Nutrients and Phytoplankton in a Shallow, Hypereutrophic Urban Lake: Prospects for Restoration
Abstract
:1. Introduction
2. Study Site
3. Materials and Methods
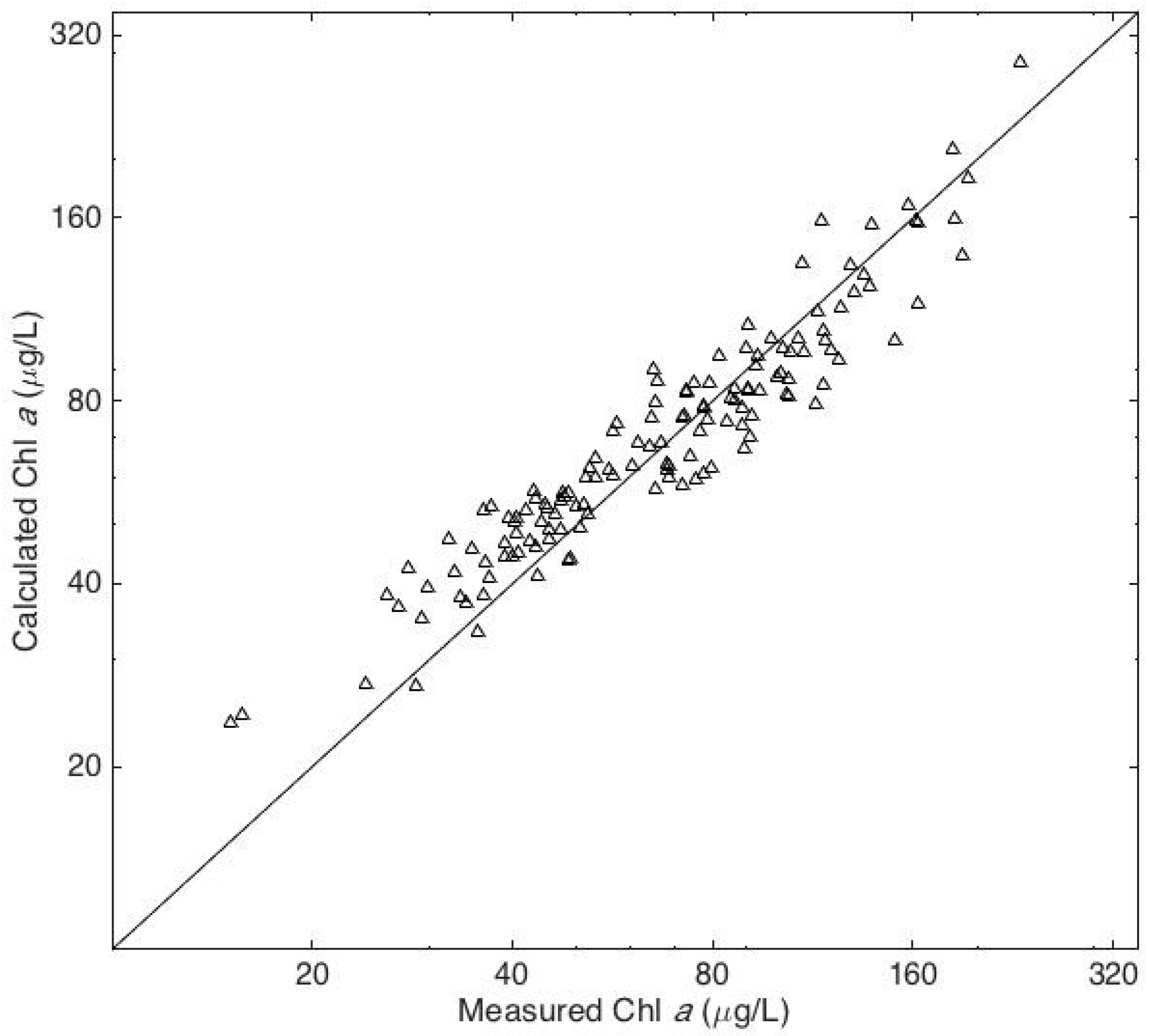
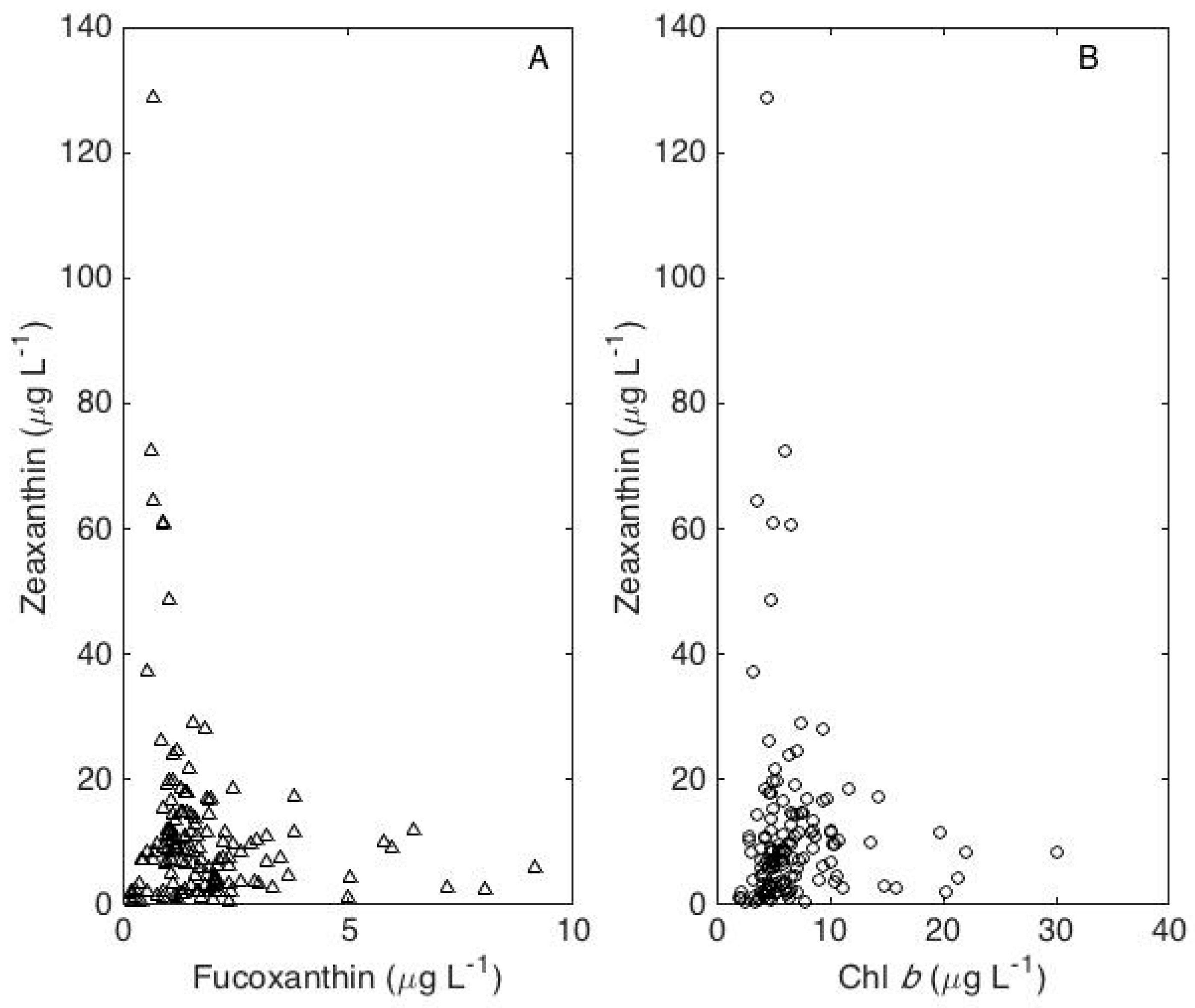
4. Results
5. Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Welch, E.B. The eventual recovery of Lake Sammamish following phosphorus diversion. J. Water Pollut. Control Fed. 1985, 57, 977–978. [Google Scholar]
- Effler, S.W.; Brooks, C.M.; Whitehead, K.A. Domestic waste inputs of nitrogen and phosphorus to Onondaga Lake, and water quality implications. Lake Reserv. Manag. 1996, 12, 127–140. [Google Scholar] [CrossRef]
- Effler, S.W.; Hennigan, R.D. Onondaga Lake, New York: Legacy of pollution. Lake Reserv. Manag. 1996, 12, 1–13. [Google Scholar] [CrossRef]
- Edmondson, W.T.; Anderson, G.C.; Peterson, D.R. Artificial eutrophication of Lake Washington. Limnol. Oceanogr. 1956, 1, 47–53. [Google Scholar] [CrossRef]
- Welch, E.B.; Michaud, J.P.; Perkins, M.A. Alum control of internal phosphorus loading in a shallow lake. Water Resour. Bull. 1982, 18, 929–936. [Google Scholar] [CrossRef]
- Welch, E.B.; Rock, C.A.; Howe, R.C.; Perkins, M.A. Lake Sammamish response to waste-water diversion and increasing urban runoff. Water Res. 1980, 14, 821–828. [Google Scholar] [CrossRef]
- Vanderdoes, J.; Verstraelen, P.; Boers, P.; Vanroestel, J.; Roijackers, R.; Moser, G. Lake restoration with and without dredging of phosphorus-enriched upper sediment layers. Hydrobiologia 1992, 233, 197–210. [Google Scholar] [CrossRef]
- James, R.T.; Pollman, C.D. Sediment and nutrient management solutions to improve the water quality of Lake Okeechobee. Lake Reserv. Manag. 2011, 27, 28–40. [Google Scholar] [CrossRef]
- Knaus, R.; Malone, R. A historical overview of a successful lakes restoration project in Baton Rouge, Louisiana. Lake Reserv. Manag. 1984, 1, 412–415. [Google Scholar] [CrossRef]
- Norris, B. Composition and Physiological Changes of the University Lake Ecosystem Phytoplankton Community: Impacts of Seasonal and Episodic Events. Master’s Thesis, Environmental Sciences, Louisiana State University, Baton Rouge, LA, USA, 2012. [Google Scholar]
- Anonymous. The LSU Lakes are Dying. Can They be Saved? 225 Magazine, January 2010; 33–37. [Google Scholar]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Lagoutte, B.; Tunkelrott, M.-L.; Feraudet-Tarisse, C.; Dano, J.; Volland, H. Fast and Direct Extraction of Cell-associated Hepatotoxins from Toxic Cyanobacteria. Water Environ. Res. 2014, 86, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Mesmer, R. Impact of Urban Runoff on Phosphorus, Nitrogen, and Dissolved Oxygen in a Shallow Subtropical Lake. Master’s Thesis, Renewable Natural Resources, Louisiana State University, Baton Rouge, LA, USA, 2010. [Google Scholar]
- Bidigare, R.R.; Van Heukelem, L.; Trees, C.C. Analysis of algal pigments by high-performance liquid chromatography. In Algal Culturing Techniques; Andersen, R.A., Ed.; Academic Press: New York, NY, USA, 2005; pp. 327–345. [Google Scholar]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analysis, 2nd ed.; Bulletins of the Fisheries Research Board of Canada; Fisheries Research Board of Canada: Ottawa, ON, Canada, 1972; Volume 167, p. 310. [Google Scholar]
- Chalup, M.S.; Laws, E.A. A test of the assumptions and predictions of recent microalgal growth models with the marine phytoplankter Pavlova lutheri. Limnol. Oceanogr. 1990, 35, 583–596. [Google Scholar] [CrossRef]
- Laws, E.A.; Redalje, D.G.; Karl, D.M.; Chalup, M.S. A theoretical and experimental examination of the predictions of two recent models of phytoplankton growth. J. Theor. Biol. 1983, 105, 469–491. [Google Scholar] [CrossRef]
- Laws, E.A. Photosynthetic quotients, new production and net community production in the open ocean. Deep Sea Res. I 1991, 38, 143–167. [Google Scholar] [CrossRef]
- Letelier, R.M.; Bidigare, R.R.; Hebel, D.V.; Ondrusek, M.; Winn, C.D.; Karl, D.M. Temporal variability of phytoplankton community structure based on pigment analysis. Limnol. Oceanogr. 1993, 38, 1420–1437. [Google Scholar] [CrossRef]
- Paerl, H.W.; Valdes, L.M.; Pinckney, J.L.; Piehler, M.F.; Dyble, J.; Moisander, P.H. Phytoplankton photopigments as indicators of estuarine and coastal eutrophication. Bioscience 2003, 53, 953–964. [Google Scholar] [CrossRef]
- Smith, G.M. The Freshwater Algae of the United States, 2nd ed.; McGraw-Hill: New York, USA, 1950; p. 719. [Google Scholar]
- Descy, J.; Sarmiento, H.; Wiggins, H. Variability of phytoplankton pigment ratios across aquatic environments. Eur. J. Phycol. 2009, 44, 319–330. [Google Scholar] [CrossRef]
- Hutchinson, G.E. Volume II: Introduction to Lake Biology and the Limnoplankton. In A Treatise on Limnology; John Wiley & Sons: New York, NY, USA, 1967; Volume 2, p. 1115. [Google Scholar]
- Riemann, B.; Simonsen, P.; Stensgaard, L. The carbon and chlorophyll content of phytoplankton from various nutrient regimes. J. Plankton Res. 1989, 11, 1037–1045. [Google Scholar] [CrossRef]
- Laws, E.A.; Bannister, T.T. Nutrient-and light-limited growth of Thalassiosira fluviatilis in continuous culture, with implications for phytoplankton growth in the ocean. Limnol. Oceanogr. 1980, 25, 457–473. [Google Scholar] [CrossRef]
- Pinckney, J.L.; Richardson, T.L.; Millie, D.F.; Paerl, H.W. Application of photopigment biomarkers for quantifying microalgal community composition and in situ growth rates. Org. Geochem. 2001, 32, 585–595. [Google Scholar] [CrossRef]
- Atlas, D.A.; Bannister, T.T. Dependence of mean spectral extinction coefficient of phytoplankton on depth, water color, and species. Limnol. Oceanogr. 1980, 25, 157–159. [Google Scholar] [CrossRef]
- Rabalais, N.N.; Diaz, R.J.; Levin, L.A.; Turner, R.E.; Gilbert, D.; Zhang, J. Dynamics and distribution of natural and human-caused hypoxia. Biogeosciences 2010, 7, 585–619. [Google Scholar] [CrossRef]
- Sunda, W.G.; Hardison, D.R. Ammonium uptake and growth limitation in marine phytoplankton. Limnol. Oceanogr. 2007, 52, 2496–2506. [Google Scholar] [CrossRef]
- Harrison, P.J.; Conway, H.L.; Dugdale, R.C. Marine diatoms grown in chemostats under silicate or ammonium limitation.1. Cellular chemical composition and steady-state growth kinetics of Skeletonema costatum. Mar. Biol. 1976, 35, 177–186. [Google Scholar] [CrossRef]
- Laws, E.A.; Pei, S.; Bienfang, P.; Grant, S. Phosphate-limited growth and uptake kinetics of the marine prasinophyte Tetraselmis suecica (Kylin) Butcher. Aquaculture 2011, 322–323, 117–121. [Google Scholar] [CrossRef]
- Laws, E.A.; Pei, S.F.; Bienfang, P.; Grant, S.; Sunda, W.G. Phosphate-limited growth of Pavlova lutheri (prymnesiophyceae) in continuous culture: Determination of growth-rate-limiting substrate concentrations with a sensitive bioassay procedure. J. Phycol. 2011, 47, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 26, 31–36. [Google Scholar] [CrossRef]
- Thomson-Bulldis, A.L.; Karl, D. Application of a novel method for phosphorus determinations in the oligotrophic North Pacific Ocean. Limnol. Oceanogr. 1998, 43, 1565–1577. [Google Scholar] [CrossRef]
- Schindler, D.W. Factors regulating phytoplankton production and standing crop in the world’s freshwaters. Limnol. Oceanogr. 1978, 23, 478–486. [Google Scholar] [CrossRef]
- Schindler, D.W. Evolution of phosphorus limitation in lakes. Science 1977, 195, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Laws, E.A. Food-web structure and planktonic predator-prey relationships in two eutrophic European lakes: Stability constraints on carbon fluxes. Limnol. Oceanogr. 2008, 53, 760–772. [Google Scholar] [CrossRef]
- Caperon, J. Time lag in population growth response of Isochrysis galbana to a variable nitrate environment. Ecology 1969, 50, 188–192. [Google Scholar] [CrossRef]
- Edmondson, W.T.; Lehman, J.T. The effect of changes in the nutrient income on the condition of Lake Washington. Limnol. Oceanogr. 1981, 26, 1–29. [Google Scholar] [CrossRef]
- Edmondson, W.T. Eutrophication effects on the food chains of lakes. Memorie dell’Istituto Italiano di Idrobiologia 1993, 52, 113–132. [Google Scholar]
- Cooke, G.D.; Welch, E.B.; Peterson, S.A.; Nichols, S.A. Restoration and Management of Lakes and Reservoirs, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2005; p. 616. [Google Scholar]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norris, B.; Laws, E.A. Nutrients and Phytoplankton in a Shallow, Hypereutrophic Urban Lake: Prospects for Restoration. Water 2017, 9, 431. https://doi.org/10.3390/w9060431
Norris B, Laws EA. Nutrients and Phytoplankton in a Shallow, Hypereutrophic Urban Lake: Prospects for Restoration. Water. 2017; 9(6):431. https://doi.org/10.3390/w9060431
Chicago/Turabian StyleNorris, Brianne, and Edward A. Laws. 2017. "Nutrients and Phytoplankton in a Shallow, Hypereutrophic Urban Lake: Prospects for Restoration" Water 9, no. 6: 431. https://doi.org/10.3390/w9060431
APA StyleNorris, B., & Laws, E. A. (2017). Nutrients and Phytoplankton in a Shallow, Hypereutrophic Urban Lake: Prospects for Restoration. Water, 9(6), 431. https://doi.org/10.3390/w9060431




