ambroise, Québec); (5) a mixture of all three tree species. We, then, seeded all plantation plots with clovers (Trifolium spp.) at a rate of 50 kg·ha−1. A clover treatment (without tree) represents the business-as-usual reclamation scenario at the Niobec site.

Table 1.
Chemical characteristics of amendments used to reclaim tailing facilities at the Niobec and Mont-Wright tailing storage facilities.
For the Mont-Wright site, reclamation began on the tailing storage facility in 2013. About half of the reclaimed block received a “Norco” treatment representing a mixture of chicken manure (5 Mg·ha−1), hay, and herbaceous seeds (grass and forb seeds spread at a rate of 220 kg·ha−1; see Juge and Cossette [49] for the composition of the seed mix). We established our experimental plots on the Mont-Wright tailing facilities in 2015 and applied a randomized block design with six amendment treatments, repeated in three blocks (156 m × 25 m; see Table 1 for the chemical characteristics of the applied amendments). Each plot measured 26 m × 25 m. The amendments included topsoil, PMS, and the Norco treatment (chicken manure). The topsoil was collected in 2015 from another area of the tailing facility, and we applied the topsoil to the appropriate plots to a depth of approximately 10 cm using an all-terrain vehicle, as done for the Niobec site. PMS had been landfilled with ash before its recovery and application (the PMS was obtained from Resolute Forest Products, Baie-Comeau, Québec; see Table 1 for PMS chemical characteristics). We removed the woody debris (stumps and branches) from all plots.
The six treatments were randomly assigned to six main plots. These treatments consisted of (1) a five-year Norco mixture (N5; plots reclaimed with Norco in 2013), (2) PMS applied at a rate of 50 Mg(dry)·ha−1, (3) PMS applied at a rate of 50 Mg(dry)·ha−1 on top of the N5 treatment (PMS50+N5), (4) topsoil, (5) topsoil on top of the N5 treatment (topsoil+N5), and (6) a three-year Norco mixture (N3; plots amended with Norco in 2015). The application of the Norco treatment alone is the business-as-usual approach used at the Mont-Wright site.
We selected a combination of local tree and shrubs species (jack pine (Pinus banksiana Lambert), green alder (Alnus alnobetula subsp. crispa (Aiton) Raus), and a hybrid poplar (Populus sp. Clone 915318)) on the basis of a preliminary greenhouse-based study investigating tree survival and growth (results not shown)49. We planted this mixture of species on all experimental units. Jack pine and hybrid poplar were obtained from the MFFP (Normandin nursery). We obtained green alder from the Girardville nursery (Girardville, Québec), and these alders were inoculated with Frankia (an N-fixing bacteria) at the Université du Sherbrooke, Québec.
2.3. Vegetation Survey
We conducted test replicates to determine the appropriate quadrat size for vegetation samplings. We confirmed that a 1 m2 quadrat makes accurate estimates for our sites. A quadrat was set in the center of each experimental unit on the tailing sites. At the reference sites, we established three quadrats aligned along cardinal directions (north, south, and west) at 3 m from the site center. Within each quadrat, we assessed plant communities between June and August 2018 by visually determining the cover (%) of these species six years (Niobec) and three years (Mont-Wright) post-reclamation. We assessed the combined cover; therefore, the percent cover for a quadrat can exceed 100%. Species were identified to the lowest possible taxonomic level. The inventory included vascular plants, mosses, and lichens. For both sites, the same person conducted all surveys. We collected a sample of each species to confirm its identification in the laboratory (at the vegetation and animal ecology lab at the Université du Québec à Chicoutimi, the bryology lab at Université du Québec en Abitibi-Témiscamingue, and the Louis-Marie Herbarium at Université Laval).
2.4. Statistical Analysis
Statistical analyses were conducted using R software, version 3.6.1 [50]. Total percent cover, species richness (S), Pielou’s evenness (J′), and Simpson’s diversity (1−D) were calculated using the “vegan” package [51]. We assessed the data for homogeneity of variance and transformed data when necessary (only richness at Mont-Wright was log-transformed). Sources of variation for the Niobec site were: (1) number of blocks (n = 4; random), (2) amendment application (PMS35+topsoil and topsoil; n = 2; fixed), and (3) the type of woody species used in plantations (L. laricina; P. resinosa; B. papyrifera; a mixture of these tree species; the no plantation control; n = 5; fixed). Sources of variation at the Mont-Wright site were: (1) number of blocks (n = 3; random) and (2) treatments applied (PMS50, PMS+N5, topsoil, topsoil+N5, N3, N5; n = 6; fixed).
We used ANOVA to test for differences between plant community responses in terms of total percent cover, S, J′, 1−D, and functional groups (grasses, forbs, and mosses). We ran post hoc tests (estimated marginal means (least-squares means)) when the effects were significant (p < 0.05). We analyzed community structures via multivariate analyses based on Bray–Curtis dissimilarity distances and matrices. Species assemblage data were transformed through square-root transformation, as suggested by Clarke and Warwick [52]. To reduce noise in the dataset, we removed single-occurrence taxa before performing our analyses. We tested for differences in community structure among treatments using permutational multivariate analysis of variance (PERMANOVA). We then used non-metric multidimensional scaling (NMDS) to display dissimilarities between samples on a two-dimensional ordination. Finally, we ran SIMPER to identify the discriminant species that could explain differences in diversity between treatments at the Niobec site. We did not perform any statistical analyses on the reference sites because these sites were not part of the experimental design. The results measured at the references sites were used as indicators to make comparisons between natural disturbed sites and reclaimed mine sites.
3. Results
We identified 60 taxa at the Niobec site, including both native and non-native species (see Appendix A for the species list). Forbs belonged to 12 families, the most abundant family being Asteraceae. We also identified ten grasses, nine moss, and three woody species that differed from those normally used in the plantations. At the Mont-Wright site, we identified 38 species, a site characterized by a high richness of grass (8) and moss (11) species. The identified forbs belonged to eight families (mostly native Ericaceae), and we found two lichen genera (see Appendix A for the species list).
3.1. Influence of Amendments and Tree Plantations on Plant Community Response at the Niobec Site
Tree plantations and the interaction between tree plantations and amendment application had no influence on plant community response at the Niobec site. However, the amendment application alone influenced total percent cover, evenness (J’), and diversity (1−D) (Table 2). Plots reclaimed with both topsoil and PMS contained significantly higher plant cover than plots treated only with topsoil (70.5% vs. 41.9%, respectively, p < 0.001) (Figure 1). Amendment application did not influence richness; however, evenness and diversity decreased with both topsoil and PMS application compared with topsoil application alone (Table 2; Figure 1). This pattern suggests that diversity declined because of the decrease in evenness related to the dominance of some species rather than a change in species number.

Table 2.
Summary of two-way ANOVA of the effect of amendments (topsoil+PMS35 and topsoil) and tree plantations (L. laricina, P. resinosa, and B. papyrifera, a mixture of these tree species, and the no plantation control) on total percent cover, richness (S), Pielou’s evenness (J′), and Simpson’s diversity index (1−D) at Niobec.
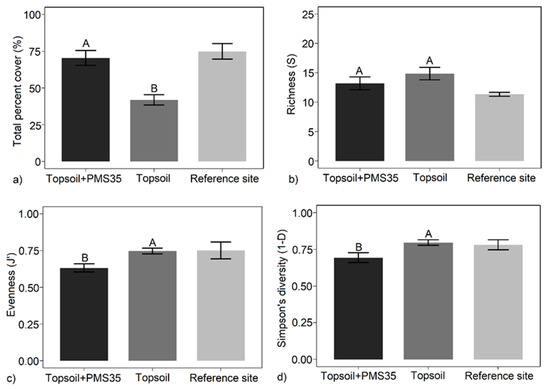
Figure 1.
Mean: (a) total percent cover; (b) richness (S); (c) Pielou’s evenness (J’); (d) Simpson’s diversity (1−D) in relation to the amendment applications (topsoil+PMS35 and topsoil) (±SE; n = 4) at Niobec and its reference sites. Letters represent statistical differences between treatments following post hoc tests, and brackets on each bar correspond to the standard error (the reference site was not included in the statistical analysis).
Total plant cover in plots amended with combined topsoil and PMS was most similar to that on the reference site (Figure 1). However, evenness and diversity on plots amended with topsoil only were more similar to those for the reference plots than for plots amended with a combination of topsoil and PMS (Figure 1).
PERMANOVA revealed community structure based on Bray–Curtis dissimilarities differed between plots that received a mixture of PMS and topsoil and those that received topsoil only (p < 0.001, Table 3). The interaction between tree plantation and amendment application did not significantly influence community structure. The NMDS representation of the community structure (Figure 2) shows a visually acceptable representation (stress = 0.222) of differences between community structures on the basis of amendment treatments. SIMPER found 13 species that explained 72.2% of the dissimilarity between treatments (topsoil+PMS35 and topsoil) (Table 4). These species included the invasive species Tussilago farfara, Sonchus arvensis, Vicia cracca, and Cirsium arvensis as well as taxa of the Poaceae family (grasses) and the moss species Brachythecium campestre (Table 4; Figure 3).

Table 3.
PERMANOVA testing of community structure in relation to the effect of amendment application (PMS35+topsoil and topsoil) and tree plantation (L. laricina, B. papyrifera, P. resinosa, a mix of the three species, and the no-tree control) at the Niobec site.
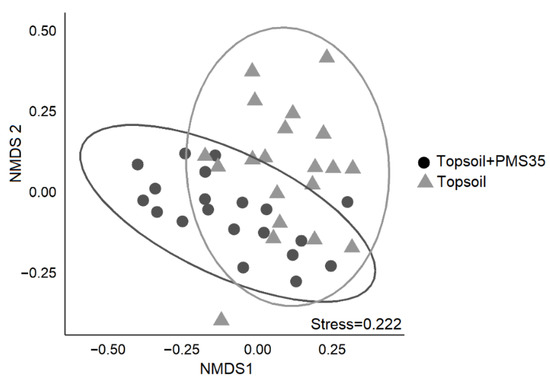
Figure 2.
Non-metric multidimensional scaling (NMDS) representation of community structure in relation to the effect of amendment application (topsoil+PMS35 and topsoil) at the Niobec site. Ellipses represent 95% confidence intervals.

Table 4.
Dissimilarity (%) of the species assemblages between amendment treatments (topsoil+PMS35 and topsoil) at the Niobec site using SIMPER analysis of square-root transformed data.
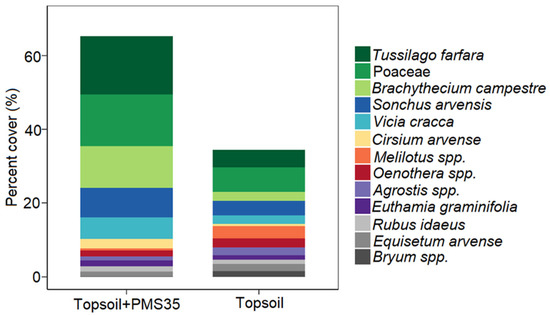
Figure 3.
SIMPER analysis of percent cover of species having the greatest contribution to dissimilarity at the Niobec site between plots amended with both topsoil and PMS (topsoil+PMS35) and plots amended with topsoil only.
3.2. Influence of Amendment on Plant Community Response at Mont-Wright
Amendment application at Mont-Wright significantly influenced the total percent cover, J′, and 1−D (Table 5). The application of PMS only, topsoil only, and the N3 treatment produced similar percent covers (Figure 4). The treatments that included the five-year Norco treatment (N5, PMS50+N5, and Topsoil+N5) produced the highest total percent cover (Figure 4). However, these treatments produced a lower evenness and diversity compared with treatments that did not include the use of Norco. PMS50+N5 produced the most distinct plant community response (total percent cover, evenness, and diversity) relative to the reference site (Figure 4).

Table 5.
Summary of one-way ANOVA of the effect of amendment application (PMS50, PMS50+N5, topsoil, topsoil+N5, N3, N5) on total percent cover, richness (S), Pielou’s evenness (J′), and Simpson’s index (1−D) at the Mont-Wright site.
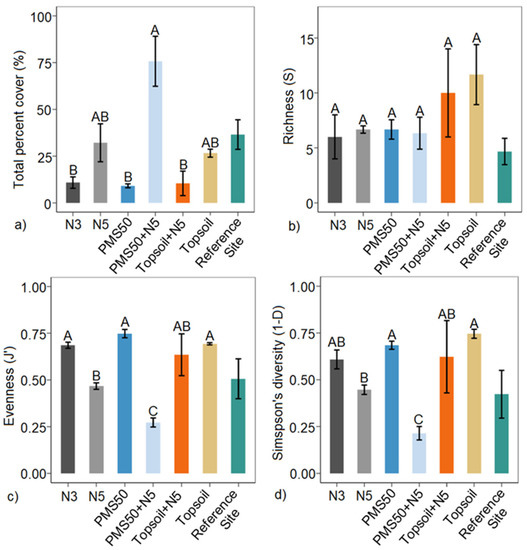
Figure 4.
Mean (a) total percent cover, (b) richness (S), (c) Pielou’s evenness (J′), and (d) Simpson’s diversity (1−D) in relation to reclamation treatments (PMS50, PMS50+N5, topsoil, topsoil+N5, N3, and N5) (±SE; n = 3) at the Mont-Wright site. Letters represent statistical differences between treatments following post hoc tests, and brackets on each bar correspond to the standard error. The reference site was not included in the statistical model.
PERMANOVA revealed that community structure differed significantly among treatments (p < 0.001, Table 6), and NMDS illustrated that community structure in treatments having a topsoil amendment differed most from the other treatments (Figure 5). Although we could not calculate the 95% confidence interval ellipses because of too few data points, the stress index value of 0.145 confirmed the NMDS as a good visual representation of community dissimilarity.

Table 6.
Summary of PERMANOVA of the effect of amendment application (N3, N5, PMS50, PMS50+N5, topsoil, and topsoil+N5) on species assemblages at the Mont-Wright site.
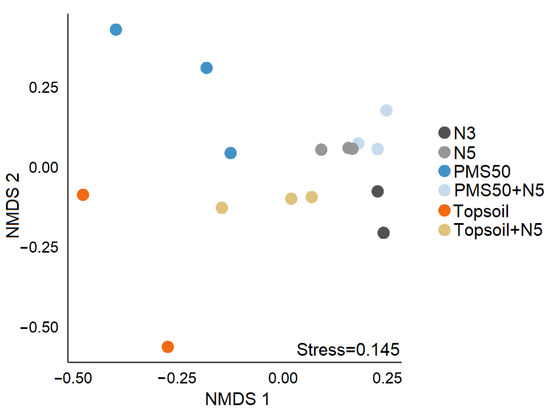
Figure 5.
Non-metric multidimensional scaling (NMDS) representation of community structure on the according to amendment application (N3, N5, PMS50, PMS50+N5, topsoil, topsoil+N5) at the Mont-Wright site.
3.3. Influence of Amendment on Functional Groups
At the Niobec site, grasses (p = 0.050) and mosses (p = 0.698) shared similar percent covers for both reclamation treatments. However, the abundance of forbs was significantly higher in plots amended with both PMS and topsoil than in plots amended with topsoil only (p = 0.008; Figure 6). At the Mont-Wright site, forbs percent cover was similar for all treatment plots (p = 0.3469). The PMS50+N5 mixture produced a higher abundance of grasses (p < 0.001) relative to the other treatments, whereas plots having topsoil mixed with 5-year-old Norco (Topsoil+N5) showed a higher abundance of mosses (p = 0.008; Figure 6).
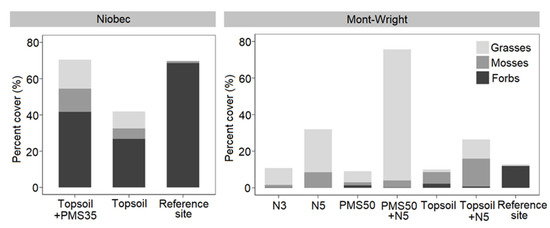
Figure 6.
Total percent cover of functional groups (grasses, forbs, and mosses) in relation to reclamation treatments at the Niobec (treatments: topsoil+PMS35, topsoil, reference sites) (n = 4) and Mont-Wright (treatments: PMS50, PMS50+N5, topsoil, topsoil+N5, N3, N5, and reference sites) (n = 3) sites.
Plant communities at both reference sites were dominated by forbs with limited to quasi-absence of mosses and grasses (Figure 6). Although forbs were the most abundant functional group at the Niobec site, the Mont-Wright mining site contained few forbs.
4. Discussion
4.1. Total Percent Cover
At the Niobec site, adding PMS to topsoil increased total percent plant cover relative to only topsoil as an amendment. This response could be attributed to the high mineralizable organic N content in PMS (C:N ratio of 23.3), as N-addition can increase plant productivity in terrestrial ecosystems [32,53,54]. Young et al. [2] showed a high utilization of organic N by plants following a 5.3 Mg·ha−1 PMS application (their PMS having a similar C:N ratio as ours) mixed with a low fertilizer rate on gold tailings in Manitoba, Canada. They also measured a positive response of total plant cover to the PMS treatment, which they attributed to increased aggregate stability and microbial activity related to the organic C provided by the PMS. Microbial activity is often used as an indicator of soil fertility because it induces biogeochemical processes, such as N mineralization, that favour plant growth [8,54,55]. In addition, Shipitalo and Bonta [55] measured an increase in plant growth at a higher PMS application rate (136 dry Mg·ha−1) on coal tailings in Ohio, United States. The authors noted that PMS significantly reduced runoff and soil erosion, and they observed no nutrient loss.
At the Mont-Wright sites, plots amended with the five-year Norco treatment had a higher total percent cover of plants than other treatment plots that were amended three years before measurements. This response could be attributed to the longer elapsed time after the N5 treatment application because organic amendments require biological processes to make nutrients available for plant uptake [56]. The slow nutrient release associated with organic amendments, especially at this more northern site, could also explain why the use of topsoil only, PMS only, and the three-year Norco treatment had similar total plant coverage [29]. Cold temperatures limit nutrient release, as microbial metabolism and the subsequent decomposition rate of organic matter are reduced in cooler conditions [57,58]. The presence of seeds within the Norco treatment did not influence the success of plant establishment after 3 years, as plots with the three-year Norco treatment had a similar total percent cover as the other treatments.
4.2. Richness, Evenness, and Diversity
At both sites, plots in which amendments produced the highest total percent cover also had the lowest evenness and diversity values. Although amendment application influenced some plant community responses, it did not influence richness. Other studies reported no change in richness on mining sites or degraded grasslands following nutrient addition [33,59,60]. This response does not coincide with the generally accepted “humpbacked model” (HBM), which states that increasing net primary production eventually leads to a decrease in richness [32,54,61]. Adler et al. [62], who also did not observe the humpback pattern of richness within their multiple sites, suggested that there might be better drivers for richness than productivity, such as resources supply rate, disturbance, habitat heterogeneity, biogeographic, and assembly history. Species that colonize nutrient-poor sites also react differently to nutrient addition than species that colonize richer sites [63]. Species that establish in nutrient-poor conditions are equivalent competitors for nutrients; therefore, an increase in primary production allows their coexistence and delays competitive exclusion [64]. Species richness tends to decrease more slowly with nutrient addition in such nutrient-poor sites because the effect of this added resource is initially expressed by a change in species abundance, which leads to an initial decrease in evenness and diversity [31,64].
At the Niobec site, the application of PMS on topsoil increased the abundance of invasive forbs, grasses, and the moss Brachythecium campestre. These species appear well adapted to colonize tailings, as reported at many reclaimed mine sites [24,37]. Similarly, at Mont-Wright, mosses known to colonize perturbed sites, such as Ceratodon purpureus, Polytrichum juniperinum, and Polytrichum piliferum, grew well [37,65].
4.3. Functional Groups
At the Niobec site, forbs grew better on plots amended with topsoil and PMS than on plots amended with topsoil only. This response related to the enhanced presence of invasive species, mostly forbs, according to the SIMPER analysis. At Mont-Wright, the 5-year Norco and PMS (PMS50+N5) enhanced grass abundance. This enhanced grass response related to the highest soil nutrient content (results not shown), stemming from this amendment and the applied seed mix being composed mostly of grasses. Nutrients enhance the growth of C3 grasses and reduce the growth of forbs and legumes [66]. Topsoil mixed with the Norco mixture enhanced moss abundance. The heterogeneous microtopography created by the topsoil could have contributed to the increase in performance of this functional group. The microtopography may have created microclimates that retained humidity, producing conditions that are more favorable for mosses [37,67]. It should also be noted that wind erosion was particularly high at the Mont-Wright site, as evidenced by the two excluded plots being buried by off-experiment tailings because of wind.
Both sites differed in their functional-group abundance relative to their respective reference sites. The relatively older communities of the reference sites and the poorer environmental conditions on tailings probably contributed to the differences in plant diversity between the experimental and reference sites. Results also show that topsoil use on tailings increases moss abundance. Although mosses are associated with a healthy ecosystem under natural succession within the boreal forest, and they have been frequently reported, less is known about their presence on tailings [37,43]. Errington and Pinno [44] also found distinct communities on mining sites reclaimed with either a forest floor–mineral mix or peat–mineral mix. Dhar et al. [37] found that the differences between sites could be attributed to the biological legacies of fires (surviving trees, snags, logs, patches of intact vegetation, seed banks in tree crowns, or the soil), which lead to the development of competitive and structured communities that are more resistant to species invasion than tailing communities lacking this predisturbance memory.
4.4. Tree Plantations
Very little empirical evidence has shown that plantations contribute to reclaiming biodiversity on mine sites, and plant community responses vary among studies. Nonetheless, reviews by Barbier et al. [25] and Bremer and Farley [23] present a more positive effect on the biodiversity of native tree plantations on degraded lands compared with other plantation types in other ecosystems. Our results at the Niobec site do not support this positive influence, however, as our plantations did not affect any of the measured plant community responses (total percent cover, S, J′, and 1−D). Felton et al. [68] identified that factors such as plantation characteristics, proximity to native vegetation, and previous land use influence biodiversity establishment in plantations, which could explain the lack of a general pattern among studies. For instance, tree spacing can play an important role in mediating plant facilitation by changing microclimatic conditions in the understorey, such as temperature, moisture, and light availability, which influence plant community composition [20,22,25,43,65].
We also observed no short-term differences in plant diversity between mixed and pure plantations. There is little evidence that mixed plantations favor a higher diversity than monocultures on mine sites [69]. Further research is required, however, to assess the longer-term influence of different plantation types on plant diversity and evaluate the potential of this reclamation method on tailings. It should be noted that six years post-planting at the Niobec site, we did not observe any canopy closure and the associated light interception (results not shown).
Finally, although plantations may not increase plant diversity, it should be noted that their use could provide other benefits, such as facilitating the establishment of other native tree species, integrating tailings into the surrounding forested landscapes, providing wildlife habitat, and increasing carbon sequestration [18,24,70].
5. Conclusions
Our study showed that the application of an organic amendment had a significant influence on plant community response (total percent cover, J′, and 1−D) at both mine tailing sites (Niobec and Mont-Wright mines, Québec), whereas plantations did not produce any community response at the Niobec site. The application of PMS on topsoil produced less diverse communities than the use of the business-as-usual topsoil-only minimal method, six years after the reclamation. At the Mont-Wright site, the early response of vegetation (three years post-reclamation) showed that the combination of the Norco treatment (chicken manure, hay, and herbaceous seeds) with topsoil and the application of topsoil only or biosolids only resulted in the highest values of evenness (J′) and diversity (1−D).
Plant communities on tailings were distinct from those found on post-fire forest reference stands. We measured a higher proportion of mosses and grasses at the Niobec site than its reference site. At Mont-Wright, topsoil enhanced the abundance of mosses, and plots that received the Norco treatment mixed with PMS contained a high abundance of grasses. Natural sites contained mostly forbs. Our results provide valuable insight regarding the influence of different reclamation methods on the plant communities of reclaimed mine sites. Further research should explore the longer-term influences of these amendments in other climate zones and applied at different rates, including cost–benefit analyses on the selection of different organic amendments for tailing reclamation.
Author Contributions
Conceptualization, J.-F.B.; methodology, A.G.; software, A.G.; validation, J.-F.B. and P.S.; formal analysis, A.G.; resources, J.-F.B. and N.J.F.; data curation, A.G.; writing—original draft preparation, A.G.; writing—review and editing, J.-F.B., P.S. and N.J.F.; supervision, J.-F.B. and P.S.; project administration, J.-F.B.; funding acquisition, J.-F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Sciences and Engineering Research Council of Canada grant number CRDPJ 488866-15.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data and analysis scripts used in this study will be available on FigShare (doi:10.6084/m9.figshare.13205909).
Acknowledgments
We thank Sébastien Roy, Université de Sherbrooke, for his work inoculating the alder with Frankia.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
List of the species found at Niobec and Mont-Wright.
Table A1.
List of the species found at Niobec and Mont-Wright.
| Niobec | Mont-Wright | ||
|---|---|---|---|
| Taxon | Functional Type | Taxon | Functional Type |
| Tussilago farfara Linnaeus | Forb | Vicia cracca Linnaeus | Forb |
| Cirsium arvense (L.) Scopoli | Forb | Rumex acetosella Linnaeus | Forb |
| Euthamia graminifolia (L.) Nuttall | Forb | Vaccinium oxycoccos Linnaeus | Forb |
| Sonchus arvensis Linnaeus | Forb | Vaccinium angustifolium Aiton | Forb |
| Oenothera Linnaeus | Forb | Chamaenerion angustifolium (L.) Scopoli | Forb |
| Taraxacum officinale F.H. Wiggers | Forb | Gaultheria hispidula (L.) Muhl. ex Big. | Forb |
| Vicia cracca Linnaeus | Forb | Equisetum pratense Ehrhart | Forb |
| Solidago rugosa Miller | Forb | Rhododendron canadense (L.) Torrey | Forb |
| Solidago canadensis Linnaeus | Forb | Kalmia polifolia Wangenheim | Forb |
| Leucanthemum vulgare Lamarck | Forb | Ranunculus acris Linnaeus | Forb |
| Erigeron philadelphicus Linnaeus | Forb | Taraxacum officinale F.H. Wiggers | Forb |
| Prunella vulgaris Linnaeus | Forb | Trifolium repens Linnaeus | Forb |
| Equisetum arvense Linnaeus | Forb | Stellaria graminea Linnaeus | Forb |
| Melilotus spp. Miller | Forb | Unknown plant 1 | Forb |
| Fragaria spp. Linnaeus | Forb | Unknown plant 2 | Forb |
| Plantago lanceolata Linnaeus | Forb | Unknown plant 3 | Forb |
| Pilosella aurantiaca (L.) F.W. Schultz and Schultz Bip. | Forb | Unknown plant 4 | Grass |
| Trifolium spp. Linnaeus | Forb | Poaceae Barnhart | Grass |
| Medicago lupulina Linnaeus | Forb | Phleum pratense Linnaeus | Grass |
| Urtica dioica Linnaeus | Forb | Agrostis spp. Linnaeus | Grass |
| Rubus idaeus Linnaeus | Forb | Festuca spp. Linnaeus | Grass |
| Rubus pubescens Rafinesque | Forb | Poa spp. Linnaeus | Grass |
| Geum spp. Linnaeus | Forb | Bromus spp. Linnaeus | Grass |
| Verbascum thapsus Linnaeus | Forb | Elymus repens (L.) Gould | Grass |
| Achillea millefolium Linnaeus | Forb | Avenella flexuosa (L.) Drejer | Grass |
| Ranunculus acris Linnaeus | Forb | Anthoxanthum odoratum L. | Grass |
| Vaccinium angustifolium Aiton | Forb | Ceratodon purpureus (Hedw.) Brid. | Moss |
| Fallopia convolvulus (L.) Á. Löve | Forb | Polytrichum juniperinum Hedw. | Moss |
| Thalictrum spp. Linnaeus | Forb | Polytrichum piliferum Hedw. | Moss |
| Unknown plant 1 | Forb | Dicranum condensatum Hedw. | Moss |
| Unknown plant 2 | Forb | Pleurozium schreberi (Willd ex Brid.) Mitt | Moss |
| Unknown plant 3 | Forb | Pohlia nutans (Hedw.) Lindb. | Moss |
| Unknown plant 4 | Forb | Pohlia camptotrachela (Renauld and Cardot) Broth. | Moss |
| Unknown plant 5 | Forb | Pogonatum urnigerum (Hedw.) P.Beauv. | Moss |
| Unknown plant 6 | Forb | Pogonatum dentatum (Menzies ex Brid.) Brid. | Moss |
| Unknown plant 7 | Forb | Racomitrium canescens (Hedw.) Brid. | Moss |
| Unknown plant 8 | Forb | Sphagnum spp. Linnaeus | Moss |
| Unknown plant 9 | Forb | Cladoniae spp. | Lichen |
| Poaceae Barnhart | Grass | Peltigera spp. | Lichen |
| Phleum pratense Linnaeus | Grass | ||
| Agrostis spp. Linnaeus | Grass | ||
| Festuca spp. Linnaeus | Grass | ||
| Calamagrostis canadensis (Michaux) Palisot de Beau. | Grass | ||
| Elymus repens (Linnaeus) Gould | Grass | ||
| Hordeum jubatum Linnaeus | Grass | ||
| Phalaris arundinacea Linnaeus | Grass | ||
| Poa spp. Linnaeus | Grass | ||
| Carex bebbii (L.H. Bailey) Olney ex Fernald | Grass | ||
| Carex spp. Linnaeus | Grass | ||
| Abies balsamea (Linnaeus) Miller | Tree | ||
| Picea mariana (Miller) Britton, Sterns and Poggenburgh | Tree | ||
| Thuja occidentalis Linnaeus | Tree | ||
| Brachythecium campestre (Müll.Hal.) Schimp. | Moss | ||
| Pohlia nutans (Hedw.) Lindb. | Moss | ||
| Barbula convoluta Hedw. | Moss | ||
| Hypnum cupressiforme Hedw. | Moss | ||
| Ceratodon purpureus (Hedw.) Brid. | Moss | ||
| Thuidium recognitum (Hedw.) Lind. | Moss | ||
| Aneura pinguis (L.) Dumort. | Moss | ||
| Unknown plant 10 | Moss | ||
References
- Aubertin, M.; Bussière, B.; Bernier, L.; Chapuis, R.; Julien, M.; Belem, T.; Simon, R.; Mbonimpa, M.; Benzaazoua, M.; Li, L. La gestion des rejets miniers dans un contexte de développement durable et de protection de l’environnement. In Congrès Annuel de la Société Canadienne de Génie Civile, 5 June 2002; the Canadian Society for Civil Engineering: Montréal, QC, Canada, 2002. [Google Scholar]
- Young, I.; Renault, S.; Markham, J. Low Levels of organic Amendments improve fertility and plant cover on non-acid generating gold mine tailings. Ecol. Eng. 2015, 74, 250–257. [Google Scholar] [CrossRef]
- Bradshaw, C.J.A.; Warkentin, I.G.; Sodhi, N.S. Urgent preservation of boreal carbon stocks and biodiversity. Trends Ecol. Evol. 2009, 24, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Gastauer, M.; Silva, J.R.; Caldeira Junior, C.F.; Ramos, S.J.; Souza Filho, P.W.M.; Furtini Neto, A.E.; Siqueira, J.O. Mine land rehabilitation: Modern ecological approaches for more sustainable mining. J. Clean. Prod. 2017, 172, 1409–1422. [Google Scholar] [CrossRef]
- Antonelli, P.; Fraser, L.; Gardner, W.; Broersma, K.; Phillips, M. Long-term carbon sequestration potential of biosolids-amended copper and molybdenum mine tailings following mine site reclamation. Ecol. Eng. 2018, 117, 38–49. [Google Scholar] [CrossRef]
- Page-Dumroese, D.; Ott, M.; Strawn, D.; Tirocke, J. Using organic amendments to restore soil physical and chemical properties of a mine site in northeastern Oregon, USA. Appl. Eng. Agric. 2018, 34, 43–55. [Google Scholar] [CrossRef]
- Vega, F.; Vega, F.; Covelo, E.; Andrade, M. Limiting factors for reforestation of mine spoils from Galicia (Spain). Land Degrad. Dev. 2005, 16, 27. [Google Scholar] [CrossRef]
- Sheoran, V.; Sheoran, A.; Poonia, P. Soil Reclamation of Abandoned Mine Land by Revegetation: A review. Int. J. Soil Sediment Water 2010, 3, 1–13. Available online: https://scholarworks.umass.edu/intljssw/vol3/iss2/13 (accessed on 10 May 2021).
- Gardner, W.C.; Anne, N.M.; Broersma, K.; Chanasyk, D.S.; Jobson, A.M. Influence of biosolids and fertiliser amendments on element concentrations and revegetation of copper mine tailings. Can. J. Soil Sci. 2012, 92, 89–102. [Google Scholar] [CrossRef] [Green Version]
- López-Marcos, D.; Turrión, M.B.; Martínez-Ruiz, A. Linking soil variability with plant community composition along a mine-slope topographic gradient: Implications for restoration. AMBIO 2020, 49, 337–349. [Google Scholar] [CrossRef]
- Tordoff, G.M.; Baker, A.J.M.; Willis, A.J. Current approaches to the revegetation and reclamation of metalliferous mine wastes. Chemosphere 2000, 41, 219–228. [Google Scholar] [CrossRef]
- Cooke, J.A.; Johnson, M.S. Ecological restoration of land with particular reference to the mining of metals and industrial minerals: A review of theory and practice. Environ. Rev. 2002, 10, 41–71. [Google Scholar] [CrossRef] [Green Version]
- Tandy, S.; Wallace, H.L.; Jones, D.L.; Nason, M.A.; Williamson, J.C.; Healey, J.R. Can a mesotrophic grassland community be restored on a post-industrial sandy site with compost made from waste materials? Biol. Conserv. 2011, 144, 500–510. [Google Scholar] [CrossRef]
- Alday, J.G.; Marrs, R.H.; Martínez-Ruiz, C. Vegetation convergence during early succession on coal wastes: A 6-year permanent plot study. J. Veg. Sci. 2011, 22, 1072–1083. [Google Scholar] [CrossRef]
- Alday, J.G.; Zaldívar, P.; Torroba-Balmori, P.; Fernández-Santos, B.; Martínez-Ruiz, C. Natural forest expansion on reclaimed coal mines in Northern Spain: The role of native shrubs as suitable microsites. Environ. Sci. Pollut. Res. 2016, 23, 13606–13616. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Chaney, R. Use of amendments to restore ecosystem function to metal mining-impacted sites: Tools to evaluate efficacy. Curr. Pollut. Rep. 2016, 2, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Minister of Energy and Natural Resources (MERN). Guidelines for Preparing Mine Closure Plans in Québec; Ministère du Développement durable, Direction de la restauration des sites miniers: Quebec City, QC, Canada, 2017. [Google Scholar]
- Ussiri, D.A.N.; Lal, R. Carbon sequestration in reclaimed minesoils. CRC Crit. Rev. Plant Sci. 2005, 24, 151–165. [Google Scholar] [CrossRef]
- Larchevêque, M.; Desrochers, A.; Bussière, B.; Cimon, D. Planting trees in soils above non-acid-generating wastes of a boreal gold mine. Écoscience 2014, 21, 217–231. [Google Scholar] [CrossRef]
- Lieffers, V.J.; Stadt, K.J. Growth of understory Picea glauca, Calamagrostis canadensis, and Epilobium angustifolium in relation to overstory light transmission. Can. J. For. Res. 1994, 24, 1193–1198. [Google Scholar] [CrossRef]
- Brockerhoff, E.; Jactel, H.; Parrotta, J.; Quine, C.; Sayer, J. Plantation forests and biodiversity: Oxymoron or opportunity? Biodivers. Conserv. 2008, 17, 925–951. [Google Scholar] [CrossRef]
- Paquette, A.; Messier, C.; Perinet, P.; Cogliastro, A. Simulating light availability under different hybrid poplar clones in a mixed intensive plantation system. For. Sci. 2008, 54, 481–489. [Google Scholar] [CrossRef]
- Bremer, L.; Farley, K. Does plantation forestry restore biodiversity or create green deserts? A synthesis of the effects of land-use transitions on plant species richness. Biodivers. Conserv. 2010, 19, 3893–3915. [Google Scholar] [CrossRef] [Green Version]
- Bouchard, H.; Guittonny, M.; Brais, S. Early recruitment of boreal forest trees in hybrid poplar plantations of different densities on mine waste rock slopes. For. Ecol. Manag. 2018, 429, 520–533. [Google Scholar] [CrossRef]
- Barbier, S.; Gosselin, F.; Balandier, P. Influence of tree species on understory vegetation diversity and mechanisms involved-a critical review for temperate and boreal forests. For. Ecol. Manag. 2008, 254, 1–15. [Google Scholar] [CrossRef]
- Gagnon, A.; Ploughe, L.W.; Harris, M.P.; Gardner, W.C.; Pypker, T.; Fraser, L.H. Grassland reclamation of a copper mine tailings facility: Long term effects of biosolids on plant community responses. Appl. Veg. Sci. 2021, 24, 1–10. [Google Scholar] [CrossRef]
- Haering, K.; Daniels, W.; Feagley, S.E. Reclaiming Mined Lands with Biosolids, Manures, and Papermill Sludges. In Reclamation of Drastically Disturbed Lands. January 1th 2000; Barnhisel, R.I., Darmody, R.G., Lee Daniels, W., Eds.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, Wisconsin, USA, 2000; pp. 615–644. Available online: doi:10.2134/agronmonogr41.c24 (accessed on 25 March 2021).
- Shipitalo, M.; Bonta, J. Impact of using paper mill sludge for surface-mine reclamation on runoff water quality and plant growth. J. Environ. Qual. 2008, 37, 2351–2359. [Google Scholar] [CrossRef] [Green Version]
- Larney, F.; Angers, D. The role of organic amendments in soil reclamation: A review. Can. J. Soil Sci. 2012, 92, 19–38. [Google Scholar] [CrossRef]
- Brown, R.L.; Naeth, M.A. Woody debris amendment enhances reclamation after oil sands mining in Alberta, Canada. Restor. Ecol. 2014, 22, 40–48. [Google Scholar] [CrossRef]
- DiTommaso, A.; Aarssen, L. Resource manipulations in natural vegetation: A review. Vegetatio 1989, 84, 9–29. Available online: https://www.jstor.org/stable/20038508 (accessed on 10 May 2021). [CrossRef]
- Fujimaki, R.; Sakai, A.; Kaneko, N. Ecological risks in anthropogenic disturbance of nitrogen cycles in natural terrestrial ecosystems. Ecol. Res. 2009, 24, 955–964. [Google Scholar] [CrossRef]
- Borden, R.K.; Black, R. Biosolids application and long-term noxious weed dominance in the western United States. Restor. Ecol. 2011, 19, 639–647. [Google Scholar] [CrossRef]
- Girard, S. Utilisation D’amendements Organiques pour le Reboisement du Parc de Résidus Miniers Sans Rejet Acide du Mont-Wright. Master’s Thesis, Université du Québec à Chicoutimi, Saguenay, QC, Canada, 2017. [Google Scholar]
- Rivera, D.; Jáuregui, B.M.; Peco, B. The fate of herbaceous seeds during topsoil stockpiling: Restoration potential of seed banks. Ecol. Eng. 2012, 44, 94–101. [Google Scholar] [CrossRef]
- Brown, S.; Mahoney, M.; Sprenger, M. A comparison of the efficacy and ecosystem impact of residual-based and topsoil-based amendments for restoring historic mine tailings in the Tri-State mining district. Sci. Total Environ. 2014, 485-486, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.; Comeau, P.G.; Karst, J.; Pinno, B.D.; Chang, S.X.; Naeth, A.M.; Vassov, R.; Bampfylde, C. Plant community development following reclamation of oil sands mine sites in the boreal forest: A review. Environ. Rev. 2018, 26, 286–298. [Google Scholar] [CrossRef]
- Faubert, P.; Barnabé, S.; Bouchard, S.; Côté, R.; Villeneuve, C. Pulp and paper mill sludge management practices: What are the challenges to assess the impacts on greenhouse gas emissions? Resour. Conserv. Recycl. 2016, 108, 107–133. [Google Scholar] [CrossRef] [Green Version]
- Chinchu, C.; Sumi, S. Pulp and paper mill fly ash: A review. Sustainability 2019, 11, 4394. [Google Scholar] [CrossRef] [Green Version]
- La Roi, G.H.; Stringer, M.H.L. Ecological studies in the boreal spruce-fir forest of the North American taiga. ii. Analysis of the bryophyte flora. Can. J. Bot. 1976, 54, 619–643. [Google Scholar] [CrossRef]
- Zouaoui, S. Dynamique des Lichens Terricoles du Genre Cladina Après les Feux et les Coupes dans le Domaine de la Pessière à Mousses. Ph.D. Thesis, Université de Montréal, Montréal, QC, Canada, 2011. [Google Scholar]
- Arseneault, J.; Fenton, N.; Bergeron, Y. Effets de l’ouverture de la canopée sur la diversité des bryophytes associées aux débris ligneux grossiers dans la pessière à mousse. Master’s Thesis, Université du Québec à Montréal, Montréal, QC, Canada, 2012. [Google Scholar]
- Bergeron, Y.; Fenton, N.J. Boreal forests of eastern Canada revisited: Old growth, nonfire disturbances, forest succession, and biodiversity. Botany 2012, 90, 509–523. [Google Scholar] [CrossRef]
- Errington, R.C.; Pinno, B.D. Early successional plant community dynamics on a reclaimed oil sands mine in comparison with natural boreal forest communities. Écoscience 2015, 22, 133–144. [Google Scholar] [CrossRef]
- Minister of Forests, Wildlife and Parks (MFFP). Zones de Végétation et Domaines Bioclimatiques du Québec. Available online: https://mffp.gouv.qc.ca/forets/inventaire/inventaire-zones-carte.jsp (accessed on 6 May 2019).
- Environment and Climate Change Canada (ECCC). Canadian Climate Normals 1971–2000 Station Data. 2019a, Station: Bagotville A. Available online: https://climate.weather.gc.ca/climate_normals/results_1981_2010_e.html?searchType=stnName&txtStationName=Bagotville&searchMethod=contains&txtCentralLatMin=0&txtCentralLatSec=0&txtCentralLongMin=0&txtCentralLongSec=0&stnID=5889&dispBack=1 (accessed on 14 November 2020).
- Environment and Climate Change Canada (ECCC). Canadian Climate Normals 1971–2000 Station Data. 2019b (last updated October 22 2019, Station: Wabush Lake A. Available online: https://climate.weather.gc.ca/climate_normals/ (accessed on 21 November 2019).
- CNRC. Biosolids Management Programs. 2003. 52p. Available online: https://fcm.ca/sites/default/files/documents/resources/guide/infraguide-biosolids-management-programs-mamp.pdf (accessed on 10 May 2021).
- Juge, C.; Cossette, N. Sustainable revegetation of iron mine tailings of north-eastern Québec and Labrador: Choice of plant species, creation of a living soil and root microbial symbioses. Can. Reclam. 2015, 2, 28–33. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Oksanen, J.; Blanchet, F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.; O’Hara, R.; Simpson, G.; Solymos, P.; et al. vegan: Community Ecology Package. R Package Version 2.5-6. 2019. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 14 May 2021).
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed.; PRIMER-E, Ltd., Plymouth Marine Laboratory: Plymouth, England, UK, 2001. [Google Scholar]
- Suding, K.N.; Collins, S.L.; Gough, L.; Clark, C.; Cleland, E.E.; Gross, K.L.; Milchunas, D.G.; Pennings, S. Functional- and abundance-based mechanisms explain diversity loss due to N fertilisation. Proc. Natl. Acad. Sci. USA 2005, 102, 4387–4392. [Google Scholar] [CrossRef] [Green Version]
- Clark, C.M.; Tilman, D. Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands. Nature 2008, 451, 712–715. [Google Scholar] [CrossRef]
- Amézketa, E. Soil aggregate stability: A review. J. Sustain. Agric. 1999, 14, 83–151. [Google Scholar] [CrossRef]
- Hagemann, N.; Harter, J.; Behrens, S. Elucidating the Impacts of Biochar Applications on Nitrogen Cycling Microbial Communities. In Biochar Application; Elsevier: Amsterdam, Netherlands, 2016; pp. 163–198. Available online: doi:10.1016/B978-0-12-803433-0.00007-2 (accessed on 26 March 2021).
- Waksman, S.A.; Gerretsen, F.C. Influence of temperature and moisture upon the nature and extent of decomposition of plant residues by microorganisms. Ecology 1931, 12, 33–60. [Google Scholar] [CrossRef]
- Honeycutt, C.W.; Potaro, L.J. Field evaluation of heat units for predicting crop residue carbon and nitrogen mineralization. Plant Soil 1990, 125, 213–220. [Google Scholar] [CrossRef]
- Díaz, S.; Lavorel, S.; Bello, F.; Quétier, F.; Grigulis, K.; Robson, T. Incorporating plant functional diversity effects in ecosystem service assessments. Proc. Natl. Acad. Sci. USA 2007, 104, 20684–20689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wójcikowska-Kapusta, A.; Urban, D.; Baran, S.; Bik-Małodzińska, M.; Żukowska, G.; Pawłowski, A.; Czechowska-Kosacka, A. Evaluation of the influence of composts made of sewage sludge, ash from power plant, and sawdust on floristic composition of plant communities in the plot experiment. Environ. Prot. Eng. 2017, 43, 129. [Google Scholar] [CrossRef]
- Grime, J.P. Competitive exclusion in herbaceous vegetation. Nature 1973, 242, 344–347. [Google Scholar] [CrossRef]
- Adler, P.B.; Seabloom, E.W.; Borer, E.T.; Hillebrand, H.; Hautier, Y.; Hector, A.; Harpole, W.S.; O’Halloran, L.R.; Grace, J.B.; Anderson, T.M.; et al. Productivity is a poor predictor of plant species richness. Science 2011, 333, 1750–1753. [Google Scholar] [CrossRef] [Green Version]
- Chapin, F.S.; Vitousek, P.M.; Van Cleve, K. The nature of nutrient limitation in plant communities. Am. Nat. 1986, 127, 48–58. [Google Scholar] [CrossRef]
- Huberty, L.E.; Gross, K.L.; Miller, C.J. Effects of nitrogen addition on successional dynamics and species diversity in Michigan old-fields. J. Ecol. 1998, 86, 794–803. [Google Scholar] [CrossRef]
- Venier, L.A.; Thompson, I.D.; Fleming, R.; Malcolm, J.; Aubin, I.; Trofymow, J.A.; Langor, D.; Sturrock, R.; Patry, C.; Outerbridge, R.O.; et al. Effects of natural resource development on the terrestrial biodiversity of Canadian boreal forests. Environ. Rev. 2014, 22, 457–490. [Google Scholar] [CrossRef]
- Pan, J.J.; Ammerman, D.; Mitchell, R.J. Nutrient Amendments in a Temperate Grassland have Greater Negative Impacts on Early Season and Exotic Plant Species. Plant Ecol. 2011, 212, 853–864. Available online: https://www.jstor.org/stable/41508773 (accessed on 16 February 2021). [CrossRef]
- Vitt, D.; Crandall-Stotler, B.; Wood, A. Bryophytes: Survival in a dry world through tolerance and avoidance. In Plant Ecology and Evolution in Harsh Environments; Rajakaruna, N., Boyd, R., Harris, T., Eds.; Nova Publishers: Hauppauge, NY, USA, 2014; pp. 267–295. [Google Scholar]
- Felton, A.; Knight, E.; Wood, J.; Zammit, C.; Lindenmayer, D. A meta-analysis of fauna and flora species richness and abundance in plantations and pasture lands. Biol. Conserv. 2010, 143, 545–554. [Google Scholar] [CrossRef] [Green Version]
- Cavard, M.; Bergeron, C. Importance of mixedwoods for biodiversity conservation: Evidence for understory plants, songbirds, soil fauna, and ectomycorrhizae in northern forests. Environ. Rev. 2011, 19, 142–161. [Google Scholar] [CrossRef]
- Carnevale, N.J.; Montagnini, F. Facilitating regeneration of secondary forests with the use of mixed and pure plantations of indigenous tree species. For. Ecol. Manag. 2002, 163, 217–227. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).