Wheat Straw as a Bio-Sorbent for Arsenate, Chromate, Fluoride, and Nickel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of the Wheat Straw
2.2. Sorption/Desorption Experiments
2.3. Data Analyses
3. Results and Discussion
3.1. As(V), Cr(VI), F−, and Ni2+ Sorption as a Function of Concentrations Added
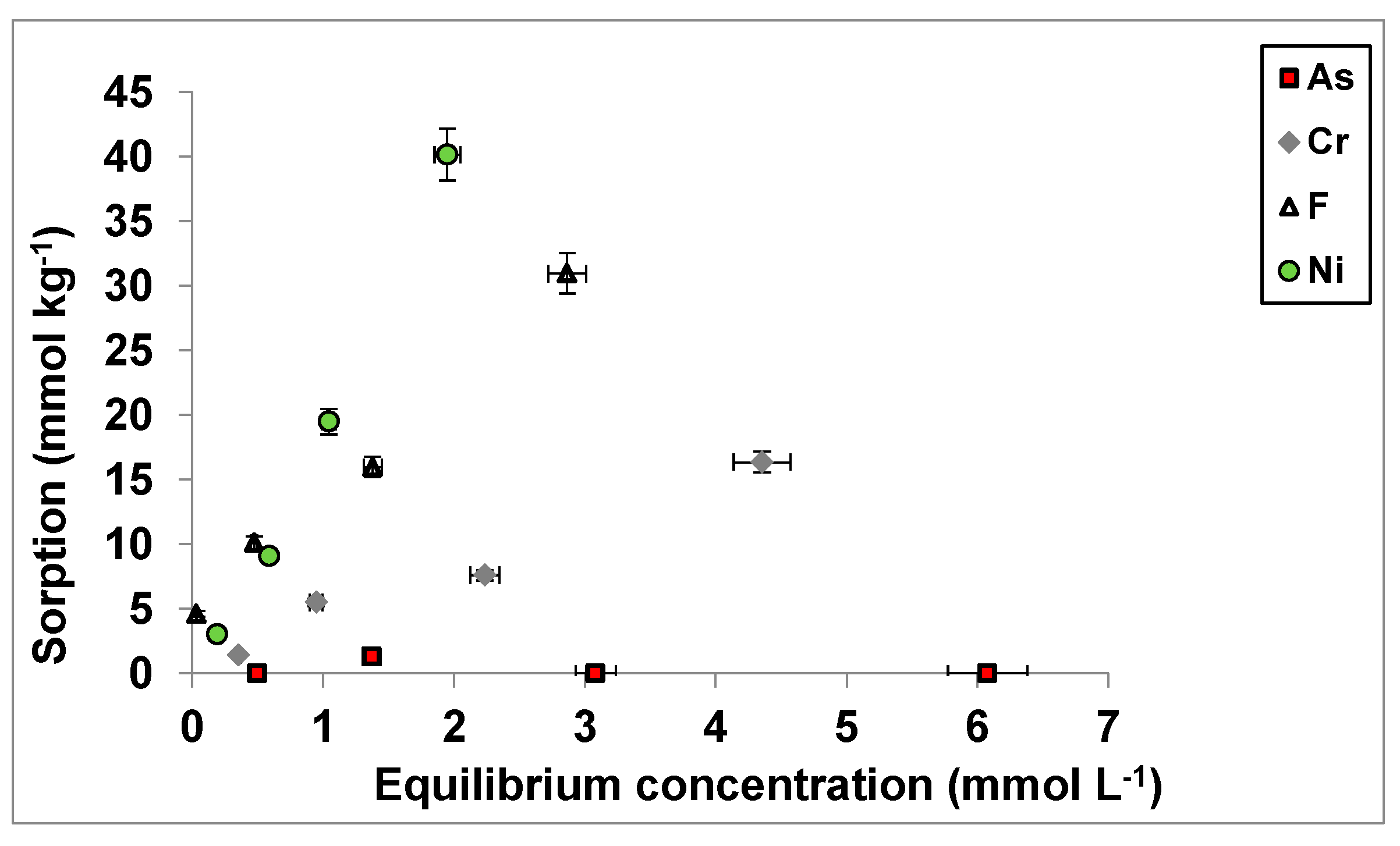
3.2. As(V), Cr(VI), F− and Ni2+ Sorption Curves
3.3. As, Cr, F, and Ni Desorption from Wheat Straw
3.4. Implications of the Research
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smith, E.; Naidu, R.; Alston, A.M. Arsenic in the soil environment, a review. Adv. Agron. 1998, 64, 149–195. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, X.; Tang, J.; Liu, W.; Yang, H. Review of arsenic geochemical characteristics and its significance on arsenic pollution studies in karst groundwater, Southwest China. Appl. Geochem. 2016, 77, 80–88. [Google Scholar] [CrossRef]
- He, L.; Wang, M.; Zhang, G.; Qiu, G.; Cai, D.; Wu, Z.; Zhang, X. Remediation of Cr(VI) contaminated soil using long-duration sodium thiosulfate supported by micro–nano networks. J. Hazard. Mater. 2015, 294, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Rawajfih, Z.; Nsour, N. Thermodynamic analysis of sorption isotherms of chromium (VI) anionic species on reed biomass. J. Chem. Thermodyn. 2008, 40, 846–851. [Google Scholar] [CrossRef]
- Loganathan, P.; Hedley, M.J.; Wallace, G.C.; Roberts, A.H.C. Fluoride accumulation in pasture forages and soils following long-term applications of phosphorus fertilizers. Environ. Pollut. 2001, 115, 275–282. [Google Scholar] [CrossRef]
- Yesilnacar, M.I.; Yetis, A.D.; Dülgergil, C.T.; Kumral, M.; Atasoy, A.D.; Dogan, T.R.; Tekiner, S.I.; Bayhan, I.; Aydogdu, M. Geomedical assessment of an area having high-fluoride groundwater in southeastern Turkey. Environ. Earth 2016, 75, 162–175. [Google Scholar] [CrossRef]
- Matraszek, R.; Hawrylak-Nowak, B.; Chwil, S.; Chwil, M. Macronutrient composition of nickel-treated wheat under different sulfur concentrations in the nutrient solution. Environ. Sci. Pollut. Res. 2016, 23, 5902–5914. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Fernández-Sanjurjo, M.J.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Fernández-Calviño, D. Perspectives on the use of by-products to treat soil and water pollution. Microporous Mesoporous Mater. 2015, 210, 199–201. [Google Scholar] [CrossRef]
- Quintáns-Fondo, A.; Ferreira-Coelho, G.; Paradelo-Núñez, R.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Promoting sustainability in the mussel industry: Mussel shell recycling to fight fluoride pollution. J. Clean. Prod. 2016, 131, 485–490. [Google Scholar] [CrossRef]
- Otero, M.; Cutillas-Barreiro, L.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Cr(VI) sorption/desorption on untreated and mussel-shell-treated soil materials: Fractionation and effects of pH and chromium concentration. Solid Earth 2015, 6, 373–382. [Google Scholar] [CrossRef]
- Seco-Reigosa, N.; Bermúdez-Couso, A.; Garrido-Rodríguez, B.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. As(V) retention on soils and forest by-products and other waste materials. Environ. Sci. Pollut. Res. 2013, 20, 6574–6583. [Google Scholar] [CrossRef] [PubMed]
- Seco-Reigosa, N.; Cutillas-Barreiro, L.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Mixtures including wastes from the mussel shell processing industry: Retention of arsenic, chromium and mercury. J. Clean. Prod. 2014, 84, 680–690. [Google Scholar] [CrossRef]
- Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Cutillas-Barreiro, L.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M. Cr(VI) Sorption/Desorption on Pine Sawdust and Oak Wood Ash. Int. J. Environ. Res. Public Health 2015, 12, 8849–8860. [Google Scholar] [CrossRef] [PubMed]
- Malik, D.S.; Jain, C.K.; Yadav, A.K. Removal of heavy metals from emerging cellulosic low-cost adsorbents: A review. Appl. Water Sci. 2016, 7, 1–24. [Google Scholar] [CrossRef]
- Searle, S.Y.; Malins, C.J. Waste and residue availability for advanced biofuel production in EU Member States. Biomass Bioenergy 2016, 89, 2–10. [Google Scholar] [CrossRef]
- Tye, Y.Y.; Lee, K.T.; Abdullah, W.N.W.; Leh, C.P. The world availability of non-wood lignocellulosic biomass for the production of cellulosic ethanol and potential pretreatments for the enhancement of enzymatic saccharification. Renew. Sustain. Energy Rev. 2016, 60, 155–172. [Google Scholar] [CrossRef]
- Coelho, G.F.; GonÇalves, A.C., Jr.; Nóvoa-Muñoz, J.C.; Fernández-Calviño, D.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Competitive and non-competitive cadmium, copper and lead sorption/desorption on wheat straw affecting sustainability in vineyards. J. Clean. Prod. 2016, 139, 1496–1503. [Google Scholar] [CrossRef]
- Doan, H.D.; Lohi, A.; Dang, V.B.H.; Dang-Vu, T. Removal of Zn+2 and Ni+2 by adsorption in a fixed bed of wheat straw. Process Saf. Environ. Prot. 2008, 86, 259–267. [Google Scholar] [CrossRef]
- Fernández, C.; Vega, J.A. Are erosion barriers and straw mulching effective for controlling soil erosion after a high severity wildfire in NW Spain? Ecol. Eng. 2016, 87, 132–138. [Google Scholar] [CrossRef]
- Vega, J.A.; Fernández, C.; Fonturbel, T. Comparing the effectiveness of seeding and mulching + seeding in reducing soil erosion after a high severity fire in Galicia (NW Spain). Ecol. Eng. 2015, 74, 206–212. [Google Scholar] [CrossRef]
- Dang, V.B.H.; Doan, H.D.; Dang-Vu, T.; Lohi, A. Equilibrium and kinetics of biosorption of cadmium(II) and copper(II) ions by wheat straw. Bioresour. Technol. 2009, 100, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Mahmood-ul-Hassan, M.; Suthar, V.; Rafique, E.; Ahmad, R.; Yasin, M. Kinetics of cadmium, chromium, and lead sorption onto chemically modified sugarcane bagasse and wheat straw. Environ. Monit. Assess. 2015, 187, 470. [Google Scholar] [CrossRef] [PubMed]
- Pehlivan, E.; Altun, T.; Parlayıcı, S. Utilization of barley straws as biosorbents for Cu2+ and Pb2+ ions. J. Hazard. Mater. 2009, 164, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Huang, D.; Zhu, H.; Zhu, Q.H.; Wang, J.Y.; Luo, Z.C.; Xu, C.; Shen, X.; He, Y.B. Effect of rice straw mulching on migration and transportation of Cd, Cu, Zn, and Ni in surface runoff under simulated rainfall. J. Soils Sediments 2016, 16, 2021–2029. [Google Scholar] [CrossRef]
- Fernández-Pazos, M.T.; Garrido-Rodríguez, B.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A.; Álvarez, E. Cr(VI) adsorption and desorption on soils and bio-sorbents. Water Air Soil Pollut. 2013, 224, 1–12. [Google Scholar] [CrossRef]
- Quintáns-Fondo, A.; Ferreira-Coelho, G.; Paradelo-Núñez, R.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. As(V)/Cr(VI) pollution control in soils, hemp waste and other by-products: Competitive sorption trials. Environ. Sci. Pollut. Res. 2016, 23, 19182–19192. [Google Scholar] [CrossRef] [PubMed]
- Paradelo, R.; Conde-Cid, M.; Arias-Estévez, M.; Nóvoa-Muñoz, J.C.; Álvarez-Rodríguez, E.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A. Removal of anionic pollutants by pine bark is influenced by the mechanism of retention. Chemosphere 2017, 167, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Seco-Reigosa, N.; Cutillas-Barreiro, L.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Álvarez-Rodríguez, E.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A. Adsorption, desorption and fractionation of As(V) on untreated and mussel shell-treated granitic material. Solid Earth 2015, 6, 337–346. [Google Scholar] [CrossRef]
- Gago, C.; Romar, A.; Fernández-Marcos, M.L.; Álvarez, E. Fluoride sorption and desorption on soils located in the surroundings of an aluminium smelter in Galicia (NW Spain). Environ. Earth Sci. 2014, 72, 4105–4114. [Google Scholar] [CrossRef]
- Castillo, A.M.N.; Soriano, J.J.; García-Delgado, R.A. Changes in chromium distribution during the electrodialytic remediation of Cr (VI)-contaminated soil. Environ. Geochem. Health 2008, 30, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Pittman, C.U. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Li, Q.; Gao, S.A.; Shang, J.K. Strong adsorption of arsenic species by amorphous zirconium oxide nanoparticles. J. Ind. Eng. Chem. 2012, 18, 1418–1427. [Google Scholar] [CrossRef]
- Ucun, H.; Bayhan, Y.K.; Kaya, Y.; Cakici, A.; Algur, O.F. Biosorption of chromium (VI) from aqueous solution by cone biomass of Pinus sylvestris. Bioresour. Technol. 2002, 85, 155–158. [Google Scholar] [CrossRef]
- Gago, C.; Romar, A.; Fernández-Marcos, M.L.; Álvarez, E. Fluorine sorption by soils developed from various parent materials in Galicia (NW Spain). J. Colloid Interface Sci. 2012, 374, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Valdivieso, A.L.; Bahena, J.L.R.; Song, S.; Urbina, R.H. Temperature effect on the zeta potential and fluoride adsorption at the α-Al2O3/aqueous solution interface. J. Colloid Interface Sci. 2006, 298, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Arnesen, A.K.M.; Krogstad, T. Sorption and desorption of fluoride in soil polluted from the aluminium smelter at Ardal in Western Norway. Water Air Soil Pollut. 1998, 103, 357–373. [Google Scholar] [CrossRef]
- Boddu, V.; Krishnaiah, A.; Talbot, J.; Smith, E. Removal of hexavalent chromium from wastewater using a new composite chitosan biosorbent. Environ. Sci. Technol. 2003, 37, 4449–4456. [Google Scholar] [CrossRef] [PubMed]
- Harrington, L.F.; Cooper, E.N.; Vasudevan, D. Fluoride sorption and associated aluminum release in variable charge soil. J. Colloid Interface Sci. 2003, 267, 302–313. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Mohan, D.; Sharma, R.; Sing, V.K.; Steele, P.; Pitman, C.U. Fluoride removal from water using bio-char, a Green waste, low-cost adsorbent: Equilibrium uptake and sorption dynamics modelling. Ind. Eng. Chem. Res. 2012, 51, 900–914. [Google Scholar] [CrossRef]
- Khezami, L.; Capart, R. Removal of chromium (VI) from aqueous solution by activated carbons: Kinetic and equilibrium studies. J. Hazard. Mater. 2005, 123, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Krishnami, K.K.; Meng, X.; Christodoulatos, B.; Boddum, V.M. Biosorption mechanism of nine different heavy metals onto biomatrix from rice husk. J. Hazard. Mater. 2008, 153, 1222–1234. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Pan, K.; Tariq, A.; Azizullah, A.; Sun, F.; Li, Z.; Xiong, Q. Adsorptive Removal of Toxic Chromium from Waste-Water Using Wheat Straw and Eupatorium adenophorum. PLoS ONE 2016, 11, e0167037. [Google Scholar] [CrossRef] [PubMed]
- Rebouh, S.; Bouhedda, M.; Hanini, S. Neuro-fuzzy modeling of Cu(II) and Cr(VI) adsorption from aqueous solution by wheat straw. Desalin. Water Treat. 2015, 57, 1–16. [Google Scholar] [CrossRef]
- Booran, S.K.; Doan, H.D.; Lohi, A. Recovery of Zn(II) and Ni(II) Binary from Wastewater Using Integrated Biosorption and Electrodeposition. Clean Soil Air Water 2015, 43, 368–374. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Niazi, N.K.; Bibi, I.; Murtaza, G.; Kunhikrishnan, A.; Seshadri, B.; Shahid, M.; Ali, S.; Bolan, N.S.; Ok, Y.S.; et al. Remediation of arsenic-contaminated water using agricultural wastes as biosorbents. Crit. Rev. Environ. Sci. Technol. 2016, 46, 467–499. [Google Scholar] [CrossRef]
- Farooq, U.; Kozinski, J.A.; Khan, M.A.; Athar, M. Biosorption of heavy metal ions using wheat based biosorbents—A review of the recent literature. Bioresour. Technol. 2010, 101, 5043–5053. [Google Scholar] [CrossRef] [PubMed]
- Lingamdinne, L.P.; Chang, Y.Y.; Yang, J.K.; Singh, J.; Choi, E.H.; Shiratani, M.; Koduru, J.R.; Attri, P. Biogenic reductive preparation of magnetic inverse spinel iron oxide nanoparticles for the adsorption removal of heavy metals. Chem. Eng. J. 2017, 307, 74–84. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Lingamdinne, L.P.; Koduru, J.R.; Jyothi, R.K.; Chang, Y.Y.; Yang, J.K. Factors affect on bioremediation of Co(II) and Pb(II) onto Lonicera japonica flowers powder. Desalin. Water Treat. 2016, 57, 13066–13080. [Google Scholar] [CrossRef]
- Lesmana, S.O.; Febriana, N.; Soetaredjo, F.E.; Sunarso, J.; Ismadji, S. Studies on potential applications of biomass for the separation of heavy metals from water and wastewater. Biochem. Eng. J. 2009, 44, 19–41. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Babalola, O.O. A New Strategy for Heavy Metal Polluted Environments: A Review of Microbial Biosorbents. Int. J. Environ. Res. Public Health 2017, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
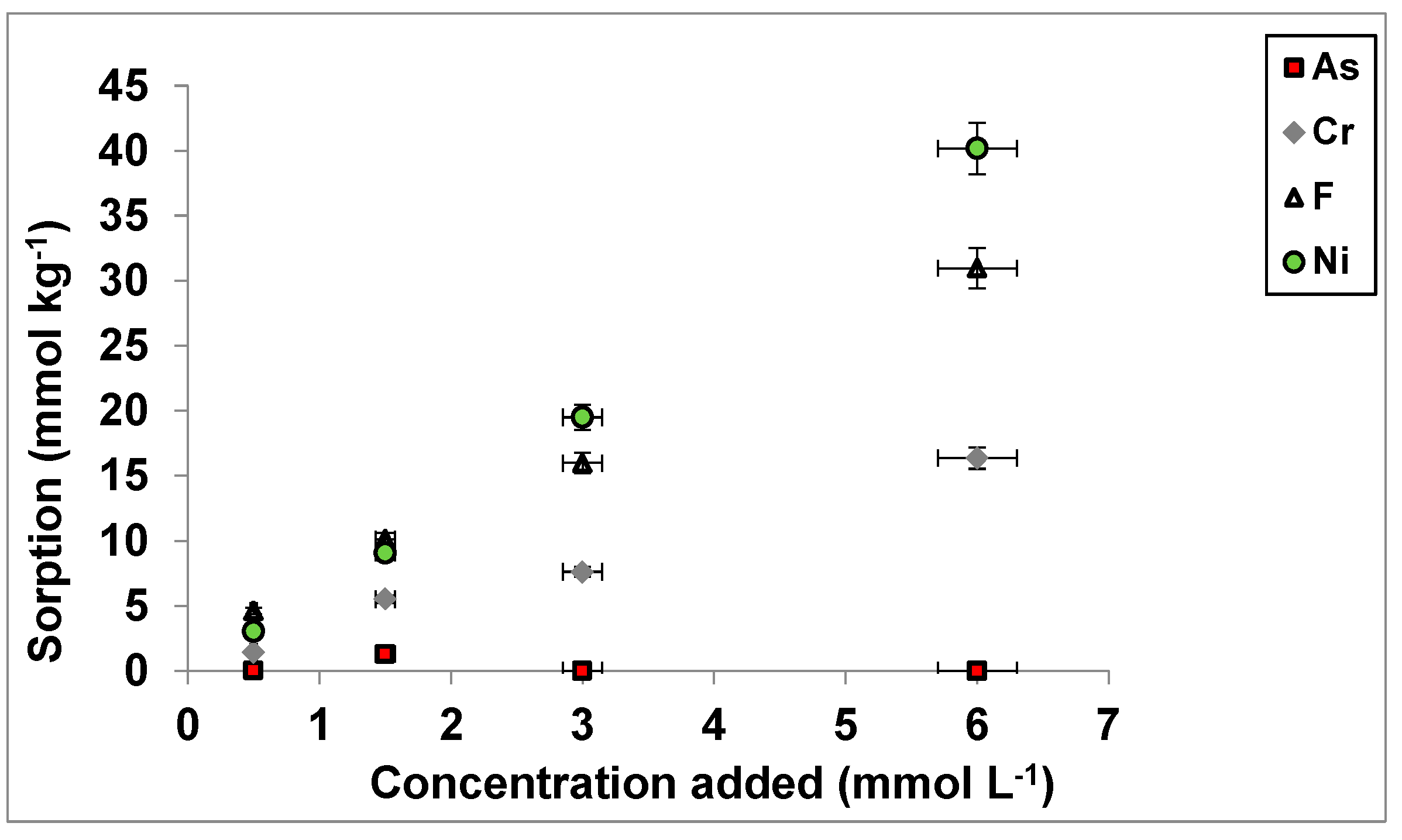
| C0 | 0.5 | 1.5 | 3.0 | 6.0 |
|---|---|---|---|---|
| Sorption (%) | ||||
| As(V) | 0.3 | 8.5 | 0 | 0 |
| Cr(VI) | 29.1 | 36.9 | 25.5 | 27.4 |
| F− | 93.4 | 68.4 | 54.1 | 52.3 |
| Ni2+ | 61.3 | 60.7 | 65.2 | 67.5 |
| C0 | As(V) | Cr(VI) | F− | Ni2+ |
|---|---|---|---|---|
| mmol·L−1 | pH | |||
| 0.5 | 7.0 (±0.1) | 6.2 (±0.1) | 8.0 (±0.2) | 5.5 (±0.1) |
| 1.5 | 7.4 (±0.1) | 6.3 (±0.1) | 7.9 (±0.2) | 5.7 (±0.1) |
| 3 | 7.5 (±0.1) | 6.4 (±0.1) | 7.1 (±0.1) | 5.8 (±0.1) |
| 6 | 7.6 (±0.2) | 6.6 (±0.1) | 7.1 (±0.1) | 5.9 (±0.1) |
| mmol·L−1 | DOC (mg·L−1) | |||
| 0.5 | 423.2 (±11.5) | 256.4 (±8.3) | 337.8 (±9.2) | 289.5 (±8.7) |
| 1.5 | 494.3 (±14.2) | 272.3 (±8.7) | 355.4 (±9.4) | 213.6 (±6.8) |
| 3.0 | 486.0 (±13.8) | 298.3 (±9.1) | 379.9 (±10.1) | 192.2 (±5.9) |
| 6.0 | 487.6 (±13.6) | 319.3 (±9.4) | 403.3 (±11.6) | 158.6 (±5.1) |
| Pollutant | KF (mmol·kg−1) | n | R2 |
|---|---|---|---|
| As | - | - | - |
| Cr | 4.3 ± 0.7 | 0.89 ± 0.13 | 0.978 |
| F | 15.1 ± 1.7 | 0.65 ± 0.12 | 0.970 |
| Ni | 18.2 ± 0.3 | 1.19 ± 0.03 | 0.999 |
| C0 | As | Cr | F | Ni |
|---|---|---|---|---|
| mmol·L−1 | Desorption | |||
| 0.5 | 0.17 (1.74) | 0.14 (1.39) | 0.23 (2.24) | 0.10 (0.99) |
| 1.5 | 0.60 (5.99) | 0.46 (4.59) | 0.55 (5.40) | 0.28 (2.81) |
| 3.0 | - | 0.92 (9.19) | 1.58 (15.65) | 0.53 (5.31) |
| 6.0 | - | 2.29 (22.76) | 3.51 (34.67) | 1.06 (10.51) |
| C0 | As | Cr | F | Ni |
|---|---|---|---|---|
| mmol·L−1 | pH | |||
| 0.5 | 6.24 (±0.1) | 5.74 (±0.1) | 5.45 (±0.1) | 5.51 (±0.1) |
| 1.5 | 6.14 (±0.1) | 5.77 (±0.1) | 5.54 (±0.1) | 5.65 (±0.1) |
| 3.0 | - | 6.16 (±0.1) | 5.6 (±0.1)0 | 5.50 (±0.1) |
| 6.0 | - | 6.35 (±0.1) | 6.19 (±0.2) | 5.8 (±0.1) |
| C0 | As | Cr | F | Ni |
| mmol·L−1 | DOC (mg·L−1) | |||
| 0.5 | 194.7 (±8.4) | 108.5 (±4.1) | 114.7 (±3.8) | 111.2 (±2.7) |
| 1.5 | 249.2 (±9.6) | 121.0 (±4.3) | 127.6 (±3.2) | 114.2 (±3.1) |
| 3.0 | - | 216.3 (±6.9) | 135.0 (±4.2) | 115.5 (±2.9) |
| 6.0 | - | 249.0 (±6.7) | 168.9 (±4.9) | 80.8 (±1.2) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romar-Gasalla, A.; Coelho, G.F.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Wheat Straw as a Bio-Sorbent for Arsenate, Chromate, Fluoride, and Nickel. Water 2017, 9, 690. https://doi.org/10.3390/w9090690
Romar-Gasalla A, Coelho GF, Nóvoa-Muñoz JC, Arias-Estévez M, Fernández-Sanjurjo MJ, Álvarez-Rodríguez E, Núñez-Delgado A. Wheat Straw as a Bio-Sorbent for Arsenate, Chromate, Fluoride, and Nickel. Water. 2017; 9(9):690. https://doi.org/10.3390/w9090690
Chicago/Turabian StyleRomar-Gasalla, Aurora, Gustavo F. Coelho, Juan Carlos Nóvoa-Muñoz, Manuel Arias-Estévez, María J. Fernández-Sanjurjo, Esperanza Álvarez-Rodríguez, and Avelino Núñez-Delgado. 2017. "Wheat Straw as a Bio-Sorbent for Arsenate, Chromate, Fluoride, and Nickel" Water 9, no. 9: 690. https://doi.org/10.3390/w9090690
APA StyleRomar-Gasalla, A., Coelho, G. F., Nóvoa-Muñoz, J. C., Arias-Estévez, M., Fernández-Sanjurjo, M. J., Álvarez-Rodríguez, E., & Núñez-Delgado, A. (2017). Wheat Straw as a Bio-Sorbent for Arsenate, Chromate, Fluoride, and Nickel. Water, 9(9), 690. https://doi.org/10.3390/w9090690









