Soil Quality Assessment Using Multivariate Approaches: A Case Study of the Dakhla Oasis Arid Lands
Abstract
:1. Introduction
2. Materials and Methods
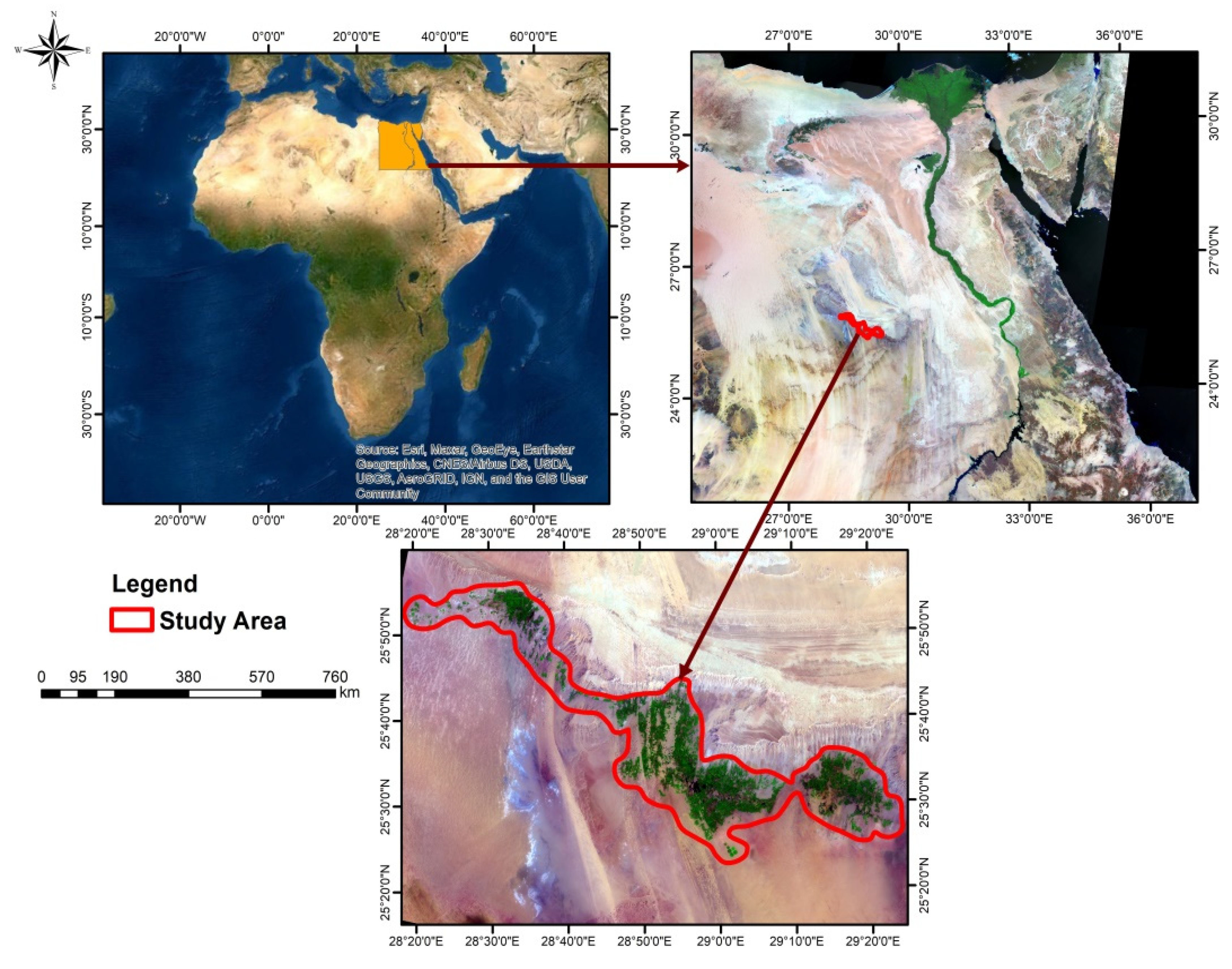
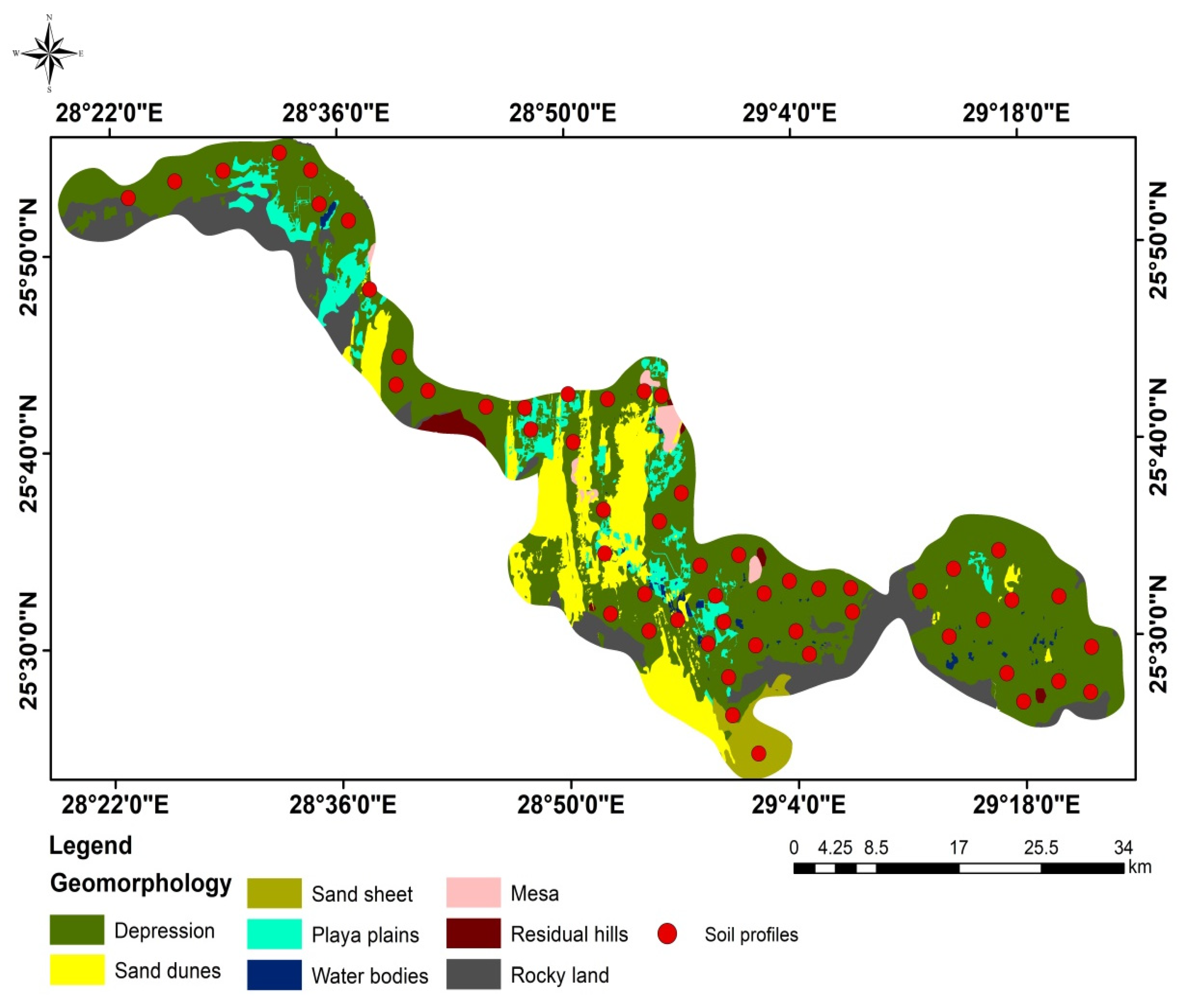
2.1. Site Description
2.2. Soil Sampling and Analysis
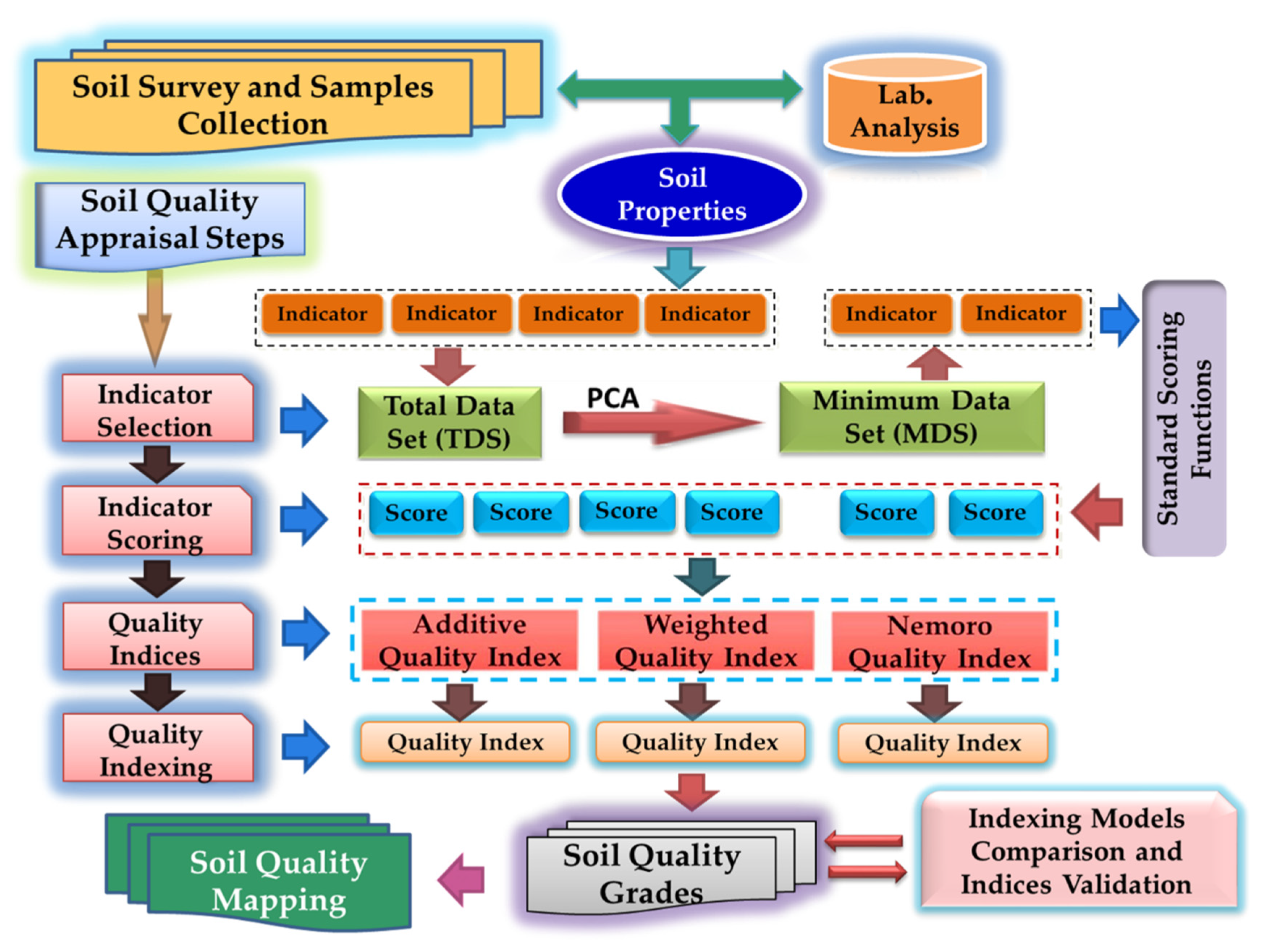
2.3. Soil Quality (SQ) Appraisal
2.3.1. Indicator Selection
- Total Data Set (TDS);
- 2.
- Minimum Data et (MDS).
2.3.2. Indicator Scoring
2.3.3. Developing Soil Quality Indices (SQIs)
- Additive quality index (AQI);
- 2.
- Weighted additive quality index (WQI);
- 3.
- Nemoro quality index (NQI).
2.3.4. Soil Quality Classes
2.4. Comparison between Indexing Models
2.5. Statistical Analysis and Spatial Interpolation
3. Results
3.1. Descriptive Statistics of Soil Indicators
3.2. Correlations among Soil Physicochemical Indicators
3.3. Principal Component Analysis (PCA) and MDS Selection Establishment
3.4. Soil Quality Assessment
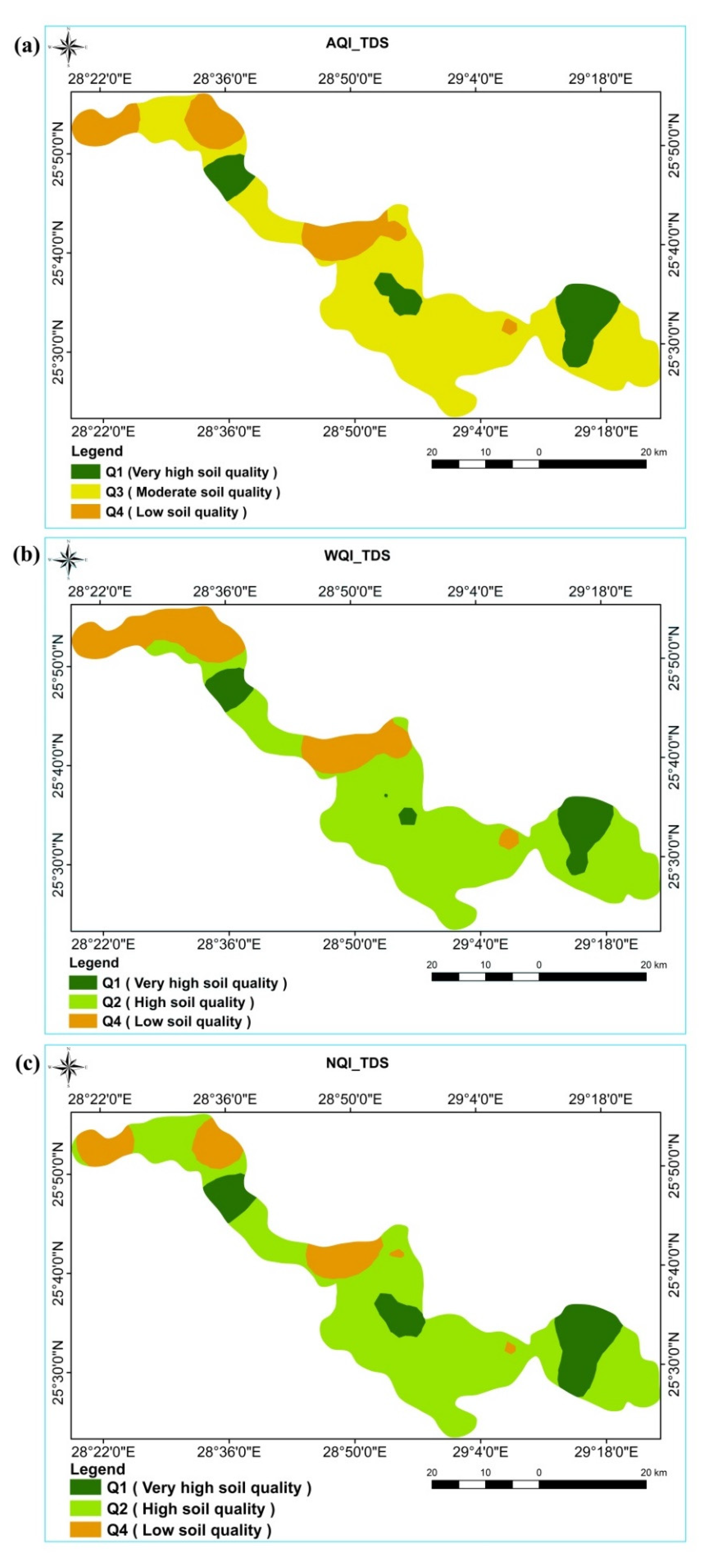
3.4.1. According to TDS
3.4.2. According to MDS
3.5. Comparison of Indices
4. Discussion
4.1. Soil Physicochemical Indicators Characterization
4.2. Soil Indicators Relationships
4.3. Soil Quality
4.4. Indices Validation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Embabi, N.S. The karstified carbonate platforms in the Western Desert. In Landscapes and Landforms of Egypt: Landforms and Evolution, 1st ed.; Embabi, N.S., Ed.; Springer: Cham, Switzerland, 2018; pp. 85–104. [Google Scholar]
- Gad, A.A. Land capability classification of some western desert Oases, Egypt, using remote sensing and GIS. Egypt. J. Remote Sens. Space Sci. 2015, 18, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Ghani, M.M.; Huerta-Martínez, F.M.; Hongyan, L.; Qureshi, R. The desert of Egypt. In Plant Responses to Hyperarid Desert Environments, 1st ed.; Abd El-Ghani, M.M., Huerta-Martínez, F.M., Hongyan, L., Qureshi, R., Eds.; Springer: Cham, Switzerland, 2017; pp. 11–20. [Google Scholar]
- Kato, H.; Kimura, R.; Elbeih, S.F.; Iwasaki, E.; Zaghloul, E.A. Land use change and crop rotation analysis of a government well district in Rashda village—Dakhla Oasis, Egypt based on satellite data. Egypt. J. Remote Sens. Space Sci. 2012, 15, 185–195. [Google Scholar] [CrossRef] [Green Version]
- Abdel Meguid, M. Key features of the Egypt’s water and agricultural resources. In Conventional Water Resources and Agriculture in Egypt, 1st ed.; Negm, A.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 39–99. [Google Scholar] [CrossRef]
- Khalifa, H.E.; Moussa, H.A. Soil and agriculture after the Aswan High Dam. In Irrigated Agriculture in Egypt: Past, Present and Future, 1st ed.; Satoh, M., Aboulroos, S., Eds.; Springer: Cham, Switzerland, 2017; pp. 81–124. [Google Scholar]
- Hamdi, H.; Abdelhafez, S. Agriculture and soil survey in Egypt. In Soil Resources of Southern and Eastern Mediterranean Countries; Zdruli, P., Steduto, P., Lacirignola, C., Montanarella, L., Eds.; CIHEAM-IAMB: Bari, Italy, 2001; pp. 111–130. [Google Scholar]
- Hussein, A.M. Characterization of some soil families in Dakhla Oasis. Azhar J. Agric. Res. 1993, 18, 339–352. [Google Scholar]
- Selmy, S.A.H. Studies on some Shale-Derived Soils in the New Valley, Egypt. Master’s Thesis, Assiut University, Assiut, Egypt, 2005. [Google Scholar]
- Karlen, D.L.; Mausbach, M.J.; Doran, J.W.; Kline, R.G.; Harris, R.F.; Schuman, G.E. Soil quality: A concept, definition, and framework for evaluation. Soil Sci. Soc. Am. J. 1997, 61, 4–10. [Google Scholar] [CrossRef] [Green Version]
- De La Rosa, D. Soil quality evaluation and monitoring based on land evaluation. Land Degrad. Dev. 2005, 16, 551–559. [Google Scholar] [CrossRef]
- Schloter, M.; Nannipieri, P.; Sørensen, S.J.; Elsas, J.D. Microbial indicators for soil quality. Biol. Fertil. Soils 2018, 54, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Karlen, D.L.; Cambardella, C.A.; Kovar, J.L.; Colvin, T.S. Soil quality response to long-term tillage and crop rotation practices. Soil Tillage Res. 2013, 133, 54–64. [Google Scholar] [CrossRef] [Green Version]
- Vasu, D.; Singh, S.K.; Ray, S.K.; Duraisami, V.P.; Tiwary, P.; Chandran, P.; Nimkar, A.M.; Anantwar, S.G. Soil quality index (SQI) as a tool to evaluate crop productivity in semi-arid Deccan plateau, India. Geoderma 2016, 282, 70–79. [Google Scholar] [CrossRef]
- Guo, L.L.; Sun, Z.G.; Ouyang, Z.; Han, D.R.; Li, F.D. A comparison of soil quality evaluation methods for Fluvisol along the lower Yellow River. Catena 2017, 152, 135–143. [Google Scholar] [CrossRef]
- Brejda, J.; Moorman, T.; Karlen, B.; Dao, T. Identification of regional soil quality factors and indicators: I. Central and southern high plains. Soil Sci. Soc. Am. J. 2000, l64, 2115–2124. [Google Scholar] [CrossRef]
- Marzaioli, R.; D’Ascoli, R.; De Pascale, R.A.; Rutigliano, F.A. Soil quality in a Mediterranean area of Southern Italy as related to different land use types. Appl. Soil Ecol. 2010, 44, 205–212. [Google Scholar] [CrossRef]
- Qi, Y.; Darilek, J.L.; Huang, B.; Zhao, Y.; Sun, W.; Gu, Z. Evaluating soil quality indices in an agricultural region of Jiangsu Province, China. Geoderma 2009, 149, 325–334. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lal, R. Comparison of soil quality index using three methods. PLoS ONE 2014, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Nabiollahi, K.; Taghizadeh-Mehrjardi, R.; Kerry, R.; Moradian, S. Assessment of soil quality indices for salt-affected agricultural land in Kurdistan Province, Iran. Ecol. Indic. 2017, 83, 482–494. [Google Scholar] [CrossRef]
- Zhou, M.; Xiao, Y.; Li, Y.; Zhang, X.; Wang, G.; Jin, J.; Ding, G.; Liu, X. Soil quality index evaluation model in responses to six-year fertilization practices in Mollisols. Arch. Agron. Soil Sci. 2020. [Google Scholar] [CrossRef]
- Karaca, S.; Dengiz, O.; Demirağ Turan, I.; Özkan, B.; Dedeoğlu, M.; Gülser, F.; Sarging, B.; Demirkaya, S.; Ay, A. An assessment of pasture soils quality based on multi-indicator weighting approaches in semi-arid ecosystem. Ecol. Indic. 2021, 121. [Google Scholar] [CrossRef]
- Rahmanipour, F.; Marzaioli, R.; Bahrami, H.A.; Fereidouni, Z.; Bandarabadi, S.R. Assessment of soil quality indices in agricultural lands of Qazvin Province, Iran. Ecol. Indic. 2014, 40, 19–26. [Google Scholar] [CrossRef]
- Raiesi, F. A minimum data set and soil quality index to quantify the effect of land use conversion on soil quality and degradation in native rangelands of upland arid and semiarid regions. Ecol. Indic. 2017, 75, 307–320. [Google Scholar] [CrossRef]
- Rezaee, L.; Moosavi, A.A.; Davatgar, N.; Sepaskhah, A.R. Soil quality indices of paddy soils in Guilan province of northern Iran: Spatial variability and their influential parameters. Ecol. Indic. 2020, 117, 106566. [Google Scholar] [CrossRef]
- Doran, J.W.; Parkin, B.T. Defining and assessing soil quality. In Defining Soil Quality for a Sustainable Environment; Doran, J.W., Coleman, D.C., Bezdicek, D.F., Stewart, B.A., Eds.; Soil Science Society of America: Madison, WI, USA, 1994; Volume 35, pp. 1–21. [Google Scholar]
- Chen, Y.D.; Wang, H.Y.; Zhou, J.M.; Xing, L.; Zhu, B.S.; Zhao, Y.C.; Chen, X.Q. Minimum data set for assessing soil quality in farmland of Northeast China. Pedosphere 2013, 23, 564–576. [Google Scholar] [CrossRef]
- Bo, Y.J.; Zhu, Q.K.; Bao, Y.X.; Zhao, W.J.; Zhao, Y.M.; Zhang, H.Z. A comparative study on three methods of soil quality evaluation of microtopography in the semi-arid Loess Plateau, China. J. Environ. Biol. 2015, 36, 325–330. [Google Scholar] [PubMed]
- Pascazioa, S.; Crecchioa, C.; Scagliolaa, M.; Mininnib, A.N.; Dichiob, B.; Xiloyannisb, C.; Sofoc, A. Microbial-based soil quality indicators in irrigated and rainfed soilportions of Mediterranean olive and peach orchards undersustainable management. Agric. Water Manag. 2018, 195, 172–179. [Google Scholar] [CrossRef]
- Armenise, E.; Redmile-Gordon, M.A.; Stellacci, A.M.; Ciccarese, A.; Rubino, P. Developing a soil quality index to compare soil fitness for agricultural use under different managements in the Mediterranean environment. Soil Tillage Res. 2013, 130, 91–98. [Google Scholar] [CrossRef]
- Biswas, S.; Hazra, G.C.; Purakayastha, T.J.; Saha, N.; Mitran, T.; Roy, S.S.; Basak, N.; Mandal, B. Establishment of critical limits of indicators and indices of soil quality in rice-rice cropping systems under different soil orders. Geoderma 2017, 292, 34–48. [Google Scholar] [CrossRef]
- Reeves, D.W. The role of soil organic matter in maintaining soil quality in continuous cropping systems. Soil Tillage Res. 1997, 43, 131–167. [Google Scholar] [CrossRef]
- Goovaerts, P. Geostatistics in soil science: State of the art and perspectives. Geoderma 1999, 89, 1–45. [Google Scholar] [CrossRef]
- Delbari, M.; Afrasiab, P.; Loiskandl, W. Using sequential Gaussian simulation to assess the field-scale spatial uncertainty of soil water content. Catena 2009, 79, 163–169. [Google Scholar] [CrossRef]
- Delbari, M.; Loiskandl, W.; Afrasiab, P. Uncertainty assessment of soil organic carbon content spatial distribution using geostatistical stochastic simulation. Soil Res. 2010, 48, 27–35. [Google Scholar] [CrossRef]
- Kravchenko, A.; Bullock, D.G. A comparative study of interpolation methods for mapping soil properties. Agronomy 1999, 91, 393–400. [Google Scholar] [CrossRef]
- Delbari, M.; Afrasiab, P.; Loiskandl, W. Geostatistical analysis of soil texture fractions on the field scale. Soil Water Res. 2011, 6, 173–189. [Google Scholar] [CrossRef] [Green Version]
- Robinson, T.P.; Metternicht, G. Testing the performance of spatial interpolation techniques for mapping soil properties. Comput. Electron. Agric. 2006, 50, 97–108. [Google Scholar] [CrossRef]
- Bogunovic, I.; Trevisani, S.; Seput, M.; Juzbasic, D.; Durdevic, B. Short-range and regional spatial variability of soil chemical properties in an agro-ecosystem in eastern Croatia. Catena 2017, 154, 50–62. [Google Scholar] [CrossRef]
- Rosemary, F.; Indraratne, S.P.; Weerasooriya, R.; Mishra, U. Exploring the spatial variability of soil properties in an Alfisol soil catena. Catena 2017, 150, 53–61. [Google Scholar] [CrossRef]
- Egyptian Meteorological Authority (EMA). The Normals for Dakhla Station, (1960–2010); Ministry of Civil Aviation: Cairo, Egypt, 2011.
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014; p. 360. [Google Scholar]
- FAO. Guidelines for Soil Description, 4th ed.; Food and Agriculture Organization of the United Nation: Rome, Italy, 2006; p. 97. [Google Scholar]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H. Methods of Soil Analysis: Part. 3-Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America and American Society of Agronomy: Madison, WI, USA, 1996; p. 1390. [Google Scholar]
- Jacob, H.D.; Clarke, G.T. Methods of Soil Analysis: Part 4 Physical Methods; Jacob, H.D., Clarke, G.T., Eds.; Soil Science Society of America: Madison, WI, USA, 2002; p. 1692. [Google Scholar]
- FAO. Irrigation Water Management. Training Manual No. 1—Introduction to Irrigation; Brouwer, C., Goffeau, A., Heibloem, M., Eds.; Food and Agriculture Organization of the United Nation: Rome, Italy, 1985. [Google Scholar]
- Karlen, D.; Wollenhaupt, N.C.; Erbach, D.C.; Berry, E.C.; Swan, J.B.; Eash, N.S.; Jordahl, J.L. Crop residue effects on soil quality following 10-years of no-till corn. Soil Tillage Res. 1994, 31, 149–167. [Google Scholar] [CrossRef]
- Karlen, D.; Wollenhaupt, N.C.; Berry, E.C.; Swan, J.B.; Eash, N.S.; Jordahl, J.L. 1994b. Long-term tillage effects on soil quality. Soil Tillage Res. 1994, 32, 313–327. [Google Scholar] [CrossRef]
- FAO. Management of gypsiferous soils. In Soils Bulletin 62; Food and Agriculture Organization of the United Nation: Rome, Italy, 1990. [Google Scholar]
- Yu, P.; Liu, S.; Zhang, L.; Li, Q.; Zhou, D. Selecting the minimum data set and quantitative soil quality indexing of alkaline soils under different land uses in northeastern China. Sci. Total Environ. 2018, 616–617, 564–571. [Google Scholar] [CrossRef] [PubMed]
- McGarigal, K.; Cushman, S.A.; Stafford, S. Multivariate Statistics for Wildlife and Ecology Research, 1st ed.; McGarigal, K., Cushman, S.A., Stafford, S., Eds.; Springer: New York, NY, USA, 2013; p. 233. [Google Scholar]
- Sánchez-Navarro, A.; Gil-Vázquez, J.M.; Delgado-Iniesta, M.J.; Marín-Sanleandro, P.; Blanco-Bernardeau, A.; Ortiz-Silla, R. Establishing an index and identification of limiting parameters for characterizing soil quality in Mediterranean ecosystems. Catena 2015, 131, 35–45. [Google Scholar] [CrossRef]
- Ferretti, F.; Saltelli, A.; Tarantola, S. Trends in sensitivity analysis practice in the last decade. Sci. Total Environ. 2016, 568, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Cude, C.G. Oregon Water Quality Index: A Tool for Evaluating Water Quality Management Effectiveness. J. Am. Water Resour. Assoc. 2001, 37, 125–137. [Google Scholar] [CrossRef]
- Pianosi, F.; Beven, K.; Freer, J.; Hall, J.W.; Rougier, J.; Stephenson, D.B.; Wagener, T. Sensitivity analysis of environmental models: A systematic review with practica workflow. Environ. Model. Softw. 2016, 79, 214–223. [Google Scholar] [CrossRef]
- Hamed, M.H.; Khalafallh, M.Y. Available nutrients and some soil properties of El-Qasr soils, El-Dakhla Oasis, Egypt. Int. J. Environ. Agric. Biotechnol. 2017, 2, 3243–3249. [Google Scholar] [CrossRef] [Green Version]
- Soil Science Division Staff. Soil Survey Manual; Ditzler, C., Scheffe, K., Monger, H.C., Eds.; Government Printing Office: Washington, DC, USA, 2017; p. 587.
- FAO. Salt-affected soils and their management. In Soils Bulletin 39; Food and Agriculture Organization of the United Nation: Rome, Italy, 1988. [Google Scholar]
- Jafari, M.; Tavili, A.; Panahi, F.; Zandi Esfahan, E.; Ghorbani, M. Characteristics of arid and desert ecosystems. In Reclamation of Arid Lands, 1st ed.; Jafari, M., Tavili, A., Panahi, F., Zandi Esfahan, E., Ghorbani, M., Eds.; Springer: Cham, Switzerland, 2018; pp. 21–91. [Google Scholar]
- Schoenholtz, S.H.; Van Miegroet, H.; Burger, J.A. A review of chemical and physical properties as indicators of forest soil quality: Challenges and opportunities. For. Ecol. Manag. 2001, 138, 335–356. [Google Scholar] [CrossRef]
- Hashemi, S.S.; Baghernejad, M.; Khademi, H. Micromorphology of gypsum crystals in southern Iranian soils under different moisture regimes. J. Agric. Sci. Technol. 2011, 13, 273–288. [Google Scholar]
- Hazelton, P.; Murphy, B. Interpreting Soil Test Results: What Do All the Numbers Mean? 3rd ed.; Hazelton, P., Murphy, B., Eds.; CSIRO Publishing: Victoria, Australia, 2017; p. 200. [Google Scholar]
- Hudson, B.D. Soil organic-matter and available water capacity. J. Soil Water Conserv. 1994, 49, 189–194. [Google Scholar]
- Saxton, K.E.; Rawl, W.J.; Romberger, J.S.; Papendick, R.I. Estimating generalized soil-water characteristics from texture. Soil Sci. Soc. Am. J. 1986, 50, 1031–1036. [Google Scholar] [CrossRef]
- Naghdi, R.; Solgi, A.; Labelle, E.R.; Nikooy, M. Combined effects of soil texture and machine operating trail gradient on changes in forest soil physical properties during ground-based skidding. Pedosphere 2020, 30, 508–516. [Google Scholar] [CrossRef]
- Hao, X.; Ball, B.C.; Culley, J.L.B.; Carter, M.R.; Parkin, G.W. Soil density and porosity. In Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorichpp, E.G., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 743–760. [Google Scholar]
- Chang, Y.; Rossi, L.; Zotarelli, L.; Gao, B.; Shahid, M.; Sarkhosh, A. Biochar improves soil physical characteristics and strengthens root architecture in Muscadine grape (Vitis rotundifolia L.). Chem. Biol. Technol. Agric. 2021, 8, 7. [Google Scholar] [CrossRef]
- Blume, H.P.; Brümme, G.W.; Fleige, H.; Horn, R.; Kandeler, E.; Kögel-Knabner, I.; Kretzschmar, R.; Stahr, K.; Wilke, B.M. Chemical properties and processes. In Scheffer/Schachtschabel Soil Science, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 123–174. [Google Scholar]
- Blume, H.P.; Brümme, G.W.; Fleige, H.; Horn, R.; Kandeler, E.; Kögel-Knabner, I.; Kretzschmar, R.; Stahr, K.; Wilke, B.M. Physical properties and processes. In Scheffer/Schachtschabel Soil Science, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 175–283. [Google Scholar]
- McCarty, L.B.; Hubbard, L.R.; Quisenberry, V. Soil physical and moisture properties. In Applied Soil Physical Properties, Drainage and Irrigation Strategies, 1st ed.; McCarty, L.B., Hubbard, L.R., Quisenberry, V., Eds.; Springer: Cham, Switzerland, 2016; pp. 1–72. [Google Scholar]
- Saedi, T.; Shorafa, M.; Gorji, M.; Khalili Moghadam, B. Indirect and direct effects of soil properties on soil splash erosion rate in calcareous soils of the central Zagross, Iran: A laboratory study. Geoderma 2016, 271, 1–9. [Google Scholar] [CrossRef]
- Khaledian, Y.; Brevik, E.C.; Pereira, P.; Cerda, A.; Fattah, M.A.; Tazikeh, H. Modeling soil cation exchange capacity in multiple countries. Catena 2017, 158, 194–200. [Google Scholar] [CrossRef] [Green Version]
- Zech, W. Geology and soils. In Tropical Forestry Handbook, 2nd ed.; Pancel, L., Köhl, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 93–283. [Google Scholar]
| Indicator | Associated Soil Function | SSF | Optimal Range | Limits References |
|---|---|---|---|---|
| Depth | Rooting relations and erosion | MB | ||
| Gravel | Fertility, structure, erosion & water retention | LB | ||
| Sand | LB | [27] | ||
| Silt | MB | |||
| Clay | OR | 15–45% | [30] | |
| OM | MB | |||
| WHC | Water storage and availability | MB | ||
| IR | OR | 0.4–0.8 in h−1 | [46] | |
| BD | Soil structure | LB | ||
| TP | Water and air movement | OR | 40–60% | [47,48] |
| ECe | Microbial activity and plant growth | OR | 0.2–2 dS m−1 | [23] |
| pH | Nutrients and rooting relations | OR | 6.5–7.5 | [20] |
| CEC | Nutrient retention | MB | ||
| ESP | Water infiltration and movement | LB | ||
| CaCO3 Gypsum | Root penetration and water relations | OR | 10–100 g kg−1 | [49] |
| OR | 30–100 g kg−1 | [49] |
| Property | Range | Minimum | Maximum | Mean | SD | CV% | Skewness | Kurtosis |
|---|---|---|---|---|---|---|---|---|
| Slope (%) | 2.2 | 0.0 | 2.2 | 0.4 | 0.6 | 148.0 | 2.3 | 5.0 |
| Profile depth (cm) | 70.0 | 80.0 | 150.0 | 130 | 19.0 | 15.3 | −0.5 | −0.5 |
| Gravel (%) | 12.4 | 0.0 | 12.4 | 3.4 | 3.4 | 100.0 | 1.1 | 0.7 |
| Sand (%) | 53.1 | 18.9 | 72.0 | 48.7 | 15.6 | 32.1 | −0.3 | −0.8 |
| Silt (%) | 34.3 | 12.3 | 46.6 | 23.0 | 8.8 | 38.3 | 1.2 | 1.0 |
| Clay (%) | 53.4 | 5.3 | 58.7 | 28.3 | 14.0 | 49.6 | 0.5 | −0.5 |
| WHC (%) | 23.9 | 15.3 | 39.2 | 26.9 | 7.6 | 28.1 | 0.3 | −1.3 |
| IR (in h−1) | 2.1 | 0.08 | 2.13 | 0.5 | 1.2 | 92.2 | 2.0 | 4.7 |
| BD (Mg m−3) | 0.7 | 1.1 | 1.8 | 1.5 | 0.4 | 26.1 | 0.5 | −0.2 |
| Porosity (%) | 70.1 | 12.4 | 72.3 | 35.3 | 17.6 | 49.8 | 0.5 | −0.5 |
| ESP (%) | 32.3 | 2.3 | 34.6 | 15.6 | 7.9 | 50.8 | 0.3 | 0.2 |
| CaCO3 (g kg−1) | 495.5 | 53.1 | 548.6 | 156.1 | 112.2 | 71.9 | 2.2 | 5.5 |
| Gypsum (g kg−1) | 72.1 | 19.2 | 91.3 | 41.6 | 20.8 | 50.1 | 1.3 | 0.8 |
| pH (1:1) | 1.5 | 7.1 | 8.6 | 7.6 | 0.4 | 5.2 | 0.5 | −0.5 |
| ECe (dSm−1) | 163.5 | 2.3 | 165.8 | 27.1 | 38.7 | 142.8 | 2.5 | 6.5 |
| OM (g kg−1) | 19.4 | 1.0 | 20.4 | 6.4 | 5.2 | 80.9 | 1.6 | 2.2 |
| CEC (Cmol(+)kg−1) | 43.8 | 3.4 | 47.2 | 19.9 | 13.0 | 65.4 | 0.8 | −0.4 |
| Texture class | C; SCL; SiL; L; SL | |||||||
| Indicator | Slope | Depth | Gravel | Sand | Silt | Clay | WHC | IR | Bd | Porosity | ECe | pH | CEC | ESP | CaCO3 | Gypsum | OM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slope | 1.000 | ||||||||||||||||
| Depth | −0.178 | 1.000 | |||||||||||||||
| Gravel | −0.369 * | 0.004 | 1.000 | ||||||||||||||
| Sand | −0.351 * | 0.107 | 0.402 * | 1.000 | |||||||||||||
| Silt | −0.013 | 0.252 | −0.156 | −0.452 ** | 1.000 | ||||||||||||
| Clay | 0.399 * | −0.278 | −0.349 * | −0.828 ** | −0.126 | 1.000 | |||||||||||
| WHC | 0.388 * | −0.129 | −0.205 | −0.387 * | −0.142 | 0.519 ** | 1.000 | ||||||||||
| IR | −0.105 | 0.037 | 0.020 | 0.338 * | 0.236 | −0.525 ** | −0.142 | 1.000 | |||||||||
| BD | −0.036 | 0.057 | 0.283 | 0.118 | −0.175 | −0.021 | −0.307 | −0.113 | 1.000 | ||||||||
| Porosity | −0.141 | 0.130 | 0.100 | −0.204 | 0.506 ** | −0.091 | 0.202 | 0.162 | −0.184 | 1.000 | |||||||
| ECe | −0.260 | −0.079 | 0.271 | 0.254 | −0.275 | −0.109 | −0.089 | −0.252 | −0.369 * | −0.161 | 1.000 | ||||||
| pH | −0.417 * | −0.008 | 0.239 | 0.300 * | −0.216 | −0.199 | −0.229 | −0.230 | −0.213 | −0.102 | 0.780 ** | 1.000 | |||||
| CEC | 0.628 ** | −0.300 * | −0.340 * | −0.756 ** | −0.068 | 0.883 ** | 0.688 ** | −0.452 | −0.078 | 0.095 | −0.144 | −0.236 | 1.000 | ||||
| ESP | −0.078 | 0.133 | −0.120 | −0.181 | 0.488 ** | −0.106 | −0.274 | −0.188 | −0.274 | 0.425 * | 0.133 | 0.251 | 0.000 | 1.000 | |||
| CaCO3 | −0.273 | 0.187 | 0.506 ** | 0.558 ** | −0.100 | −0.558 ** | −0.305 * | 0.511 ** | 0.231 | −0.043 | −0.031 | −0.030 | −0.531 ** | −0.272 | 1.000 | ||
| Gypsum | −0.068 | −0.096 | 0.307 * | −0.211 | −0.001 | 0.235 | 0.177 | −0.083 | 0.055 | 0.159 | 0.307 * | 0.078 | 0.267 | −0.004 | −0.073 | 1.000 | |
| OM | 0.629 ** | −0.245 | −0.400 * | −0.614 ** | −0.025 | 0.698 ** | 0.603 ** | −0.279 | −0.064 | −0.080 | −0.188 | −0.140 | 0.823 ** | −0.002 | −0.454 ** | −0.029 | 1.000 |
| Principal Component | PC1 | PC2 | PC3 | PC4 | Communalities | Indicator Weight | ||
|---|---|---|---|---|---|---|---|---|
| Eigenvalue | 7.05 | 2.50 | 1.98 | 1.58 | Total dataset | Minimum dataset | ||
| Variance (%) | 44.05 | 15.63 | 12.35 | 9.85 | ||||
| Cumulative (%) | 44.05 | 59.68 | 72.03 | 81.88 | ||||
| Indicator | Eigenvectors | Communality | Variance | Communality | ||||
| Depth | 0.087 | 0.030 | −0.747 | 0.268 | 0.639 | 0.049 | 0.151 | 0.222 |
| Gravel | −0.268 | 0.802 | 0.079 | 0.088 | 0.729 | 0.056 | 0.191 | 0.273 |
| Sand | −0.971 | 0.117 | 0.150 | −0.067 | 0.984 | 0.075 | 0.538 | 0.242 |
| Silt | 0.813 | 0.419 | −0.260 | −0.121 | 0.918 | 0.070 | ||
| Clay | 0.887 | −0.364 | 0.029 | 0.158 | 0.945 | 0.072 | ||
| WHC | 0.936 | −0.254 | −0.040 | 0.160 | 0.968 | 0.074 | ||
| IR | −0.738 | 0.434 | −0.053 | 0.304 | 0.828 | 0.063 | ||
| BD | −0.928 | 0.349 | 0.035 | −0.009 | 0.985 | 0.075 | ||
| TP | 0.928 | −0.349 | −0.036 | 0.009 | 0.985 | 0.075 | ||
| ECe | −0.166 | 0.114 | 0.729 | −0.438 | 0.764 | 0.058 | ||
| pH | −0.247 | 0.572 | 0.279 | 0.060 | 0.469 | 0.036 | ||
| CEC | 0.810 | −0.324 | 0.114 | 0.215 | 0.821 | 0.063 | ||
| ESP | −0.066 | −0.198 | 0.208 | −0.915 | 0.924 | 0.071 | 0.120 | 0.262 |
| CaCO3 | −0.475 | 0.573 | −0.166 | 0.115 | 0.596 | 0.045 | ||
| Gypsum | 0.177 | 0.097 | 0.740 | 0.277 | 0.726 | 0.055 | ||
| OM | 0.654 | −0.465 | 0.107 | 0.407 | 0.82 | 0.063 | ||
| Index | Indicator | Soil Quality Grade | ||||
|---|---|---|---|---|---|---|
| Very Low | Low | Moderate | High | Very High | ||
| Q5 | Q4 | Q3 | Q2 | Q1 | ||
| AQI | TDS | <0.48 | 0.48–0.55 | 0.55–0.62 | 0.62–0.69 | >0.69 |
| MDS | <0.30 | 0.30–0.40 | 0.40–0.50 | 0.50–0.60 | >0.60 | |
| WQI | TDS | <0.46 | 0.46–0.53 | 0.53–0.60 | 0.60–0.67 | >0.67 |
| MDScom | <0.33 | 0.33–0.46 | 0.46–0.59 | 0.59–0.72 | >0.72 | |
| MDSvar | <0.29 | 0.29–0.39 | 0.39–0.49 | 0.49–0.59 | >0.59 | |
| NQI | TDS | <0.32 | 0.32–0.37 | 0.37–0.42 | 0.42–0.47 | >0.47 |
| MDS | <0.17 | 0.17–0.23 | 0.23–0.29 | 0.29–0.35 | >0.35 | |
| Indicator | Total Dataset (TDS) | Minimum Dataset (MDS) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Index | AQI | WQI | NQI | AQI | WQIcom | WQIvar | NQI | |||||||
| Minimum | 0.48 | Q4 | 0.46 | Q4 | 0.32 | Q4 | 0.28 | Q5 | 0.27 | Q5 | 0.28 | Q5 | 0.11 | Q5 |
| Maximum | 0.80 | Q1 | 0.78 | Q1 | 0.55 | Q1 | 0.76 | Q1 | 0.78 | Q1 | 0.75 | Q1 | 0.41 | Q1 |
| Mean | 0.61 | Q3 | 0.60 | Q2 | 0.42 | Q2 | 0.54 | Q2 | 0.48 | Q3 | 0.53 | Q2 | 0.28 | Q3 |
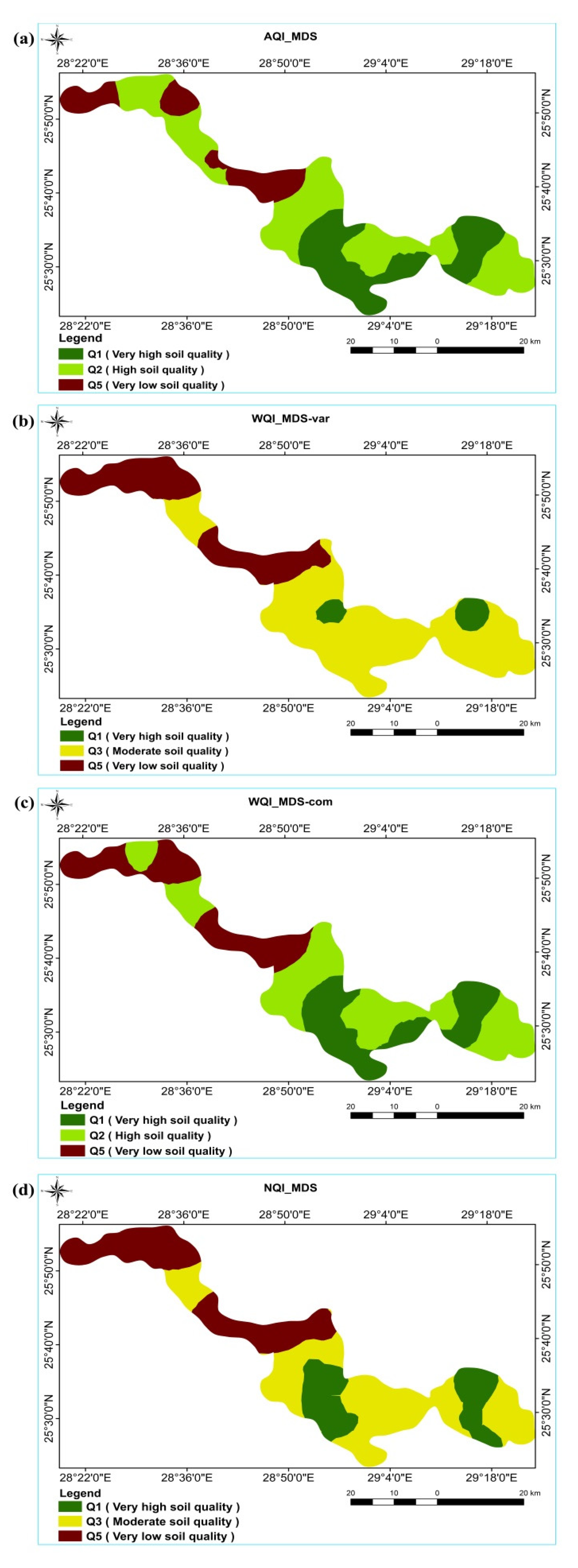
| Soil Quality Grade | Area | |||||||||||||
| km2 | % | km2 | % | km2 | % | km2 | % | km2 | % | km2 | % | km2 | % | |
| V. high (Q1) | 200.0 | 13.7 | 115.2 | 7.9 | 236.0 | 16.2 | 387.0 | 26.5 | 337.0 | 23.0 | 72.0 | 4.9 | 273.1 | 18.7 |
| High (Q2) | - | - | 985.5 | 67.4 | 1013.6 | 69.3 | 818.8 | 56.0 | 764.3 | 52.3 | - | - | - | - |
| Moderate (Q3) | 984.0 | 67.3 | - | - | - | - | - | - | - | - | 966.3 | 66.1 | 742.3 | 50.8 |
| Low (Q4) | 277.6 | 19.0 | 360.9 | 24.7 | 212.0 | 14.5 | - | - | - | - | - | - | - | - |
| V. low (Q5 | - | - | - | - | - | - | 255.2 | 17.5 | 360.2 | 24.7 | 423.4 | 29.0 | 446.2 | 30.5 |
| Source of Variance | Sum of Squares | Df | Mean Square | F | Significant | SI | R2 | ||
|---|---|---|---|---|---|---|---|---|---|
| Between Groups | 2.434 | 6 | 0.406 | 25.439 | 0.000 | ||||
| Within Groups | 3.684 | 231 | 0.016 | ||||||
| Total | 6.118 | 237 | |||||||
| Index | AQI-TDS | AQI-MDS | WQI-TDS | WQI-MDS-var | WQI-MDS-com | NQI-TDS | NQI-MDS | ||
| AQI-TDS | 1 | 1.7 | 0.75 | ||||||
| AQI-MDS | 0.609 ** | 1 | 2.7 | ||||||
| WQI-TDS | 0.997 ** | 0.556 ** | 1 | 1.7 | 0.88 | ||||
| WQI-MDS-var | 0.917 ** | 0.774 ** | 0.902 ** | 1 | 2.9 | ||||
| WQI-MDS-com | 0.595 ** | 0.999 ** | 0.543 ** | 0.766 ** | 1 | 2.7 | 0.74 | ||
| NQI-TDS | 0.992 ** | 0.565 ** | 0.992 ** | 0.874 ** | 0.552 ** | 1 | 1.8 | 0.77 | |
| NQI-MDS | 0.588 ** | −0.134 | 0.632 ** | 0.479 ** | −0.142 | 0.614 ** | 1 | 1.6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selmy, S.A.H.; Abd Al-Aziz, S.H.; Jiménez-Ballesta, R.; Jesús García-Navarro, F.; Fadl, M.E. Soil Quality Assessment Using Multivariate Approaches: A Case Study of the Dakhla Oasis Arid Lands. Land 2021, 10, 1074. https://doi.org/10.3390/land10101074
Selmy SAH, Abd Al-Aziz SH, Jiménez-Ballesta R, Jesús García-Navarro F, Fadl ME. Soil Quality Assessment Using Multivariate Approaches: A Case Study of the Dakhla Oasis Arid Lands. Land. 2021; 10(10):1074. https://doi.org/10.3390/land10101074
Chicago/Turabian StyleSelmy, Salman A. H., Salah H. Abd Al-Aziz, Raimundo Jiménez-Ballesta, Francisco Jesús García-Navarro, and Mohamed E. Fadl. 2021. "Soil Quality Assessment Using Multivariate Approaches: A Case Study of the Dakhla Oasis Arid Lands" Land 10, no. 10: 1074. https://doi.org/10.3390/land10101074
APA StyleSelmy, S. A. H., Abd Al-Aziz, S. H., Jiménez-Ballesta, R., Jesús García-Navarro, F., & Fadl, M. E. (2021). Soil Quality Assessment Using Multivariate Approaches: A Case Study of the Dakhla Oasis Arid Lands. Land, 10(10), 1074. https://doi.org/10.3390/land10101074









