Exploring Assembly Trajectories of Abandoned Grasslands in Response to 10 Years of Mowing in Sub-Mediterranean Context
Abstract
:1. Introduction
- (I)
- Does mowing foster in the short term the divergence of spatial niche occupation strategies?
- (II)
- Does mowing reduce in the long term the convergence of temporal niche occupation strategies?
- (III)
- Does mowing favor the divergence of CSR strategies, leading to more ruderal communities, and how does this change reflect on leaf traits?
2. Materials and Methods
2.1. Study Area
2.2. Sampling Design
2.3. Data Collection
2.4. Statistical Analysis
3. Results
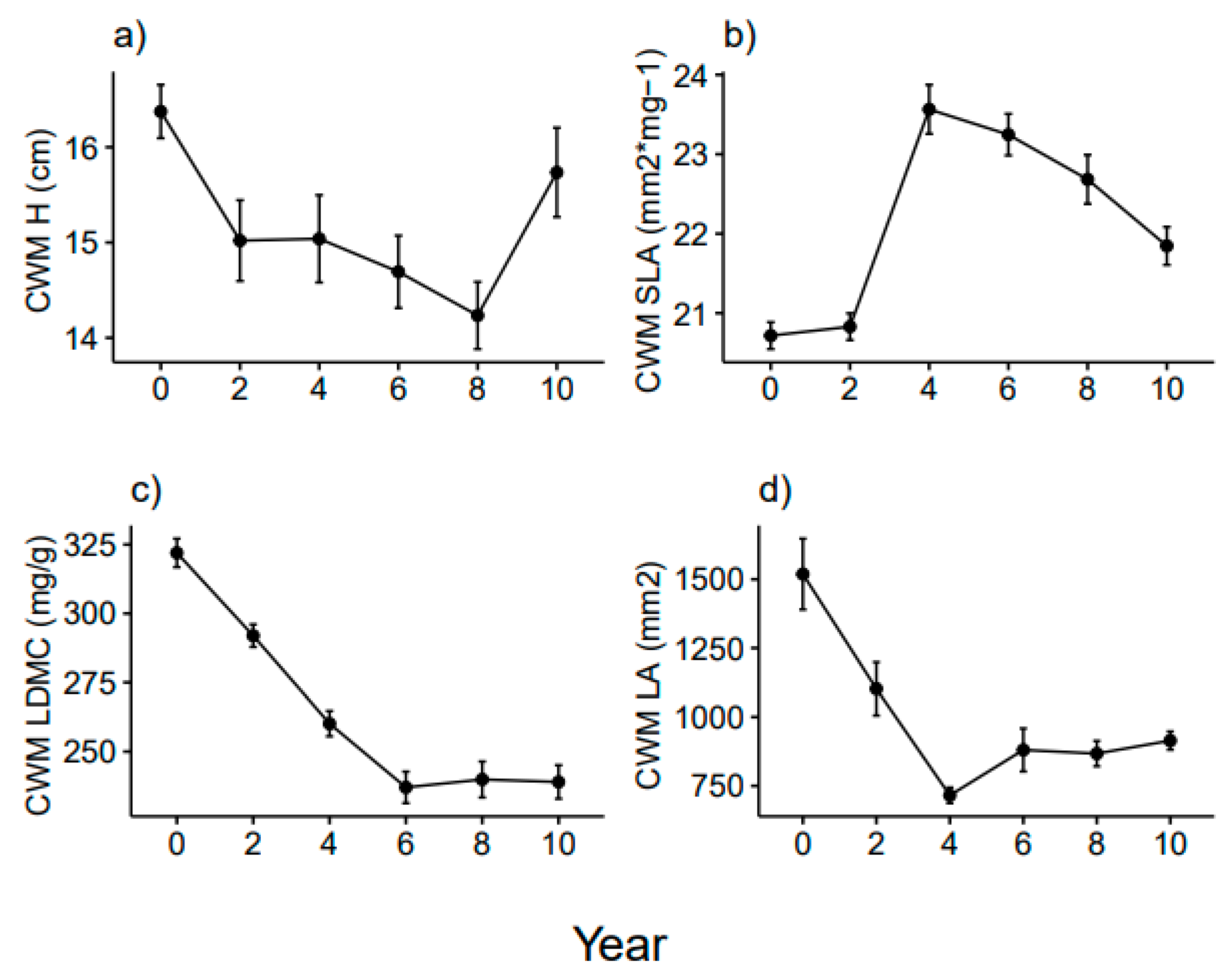
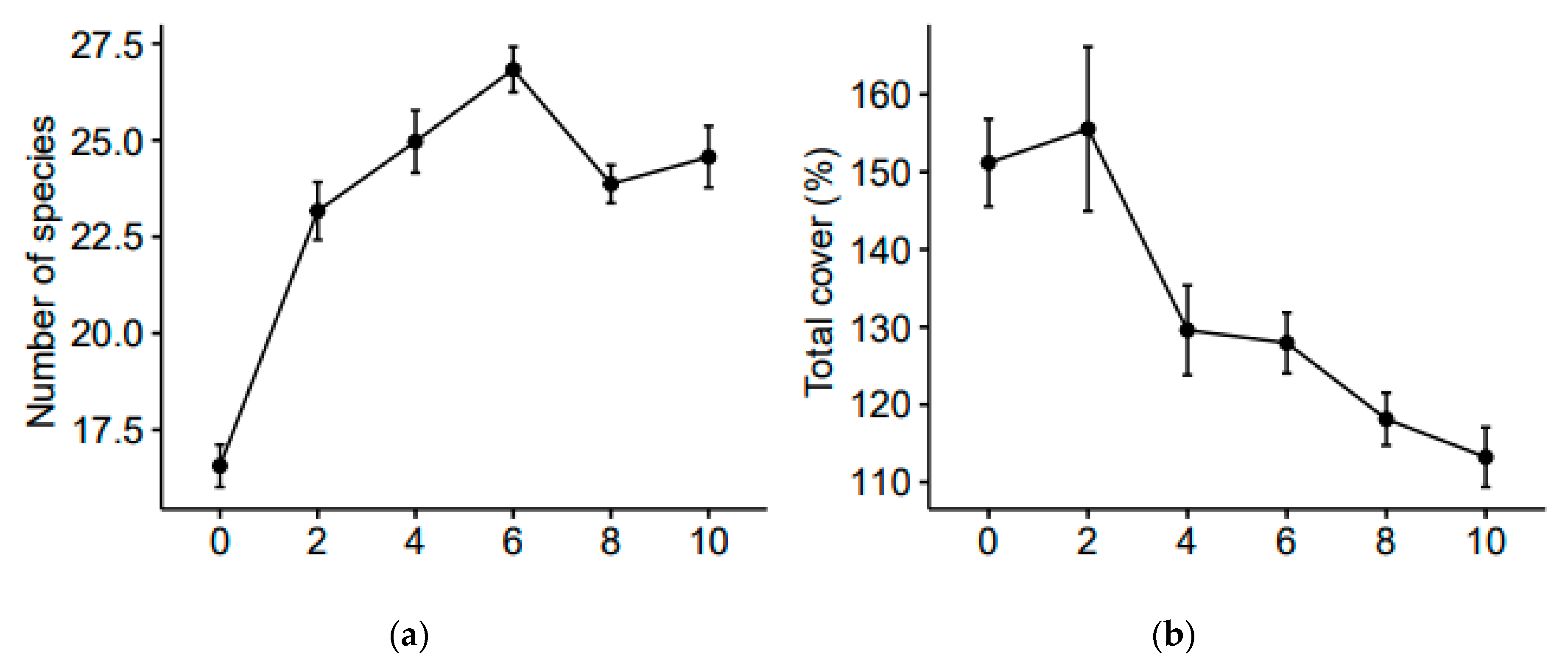
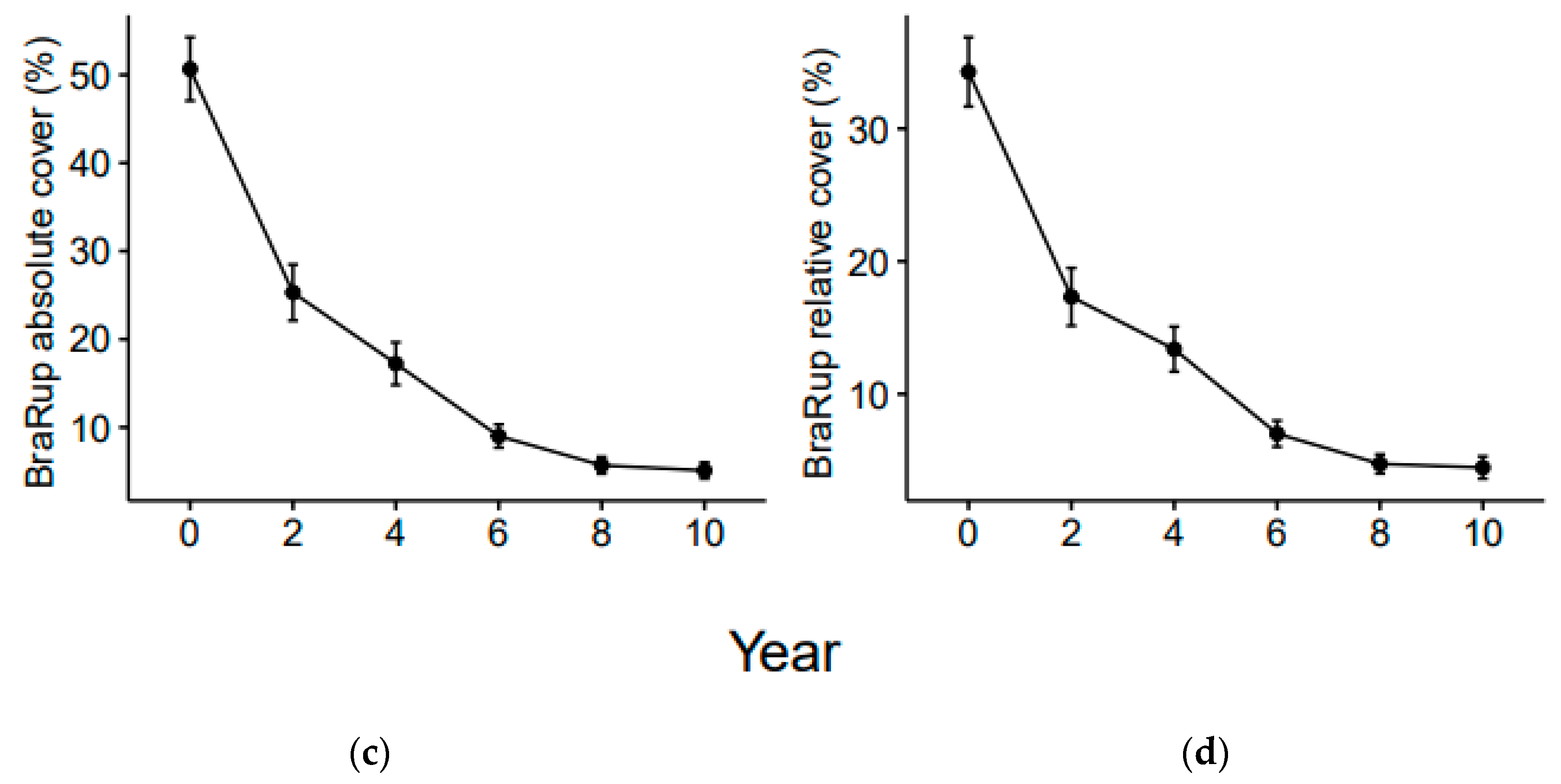
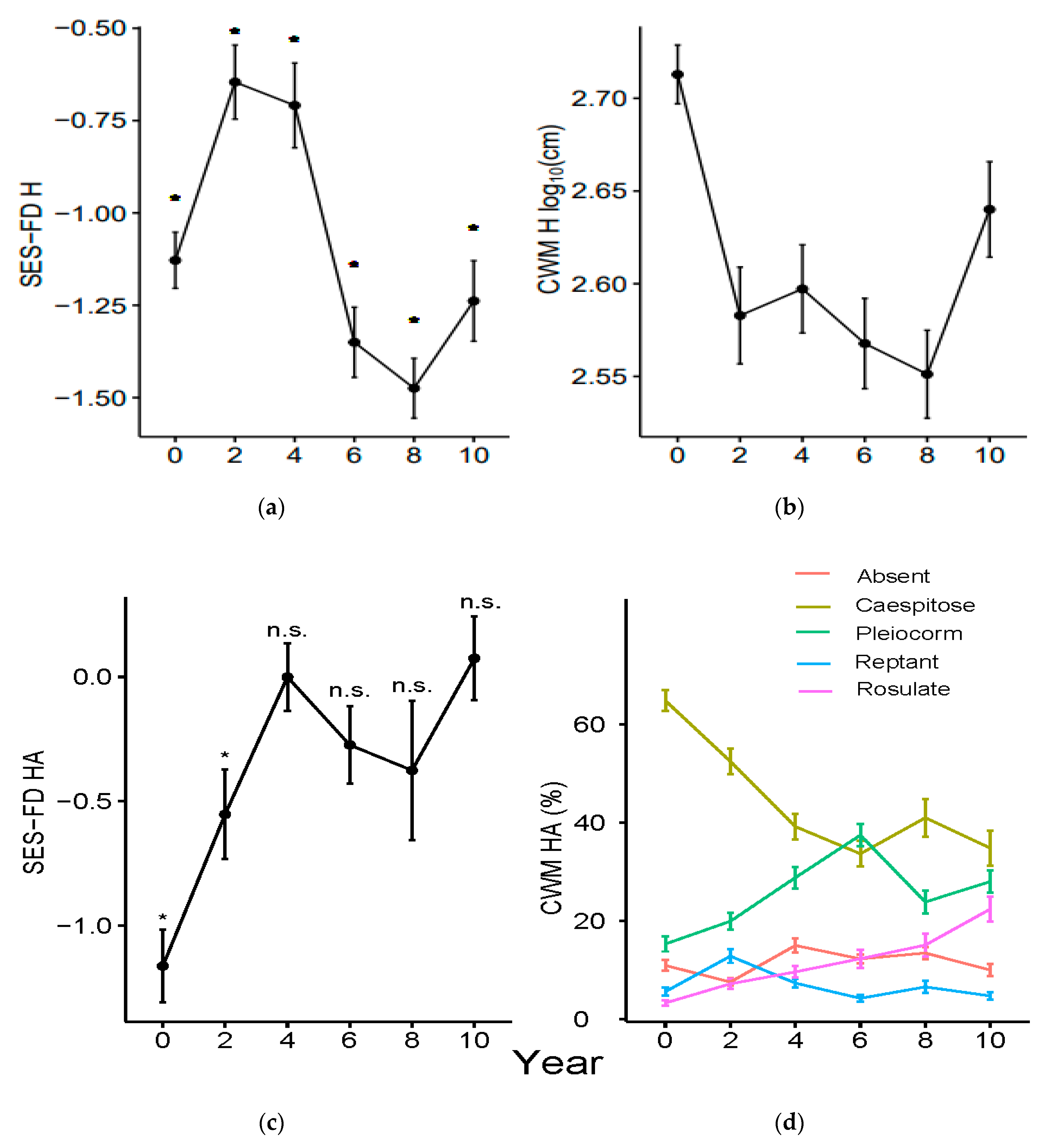
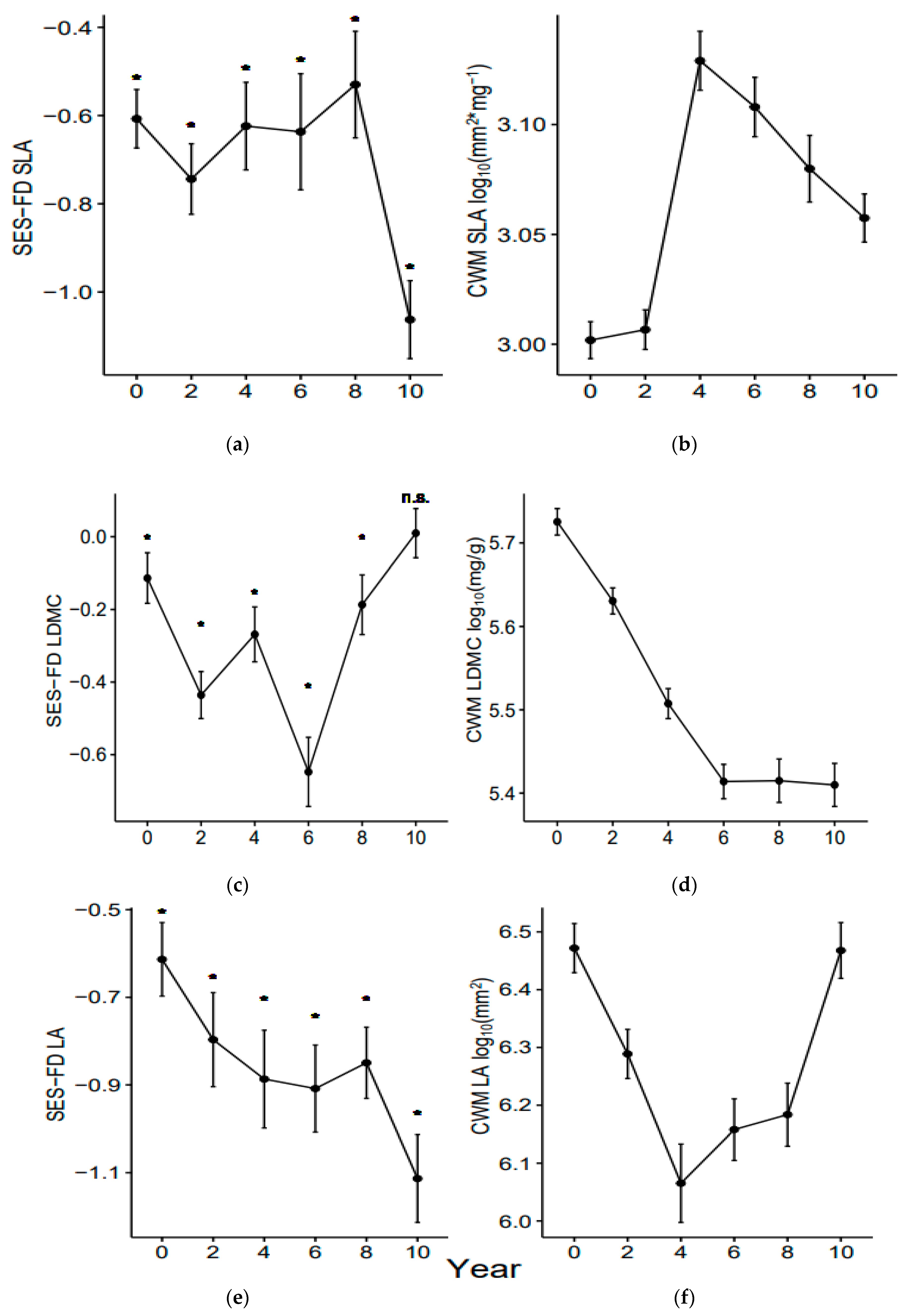
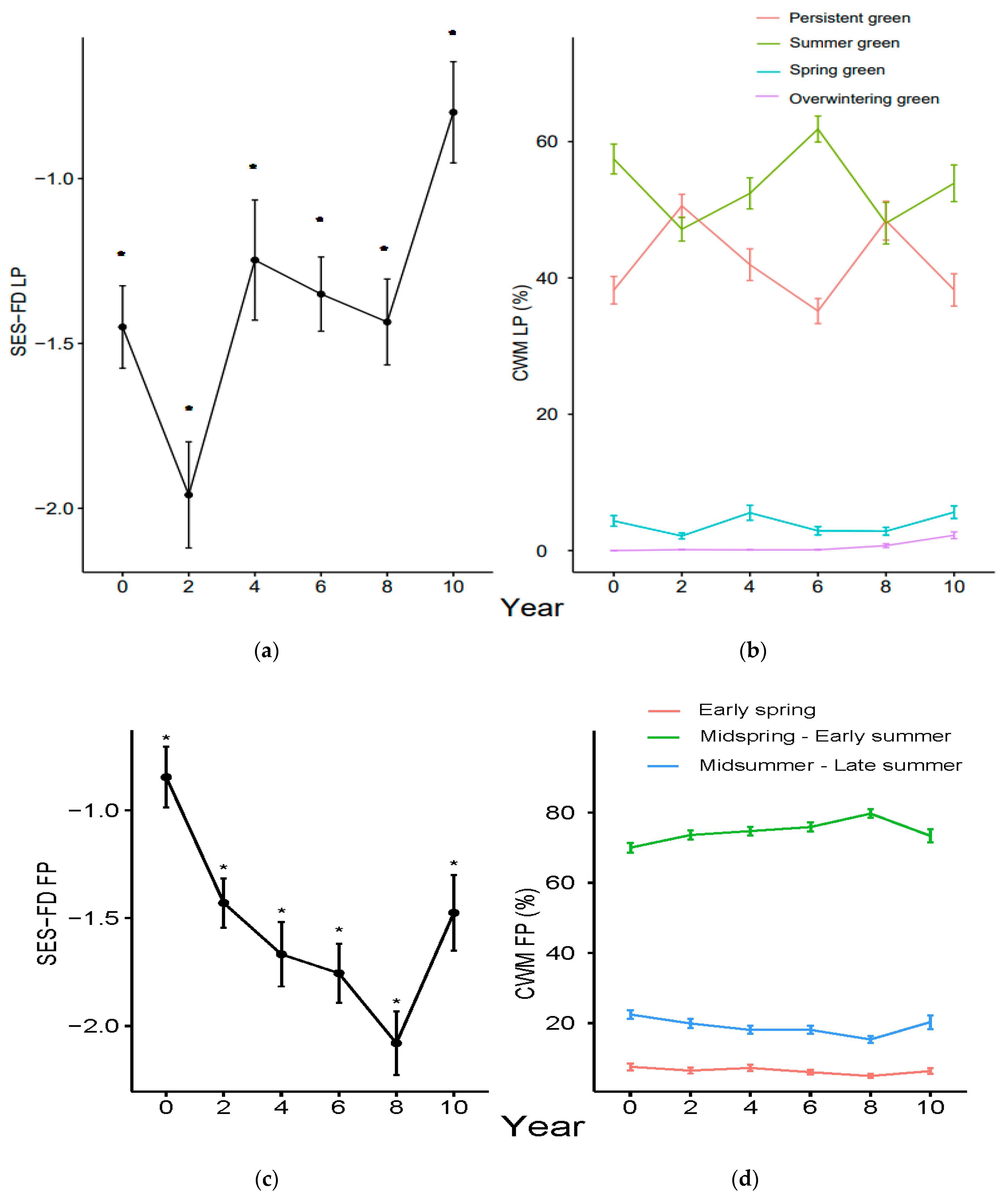
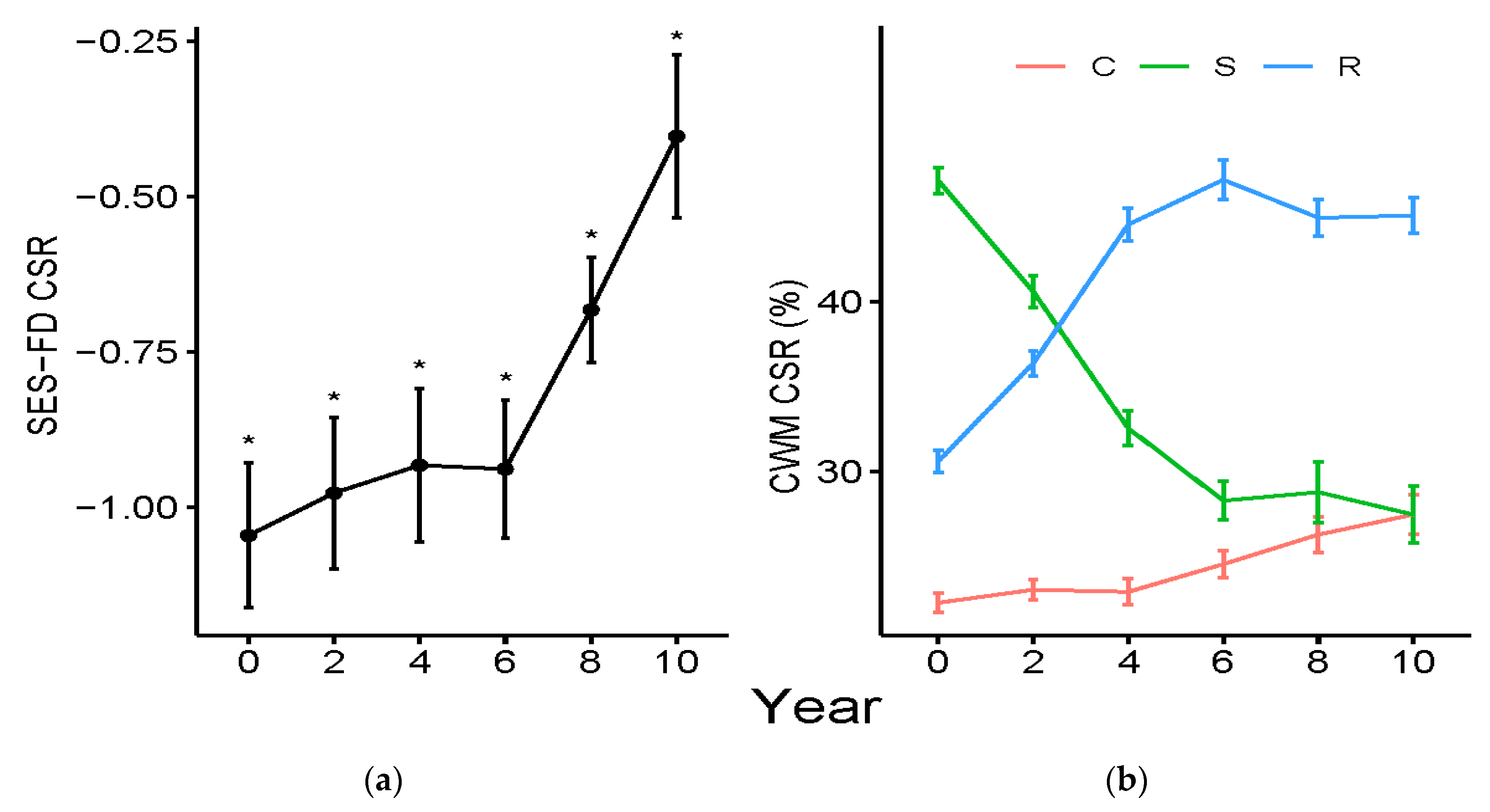
3.1. Functional Diversity Variation
3.2. Community Weighted Mean Variation
4. Discussion
4.1. Shorter-Term Variations
4.2. Longer-Term Effects
4.3. Variation of Grime’s CSR Strategies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Dengler, J.; Janišová, M.; Török, P.; Wellstein, C. Biodiversity of Palaearctic grasslands: A synthesis. Agric. Ecosyst. Environ. 2014, 182, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, D.; Crabtree, J.R.; Wiesinger, G.; Dax, T.; Stamou, N.; Fleury, P.; Lazpita, J.; Gibon, A. Agricultural abandonment in mountain areas of Europe: Environmental consequences and policy response. J. Environ. Manag. 2000, 59, 47–69. [Google Scholar] [CrossRef]
- Poptcheva, K.; Schwartze, P.; Vogel, A.; Kleinebecker, T.; Hölzel, N. Changes in wet meadow vegetation after 20 years of different management in a field experiment (North-West Germany). Agric. Ecosyst. Environ. 2009, 134, 108–114. [Google Scholar] [CrossRef]
- Catorci, A.; Foglia, M.; Tardella, F.M.; Vitanzi, A.; Sparvoli, D.; Gatti, R.; Galli, P.; Paradisi, L. Map of changes in landscape naturalness in the Fiastra and Salino catchment basins (central Italy). J. Maps 2012, 8, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, A.; Biró, M.; Malatesta, L.; Molnár, Z.; Mugnoz, S.; Tardella, F.M.; Catorci, A. Land-use modifications and ecological implications over the past 160 years in the central Apennine mountains. Landsc. Res. 2021, 1–13. [Google Scholar] [CrossRef]
- Bracchetti, L.; Carotenuto, L.; Catorci, A. Land-cover changes in a remote area of central Apennines (Italy) and management directions. Landsc. Urban Plan. 2012, 104, 157–170. [Google Scholar] [CrossRef]
- Malavasi, M.; Carranza, M.L.; Moravec, D.; Cutini, M. Reforestation dynamics after land abandonment: A trajectory analysis in Mediterranean mountain landscapes. Reg. Environ. Chang. 2018, 18, 2459–2469. [Google Scholar] [CrossRef]
- Bonanomi, G.; Caporaso, S.; Allegrezza, M. Short-term effects of nitrogen enrichment, litter removal and cutting on a Mediterranean grassland. Acta Oecol. 2006, 30, 419–425. [Google Scholar] [CrossRef]
- Catorci, A.; Ottaviani, G.; Cesaretti, S. Functional and coenological changes under different long-term management conditions in Apennine meadows (central Italy). Phytocoenologia 2011, 41, 45. [Google Scholar] [CrossRef]
- Catorci, A.; Cesaretti, S.; Gatti, R.; Ottaviani, G. Abiotic and biotic changes due to spread of Brachypodium genuense (DC.) Roem. & Schult. in sub-Mediterranean meadows. Community Ecol. 2011, 12, 117–125. [Google Scholar] [CrossRef]
- Giarrizzo, E.; Burrascano, S.; Chiti, T.; de Bello, F.; Lepš, J.; Zavattero, L.; Blasi, C. Re-visiting historical semi-natural grasslands in the Apennines to assess patterns of changes in species composition and functional traits. Appl. Veg. Sci. 2017, 20, 247–258. [Google Scholar] [CrossRef]
- Malatesta, L.; Tardella, F.M.; Tavoloni, M.; Postiglione, N.; Piermarteri, K.; Catorci, A. Land use change in the high mountain belts of the central Apennines led to marked changes of the grassland mosaic. Appl. Veg. Sci. 2019, 22, 243–255. [Google Scholar] [CrossRef]
- Pottier, J.; Evette, A. On the relationship between clonal traits and small-scale spatial patterns of three dominant grasses and its consequences on community diversity. Folia Geobot. 2010, 45, 59–75. [Google Scholar] [CrossRef]
- De Kroon, H.; Bobbink, R. Clonal plant dominance under elevated nitrogen deposition, with special reference to Brachypodium pinnatum in chalk grassland. In The Ecology and Evolution of Clonal Plants; De Kroon, G., Ed.; Backhuys Publishers: Kerkwerve, The Netherlands, 1997; pp. 359–379. [Google Scholar]
- Catorci, A.; Cesaretti, S.; Tardella, F.M. Effect of tall-grass invasion on the flowering-related functional pattern of submediterranean hay-meadows. Plant Biosyst. 2014, 148, 1127–1137. [Google Scholar] [CrossRef]
- Kılıç, D.D.; Kutbay, H.G.; Sürmen, B.; Hüseyinoğlu, R. The classification of some plants subjected to disturbance factors (grazing and cutting) based on ecological strategies in Turkey. Rend. Lincei Sci. Fis. Nat. 2018, 29, 87–102. [Google Scholar] [CrossRef]
- Lepš, J. Scale-and time-dependent effects of fertilization, mowing and dominant removal on a grassland community during a 15-year experiment. J. Appl. Ecol. 2014, 51, 978–987. [Google Scholar] [CrossRef]
- Filibeck, G.; Sperandii, M.G.; Bragazza, L.; Bricca, A.; Chelli, S.; Maccherini, S.; Wellstein, C.; Conte, A.L.; Di Donatantonio, M.; Forte, T.G.; et al. Competitive dominance mediates the effects of topography on plant richness in a mountain grassland. Basic Appl. Ecol. 2020, 48, 112–123. [Google Scholar] [CrossRef]
- Chesson, P. Mechanisms of maintenance of species diversity. Ann. Rev. Ecolog. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef] [Green Version]
- Mayfield, M.M.; Levine, J.M. Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol. Lett. 2010, 13, 1085–1093. [Google Scholar] [CrossRef]
- Vitasović Kosić, I.; Tardella, F.M.; Grbeša, D.; Škvorc, Ž.; Catorci, A. Effects of abandonment on the functional composition and forage nutritive value of a North Adriatic dry grassland community (Ćićarija, Croatia). Appl. Ecol. Environ. Res. 2014, 12, 285–299. [Google Scholar] [CrossRef]
- Scocco, P.; Mercati, F.; Tardella, F.M.; Catorci, A. Increase of forage dryness induces differentiated anatomical response in the sheep rumen compartments. Microsc. Res. Tech. 2016, 79, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Catorci, A.; Antolini, E.; Tardella, F.M.; Scocco, P. Assessment of interaction between sheep and poorly palatable grass: A key tool for grassland management and restoration. J. Plant Interact. 2014, 9, 112–121. [Google Scholar] [CrossRef]
- Scocco, P.; Brusaferro, A.; Catorci, A. Comparison between two different methods for evaluating rumen papillae measures related to different diets. Microsc. Res. Tech. 2012, 75, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Scocco, P.; Mercati, F.; Brusaferro, A.; Ceccarelli, P.; Belardinelli, C.; Malfatti, A. Keratinization degree of rumen epithelium and body condition score in sheep grazing on Brachypodium rupestre. Vet. Ital. 2013, 49, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Tälle, M.; Deák, B.; Poschlod, P.; Valkó, O.; Westerberg, L.; Milberg, P. Grazing vs. mowing: A meta-analysis of biodiversity benefits for grassland management. Agric. Ecosyst. Environ. 2016, 222, 200–212. [Google Scholar] [CrossRef]
- Tardella, F.M.; Bricca, A.; Goia, I.G.; Catorci, A. How mowing restores montane Mediterranean grasslands following cessation of traditional livestock grazing. Agric. Ecosyst. Environ. 2020, 295, 106880. [Google Scholar] [CrossRef]
- Halassy, M.; Botta-Dukát, Z.; Csecserits, A.; Szitár, K.; Török, K. Trait-based approach confirms the importance of propagule limitation and assembly rules in old-field restoration. Restor. Ecol. 2019, 27, 840–849. [Google Scholar] [CrossRef] [Green Version]
- Klimešová, J.; Janeček, Š.; Hornik, J.; Doležal, J. Effect of the method of assessing and weighting abundance on the interpretation of the relationship between plant clonal traits and meadow management. Preslia 2011, 83, 437–453. [Google Scholar]
- Milberg, P.; Tälle, M.; Fogelfors, H.; Westerberg, L. The biodiversity cost of reducing management intensity in species-rich grasslands: Mowing annually vs. every third year. Basic Appl. Ecol. 2017, 22, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Tardella, F.M.; Malatesta, L.; Goia, I.G.; Catorci, A. Effects of long-term mowing on coenological composition and recovery routes of a Brachypodium rupestre-invaded community: Insight into the restoration of sub-Mediterranean productive grasslands. Rend. Lincei Sci. Fis. Nat. 2018, 29, 329–341. [Google Scholar] [CrossRef]
- Doležal, J.; Mašková, Z.; Lepš, J.; Steinbachová, D.; de Bello, F.; Klimešová, J.; Tackenberg, O.; Zemek, F.; Květ, J. Positive long-term effect of mulching on species and functional trait diversity in a nutrient-poor mountain meadow in Central Europe. Agric. Ecosyst. Environ. 2011, 145, 10–28. [Google Scholar] [CrossRef]
- Diaz, S.; Lavorel, S.; McIntyre, S.U.E.; Falczuk, V.; Casanoves, F.; Milchunas, D.G.; Skarpe, C.; Rusch, G.; Sternberg, M.; Noy-Meir, I.; et al. Plant trait responses to grazing–a global synthesis. Glob. Chang. Biol. 2007, 13, 313–341. [Google Scholar] [CrossRef]
- Martins, N.; Ferreira, I.C. Mountain food products: A broad spectrum of market potential to be exploited. Trends Food Sci. Technol. 2017, 67, 12–18. [Google Scholar] [CrossRef]
- Olmeda, C.; Keenleyside, C.; Tucker, G.; Underwood, E. Farming for Natura 2000 Guidance on How to Support Natura 2000 Farming Systems to Achieve Conservation Objectives, Based on Member States Good Practice Experiences; European Comission; Publications Office of the European Union: Luxembourg, 2014. [Google Scholar] [CrossRef]
- Pierce, S.; Negreiros, D.; Cerabolini, B.E.L.; Kattge, J.; Díaz, S.; Kleyer, M.; Shipley, B.; Wright, S.J.; Soudzilovskaia, N.A.; Onipchenko, V.G.; et al. A global method for calculating plant CSR ecological strategies applied across biomes world-wide. Funct. Ecol. 2017, 31, 444–457. [Google Scholar] [CrossRef]
- Grime, J.P. Vegetation classification by reference to strategies. Nature 1974, 250. [Google Scholar] [CrossRef]
- Grime, J.P. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 1977, 111, 1169–1194. [Google Scholar] [CrossRef]
- Grime, J.P. Plant Strategies, Vegetation Processes and Ecosystem Properties, 2nd ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Grime, J.P.; Pierce, S. The Evolutionary Strategies That Shape Ecosystems; Wiley-Blackwell: London, UK, 2012. [Google Scholar]
- Negreiros, D.; Le Stradic, S.; Fernandes, G.W.; Rennó, H.C. CSR analysis of plant functional types in highly diverse tropical grasslands of harsh environments. Plant Ecol. 2014, 215, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Rosenfield, M.F.; Müller, S.C.; Overbeck, G.E. Short gradient, but distinct plant strategies: The CSR scheme applied to subtropical forests. J. Veg. Sci. 2019, 30, 984–993. [Google Scholar] [CrossRef]
- Zanzottera, M.; Dalle Fratte, M.; Caccianiga, M.; Pierce, S.; Cerabolini, B.E. Community-level variation in plant functional traits and ecological strategies shapes habitat structure along succession gradients in alpine environment. Community Ecol. 2020, 21, 55–65. [Google Scholar] [CrossRef]
- Catorci, A.; Ottaviani, G.; Vitasović Kosić, I.; Cesaretti, S. Effect of spatial and temporal patterns of stress and disturbance intensities in a sub-Mediterranean grassland. Plant Biosyst. 2012, 146, 352–367. [Google Scholar] [CrossRef]
- Corazza, M.; Tardella, F.M.; Ferrari, C.; Catorci, A. Tall grass invasion after grassland abandonment influences the availability of palatable plants for wild herbivores: Insight into the conservation of the Apennine chamois Rupicapra pyrenaicaornata. Environ. Manag. 2016, 57, 1247–1261. [Google Scholar] [CrossRef] [PubMed]
- Catorci, A.; Lulli, R.; Malatesta, L.; Tavoloni, M.; Tardella, F.M. How the interplay between management and interannual climatic variability influences the NDVI variation in a sub-Mediterranean pastoral system: Insight into sustainable grassland use under climate change. Agric. Ecosyst. Environ. 2021, 314, 107372. [Google Scholar] [CrossRef]
- Rivas-Martínez, S.; Sáenz, S.R.; Penas, A. Worldwide bioclimatic classification system. Glob. Geobot. 2011, 1, 1–634. [Google Scholar] [CrossRef]
- Catorci, A.; Cesaretti, S.; Gatti, R. Biodiversity conservation: Geosynphytosociology as a tool of analysis and modelling of grassland systems. Hacquetia 2009, 8, 129–146. [Google Scholar] [CrossRef] [Green Version]
- Socher, S.A.; Prati, D.; Boch, S.; Müller, J.; Baumbach, H.; Gockel, S.; Hemp, A.; Schöning, I.; Wells, K.; Buscot, F.; et al. Interacting effects of fertilization, mowing and grazing on plant species diversity of 1500 grasslands in Germany differ between regions. Basic Appl. Ecol. 2013, 14, 126–136. [Google Scholar] [CrossRef]
- Bobbink, R.; Den Dubbelden, K.; Willems, J.H. Seasonal dynamics of phytomass and nutrients in chalk grassland. Oikos 1989, 216–224. [Google Scholar] [CrossRef]
- Wellstein, C.; Campetella, G.; Spada, F.; Chelli, S.; Mucina, L.; Canullo, R.; Bartha, S. Context-dependent assembly rules and the role of dominating grasses in semi-natural abandoned sub-Mediterranean grasslands. Agric. Ecosyst. Environ. 2014, 182, 113–122. [Google Scholar] [CrossRef] [Green Version]
- Bricca, A.; Tardella, F.M.; Tolu, F.; Goia, I.; Ferrara, A.; Catorci, A. Disentangling the effects of disturbance from those of dominant tall grass features in driving the functional variation of restored grassland in a Sub-Mediterranean context. Diversity 2019, 12, 11. [Google Scholar] [CrossRef] [Green Version]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.C.; Wright, I.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Prentice, I.C.; et al. The global spectrum of plant form and function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef]
- Klimešová, J.; De Bello, F. CLO-PLA: The database of clonal and bud bank traits of Central European flora. J. Veg. Sci. 2009, 20, 511–516. [Google Scholar] [CrossRef]
- Klimešová, J.; Latzel, V.; de Bello, F.; van Groenendael, J.M. Plant functional traits in studies of vegetation changes in response to grazing and mowing: Towards a use of more specific traits. Preslia 2008, 80, 245–253. [Google Scholar]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. Corrigendum to: New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2016, 64, 715–716. [Google Scholar] [CrossRef] [Green Version]
- Rosbakh, S.; Römermann, C.; Poschlod, P. Specific leaf area correlates with temperature: New evidence of trait variation at the population, species and community levels. Alp. Bot. 2015, 125, 79–86. [Google Scholar] [CrossRef]
- Májeková, M.; Paal, T.; Plowman, N.S.; Bryndová, M.; Kasari, L.; Norberg, A.; Weiss, M.; Bishop, T.R.; Luke, S.H.; Sam, K.; et al. Evaluating functional diversity: Missing trait data and the importance of species abundance structure and data transformation. PLoS ONE 2016, 11, e0149270. [Google Scholar] [CrossRef] [PubMed]
- Grime, J.P. Benefits of plant diversity to ecosystems: Immediate, filter and founder effects. J. Ecol. 1998, 86, 902–910. [Google Scholar] [CrossRef]
- de Bello, F.; Botta-Dukat, Z.; Lepš, J.; Fibich, P. Towards a more balanced combination of multiple traits when computing functional differences between species. Methods Ecol. Evol. 2021, 12, 443–448. [Google Scholar] [CrossRef]
- Kleyer, M.; Bekker, R.; Knevel, I.; Bakker, J.; Thompson, K.; Sonnenschein, M.; Poschlod, P.; van Groenendael, J.; Klimeš, L.; Klimešová, J.; et al. The LEDA Traitbase: A database of life-history traits of Northwest European flora. J. Ecol. 2008, 96, 1266–1274. [Google Scholar] [CrossRef]
- de Bello, F.; Lavergne, S.; Meynard, C.N.; Lepš, J.; Thuiller, W. The partitioning of diversity: Showing Theseus a way out of the labyrinth. J. Veg. Sci. 2010, 21, 992–1000. [Google Scholar] [CrossRef]
- Ricotta, C.; Moretti, M. CWM and Rao’s quadratic diversity: A unified framework for functional ecology. Oecologia 2011, 167, 181–188. [Google Scholar] [CrossRef]
- Pavoine, S.; Vallet, J.; Dufour, A.B.; Gachet, S.; Daniel, H. On the challenge of treating various types of variables: Application for improving the measurement of functional diversity. Oikos 2009, 118, 391–402. [Google Scholar] [CrossRef]
- Botta-Dukát, Z.; Czúcz, B. Testing the ability of functional diversity indices to detect trait convergence and divergence using individual-based simulation. Methods Ecol. Evol. 2016, 7, 114–126. [Google Scholar] [CrossRef]
- de Bello, F.; Šmilauer, P.; Diniz-Filho, J.A.F.; Carmona, C.P.; Lososová, Z.; Herben, T.; Götzenberger, L. Decoupling phylogenetic and functional diversity to reveal hidden signals in community assembly. Methods Ecol. Evol. 2017, 8, 1200–1211. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models: An Introduction with R; Chapman and Hall/CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Zelený, D.; Schaffers, A.P. Too good to be true: Pitfalls of using mean E llenberg indicator values in vegetation analyses. J. Veg. Sci. 2012, 23, 419–431. [Google Scholar] [CrossRef]
- Zelený, D. Which results of the standard test for community-weighted mean approach are too optimistic? J. Veg. Sci. 2018, 29, 953–966. [Google Scholar] [CrossRef]
- Green, R.H. Sampling Design and Statistical Methods for Environmental Biologists; John Wiley & Sons: Hoboken, NJ, USA, 1979. [Google Scholar]
- Underwood, A.J. On beyond BACI: Sampling designs that might reliably detect environmental disturbances. Ecol. Appl. 1994, 4, 3–15. [Google Scholar] [CrossRef]
- Janeček, Š.; Lepš, J. Effect of litter, leaf cover and cover of basal internodes of the dominant species Molinia caerulea on seedling recruitment and established vegetation. Acta Oecol. 2005, 28, 141–147. [Google Scholar] [CrossRef]
- Jensen, K.; Gutekunst, K. Effects of litter on establishment of grassland plant species: The role of seed size and successional status. Basic Appl. Ecol. 2003, 4, 579–587. [Google Scholar] [CrossRef]
- Catorci, A.; Cesaretti, S.; Gatti, R.; Tardella, F.M. Trait-related flowering patterns in submediterranean mountain meadows. Plant Ecol. 2012, 213, 1315–1328. [Google Scholar] [CrossRef]
- Catorci, A.; Piermarteri, K.; Penksza, K.; Házi, J.; Tardella, F.M. Filtering effect of temporal niche fluctuation and amplitude of environmental variations on the trait-related flowering patterns: Lesson from sub-Mediterranean grasslands. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, M.A.; Dodge, G.J.; Inouye, D.W. A phenological mid-domain effect in flowering diversity. Oecologia 2005, 142, 83–89. [Google Scholar] [CrossRef]
- Briske, D.D. Strategies of plant survival in grazed systems: A functional interpretation. In The Ecology and Management of Grazing Systems; Hodgson, I., Ed.; CAB International: Wallingford, UK, 1996; pp. 37–68. [Google Scholar]
- Klimeš, L.; Klimešová, J. The effects of mowing and fertilization on carbohydrate reserves and regrowth of grasses: Do they promote plant coexistence in species-rich meadows? In Ecology and Evolutionary Biology of Clonal Plants; Stuefer, J.F., Erschbamer, B., Huber, H., Suzuki, J.-I., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 141–160. [Google Scholar]
- da Silveira Pontes, L.; Maire, V.; Schellberg, J.; Louault, F. Grass strategies and grassland community responses to environmental drivers: A review. Agron. Sustain. Dev. 2015, 35, 1297–1318. [Google Scholar] [CrossRef]
- Tardella, F.M.; Bricca, A.; Piermarteri, K.; Postiglione, N.; Catorci, A. Context-dependent variation of SLA and plant height of a dominant, invasive tall grass (Brachypodium genuense) in sub-Mediterranean grasslands. Flora 2017, 229, 116–123. [Google Scholar] [CrossRef]
- Conti, L.; Block, S.; Parepa, M.; Münkemüller, T.; Thuiller, W.; Acosta, A.T.; van Kleunen, M.; Dullinger, S.; Essl, F.; Dullinger, I.; et al. Functional trait differences and trait plasticity mediate biotic resistance to potential plant invaders. J. Ecol. 2018, 106, 1607–1620. [Google Scholar] [CrossRef] [Green Version]

| Plant Function | Trait | Variable Type | Trait States |
|---|---|---|---|
| Competition for light and space occupancy | Plant vegetative height (H) | Continuous | - |
| Horizontal architecture (HA) | Categorical | Absent, caespitose, pleiocorm, reptant, rosulate | |
| Resource exploitation | Specific leaf area (SLA) | Continuous | - |
| Leaf dry matter content (LDMC) | Continuous | - | |
| Water-use strategy | Leaf area (LA) | Continuous | - |
| Reproduction | Flowering period (FP) | Categorical | Early and mid-spring (March–April); late spring–early summer (May–June); mid-summer–early autumn (July–September) |
| Temporal resource exploitation | Leaf phenology (LP) | Categorical | Overwintering green, persistent green, spring green, summer green |
| Persistence | Clonal position (CP) | Categorical | Above-soil, below-soil |
| Space occupancy | Clonal spread (CS) | Categorical | Absent, short-spread, long-spread |
| Trait | edf | Adj. R2 | AIC |
|---|---|---|---|
| H | 1 | 0.08 | 299.6 |
| HA | 2.6 | 0.12 | 522.0 |
| SLA | 2.3 | 0.06 | 307.13 |
| LDMC | 1.9 | 0.12 | 214.8 |
| LA | 1 | 0.05 | 292.88 |
| LP | 1 | 0.07 | 442.0 |
| FP | 2.2 | 0.15 | 438.9 |
| CS | 2 | 0.05 | 400.2 |
| CP | 2.6 | 0.25 | 457.2 |
| CSR Grime | 1.8 | 0.10 | 352.5 |
| Trait | Trait State | edf | Adj. R2 | AIC |
|---|---|---|---|---|
| H | 2.3 | 0.13 | −180.3 | |
| HA | Absent | 1.0 | <0.01 | −470.2 |
| Caespitose | 2.3 | 0.30 | −128.4 | |
| Pleiocorm | 1.8 | 0.18 | −252.8 | |
| Reptant | 1.0 | 0.04 | −511.3 | |
| Rosulate | 1.4 | 0.29 | −327.3 | |
| SLA | 2.0 | 0.25 | −452.9 | |
| LDMC | 2.6 | 0.53 | −254.3 | |
| LA | 2.7 | 0.23 | 73.6 | |
| LP | Persistent green | 1.0 | <0.01 | −226.0 |
| Summer green | 1.0 | <0.01 | −205.8 | |
| Spring green | 1.0 | <0.01 | −606.7 | |
| Overwintering green | 2.7 | 0.27 | −1032.9 | |
| FP | Early spring | 1.0 | 0.02 | −596.1 |
| Mid-spring–Early summer | 1.9 | 0.10 | −397.6 | |
| Mid-summer–Late summer | 2.1 | 0.05 | −409.0 | |
| CS | Absent | 1.3 | 0.13 | −385.5 |
| Short | 1.7 | 0.05 | −279.3 | |
| Long | 1.2 | 0.16 | −332.4 | |
| CP | Above | 1.0 | 0.24 | −381.1 |
| Below | 2.2 | 0.11 | −318.0 | |
| CSR | C | 1.3 | 0.13 | 1060.8 |
| S | 2.6 | 0.52 | 1211.9 | |
| R | 2.6 | 0.54 | 1114.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bricca, A.; Tardella, F.M.; Ferrara, A.; Panichella, T.; Catorci, A. Exploring Assembly Trajectories of Abandoned Grasslands in Response to 10 Years of Mowing in Sub-Mediterranean Context. Land 2021, 10, 1158. https://doi.org/10.3390/land10111158
Bricca A, Tardella FM, Ferrara A, Panichella T, Catorci A. Exploring Assembly Trajectories of Abandoned Grasslands in Response to 10 Years of Mowing in Sub-Mediterranean Context. Land. 2021; 10(11):1158. https://doi.org/10.3390/land10111158
Chicago/Turabian StyleBricca, Alessandro, Federico Maria Tardella, Arianna Ferrara, Tiziana Panichella, and Andrea Catorci. 2021. "Exploring Assembly Trajectories of Abandoned Grasslands in Response to 10 Years of Mowing in Sub-Mediterranean Context" Land 10, no. 11: 1158. https://doi.org/10.3390/land10111158
APA StyleBricca, A., Tardella, F. M., Ferrara, A., Panichella, T., & Catorci, A. (2021). Exploring Assembly Trajectories of Abandoned Grasslands in Response to 10 Years of Mowing in Sub-Mediterranean Context. Land, 10(11), 1158. https://doi.org/10.3390/land10111158






