Quantifying Mechanistic Detachment Parameters Due to Humic Acids in Biological Soil Crusts
Abstract
1. Introduction
2. Materials and Methods
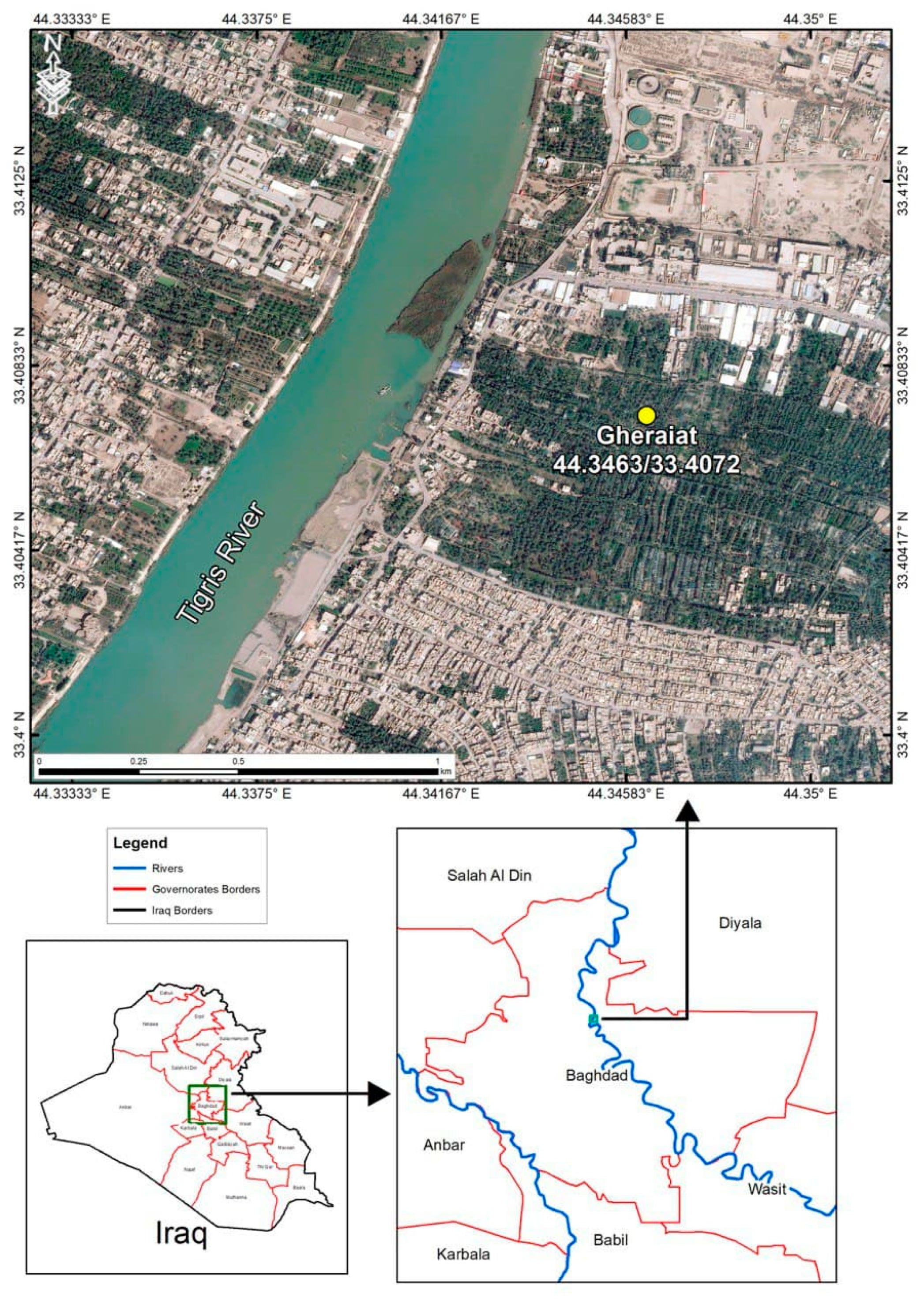
2.1. Study Area
2.2. Humic Acid and Sampling Collection
2.3. Analysis of Soil Physical-Chemical Characteristics
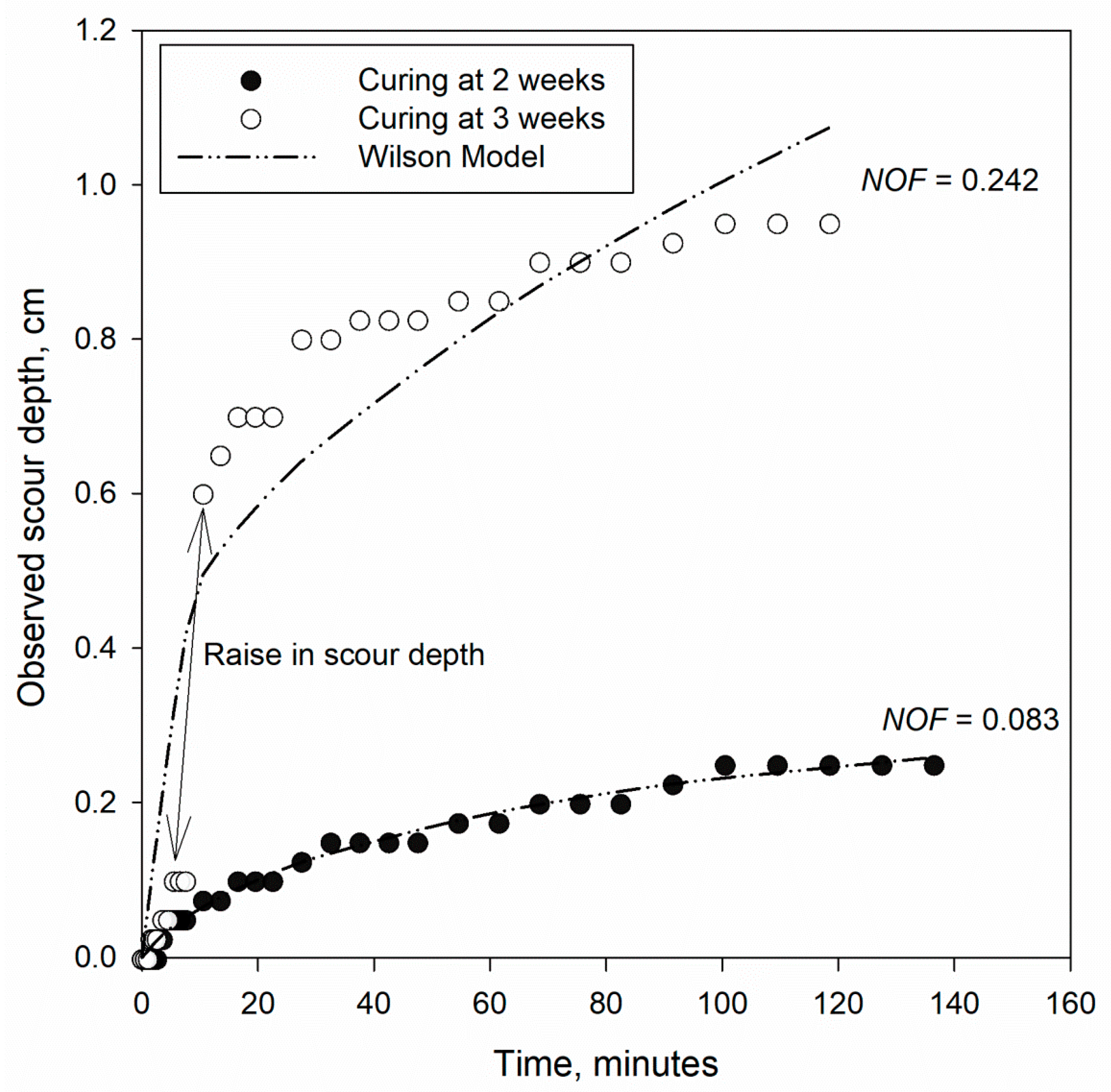
2.4. Testing Procedure
2.5. Deriving of Wilson Model Parameters
2.6. Statistical Analysis
3. Results
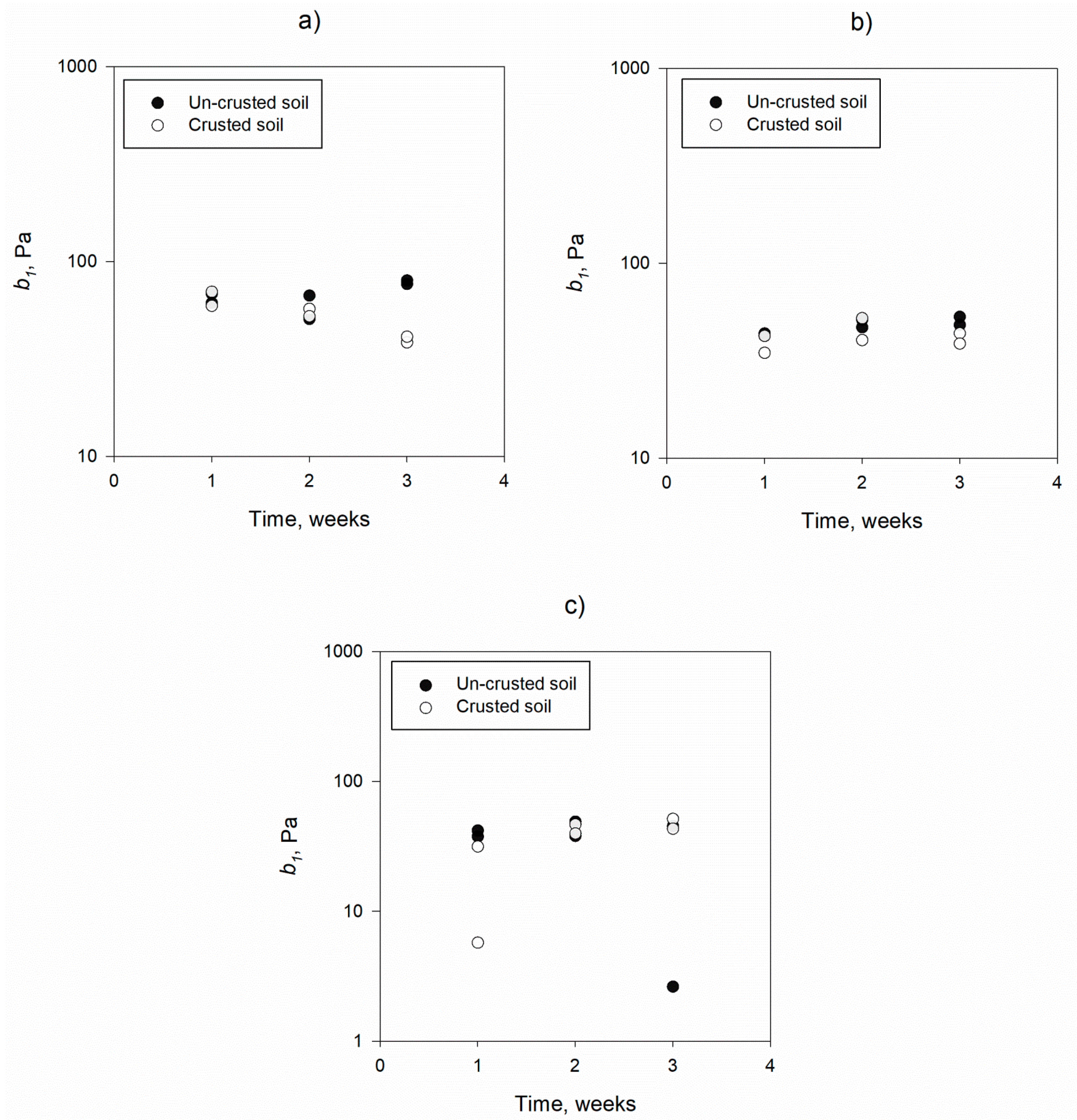
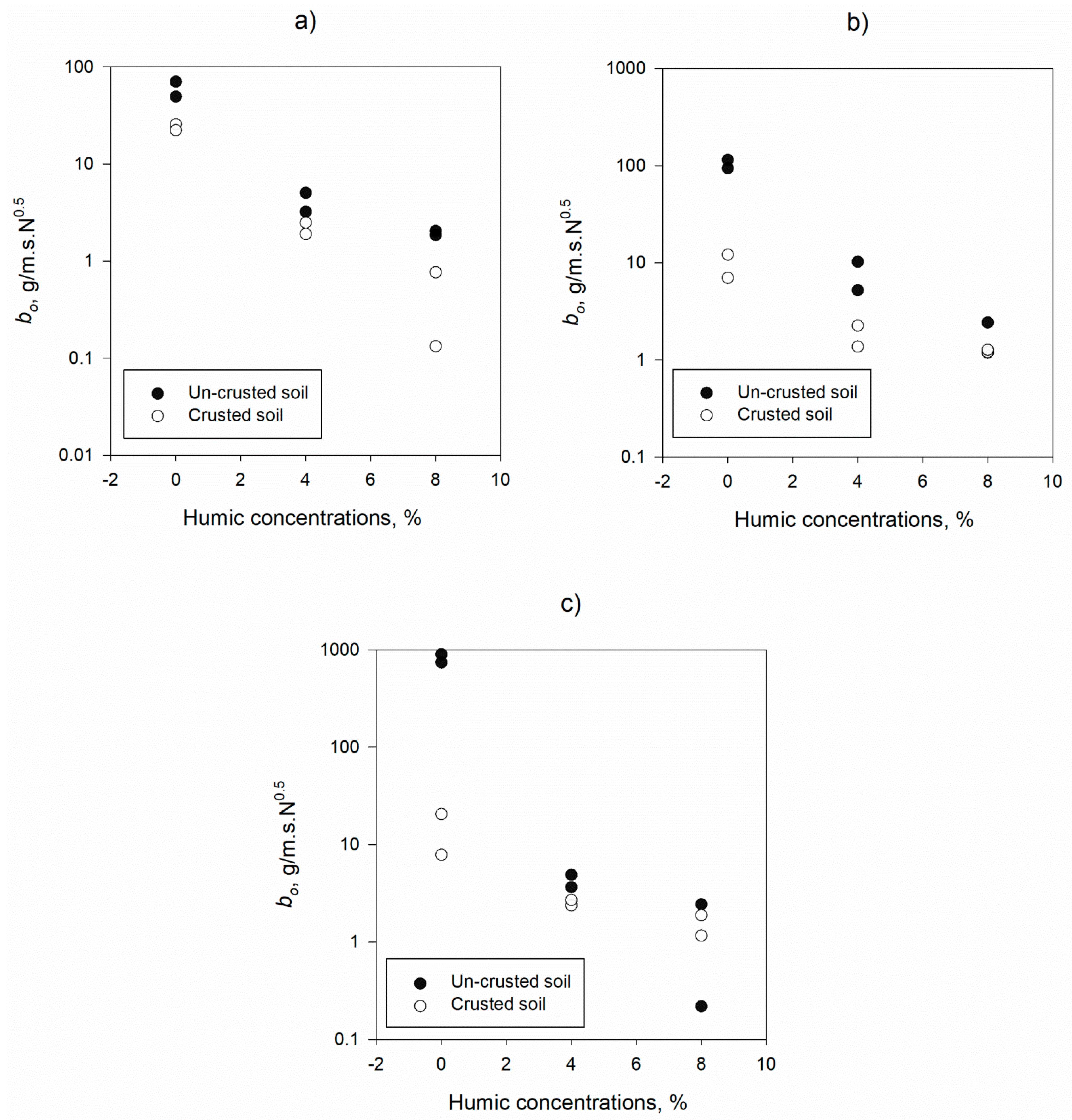
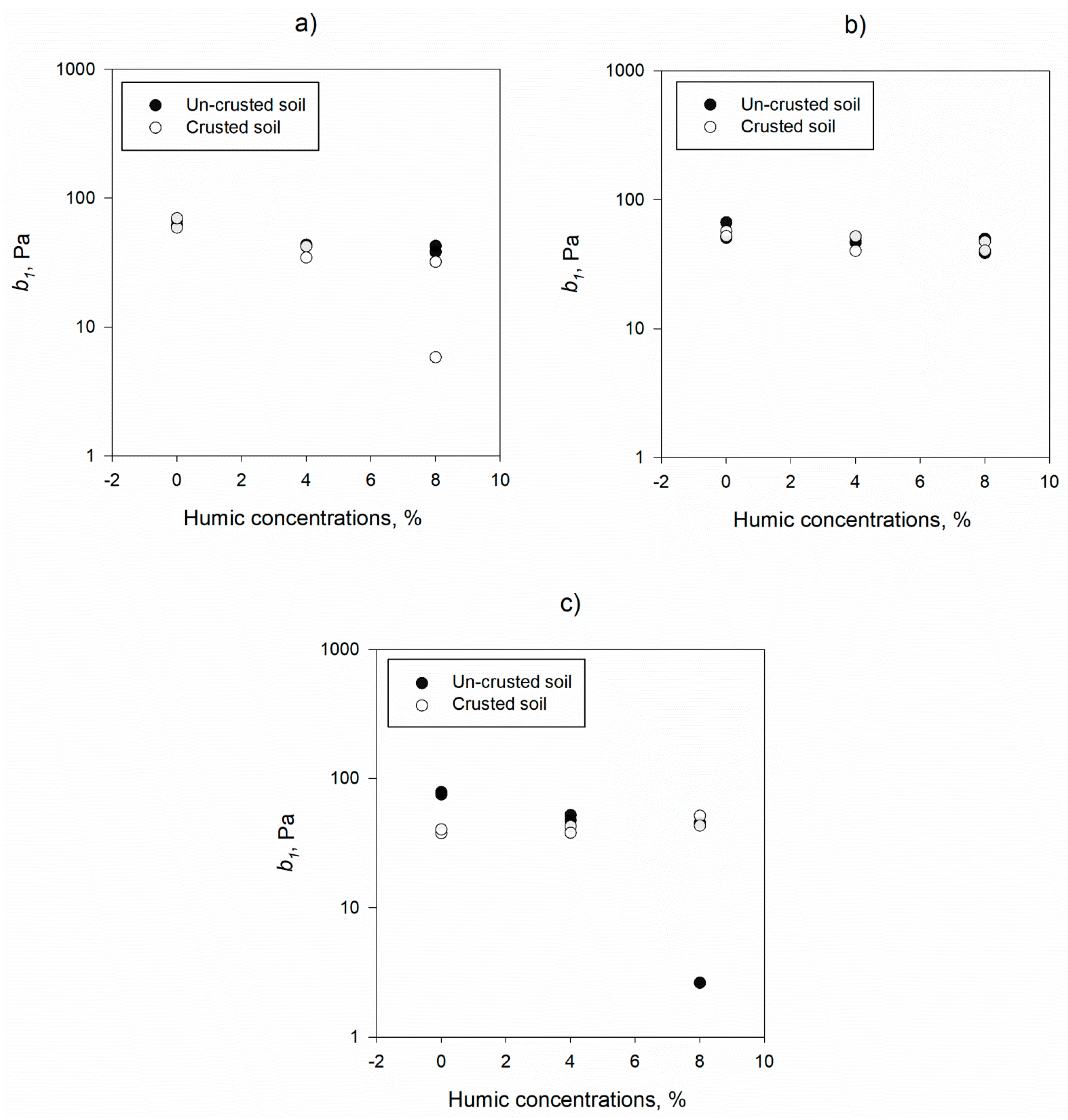
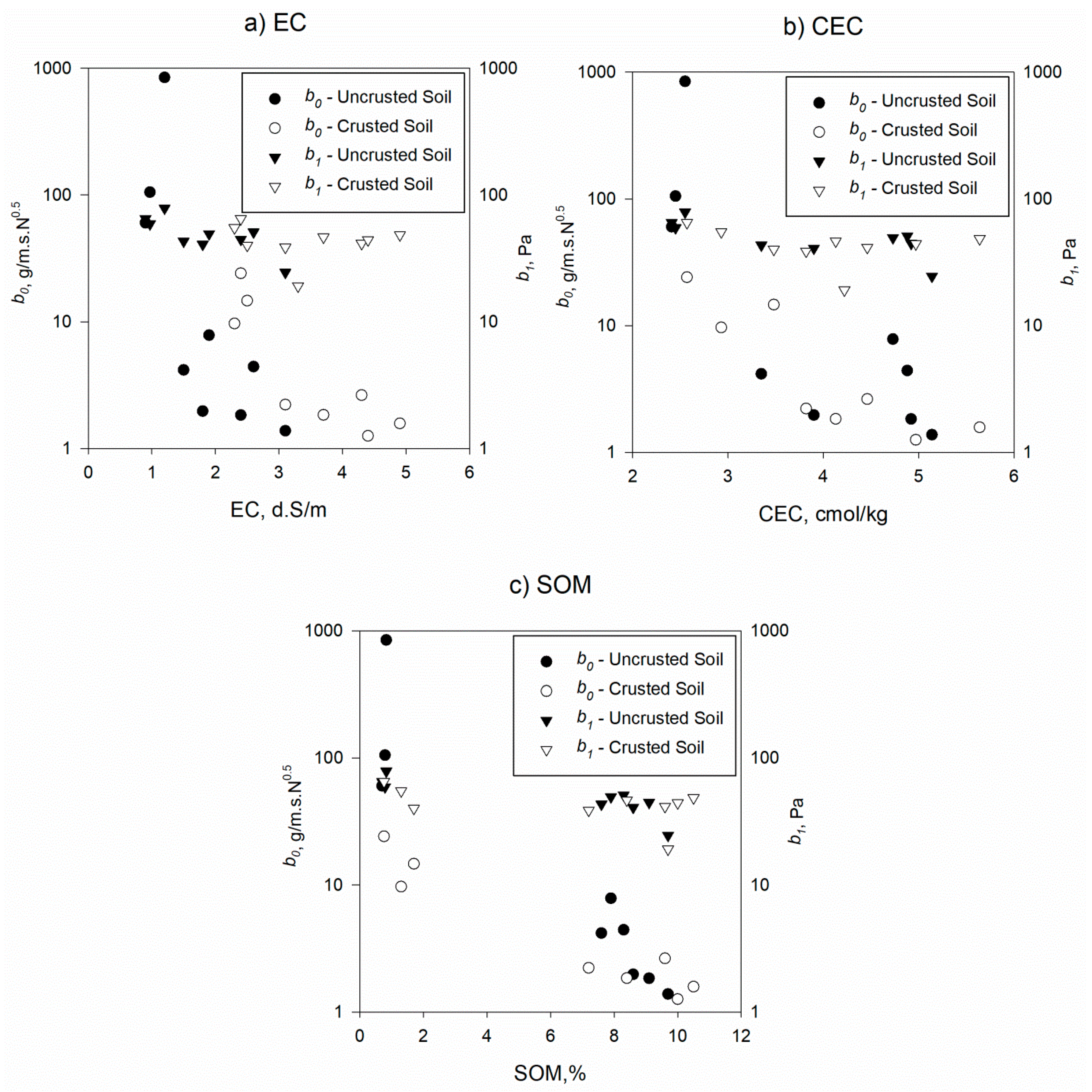
3.1. Mechanistic Detachment Parameters in Presence of Humic Acid
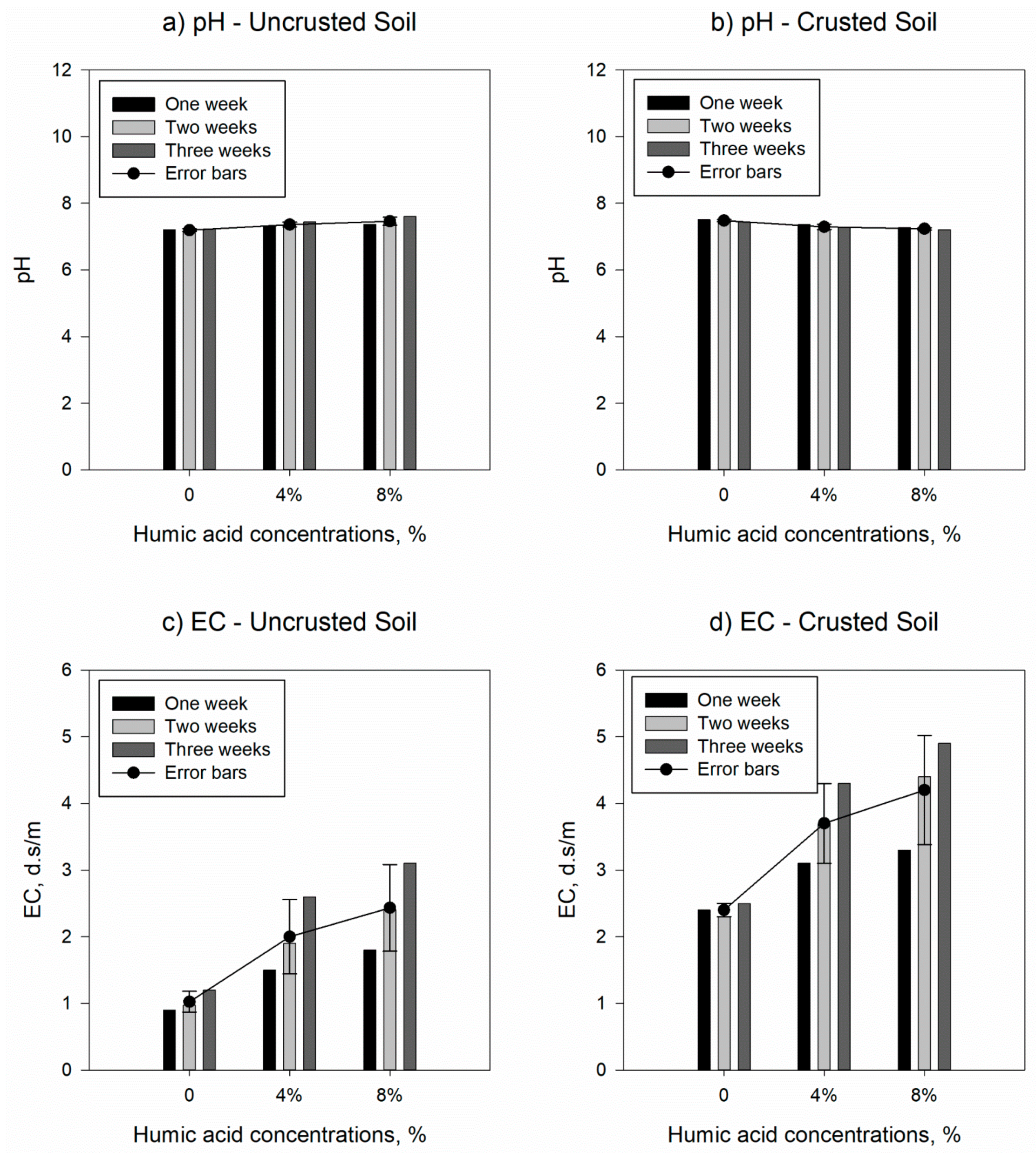
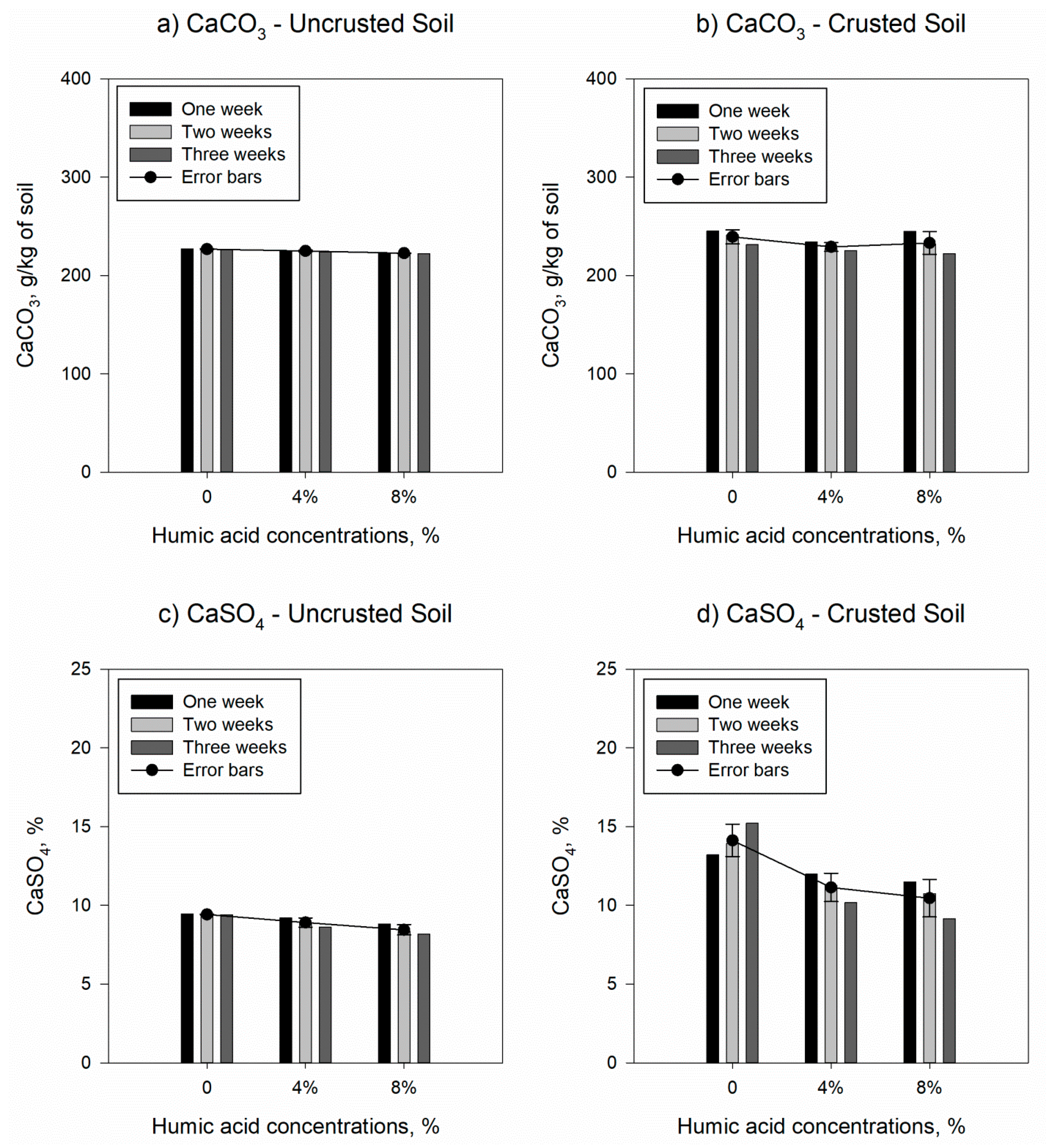
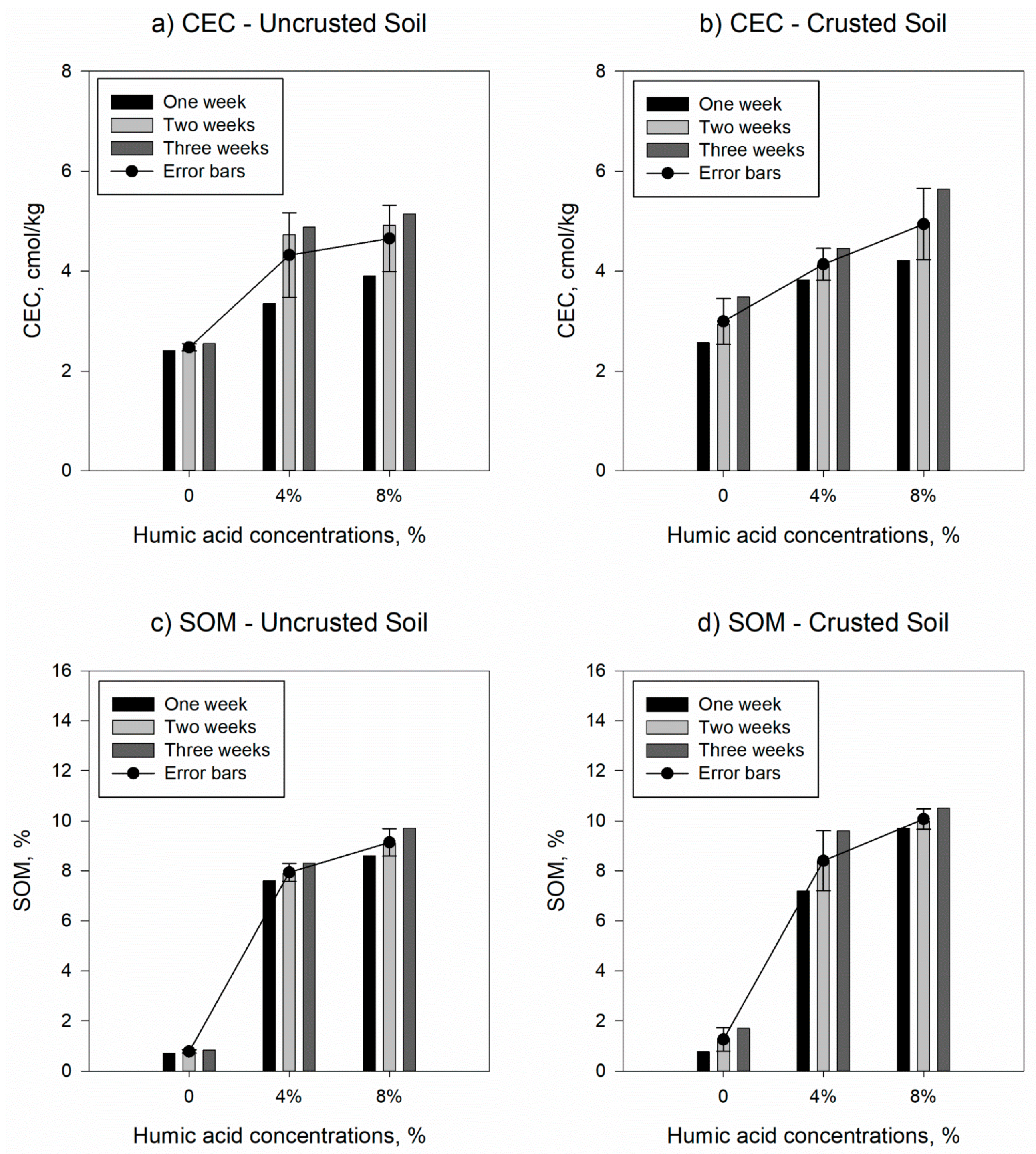
3.2. Influnce of Humic Acids on Soil Charecteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cucunubá-Melo, J.L.; Álvarez-Herrera, J.G.; Camacho-Tamayo, J.H. Identification of agronomic management units based on physical attributes of soil. J. Soil Sci. Plant Nutr. 2011, 11, 87–99. [Google Scholar] [CrossRef][Green Version]
- Tejada, M.; Gonzalez, J.L. Influence of two organic amendments on the soil physical properties, soil losses, sediments and runoff water quality. Geoderma 2008, 145, 325–334. [Google Scholar] [CrossRef]
- Huang, S.; Peng, X.; Huang, Q.; Zhang, W. Soil aggregation and organic carbon fractions affected by long-term fertilization in a red soil of subtropical China. Geoderma 2010, 154, 364–369. [Google Scholar] [CrossRef]
- Zech, W.; Senesi, N.; Guggenberger, G.; Kaiser, K.; Lehmann, J.; Miano, T.; Miltner, A.; Schroth, G. Factors controlling humification and mineralization of soil organic matter in the tropics. Geoderma 1997, 79, 117–161. [Google Scholar] [CrossRef]
- Gude, A.; Kandeler, E.; Gleixner, G. Input related microbial carbon dynamic of soil organic matter in particle size fractions. Soil Biol. Biochem. 2012, 47, 209–219. [Google Scholar] [CrossRef]
- Senesi, N.; Miano, T.M.; Brunetti, G. Methods and related problems for sampling soil and sediment organic matter: Extraction, fractionation and purification of humic substances. Quim. Anal. 1994, 13, S26. [Google Scholar]
- Santos, G.A.; Camargo, F.A.O. Fundamentos da Matéria Orgânica do sol; Alegre, G.P., Ed.; Metrópole: Porto Alegre, Brazil, 1999; p. 491. [Google Scholar]
- Janoš, P. Separation methods in the chemistry of humic substances. J. Chromatogr. A. 2003, 983, 1–18. [Google Scholar] [CrossRef]
- De Melo Benites, V.; de Oliveira Moutta, R.; da Costa Coutinho, H.L.; de Carvalho Balieiro, F. Análise discriminante de solos sob diferentes usos em área de Mata Atlântica a partir de atributos da matéria orgânica. Rev. Árvore 2010, 34, 685–690. [Google Scholar] [CrossRef][Green Version]
- Chen, J.; Gu, B.; LeBoeuf, E.J.; Pan, H.; Dai, S. Spectroscopic characterization of the structural and functional properties of natural organic matter fractions. Chemosphere 2002, 48, 59–68. [Google Scholar] [CrossRef]
- De Moraes, G.M.; da Silva Xavier, F.A.; de Sá Mendonça, E.; de Araújo Filho, J.A.; de Oliveiram, T.S. Chemical and structural characterization of soil humic substances under agroforestry and conventional systems. Rev. Bras. Ciência Do Solo 2011, 35, 1597–1608. [Google Scholar] [CrossRef]
- Clemente, J.S.; Simpson, M.J.; Simpson, A.J.; Yanni, S.F.; Whalen, J.K. Comparison of soil organic matter composition after incubation with maize leaves, roots, and stems. Geoderma 2013, 192, 86–96. [Google Scholar] [CrossRef]
- Oades, J.M.; Vassallo, A.M.; Waters, A.G.; Wilson, M.A. Characterization of organic matter in particle size and density fractions from a red-brown earth by solid state 13C NMR. Soil Res. 1987, 25, 71–82. [Google Scholar] [CrossRef]
- Rigobello, E.S.; Campos, S.X.; de Azevedo, E.R.; Dantas, A.D.B.; Vieira, E.M. Comparative characterization of humic substances extracted from freshwater and peat of different apparent molecular sizes. Rev. Ambient. Água 2017, 12, 774–785. [Google Scholar] [CrossRef][Green Version]
- Ibraheem, I.M. Cyanobacteria as alternative Biological conditioners for bioremediation of barren soil. Egypt. J. Phycol. 2007, 8, 99–116. [Google Scholar] [CrossRef]
- Zhang, G.S.; Chan, K.Y.; Oates, A.; Heenan, D.P.; Huang, G.B. Relationship between soil structure and runoff/soil loss after 24 years of conservation tillage. Soil Tillage Res. 2007, 92, 112–128. [Google Scholar] [CrossRef]
- Sarkhot, D.V.; Comerford, N.B.; Jokela, E.J.; Reeves, J.B. Effects of forest management intensity on carbon and nitrogen content in different soil size fractions of a North Florida Spodosol. Plant. Soil 2007, 294, 291–303. [Google Scholar] [CrossRef]
- Xiao-Gang, L.; Feng-Min, L.; Rengel, Z.; Zheng-Yan, Z.; Singh, B. Soil physical properties and their relations to organic carbon pools as affected by land use in an Alpine Pastureland. Geoderma 2007, 139, 98–105. [Google Scholar]
- Fataftah, A.K.; Walia, D.S.; Gains, B.; Kotob, S.I. A comparative evaluation of known liquid humic acid analysis methods. Spec. Publ. Soc. Chem. 2001, 273, 337–344. [Google Scholar]
- Hashim, Z.E.; Al-Madhhachi, A.T.; Alzubaidi, L.A. Behavior of soil erodibility parameters due to biological soil crusts using jet erosion tests. Ecol. Eng. 2020, 153, 105903. [Google Scholar] [CrossRef]
- Roper, M.M.; Gupta, V. Management-practices and soil biota. Soil Res. 1995, 33, 321–339. [Google Scholar] [CrossRef]
- Bruce, R.R.; Langdale, G.W.; West, L.T.; Miller, W. Surface soil degradation and soil productivity restoration and maintenance. Soil Sci. Soc. Am. J. 1995, 59, 654–660. [Google Scholar] [CrossRef]
- Biot, Y.; Lu, X.X. Loss of yield caused by soil erosion on sandy soils in the UK. Soil Use Manag. 2007, 11, 157–162. [Google Scholar] [CrossRef]
- Jing, K.; Wang, W.Z.; Zheng, F.L. Soil Erosion and Environment in China; Science Press: Beijing, China, 2020; p. 359. [Google Scholar]
- Drahorad, S.; Felix-Henningsen, P.; Eckhardt, K.U.; Leinweber, P. Spatial carbon and nitrogen distribution and organic matter characteristics of biological soil crusts in the Negev desert (Israel) along a rainfall gradient. J. Arid Environ. 2013, 94, 18–26. [Google Scholar] [CrossRef]
- Xiao, B.; Zhao, Y.; Wang, Q.; Li, C. Development of artificial moss-dominated biological soil crusts and their effects on runoff and soil water content in a semi-arid environment. J. Arid Environ. 2015, 117, 75–83. [Google Scholar] [CrossRef]
- Gao, L.; Bowker, M.A.; Xu, M.; Sun, H.; Tuo, D.; Zhao, Y. Biological soil crusts decrease erodibility by modifying inherent soil properties on the Loess Plateau, China. Soil Biol. Biochem. 2017, 105, 49–58. [Google Scholar] [CrossRef]
- Gao, L.; Sun, H.; Xu, M.; Zhao, Y. Biocrusts resist runoff erosion through direct physical protection and indirect modification of soil properties. J. Soils Sediments 2019, 20, 133–142. [Google Scholar] [CrossRef]
- Al-Madhhachi, A.-S.T.; Hanson, G.J.; Fox, G.A.; Tyagi, A.K.; Bulut, R. Deriving parameters of a fundamental detachment model for cohesive soils from flume and jet erosion tests. Trans. ASABE 2013, 56, 489–504. [Google Scholar] [CrossRef]
- Al-Madhhachi, A.-S.T.; Fox, G.A.; Hanson, G.J.; Tyagi, A.K.; Bulut, R. Mechanistic detachment rate model to predict soil erodibility due to fluvial and seepage forces. J. Hydraul. Eng. 2014, 140, 4014010. [Google Scholar] [CrossRef]
- Al-Madhhachi, A.-S.T.; Fox, G.A.; Hanson, G.J. Quantifying the erodibility of streambanks and hillslopes due to surface and subsurface forces. Trans. ASABE 2014, 57, 1057–1069. [Google Scholar]
- Daly, E.R.; Fox, G.A.; Al-Madhhachi, A.-S.T.; Storm, D.E. Variability of fluvial erodibility parameters for streambanks on a watershed scale. Geomorphology 2015, 231, 281–291. [Google Scholar] [CrossRef]
- Khanal, A.; Fox, G.A. Detachment characteristics of root-permeated soils from laboratory jet erosion tests. Ecol. Eng. 2017, 100, 335–343. [Google Scholar] [CrossRef]
- Al-Madhhachi, A.-S.T.; Mutter, G.M.; Hasan, M.B. Predicting mechanistic detachment model due to lead-contaminated soil treated with Iraqi stabilizers. KSCE J. Civ. Eng. 2019, 23, 2898–2907. [Google Scholar] [CrossRef]
- Adnan, T.A.; Al-Madhhachi, A.-S.T.; Mohammed, E.A. Relationships of soil erodibility parameters and water quality indices along Tigris Riverbanks, Baghdad City, Iraq. Cogent Eng. 2021, 8, 1917330. [Google Scholar] [CrossRef]
- Wilson, B.N. Development of a fundamentally based detachment model. Trans. ASAE 1993, 36, 1105–1114. [Google Scholar] [CrossRef]
- Wilson, B.N. Evaluation of a fundamentally based detachment model. Trans. ASAE 1993, 36, 1115–1122. [Google Scholar] [CrossRef]
- ASTM Standards. Section 4: Construction. In Annual Book of ASTM Standards; ASTM: Philadelphia, PA, USA, 2006. [Google Scholar]
- Food and Agriculture Organization (FAO), Prognosis of Salinity and Alkalinity; FAO: Great Konya Basin, Turkey, 1975; p. 260.
- Hendershot, W.H.; Duquette, M. A simple barium chloride method for determining cation exchange capacity and exchangeable cations. Soil Sci. Soc. Am. J. 1986, 50, 605–608. [Google Scholar] [CrossRef]
- Einstein, H.A. The Bed-Load Function for Sediment Transportation in Open Channel Flows; Technical Bulletins; US Government Printing Office: Washington, DC, USA, 1950.
- Partheniades, E. Erosion and deposition of cohesive soils. J. Hydraul. Div. 1965, 91, 105–139. [Google Scholar] [CrossRef]
- SÖNMEZ, F.; Şevket, A.L.P. The Effects of Applications Humic Acids on Macronutrient, Micronutrient, Heavy Metal and Soil Properties. Yüzüncü Yıl Üniversitesi Tarım Bilim. Derg. 2019, 29, 809–816. [Google Scholar] [CrossRef][Green Version]
- Çelik, H.; Katkat, A.V.; Aşık, B.B.; Turan, M.A. Effects of soil applied humic substances to dry weight and mineral nutrients uptake of maize under calcareous soil conditions. Arch. Agron. Soil Sci. 2008, 54, 605–614. [Google Scholar] [CrossRef]
- Yilmaz, E. Changes of some soil properties by agricultural processing waste (soybean pulp) amendment. J. Food Agr. Env. 2010, 8, 1057–1060. [Google Scholar]
- Quilty, J.; Cattle, S. Use and Understanding of Organic Amendments in Australian Agriculture: A Review. Soil Res. 2011, 49, 1–26. [Google Scholar] [CrossRef]
- Khaled, H.; Fawy, H.A. Effect of different levels of humic acids on the nutrient content, plant growth, and soil properties under conditions of salinity. Soil Water Res. 2011, 6, 21–29. [Google Scholar] [CrossRef]
- Piccolo, A.; Mbagwu, J.S.C. Humic substances and surfactants effects on the stability of two tropical soils. Soil Sci. Soc. Am. J. 1994, 58, 950–955. [Google Scholar] [CrossRef]
- Piccolo, A.; Mbagwu, J.S.C. Role of hydrophobic components of soil organic matter in soil aggregate stability. Soil Sci. Soc. Am. J. 1999, 63, 1801–1810. [Google Scholar] [CrossRef]
- Gümüş, İ.; Şeker, C. Influence of humic acid applications on soil physicochemical properties. Solid Earth 2015, 7, 2481–2500. [Google Scholar]
- Drahorad, S.L.; Felix-Henningsen, P. Application of an Electronic Micropenetrometer to Assess Mechanical Stability of Biological Soil Crusts. J. Plant. Nutr. Soil Sci. 2013, 176, 904–909. [Google Scholar] [CrossRef]
- Hansen, V.; Müller-Stöver, D.; Munkholm, L.J.; Peltre, C.; Hauggaard-Nielsen, H.; Jensen, L.S. The effect of straw and wood gasification biochar on carbon sequestration, selected soil fertility indicators and functional groups in soil: An incubation study. Geoderma 2016, 269, 99–107. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, J.; Zhang, C.; Xin, X.; Li, H. The coupling effects of soil organic matter and particle interaction forces on soil aggregate stability. Soil Tillage Res. 2017, 174, 251–260. [Google Scholar] [CrossRef]
- Sootahar, M.K.; Zeng, X.; Su, S.; Wang, Y.; Bai, L.; Zhang, Y.; Li, T.; Zhang, X. The effect of fulvic acids derived from different materials on changing properties of albic black soil in the Northeast Plain of China. Molecules 2019, 24, 1535. [Google Scholar] [CrossRef]


| Soil Physical Characteristics | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Soil Texture | Initial Natural Compaction | ||||||||
| Samples | USDA classification | Sand, % | Silt, % | Clay, % | Bulk density, g/cm3 | Water content, % | Specific gravity | pH | EC, d.S/cm |
| Uncrusted soil | Loam | 34 | 26 | 40 | 1.70 | 32.12 | 2.47 | 7.20 | 0.90 |
| Crusted soil | Loam | 44 | 24 | 32 | 1.68 | 30.51 | 2.48 | 7.50 | 2.40 |
| Soil chemical characteristics, mean (standard errors) | |||||||||
| Samples | CaCO3, g/kg of soil | CaSO4, % | Ca, meq/L * | Mg, meq/L * | Na, meq/L * | SAR, % | CEC, cmol/kg | SOM, % | OC, % |
| Uncrusted soil | 227 (0.337) | 9.42 (0.018) | 6.07 (0.396) | 3.88 (0.168) | 4.16 (0.370) | 1.86 (0.135) | 2.47 (0.042) | 0.77 (0.038) | 0.45 (0.024) |
| Crusted soil | 239 (4.129) | 14.12 (0.589) | 15.77 (1.326) | 8.31 (1.087) | 3.53 (0.152) | 1.02 (0.070) | 2.99 (0.265) | 1.25 (0.272) | 0.73 (0.158) |
| Mechanistic Detachment Parameters | Humic Acid Concentrations | Uncrusted Soil | Crusted Soil | Crusted versus Uncrusted Soils | ||
|---|---|---|---|---|---|---|
| Mean | Std. Dev. | Mean | Std. Dev. | p * | ||
| b0, g/m.s.N0.5 | 0% | 337.48 | 398.51 | 16.17 | 8.00 | 0.031 |
| 4% | 5.48 | 2.53 | 2.24 | 0.50 | 0.265 | |
| 8% | 1.73 | 0.88 | 1.10 | 0.61 | 0.904 | |
| b1, Pa | 0% | 67.81 | 10.64 | 53.45 | 11.85 | 0.141 |
| 4% | 47.95 | 4.22 | 42.31 | 5.93 | 0.783 | |
| 8% | 36.70 | 17.24 | 37.38 | 16.89 | 0.857 | |
| Compounds | Uncrusted Soil with No Humic, % | Uncrusted Soil with Humic, % | Crusted Soil with No Humic, % | Crusted Soil with Humic, % |
|---|---|---|---|---|
| SiO2 | 39.27 | 38.75 | 37.70 | 34.36 |
| CaO | 16.40 | 16.20 | 16.24 | 14.76 |
| Al2O3 | 8.98 | 9.06 | 8.62 | 8.13 |
| Fe2O3 | 5.87 | 5.66 | 5.25 | 5.31 |
| MgO | 4.77 | 4.85 | 4.37 | 4.16 |
| K2O | 1.34 | 1.45 | 1.34 | 2.32 |
| Others | 3.18 | 2.68 | 2.82 | 2.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbood, A.A.; Al-Madhhachi, A.-S.T. Quantifying Mechanistic Detachment Parameters Due to Humic Acids in Biological Soil Crusts. Land 2021, 10, 1180. https://doi.org/10.3390/land10111180
Abbood AA, Al-Madhhachi A-ST. Quantifying Mechanistic Detachment Parameters Due to Humic Acids in Biological Soil Crusts. Land. 2021; 10(11):1180. https://doi.org/10.3390/land10111180
Chicago/Turabian StyleAbbood, Atheer A., and Abdul-Sahib T. Al-Madhhachi. 2021. "Quantifying Mechanistic Detachment Parameters Due to Humic Acids in Biological Soil Crusts" Land 10, no. 11: 1180. https://doi.org/10.3390/land10111180
APA StyleAbbood, A. A., & Al-Madhhachi, A.-S. T. (2021). Quantifying Mechanistic Detachment Parameters Due to Humic Acids in Biological Soil Crusts. Land, 10(11), 1180. https://doi.org/10.3390/land10111180







