Climate Variability Structures Plant Community Dynamics in Mediterranean Restored and Reference Tidal Wetlands
Abstract
:1. Introduction
2. Materials and Methods
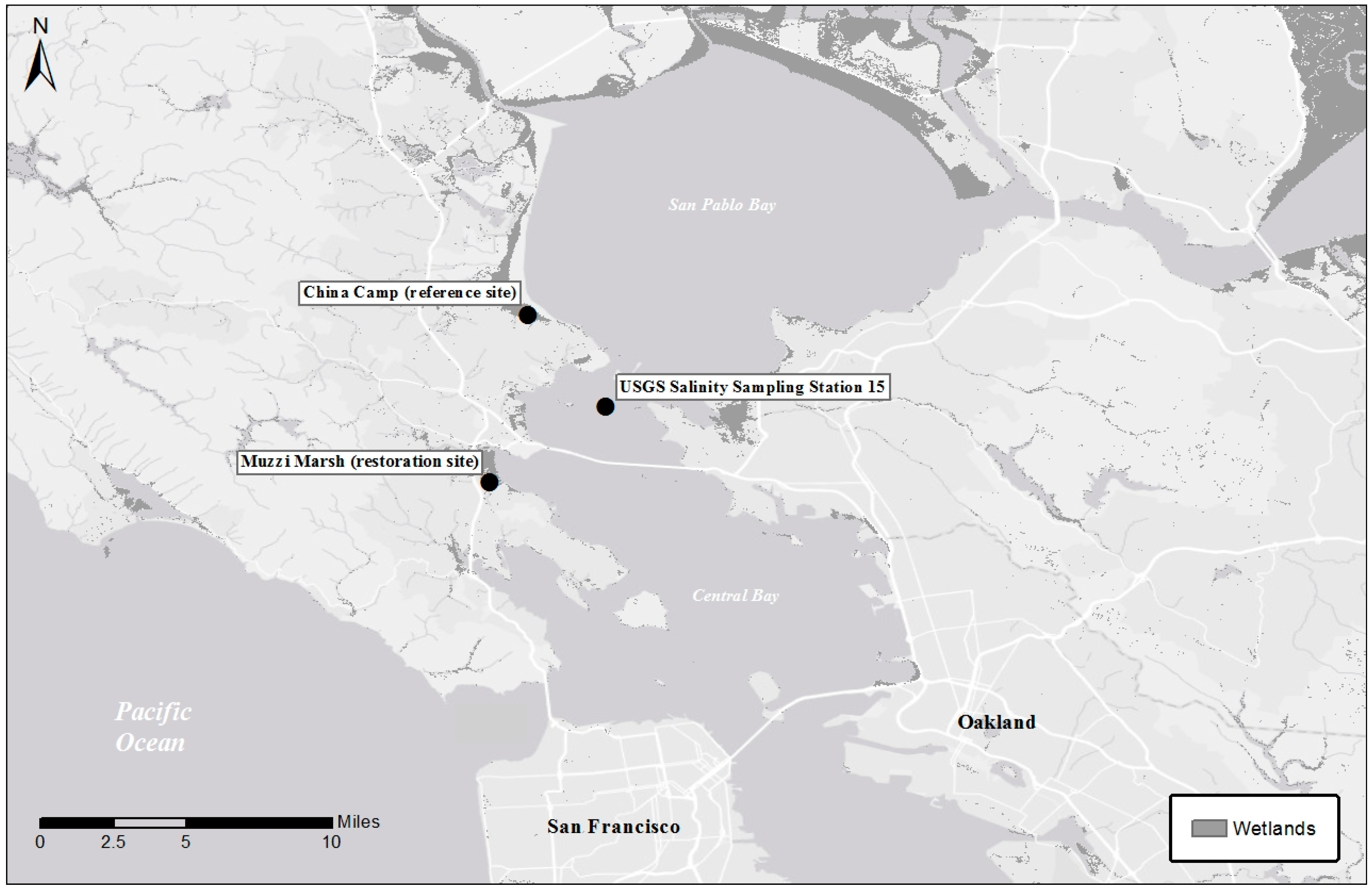
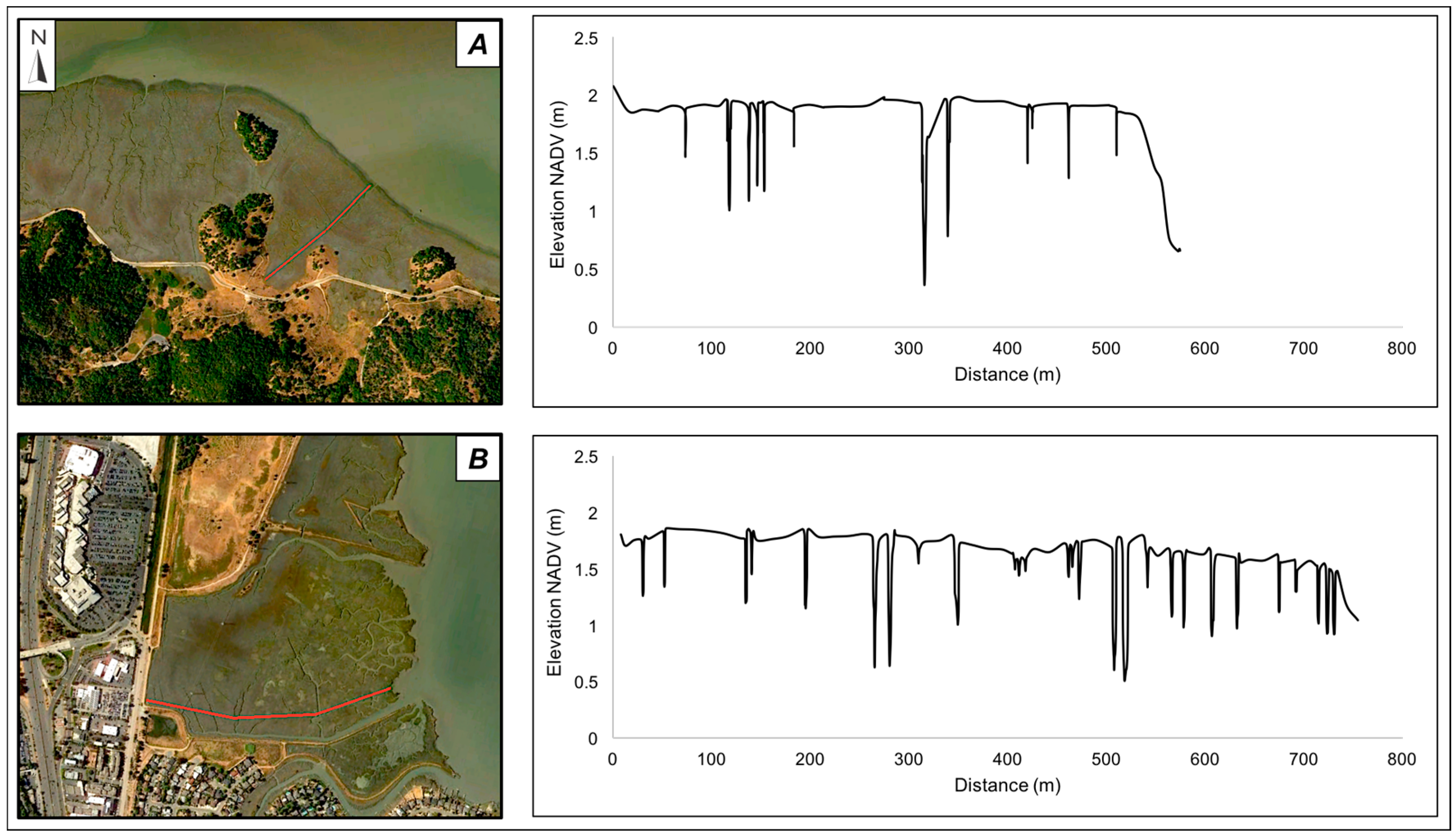
2.1. Sites
2.2. Field Data Collection
2.3. Salinity and Rainfall
2.4. Data Analysis
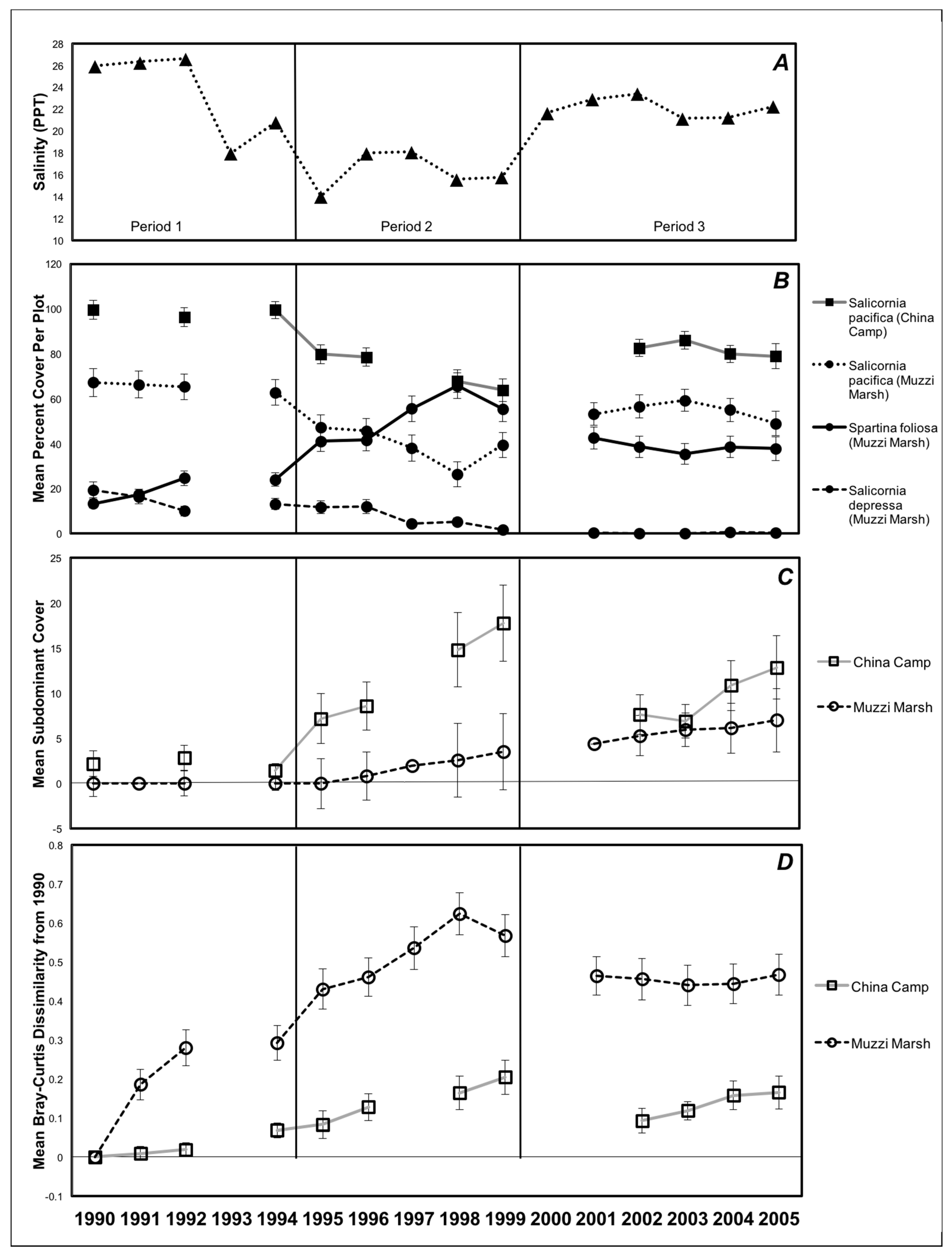
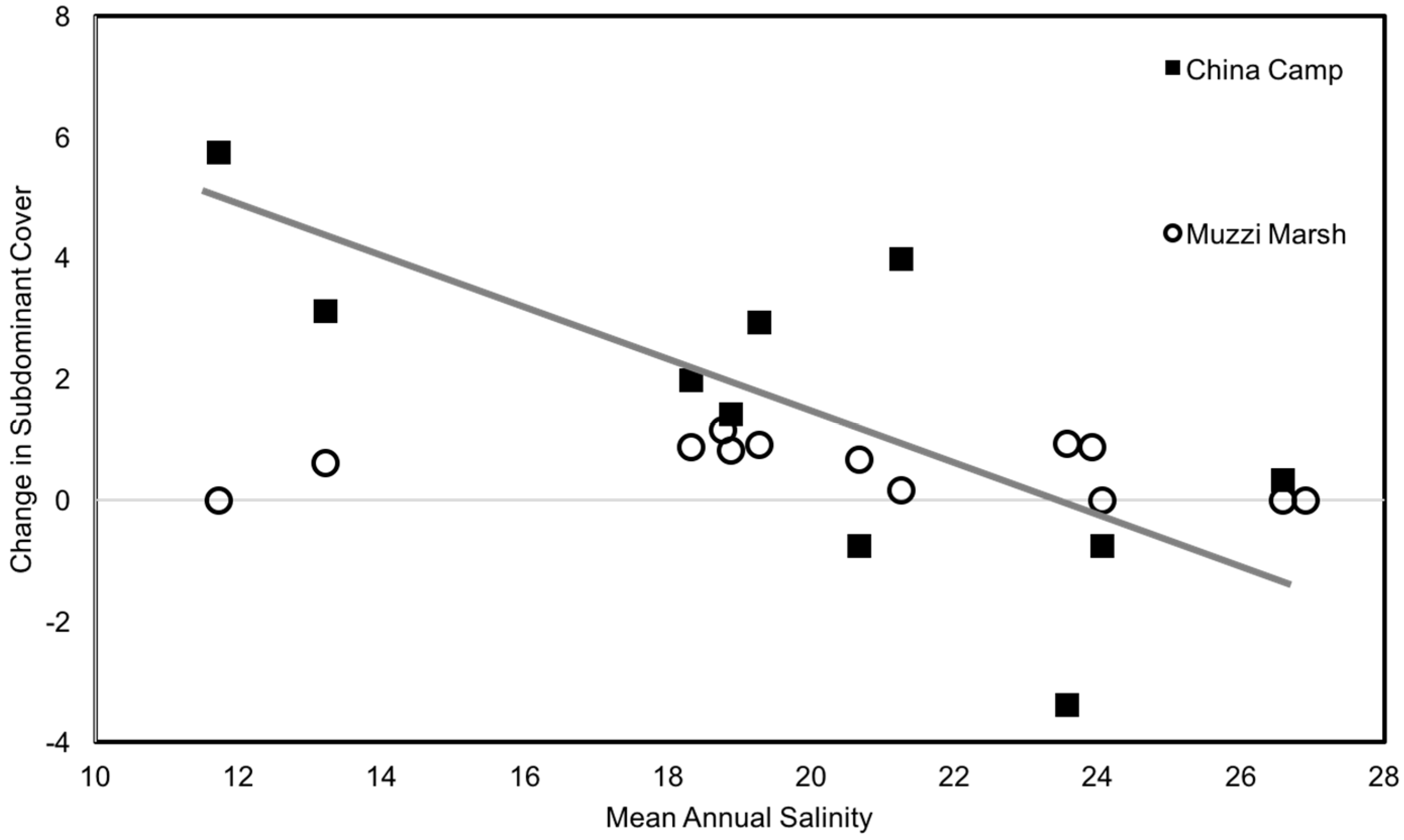
3. Results
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| SADE | SPFO | SAPA | DISP | JACA | FRSA | GRST | BOMA | Sub Dom Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | CC | MM | CC | MM | CC | MM | CC | MM | CC | MM | CC | MM | CC | MM | CC | MM | CC | MM |
| 1990 | 0.00 | 19.46 | 3.53 | 13.35 | 94.19 | 67.19 | 1.13 | 0.00 | 0.54 | 0.00 | 0.59 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 | 2.28 | 0.00 |
| 1991 | 0.00 | 16.43 | 17.30 | 66.27 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||||||
| 1992 | 0.00 | 10.04 | 3.83 | 24.67 | 93.64 | 65.29 | 1.04 | 0.00 | 0.60 | 0.00 | 0.89 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.53 | 0.00 |
| 1994 | 0.00 | 13.21 | 4.41 | 24.04 | 94.15 | 62.76 | 0.40 | 0.00 | 1.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.44 | 0.00 |
| 1995 | 0.00 | 11.75 | 4.55 | 41.06 | 89.14 | 47.19 | 4.91 | 0.00 | 0.65 | 0.00 | 0.28 | 0.00 | 0.48 | 0.00 | 0.00 | 0.00 | 6.31 | 0.00 |
| 1996 | 0.00 | 11.98 | 3.83 | 41.56 | 88.06 | 45.64 | 3.78 | 0.82 | 1.11 | 0.00 | 0.28 | 0.00 | 2.22 | 0.00 | 0.72 | 0.00 | 7.39 | 0.82 |
| 1997 | 0.00 | 4.47 | 55.61 | 38.06 | 1.81 | 0.00 | 0.00 | 0.05 | 0.00 | 1.86 | ||||||||
| 1998 | 0.00 | 5.24 | 4.34 | 65.73 | 81.35 | 26.40 | 9.52 | 2.08 | 1.12 | 0.00 | 0.22 | 0.00 | 3.44 | 0.55 | 0.00 | 0.00 | 14.31 | 2.63 |
| 1999 | 0.00 | 1.64 | 3.18 | 55.50 | 79.51 | 39.39 | 8.17 | 2.95 | 2.21 | 0.00 | 0.53 | 0.00 | 4.04 | 0.53 | 2.35 | 0.00 | 14.95 | 3.48 |
| 2001 | 0.00 | 0.37 | 42.57 | 53.17 | 2.69 | 0.00 | 0.18 | 1.01 | 0.00 | 3.88 | ||||||||
| 2002 | 0.00 | 0.11 | 3.83 | 38.65 | 89.53 | 56.51 | 1.41 | 3.14 | 2.03 | 0.13 | 0.59 | 0.25 | 2.59 | 1.21 | 0.00 | 0.00 | 6.63 | 4.73 |
| 2003 | 0.00 | 0.11 | 5.31 | 35.36 | 88.08 | 59.21 | 2.30 | 3.24 | 2.24 | 0.26 | 0.61 | 0.34 | 1.19 | 1.48 | 0.28 | 0.00 | 6.33 | 5.32 |
| 2004 | 0.00 | 0.71 | 2.61 | 38.55 | 87.08 | 55.06 | 3.17 | 4.60 | 3.03 | 0.18 | 0.92 | 0.20 | 2.20 | 0.71 | 1.00 | 0.00 | 9.31 | 5.68 |
| 2005 | 0.00 | 0.31 | 3.01 | 41.01 | 87.20 | 53.01 | 2.34 | 3.77 | 2.90 | 0.13 | 0.14 | 0.67 | 2.73 | 1.11 | 1.67 | 0.00 | 8.12 | 5.68 |
| 2015 | 0.00 | 0.00 | 2.71 | 31.67 | 94.49 | 61.45 | 0.36 | 4.94 | 1.44 | 1.43 | 0.46 | 0.24 | 0.55 | 0.27 | 0.00 | 0.00 | 2.81 | 6.88 |
| Period | p | Estimate | SE | t | Df | |
|---|---|---|---|---|---|---|
| Reference Site (China Camp) | ||||||
| 1 (1990–1994) | Year | 0.570 | −0.002 | 0.003 | −0.565 | 5 |
| Channel | 0.959 | −0.001 | 0.023 | −0.050 | 5 | |
| 2 (1994–1999) | Year | <0.000 * | 0.031 | 0.005 | 6.361 | 5 |
| Channel | 0.006 * | 0.163 | 0.058 | 2.839 | 5 | |
| 3 (1999–2005) | Year | 0.015 * | −0.009 | 0.004 | −2.461 | 5 |
| Channel | 0.006 * | 0.166 | 0.059 | 2.809 | 5 | |
| Total (1990–2005) | Year | <0.000 * | 0.006 | 0.001 | 4.981 | 5 |
| Channel | 0.006 * | 0.129 | 0.046 | 2.823 | 5 | |
| Restoration Site (Muzzi Marsh) | ||||||
| 1 (1990–1994) | Year | na | na | na | na | 5 |
| Channel | na | na | na | na | 5 | |
| 2 (1994–1999) | Year | <0.000 * | 0.0076 | 0.002 | 3.954 | 5 |
| Channel | 0.0847 | 0.025 | 0.015 | 1.713 | 5 | |
| 3 (1999–2005) | Year | <0.000 * | 0.006 | 0.002 | 3.865 | 5 |
| Channel | 0.0032 * | 0.105 | 0.035 | 3.025 | 5 | |
| Total (1990–2005) | Year | <0.000 * | 0.005 | 0.001 | 8.084 | 5 |
| Channel | 0.007 * | 0.05148 | 0.019 | 2.747 | 5 |
| Period | p | Estimate | SE | t | Df | |
|---|---|---|---|---|---|---|
| Reference Site (China Camp) | ||||||
| 1 (1990–1994) | Year | 0.089 | 0.034 | 0.020 | 1.694 | 5 |
| Channel | 0.014 * | 0.047 | 0.019 | 2.455 | 5 | |
| 2 (1994–1999) | Year | <0.000 * | 0.155 | 0.0287 | 5.394 | 5 |
| Channel | 0.022 * | 0.127 | 0.055 | 2.312 | 5 | |
| 3 (1999–2005) | Year | 0.650 | 0.023 | 0.007 | 3.381 | 5 |
| Channel | 0.046 * | 0.104 | 0.055 | 1.871 | 5 | |
| Total (1990–2005) | Year | <0.000 * | 0.055 | 0.010 | 5.776 | 5 |
| Channel | 0.001 * | 0.085 | 0.026 | 3.31 | 5 | |
| Restoration Site (Muzzi Marsh) | ||||||
| 1 (1990–1994) | Year | <0.000 * | 0.212 | 0.027 | 7.854 | 5 |
| Channel | 0.453 | 0.030 | 0.041 | 0.742 | 5 | |
| 2 (1994–1999) | Year | <0.000 * | 0.370 | 0.032 | 11.42 | 5 |
| Channel | 0.519 | 0.053 | 0.084 | 0.632 | 5 | |
| 3 (1999–2005) | Year | <0.000 * | −0.199 | 0.048 | −4.18 | 5 |
| Channel | 0.636 | 0.040 | 0.087 | 0.464 | 5 | |
| Total (1990–2005) | Year | <0.000 * | 0.174 | 0.011 | 16.456 | 5 |
| Channel | 0.034 * | 0.085 | 0.040 | 2.115 | 5 |
References
- Williams, P.B.; Orr, M.K. Physical evolution of restored breached levee salt marshes in the San Francisco Bay estuary. Restor. Ecol. 2002, 10, 527–542. [Google Scholar] [CrossRef]
- Brand, L.A.; Smith, L.M.; Takekawa, J.Y.; Athearn, N.D.; Taylor, K.; Shellenbarger, G.G.; Schoellhamer, D.H.; Spenst, R. Trajectory of early tidal marsh restoration: Elevation, sedimentation and colonization of breached salt ponds in the northern San Francisco Bay. Ecol. Eng. 2012, 42, 19–29. [Google Scholar] [CrossRef]
- Boyer, K.E.; Thornton, W.J. Natural and Restored Tidal Marsh Communities. In Ecology, Conservation, and Restoration of Tidal Marshes; University of California Press: Berkeley, CA, USA, 2012; pp. 233–252. [Google Scholar]
- Silliman, B.R.; Schrack, E.; He, Q.; Cope, R.; Santoni, A.; Van Der Heide, T.; Jacobi, R.; Jacobi, M.; Van De Koppel, J. Facilitation shifts paradigms and can amplify coastal restoration efforts. Proc. Natl. Acad. Sci. USA 2015, 112, 14295–14300. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, M.; Scheffer, M. El Niño as a window of opportunity for the restoration of degraded arid ecosystems. Ecosystems 2001, 4, 151–159. [Google Scholar] [CrossRef]
- Holmgren, M.; Stapp, P.; Dickman, C.R.; Gracia, C.; Graham, S.; Gutiérrez, J.R.; Hice, C.; Jaksic, F.; Kelt, D.A.; Letnic, M.; et al. Extreme climatic events shape arid and semiarid ecosystems. Front. Ecol. Environ. 2006, 4, 87–95. [Google Scholar] [CrossRef]
- Vaughn, K.J.; Young, T.P. Contingent conclusions: Year of initiation influences ecological field experiments, but temporal replication is rare. Restor. Ecol. 2010, 18, 59–64. [Google Scholar] [CrossRef]
- Matthews, J.W.; Peralta, A.L.; Flanagan, D.N.; Baldwin, P.M.; Soni, A.; Kent, A.D.; Endress, A.G. Relative influence of landscape vs. local factors on plant community assembly in restored wetlands. Ecol. Appl. 2009, 19, 2108–2123. [Google Scholar] [CrossRef] [PubMed]
- Grman, E.; Bassett, T.; Brudvig, L.A. Confronting contingency in restoration: Management and site history determine outcomes of assembling prairies, but site characteristics and landscape context have little effect. J. Appl. Ecol. 2013, 50, 1234–1243. [Google Scholar] [CrossRef]
- Grman, E.; Bassett, T.; Zirbel, C.R.; Brudvig, L.A. Dispersal and establishment filters influence the assembly of restored prairie plant communities. Restor. Ecol. 2015, 23, 892–899. [Google Scholar] [CrossRef]
- Brudvig, L.A. The restoration of biodiversity: Where has research been and where does it need to go? Am. J. Bot. 2011, 98, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Mateos, D.; Power, M.E.; Comín, F.A.; Yockteng, R. Structural and functional loss in restored wetland ecosystems. PLoS Biol. 2012, 10, e1001247. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Mateos, D.; Maris, V.; Béchet, A.; Curran, M. The true loss caused by biodiversity offsets. Biol. Conserv. 2015, 192, 552–559. [Google Scholar] [CrossRef]
- Zedler, J.B.; Callaway, J.C. Tracking wetland restoration: Do mitigation sites follow desired trajectories? Restor. Ecol. 1999, 7, 69–73. [Google Scholar] [CrossRef]
- Dettinger, M.D.; Cayan, D.R. Interseasonal covariability of Sierra Nevada streamflow and San Francisco Bay salinity. J. Hydrol. 2003, 277, 164–181. [Google Scholar] [CrossRef]
- Dettinger, M.D.; Ralph, F.M.; Das, T.; Neiman, P.J.; Cayan, D.R. Atmospheric rivers, floods and the water resources of California. Water 2011, 3, 445–478. [Google Scholar] [CrossRef]
- Bêche, L.A.; Connors, P.G.; Resh, V.H.; Merenlender, A.M. Resilience of fishes and invertebrates to prolonged drought in two California streams. Ecography 2009, 32, 778–788. [Google Scholar] [CrossRef]
- Cleland, E.E.; Collins, S.L.; Dickson, T.L.; Farrer, E.C.; Gross, K.L.; Gherardi, L.A.; Hallett, L.M.; Hobbs, R.J.; Hsu, J.S.; Turnbull, L.; et al. Sensitivity of grassland plant community composition to spatial vs. temporal variation in precipitation. Ecology 2013, 94, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Sitters, J.; Holmgren, M.; Stoorvogel, J.J.; López, B.C. Rainfall-Tuned Management Facilitates Dry Forest Recovery. Restor. Ecol. 2012, 20, 33–42. [Google Scholar] [CrossRef]
- Suding, K.N.; Gross, K.L.; Houseman, G.R. Alternative states and positive feedbacks in restoration ecology. Trends Ecol. Evol. 2004, 19, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, S.; Briske, D.D.; Wu, X.B.; McClaran, M.P.; Bestelmeyer, B.T.; Fernández-Giménez, M.E. Empirical assessment of state-and-transition models with a long-term vegetation record from the Sonoran Desert. Ecol. Appl. 2012, 22, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Collinge, S.K.; Ray, C.; Marty, J.T. A Long-Term Comparison of Hydrology and Plant Community Composition in Constructed Versus Naturally Occurring Vernal Pools. Restor. Ecol. 2013, 21, 704–712. [Google Scholar] [CrossRef]
- Zedler, J.B.; Doherty, J.M.; Miller, N.A. Shifting Restoration Policy to Address Landscape Change, Novel Ecosystems, and Monitoring. Ecol. Soc. 2012, 17, 36. [Google Scholar] [CrossRef]
- Bernhardt, E.S.; Sudduth, E.B.; Palmer, M.A.; Allan, J.D.; Meyer, J.L.; Alexander, G.; Follastad-Shah, J.; Hassett, B.; Jenkinson, R.; Lave, R. Restoring rivers one reach at a time: Results from a survey of US river restoration practitioners. Restor. Ecol. 2007, 15, 482–493. [Google Scholar] [CrossRef]
- Kondolf, G.M.; Anderson, S.; Lave, R.; Pagano, L.; Merenlender, A.; Bernhardt, E.S. Two decades of river restoration in California: What can we learn? Restor. Ecol. 2007, 15, 516–523. [Google Scholar] [CrossRef]
- Dudney, J.; Hallett, L.M.; Larios, L.; Farrer, E.C.; Spotswood, E.N.; Stein, C.; Suding, K.N. Lagging behind: Have we overlooked previous-year rainfall effects in annual grasslands? J. Ecol. 2016. [Google Scholar] [CrossRef]
- Dettinger, M.D. From climate-change spaghetti to climate-change distributions for 21st Century California. San Franc. Estuary Watershed Sci. 2005, 3. Available online: https://escholarship.org/uc/item/2pg6c039# (accessed on 10 March 2017). [Google Scholar]
- Zedler, J.B. Freshwater impacts in normally hypersaline marshes. Estuaries 1983, 6, 346–355. [Google Scholar] [CrossRef]
- Zedler, J.B.; Covin, J.; Nordby, C.; Williams, P.; Boland, J. Catastrophic events reveal the dynamic nature of salt-marsh vegetation in Southern California. Estuaries 1986, 9, 75–80. [Google Scholar] [CrossRef]
- Noe, G.B.; Zedler, J.B. Spatio-temporal variation of salt marsh seedling establishment in relation to the abiotic and biotic environment. J. Veg. Sci. 2001, 12, 61–74. [Google Scholar] [CrossRef]
- Callaway, R.M.; Sabraw, C.S. Effects of variable precipitation on the structure and diversity of a California salt marsh community. J. Veg. Sci. 1994, 5, 433–438. [Google Scholar] [CrossRef]
- Shumway, S.W.; Bertness, M.D. Salt stress limitation of seedling recruitment in a salt marsh plant community. Oecologia 1992, 92, 490–497. [Google Scholar] [CrossRef]
- Zedler, J.B.; Morzaria-Luna, H.; Ward, K. The challenge of restoring vegetation on tidal, hypersaline substrates. Plant Soil 2003, 253, 259–273. [Google Scholar] [CrossRef]
- Watson, E.B. Changing elevation, accretion, and tidal marsh plant assemblages in a South San Francisco Bay tidal marsh. Estuaries 2004, 27, 684–698. [Google Scholar] [CrossRef]
- Levin, L.A.; Talley, T.S. Natural and manipulated sources of heterogeneity controlling early faunal development of a salt marsh. Ecol. Appl. 2002, 12, 1785–1802. [Google Scholar] [CrossRef]
- Nomann, B.E.; Pennings, S.C. Fiddler crab–vegetation interactions in hypersaline habitats. J. Exp. Mar. Biol. Ecol. 1998, 225, 53–68. [Google Scholar] [CrossRef]
- Bertness, M.D. Fiddler Crab Regulation of Spartina alterniflora Production on a New England Salt Marsh. Ecology 1985, 66, 1042–1055. [Google Scholar] [CrossRef]
- Bortolus, A.; Schwindt, E.; Iribarne, O. Positive plant-animal interactions in the high marsh of an Argentinean coastal lagoon. Ecology 2002, 83, 733–742. [Google Scholar]
- Callaway, J.C.; Thomas Parker, V.; Vasey, M.C.; Schile, L.M. Emerging issues for the restoration of tidal marsh ecosystems in the context of predicted climate change. Madroño 2007, 54, 234–248. [Google Scholar] [CrossRef]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P.; et al. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Sanderson, E.W.; Ustin, S.L.; Foin, T.C. The influence of tidal channels on the distribution of salt marsh plant species in Petaluma Marsh, CA, USA. Plant Ecol. 2000, 146, 29–41. [Google Scholar] [CrossRef]
- Morzaria-Luna, L.; Callaway, J.C.; Sullivan, G.; Zedler, J.B. Relationship between topographic heterogeneity and vegetation patterns in a Californian salt marsh. J. Veg. Sci. 2004, 15, 523–530. [Google Scholar] [CrossRef]
- Armitage, A.R.; Boyer, K.E.; Vance, R.R.; Ambrose, R.F. Restoring assemblages of salt marsh halophytes in the presence of a rapidly colonizing dominant species. Wetlands 2006, 26, 667–676. [Google Scholar] [CrossRef]
- Diggory, Z.E.; Parker, V.T. Seed Supply and Revegetation Dynamics at Restored Tidal Marshes, Napa River, California. Restor. Ecol. 2011, 19, 121–130. [Google Scholar] [CrossRef]
- Brudvig, L.A.; Damschen, E.I.; Tewksbury, J.J.; Haddad, N.M.; Levey, D.J. Landscape connectivity promotes plant biodiversity spillover into non-target habitats. Proc. Natl. Acad. Sci. USA 2009, 106, 9328–9332. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.; Faber, P. Salt marsh restoration experience in San Francisco Bay. J. Coast. Res. 2001, 27, 203–211. [Google Scholar]
- Faber, P.M. Design Guidelines for Tidal Wetland Restoration in San Francisco Bay; Phillip Williams and Associates, LTD.: San Francisco, CA, USA, 2004. [Google Scholar]
- Cloern, J.E.; Schraga, T.S. USGS Measurements of Water Quality in San Francisco Bay (CA), 1969–2015, Version 2: U.S. Geological Survey Release; U.S. Geologic Survey: Reston, VA, USA, 2016. [Google Scholar]
- National Climate Data Center Climate Data Online; US National Oceanic and Atmospheric Administration: Silver Spring, MD, USA, 2017. Available online: https://www.ncdc.noaa.gov/cdo-web/datasets (accessed on 6 September 2016).
- McCune, B.; Grace, J.B.; Urban, D.L. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002; Volume 28. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H.; et al. Package “Vegan”. Community Ecol. Package Version 2.4.2. 2013. Available online: www.r-project.org (accessed on 12 March 2017).
- Hastie, T. GAM: Generalized Additive Models, R Package, Version 0.98. R Foundation for Statistical Computing: Vienna, Austria, 2013. Available online: www.r-project.org (accessed on 10 March 2017).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S.; Christensen, R.H.B.; Singmann, H.; Dai, B.; Grothendieck, G.; Green, P. lme4: Linear Mixed-Effects Models using “Eigen” and S4. J. Stat. Softw. 2016, 67, 1–48. [Google Scholar]
- Ward, K.M.; Callaway, J.C.; Zedler, J.B. Episodic colonization of an intertidal mudflat by native cordgrass (Spartina foliosa) at Tijuana Estuary. Estuar. Coasts 2003, 26, 116–130. [Google Scholar] [CrossRef]
- Scheffer, M.; Bascompte, J.; Brock, W.A.; Brovkin, V.; Carpenter, S.R.; Dakos, V.; Held, H.; van Nes, E.H.; Rietkerk, M.; Sugihara, G. Early-warning signals for critical transitions. Nature 2009, 461, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Suding, K.N.; Hobbs, R.J. Threshold models in restoration and conservation: A developing framework. Trends Ecol. Evol. 2009, 24, 271–279. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.L.; Zedler, J.B. Accelerating the restoration of vegetation in a southern California salt marsh. Wetl. Ecol. Manag. 2006, 14, 269–286. [Google Scholar] [CrossRef]
- Zedler, J.B.; Callaway, J.C.; Desmond, J.S.; Vivian-Smith, G.; Williams, G.D.; Sullivan, G.; Brewster, A.E.; Bradshaw, B.K. Californian salt-marsh vegetation: An improved model of spatial pattern. Ecosystems 1999, 2, 19–35. [Google Scholar] [CrossRef]
- Zedler, J.B. Success: An unclear, subjective descriptor of restoration outcomes. Ecol. Restor. 2007, 25, 162–168. [Google Scholar] [CrossRef]
- Moss, B.M. Freshwater reference states, and the mitigation of climate change. Freshw. Biol. 2015, 60, 1964–1976. [Google Scholar] [CrossRef]
- Levine, C.R.; Yanai, R.D.; Lampman, G.G.; Burns, D.A.; Driscoll, C.T.; Lawrence, G.B.; Lynch, J.A.; Schoch, N. Evaluating the efficiency of environmental monitoring programs. Ecol. Indic. 2014, 39, 94–101. [Google Scholar] [CrossRef]
- Goals Project. The Baylands and Climate Change: What We Can Do. Baylands Ecosystem Habitat Goals Science Update 2015; San Francisco Bay Area Wetlands Ecosystem Goals Project, California State Coastal Conservancy: Oakland, CA, USA, 2015. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chapple, D.E.; Faber, P.; Suding, K.N.; Merenlender, A.M. Climate Variability Structures Plant Community Dynamics in Mediterranean Restored and Reference Tidal Wetlands. Water 2017, 9, 209. https://doi.org/10.3390/w9030209
Chapple DE, Faber P, Suding KN, Merenlender AM. Climate Variability Structures Plant Community Dynamics in Mediterranean Restored and Reference Tidal Wetlands. Water. 2017; 9(3):209. https://doi.org/10.3390/w9030209
Chicago/Turabian StyleChapple, Dylan E., Phyllis Faber, Katharine N. Suding, and Adina M. Merenlender. 2017. "Climate Variability Structures Plant Community Dynamics in Mediterranean Restored and Reference Tidal Wetlands" Water 9, no. 3: 209. https://doi.org/10.3390/w9030209
APA StyleChapple, D. E., Faber, P., Suding, K. N., & Merenlender, A. M. (2017). Climate Variability Structures Plant Community Dynamics in Mediterranean Restored and Reference Tidal Wetlands. Water, 9(3), 209. https://doi.org/10.3390/w9030209





