Abstract
This study integrates molecular dynamics (MD) simulations and density functional theory (DFT) computations to elucidate the unique adsorption characteristics of phenol and para-chlorophenol onto sepiolite by examining structural deformation, electronic properties, and adsorption energetics. The hydroxyl group (-OH) of phenol mainly determines its adsorption process since it has a quite negative Mulliken charge (−0.428) and significant electrophilic reactivity (fi+ = 0.090), therefore enabling strong hydrogen bonding with the silanol (-SiOH) groups of sepiolite. By π-π interactions with the electron-rich siloxane (-Si-O-Si-) surfaces, the aromatic carbons in phenol improve stability. The close molecular structure allows minimum deformation energy (Edef = 94.18 kcal/mol), hence optimizing alignment with the sepiolite surface. The much negative adsorption energy (Eads = −349.26 kcal/mol) of phenol supports its further thermodynamic stability. Conversely, because of its copious chlorine (-Cl) component, para-chlorophenol runs against steric and electrical obstacles. The virtually neutral Mulliken charge (−0.020) limits electrostatic interactions even if the chlorine atom shows great electrophilicity (fi+ = 0.278). Chlorine’s electron-withdrawing action lowers the hydroxyl group’s (fi+ = 0.077) reactivity, hence lowering hydrogen bonding. Moreover, para-chlorophenol shows strong deformation energy (Edef = 102.33 kcal/mol), which causes poor alignment and less access to high-affinity sites. With less negative than phenol, the adsorption energy for para-chlorophenol (Eads = −317.53 kcal/mol) indicates its reduced thermodynamic affinity. Although more evident in para-chlorophenol because of the polarizable chlorine atom, van der Waals interactions do not balance its steric hindrance and reduced electrostatic interactions. With a maximum Qmax = 0.78 mmol/g, isotherm models confirm the remarkable adsorption capability of phenol in contrast to Qmax = 0.66 mmol/g for para-chlorophenol. By hydrogen bonding and π-cation interactions, phenol builds a dense and structured adsorption layer, and para-chlorophenol shows a chaotic organization with reduced site use. Supported by computational approaches and experimental validation, the results provide a comprehensive knowledge of adsorption mechanisms and provide a basis for the design of adsorbents catered for particular organic pollutants.
1. Introduction
Water pollution from organic contaminants has emerged as a significant global concern due to its serious environmental and health consequences. Phenol and its halogenated derivatives, notably para-chlorophenol, are particularly alarming pollutants due to their extensive industrial application and persistence in aquatic habitats. These compounds are frequently present in effluents from industries like pharmaceuticals, petrochemicals, and agriculture, posing considerable ecological threats and public health dangers [1,2,3]. Their toxicity, resistance to biodegradation, and propensity for bioaccumulation require the creation of effective removal technologies from wastewater. Adsorption is one of the most efficient and adaptable methods for eliminating organic contaminants from water. It is preferred for its simplicity, cost efficiency, and capacity to address a broad spectrum of pollutants [4,5,6]. The efficacy of the adsorption process is significantly influenced by the selection of adsorbent. Natural clays, including sepiolite, have garnered significant interest owing to their elevated surface area, distinctive structural characteristics, and chemical stability [7,8]. Sepiolite is a fibrous magnesium silicate clay adsorbent, distinguished by its microporous channels and many silanol (-SiOH) groups, rendering it an exceptional option for the adsorption of polar and nonpolar organic molecules [9,10]. Several comprehensive studies have been conducted on sepiolite as an adsorbent, but the chemical mechanisms governing its interactions with certain organic molecules are still inadequately understood. Phenol and para-chlorophenol are exemplary model compounds for examining these pathways because of their differing structural and electrical characteristics. The small size of phenol and its hydroxyl (-OH) group promote strong hydrogen bonding and electrostatic interactions, whereas the bulky chlorine (-Cl) substituent in para-chlorophenol causes steric hindrance and electronic polarization effects [11,12]. These distinctions render them significant for comprehending how molecule shape, electronic characteristics, and steric variables affect adsorption behavior.
Progress in computational chemistry, especially density functional theory (DFT) and molecular dynamics (MD) simulations has allowed researchers to elucidate the atomic-level interactions that dictate adsorption processes [13,14]. Integrating these computational methods with experimental adsorption research enables a more thorough comprehension of adsorption mechanisms. Fukui indices and Mulliken charge assessments obtained from DFT calculations are important in identifying reactive sites on both the adsorbate and adsorbent, while MD simulations provide valuable information regarding adsorption energetics and structural dynamics [15,16].
This work seeks to reconcile theoretical approaches by examining the adsorption mechanisms of phenol and para-chlorophenol on sepiolite. Using a synthesis of DFT calculations, MD simulations, and isotherm modeling, we clarify the impact of electrical characteristics, molecule structure, and steric parameters on adsorption efficiency. These findings further the overarching objective of enhancing adsorbent design for the effective elimination of organic contaminants from wastewater.
2. Materials and Methods
2.1. Adsorbent Model Preparation
The adsorbent model, as shown in Figure 1, was prepared using Biovia Materials Studio 2020, by cleaving the sepiolite structure along the (110) plane, a surface chosen for its high reactivity and extensive use in adsorption studies [9]. The bulk structure of sepiolite was constructed based on experimentally derived lattice parameters [1], with orthorhombic unit cell dimensions of a = 13.4 Å, b = 26.8 Å, c =5.28 Å and angles α = β = γ = 90°. Atomic positions were assigned according to crystallographic data for Mg8Si12O30(OH)4. (H2O)4∙8H2O [17] captured the fibrous channels formed by Mg-O-Si chains. To ensure sufficient surface area and reduce periodic boundary artifacts, the unit cell was scaled into a supercell by extending it U = 4 along the a-axis and V = 1 along the b-axis, resulting in overall dimensions of 53.6 Å × 26.8 Å × 5.28 Å. The scaling along the a-axis was specifically implemented to increase surface area while minimizing interactions between adsorbates, preserving the natural periodicity of the (110) plane through the choice of V = 1. To isolate the slab from periodic images and prevent artificial interactions, a vacuum layer of 30 Å was introduced along the c-axis. The crystal slab was modeled with a thickness of 30 Å, equivalent to approximately five-unit cells along the c-axis. This slab was symmetrically centered within the simulation box to eliminate edge effects and maintain structural alignment.
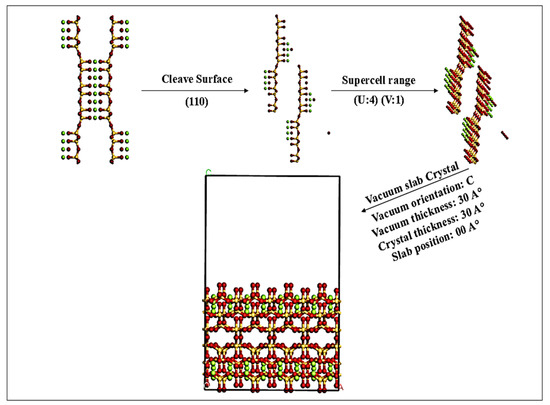
Figure 1.
The sepiolite adsorbent model preparation.
2.2. DFT Computational Details
The molecular structures of phenol and para-chlorophenol were investigated using density functional theory (DFT) with the B3LYP hybrid functional and the 6-311G basis set [18,19], selecting of B3LYP based on its extensively benchmarked against experimental thermochemical and spectroscopic data for organic and hydrogen-bonded systems, striking a reliable balance between accuracy and computational cost. The 6-311G basis set ensures that key features such as charge distribution and hydrogen-bond interactions are well described without the prohibitive expense of larger basis sets [20,21], producing optimized geometries illustrated in Figure 2. All DFT calculations were performed using the Gaussian 09W software package, with Gauss View 5 employed for analyzing and visualizing frontier molecular orbitals (FMO) [22]. Key calculated electronic parameters, including the energies of the highest occupied (EHOMO) and lowest unoccupied molecular orbitals (ELUMO), facilitated the computation of several global reactivity descriptors. These descriptors encompass the energy gap (ΔE), ionization energy (I), electron affinity (A), chemical potential (μ), hardness (η), softness (S), electronegativity (χ), global electrophilicity index (ω), nucleophilicity (ε), and back-donation energy (ΔE back-donation) [23,24,25,26], as derived from equations in Table 1.
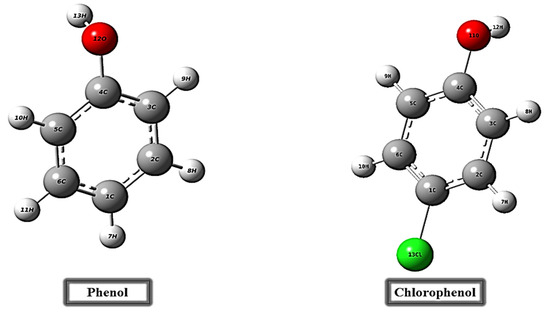
Figure 2.
The molecular structures of phenol and para-chlorophenol.

Table 1.
DFT computational equations.
To evaluate the local reactivity indicators of both compounds, we focused on the analysis of partial Mulliken atomic charges, electrostatic potential maps, the spatial distributions of HOMO and LUMO orbitals, and Fukui indices.
2.3. Adsorption MD Simulation Preparation
The molecular dynamics simulations were carried out using the COMPASSIII force field to ensure an accurate representation of interactions. Electrostatic forces were calculated using the Ewald summation method, while van der Waals interactions were handled through the atom-based summation approach [27,28]. To determine the most energetically favorable adsorption sites, the Adsorption Locator tool was employed, which identified low-energy configurations. Additionally, the sorption module was used to simulate adsorption isotherms across a range of fugacity conditions using the configurational bias Monte Carlo method [29]. The simulations were performed under the NVT ensemble at a temperature of 298 K, with a Berendsen thermostat maintaining thermal stability throughout the process. A cubic simulation box with dimensions of 30 Å × 30 Å × 30 Å was used to ensure adequate solvation of the sepiolite slab and the adsorbates. The solvent density was set to 1.0 g/cm3, replicating bulk water conditions; the system contained 500 water molecules, maintaining a neutral medium at a pH of 7. This choice was made to prevent competitive hydrogen adsorption on the sepiolite surface under acidic conditions and to avoid the deprotonation of phenol and chlorophenol, which would lead to the formation of phenolate and para-chlorophenolate species in the basic medium. Such species are known to introduce electrostatic repulsion with the negatively charged sepiolite surface, potentially reducing adsorption efficiency [30,31]. Previous experimental studies have demonstrated that the highest adsorption efficiency for phenol and para-chlorophenol occurs in neutral media (pH 6–7). This approach allowed for precise modeling of the adsorption behavior of phenol and para-chlorophenol on the sepiolite surface, providing comprehensive insights into the underlying mechanisms governing these interactions.
3. Results
3.1. DFT Results and Analysis
To gain a deeper understanding of the electronic properties and reactivity of phenol and para-chlorophenol, density functional theory (DFT) calculations were conducted. The analysis focuses on the frontier molecular orbitals, global reactivity descriptors, and related electronic parameters, as summarized in Figure 3 and Table 2. The electronic properties of phenol and para-chlorophenol were analyzed using density functional theory (DFT) to understand their potential adsorption behavior on sepiolite. The parameters derived from the HOMO-LUMO energies and reactivity indices are presented in Table 2. Phenol has a slightly larger HOMO-LUMO gap (ΔEgap = 6.352 eV) compared to para-chlorophenol (ΔEgap = 6.077 eV), which could suggest that phenol may exhibit lower polarizability and a more stable electronic structure. This characteristic might favor strong, directional interactions such as hydrogen bonding. In contrast, the chlorine substituent in para-chlorophenol raises the HOMO energy (−6.586 eV compared to −6.783 eV for phenol), potentially enhancing its electron-donating ability. Its lower LUMO energy (−0.509 eV versus −0.431 eV for phenol) indicates an improved ability to accept electrons. These factors might allow para-chlorophenol to interact with electron-deficient sites more effectively [32,33]. Regarding global reactivity indices, phenol shows slightly higher electronegativity (χ = 3.607 vs. 3.547 for para-chlorophenol), which could indicate a stronger overall attraction to electron-rich sites. However, para-chlorophenol exhibits higher softness (S = 0.33 eV vs. 0.31 eV for phenol) and lower chemical hardness (η = 3.038 eV vs. 3.176 eV for phenol), suggesting greater polarizability and a potentially more adaptable electronic structure. These characteristics may facilitate interactions with diverse adsorption sites but could also imply reduced stability in specific configurations [34,35]. Both molecules demonstrate properties that could influence their adsorption behavior in different ways. The electronic structure of phenol may favor more stable and directional interactions, while para-chlorophenol’s greater polarizability and electron-donating capacity could allow for adaptable and varied adsorption interactions.
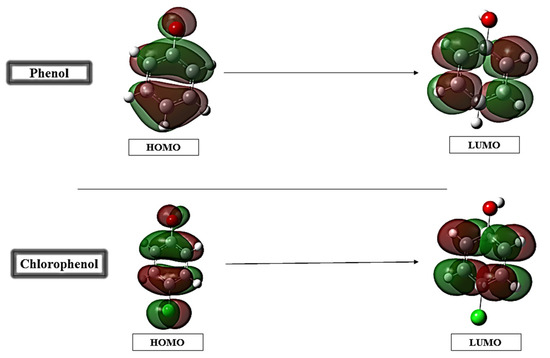
Figure 3.
Frontier molecular orbitals of phenol and para-chlorophenol.

Table 2.
The parameters derived from DFT equations.
3.2. Molecular Electrostatic Potential (MEP) Maps
The Molecular Electrostatic Potential (MEP) maps, shown in Figure 4, reveal the electrostatic distribution over the molecular surfaces of phenol and para-chlorophenol, highlighting regions critical for reactivity. The color gradient represents electron density: red for electron-rich regions prone to electrophilic attack, blue for electron-deficient areas susceptible to nucleophilic attack, and green or yellow for balanced regions with minimal reactivity [36,37]. For phenol, the oxygen atom in the hydroxyl group is surrounded by a prominent red region, indicating its electron-rich nature and strong potential for hydrogen bonding or electrostatic interactions. The hydroxyl hydrogen appears blue, reflecting its electron-deficient character, making it a site for nucleophilic interactions. The aromatic ring shows delocalized π-electron density, shaded green or yellow, suggesting limited reactivity but possible involvement in van der Waals or π-cation interactions. In para-chlorophenol, the chlorine atom reduces the electron density of the hydroxyl oxygen, weakening its hydrogen bonding potential. The aromatic ring displays noticeable polarization, with a slight reddening near the para-carbon due to resonance effects and blue shifts on adjacent positions. This asymmetry alters the molecule’s reactivity, introducing steric and electronic effects that moderate its interaction strength compared to phenol. Phenol’s stronger electron-rich regions make it more reactive for hydrogen bonding and electrostatic interactions, whereas para-chlorophenol’s reactivity is reduced due to steric hindrance, electron-withdrawing, and polarization effects caused by the chlorine substituent. This contrast directly influences their adsorption behavior on sepiolite.
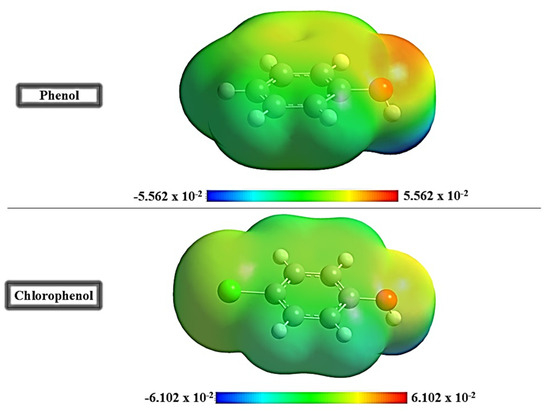
Figure 4.
The Molecular Electrostatic Potential (MEP) maps of phenol and para-chlorophenol.
3.3. Mulliken Charges and Fukui Indices
Mulliken charges and Fukui indices are critical descriptors for identifying reactive sites in molecules. They allow for an evaluation of the electrophilic and nucleophilic tendencies at specific atoms, providing insights into how these molecules interact with their environment. The results of the Mulliken charge analysis and the Fukui indices (fi+, fi−, fi°) for phenol and para-chlorophenol are presented in Figure 5 and Table 3. Typically, the atom with the highest fi+ value is preferred for nucleophilic attack (acceptance of electron), while the atom with the highest fi− value is favored for electrophilic attack (donation of electron) [38,39].
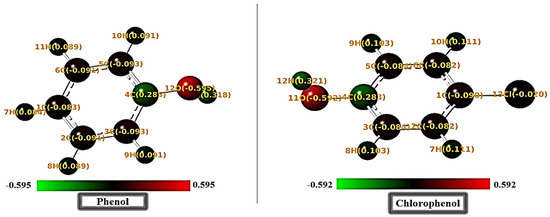
Figure 5.
Mulliken charges structural map for phenol and para-chlorophenol.

Table 3.
The Mulliken charge analysis and the Fukui indices (fi+, fi−, fi°) for phenol and para-chlorophenol.
For the hydroxyl oxygen, 12O in phenol has a charge of −0.428 and an electrophilic Fukui index (f⁺) of 0.090, indicating moderate electrophilic reactivity and a capacity for hydrogen bonding. In contrast, the hydroxyl oxygen of para-chlorophenol, 11O, exhibits a more negative charge of −0.592 and a lower f⁺ value of 0.077, suggesting reduced hydrogen bonding potential. The enhanced negative charge on 11O might favor electrostatic interactions, but steric hindrance from the chlorine substituent could limit effective binding. The chlorine atom, 13Cl, in para-chlorophenol acts as an electrophilic hotspot, with a significant f⁺ value of 0.278 and a Mulliken charge of −0.020. This site could engage with nucleophilic regions of a substrate, but its para position and steric bulk might restrict strong directional interactions. Polarization effects caused by 13Cl are evident in the aromatic ring. For instance, the adjacent carbon, 4C, in para-chlorophenol shows a positive charge of 0.283 compared to 0.114 for 4C in phenol. This polarization enhances the nucleophilicity of carbons such as 4C in para-chlorophenol, with an f− value of 0.014, compared to 0.001 for phenol’s 4C, suggesting slightly increased reactivity at this site. The hydrogen atoms bonded to the oxygen, such as 7H in phenol and 7H in para-chlorophenol, also differ in charge and reactivity. In phenol, 7H has a Mulliken charge of 0.097 and an f⁺ value of 0.093, while in para-chlorophenol, 7H shows a higher charge of 0.111 but a lower f⁺ of 0.085. This indicates that hydrogen bonding potential is weaker in para-chlorophenol, likely due to the electron-withdrawing effect of the chlorine substituent. Overall, 12O in phenol appears more capable of engaging in directional hydrogen bonding, while 11O in para-chlorophenol favors electrostatic interactions. The chlorine atom, 13Cl, introduces electrophilic competition but also disrupts interaction efficiency due to steric effects. The adjacent carbon atoms, particularly 4C, show enhanced reactivity in para-chlorophenol due to polarization, while hydrogens such as 7H in phenol demonstrate slightly stronger bonding potential. These electronic features highlight distinctive interaction tendencies for each molecule, which could influence their adsorption or reactivity in subsequent studies.
4. Adsorption MD Simulation Results
The adsorption energetics of phenol and para-chlorophenol on sepiolite were evaluated under neutral conditions to explore their interaction mechanisms and positions, as shown in Figure 6. The total energy (Etotal), adsorption energy (Eads), rigid adsorption energy (Erigid), and deformation energy (Edef) are summarized in Table 4.
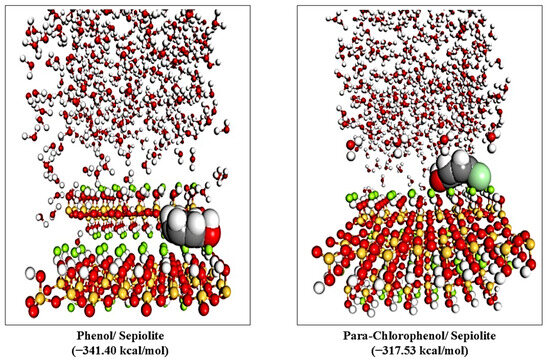
Figure 6.
Views of the adsorption of the phenol and para-chlorophenol sepiolite surface (110) in neutral medium.

Table 4.
The adsorption energetics of phenol and para-chlorophenol on sepiolite results.
Phenol exhibits a more negative Etotal (−349.26 kcal/mol) compared to para-chlorophenol (−327.39 kcal/mol), indicating its adsorption is thermodynamically and binding strength more favorable. The Eads (the energy released when a molecule binds to the surface) are significantly more negative for phenol (−341.40 kcal/mol) than for para-chlorophenol (−317.53 kcal/mol). This suggests stronger binding interactions between phenol and the sepiolite [40,41]. The Erigid (energy assuming no structural deformation) is more negative for both compounds than their actual adsorption energy, highlighting the energy penalty associated with molecular deformation during adsorption. Para-chlorophenol requires greater Edef (102.33 kcal/mol) than phenol (94.18 kcal/mol) to adapt to the sepiolite surface geometry [42].
Figure 7 shows the variations in van der Waals and electrostatic interaction energies during the adsorption of phenol and para-chlorophenol onto the adsorbent surface across the simulation steps. The electrostatic interaction energies for phenol are notably stronger (more negative), reaching approximately −170 kcal/mol, compared to para-chlorophenol, which peaks at around −140 kcal/mol. This indicates that electrostatic forces play a stronger role in the adsorption of phenol, likely due to its higher polarity and stronger interactions with the negatively charged surface of the adsorbent. In contrast, the van der Waals interaction energies show a slightly better performance for para-chlorophenol. This is attributed to the bulky chlorine atom in para-chlorophenol, which enhances dispersion forces and strengthens van der Waals interactions. The presence of chlorine, however, introduces steric effects that slightly reduce para-chlorophenol electrostatic interactions when compared to phenol.
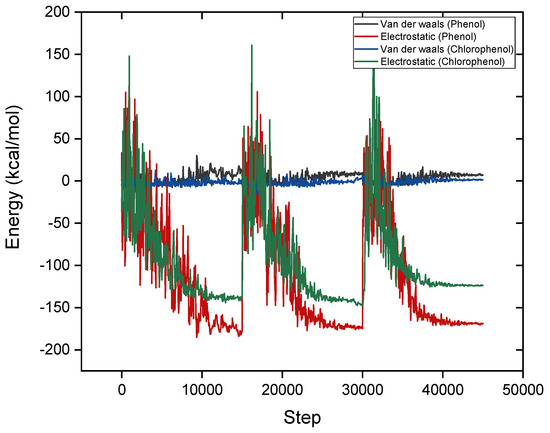
Figure 7.
The variations in van der Waals and electrostatic interaction energies during the adsorption.
5. Adsorption Isotherm Model
The adsorption isotherms of phenol and para-chlorophenol on sepiolite were analyzed using both the Freundlich and Langmuir models. The nonlinear Freundlich model provided the best fit to the data, with parameters (KfPhenol = 0.44 kPa−1, nphenol = 11, R2 = 0.99 and Kfpara-ChloroPhenol = 0.37 kPa−1, npara-ChloroPhenol = 11, R2 = 0.98). These high R2 values reflect the nonlinear Freundlich model’s ability to describe the multilayer adsorption mechanism and the heterogeneous distribution of adsorption sites on sepiolite. In contrast, the nonlinear Langmuir model, which assumes monolayer adsorption, showed poorer fits, with parameters (KLPhenol = 0.08 kPa−1, QmLphenol = 0.75 mmol/g, R2 = 0.73 and KLpara-ChloroPhenol = 0.24 kPa−1, QmLpara-ChloroPhenol = 0.62 mmol/g, R2 = 0.80) (Figure 8). This sigmoidal pattern is characteristic of multilayer adsorption on a heterogeneous surface with a finite number of active sites. The plateau observed at higher fugacities (900–1000 kPa) indicates the saturation of sepiolite’s adsorption sites, beyond which no additional adsorption occurs as a complete multilayer form. Phenol demonstrates a higher maximum adsorption capacity (Qmax = 0.78 mmol/g) compared to para-chlorophenol (Qmax = 0.66 mmol/g). This result highlights phenol’s stronger interaction with sepiolite.
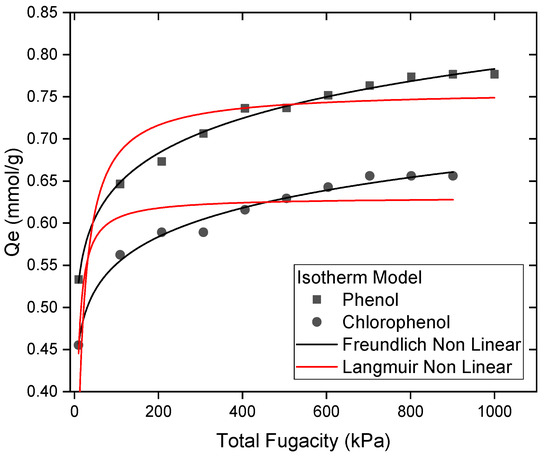
Figure 8.
The adsorption isotherms for phenol and para-chlorophenol onto sepiolite plot.
6. Proposed Mechanism
The adsorption of phenol and para-chlorophenol onto sepiolite is dictated by complex molecular interactions at the atomic level, involving site-specific affinities, molecular geometry, and the unique surface chemistry of sepiolite. The following mechanism integrates insights from isotherm models, density functional theory (DFT) calculations, and reactivity indices to provide a detailed understanding of the adsorption behavior of these molecules.
Phenol Adsorption: The hydroxyl (-OH) group of phenol serves as the primary adsorption site. Its highly negative Mulliken charge (−0.428) and significant electrophilic Fukui index (fi+ = 0.090) facilitate strong hydrogen bonding with the silanol (-SiOH) groups on the sepiolite surface. Additionally, the aromatic carbons (particularly atoms 3 and 5) exhibit high nucleophilic Fukui indices (fi− = 0.109), suggesting potential π-π interactions with the electron-rich siloxane (-Si-O-Si-) surfaces of sepiolite. The compact molecular structure of phenol (94.11 g/mol) and the flexibility of its -OH group allow it to undergo minimal structural deformation (Edef = 94.18 kcal/mol) upon adsorption. This facilitates efficient penetration into sepiolite microporous channels, optimizing hydrogen bonding and improving overall adsorption efficiency. Phenol’s strong interactions with the sepiolite surface are further supported by its thermodynamic stability, as indicated by its highly negative adsorption energy (−349.26 kcal/mol).
Para-chlorophenol Adsorption: In para-chlorophenol, the presence of a bulky chlorine (-Cl) substituent introduces steric hindrance and reduces the molecule’s ability to interact with the sepiolite surface effectively. The chlorine atom (atom 13) has a relatively high electrophilic Fukui index (fi+ = 0.278), but its near-neutral Mulliken charge (−0.020) limits its capacity for significant electrostatic interactions. Consequently, para-chlorophenol relies primarily on weaker van der Waals forces or hydrophobic partitioning for adsorption. The hydroxyl oxygen (atom 11) in para-chlorophenol retains a strongly negative Mulliken charge (−0.592) but exhibits reduced electrophilic reactivity (fi+ = 0.077) compared to phenol. This reduction is likely due to the electron-withdrawing effects of the chlorine group, which decrease the overall reactivity of the -OH group. Furthermore, the larger steric bulk of para-chlorophenol increases structural deformation energy (Edef = 102.33 kcal/mol), forcing the molecule into less favorable configurations and limiting its access to high-affinity sites within the sepiolite micropores.
Phenol demonstrates a more efficient adsorption mechanism than para-chlorophenol, as evidenced by its higher adsorption energy and stronger interaction with the sepiolite surface. The localized reactivity of phenol (fi+ = 0.090) aligns well with the hydrophilic surface of sepiolite, resulting in a compact and well-ordered adsorption layer. Phenol molecules orient their -OH groups toward the silanol or cation-exchange sites and align their aromatic rings parallel to the surface, maximizing hydrogen bonding and π-cation interactions. In contrast, para-chlorophenol adopts a disordered adsorption configuration. The bulky -Cl groups protrude away from the surface to minimize steric strain, leading to lower packing density and weaker van der Waals interactions. Despite para-chlorophenol lower HOMO-LUMO gap (ΔEGAP = 6.077 eV) and higher global electrophilicity index (ω = 2.071), its adsorption is thermodynamically less favorable due to inefficient site utilization and weaker binding forces.
The adsorption of phenol onto sepiolite is driven by the strong affinity of its hydroxyl group for silanol sites, its compact molecular structure, and its ability to form robust hydrogen bonds and π-cation interactions. Para-chlorophenol, on the other hand, faces steric and electronic challenges that reduce its adsorption efficiency. The proposed mechanism schema demonstrated in Figure 9 and Table 5 shows the comparison of phenol and para-chlorophenol adsorption mechanisms on sepiolite.
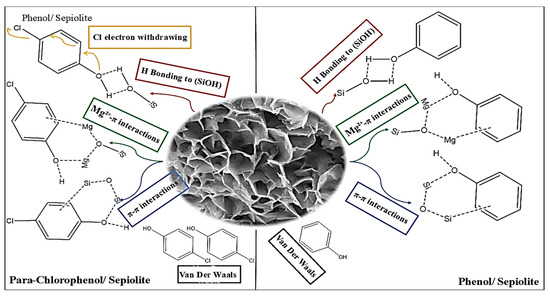
Figure 9.
Proposed mechanism schema for the adsorption of phenol and para-chlorophenol onto sepiolite.

Table 5.
Comparison of phenol and para-chlorophenol adsorption mechanisms on sepiolite.
7. Comparative Geometric Analysis
Figure 10 demonstrates the geometric analysis. Starting by the adsorption of phenol onto sepiolite, the C4–C5 bond remains at 1.397 Å before and after adsorption, preserving ring planarity and indicating that the ring itself is undistorted, while the 12O–13H bond lengthens from (0.94 Å before adsorption) to (1.207 Å after adsorption) as the oxygen 12O engages in a strong H–O⋯H hydrogen bond with a hydrogen of silanol at a distance of 2.29 Å. A secondary contact between a ring hydrogen (10H) and surface oxygen at 2.43 Å further reinforces this interaction. By contrast, para-chlorophenol’s Cl–C bond (13Cl–1C) increases only slightly from (1.173 Å before adsorption) to (1.178 Å after adsorption) and its C4–C5 distance remains unchanged, indicating preserved aromatic geometry. However, its O–H⋯O hydrogen bond is much weaker, as shown by a stretched H⋯O(silanol) separation of (3.63 Å 11O–H of the sepiolite surface). To compensate, the chlorine 13Cl atom positions itself 2.41 Å from an Mg2⁺ site, forming a moderate electrostatic or dispersion contact. Together, these measurements illustrate that phenol achieves robust anchoring through strong hydrogen bonding, whereas para-chlorophenol settles for a weaker elongated O–H bond, underscoring the steric and electronic penalties of the chlorine substituent.
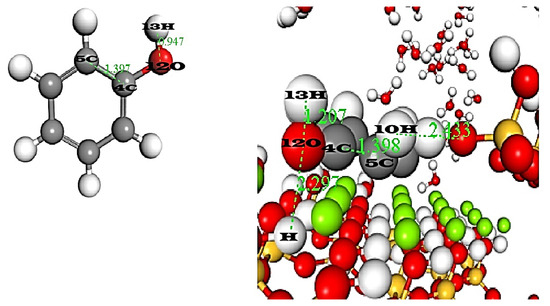
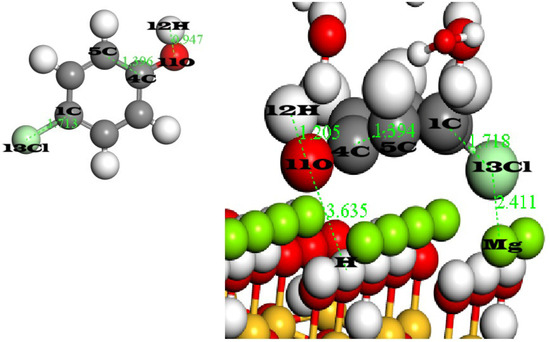
Figure 10.
The geometric analysis of phenol and para-chlorophenol before and after adsorption.
8. Comparison of Phenol/Chlorophenol Adsorption Capacities Across Different Clay
The comparison of maximum phenol/chlorophenol adsorption experimental capacities of different clay is presented in Table 6.

Table 6.
Comparison of maximum phenol/chlorophenol adsorption capacities.
9. Conclusions
- Phenol demonstrates enhanced adsorption efficiency on sepiolite relative to para-chlorophenol, attributed to robust hydrogen bonding and π-cation interactions, as indicated by DFT calculations;
- The hydroxyl group in phenol has a pronounced negative Mulliken charge (−0.428) and notable electrophilic reactivity (fi+ = 0.090), hence augmenting its interaction with silanol (-SiOH) groups;
- The substantial chlorine substituent of para-chlorophenol diminishes its hydrogen bonding capacity and imposes steric hindrance, as evidenced by its less reactive hydroxyl group (fi+ = 0.077) and almost neutral Mulliken charge (−0.020);
- Phenol exhibits reduced deformation energy (+94.18 kcal/mol), facilitating superior structural alignment with sepiolite’s active sites, in contrast to para-chlorophenol (+102.33 kcal/mol);
- Theoretical isotherm findings validate phenol’s superior adsorption capacity (Qmax = 0.78 mmol/g), indicating effective site usage and alignment;
- Nonlinear Freundlich-type behavior for both molecules indicates multilayer adsorption, with phenol establishing a denser and more organized adsorption layer.
This study synthesizes DFT insights, Fukui indices, Mulliken charge analysis, and MD simulation data to provide a complete framework for elucidating adsorption mechanisms and informing the design of customized adsorbents.
Author Contributions
Conceptualization, A.K., R.Y., R.K., and H.C.; methodology, B.B., A.K., R.Y., R.K., and L.M.; software, R.Y., H.C., and R.K.; validation, S.S., L.M., A.K., R.Y., R.K., and H.C.; formal analysis, H.C., L.M., A.K., and R.Y.; investigation, A.K. and B.B.; resources, A.K., L.M., and B.B.; data curation H.C. and A.K.; writing—original draft preparation, S.S., A.K., R.Y., R.K., and L.M.; writing—review and editing, S.S., L.M., A.K., R.Y., R.K., and H.C.; visualization, A.K., R.Y., R.K., and L.M.; supervision, A.K., R.Y., R.K., M.N.A., and L.M.; project administration, A.K., R.Y., R.K., M.N.A., and L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by (PNURSP2025R481), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors are grateful to Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R481), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ahmaruzzaman, M.; Mishra, S.R.; Gadore, V.; Yadav, G.; Roy, S.; Bhattacharjee, B.; Bhuyan, A.; Hazarika, B.; Darabdhara, J.; Kumari, K. Phenolic Compounds in Water: From Toxicity and Source to Sustainable Solutions—An Integrated Review of Removal Methods, Advanced Technologies, Cost Analysis, and Future Prospects. J. Environ. Chem. Eng. 2024, 12, 112964. [Google Scholar] [CrossRef]
- Ladeia Ramos, R.; Rezende Moreira, V.; Santos Amaral, M.C. Phenolic Compounds in Water: Review of Occurrence, Risk, and Retention by Membrane Technology. J. Environ. Manag. 2024, 351, 119772. [Google Scholar] [CrossRef] [PubMed]
- Djama, C.; Bouguettoucha, A.; Chebli, D.; Amrane, A.; Tahraoui, H.; Zhang, J.; Mouni, L. Experimental and Theoretical Study of Methylene Blue Adsorption on a New Raw Material, Cynara Scolymus—A Statistical Physics Assessment. Sustainability 2023, 15, 10364. [Google Scholar] [CrossRef]
- Satyam, S.; Patra, S. Innovations and Challenges in Adsorption-Based Wastewater Remediation: A Comprehensive Review. Heliyon 2024, 10, e29573. [Google Scholar] [CrossRef]
- Khader, E.H.; Mohammed, T.J.; Mirghaffari, N.; Salman, A.D.; Juzsakova, T. Removal of Organic Pollutants from Produced Water by Batch Adsorption Treatment|Clean Technologies and Environmental Policy. Clean Technol. Environ. Policy 2022, 24, 713–720. Available online: https://link.springer.com/article/10.1007/s10098-021-02159-z (accessed on 26 March 2025). [CrossRef]
- Benazouz, K.; Bouchelkia, N.; Moussa, H.; Boutheldja, R.; Zamouche, M.; Amrane, A.; Parvathiraja, C.; Al-Lohedan, H.A.; Bollinger, J.-C.; Mouni, L. Efficient Removal of Cu(II) from Wastewater Using Chitosan Derived from Shrimp Shells: A Kinetic, Thermodynamic, Optimization, and Modelling Study. Water 2025, 17, 851. [Google Scholar] [CrossRef]
- Zango, Z.U.; Rozaini, M.N.; Bakar, N.H.H.A.; Zango, M.U.; Haruna, M.A.; Dennis, J.O.; Alsadig, A.; Ibnaouf, K.H.; Aldaghri, O.A.; Wadi, I.A. Advancements in Clay Materials for Trace Level Determination and Remediation of Phenols from Wastewater: A Review. Separations 2023, 10, 125. [Google Scholar] [CrossRef]
- Khan, S.; Ajmal, S.; Hussain, T.; Rahman, M.U. Clay-Based Materials for Enhanced Water Treatment: Adsorption Mechanisms, Challenges, and Future Directions. J. Umm Al-Qura Univ. Appl. Sci. 2023. [Google Scholar] [CrossRef]
- Khachay, A.; Cherifi, H.; Yous, R.; Khalladi, R.; Belaid, B. Mechanistic Insights into the Adsorption of Cationic Crystal Violet onto Sepiolite: A Comprehensive Study Combining Experimental and Theoretical Approaches. J. Mol. Struct. 2025, 1325, 140944. [Google Scholar] [CrossRef]
- Junior, H.B.; da Silva, E.; Saltarelli, M.; Crispim, D.; Nassar, E.J.; Trujillano, R.; Rives, V.; Vicente, M.A.; Gil, A.; Korili, S.A.; et al. Inorganic–Organic Hybrids Based on Sepiolite as Efficient Adsorbents of Caffeine and Glyphosate Pollutants. Appl. Surf. Sci. Adv. 2020, 1, 100025. [Google Scholar] [CrossRef]
- Dehmani, Y.; Lgaz, H.; Alrashdi, A.A.; Lamhasni, T.; Abouarnadasse, S.; Chung, I.-M. Phenol Adsorption Mechanism on the Zinc Oxide Surface: Experimental, Cluster DFT Calculations, and Molecular Dynamics Simulations. J. Mol. Liq. 2021, 324, 114993. [Google Scholar] [CrossRef]
- Kuśmierek, K. The Removal of Chlorophenols from Aqueous Solutions Using Activated Carbon Adsorption Integrated with H2O2 Oxidation. React. Kinet. Mech. Catal. 2016, 119, 19–34. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, J.; Li, D.; Ao, Z.; An, T.; Wang, G. Density Functional Theory Study on the Enhanced Adsorption Mechanism of Gaseous Pollutants on Al-Doped Ti2CO2 Monolayer. Sustain. Mater. Technol. 2021, 29, e00294. [Google Scholar] [CrossRef]
- Salahshoori, I.; Mahdavi, S.; Moradi, Z.; Otadi, M.; Zare Kazemabadi, F.; Nobre, M.A.L.; Ali Khonakdar, H.; Baghban, A.; Wang, Q.; Mohammadi, A.H. Advancements in Molecular Simulation for Understanding Pharmaceutical Pollutant Adsorption: A State-of-the-Art Review. J. Mol. Liq. 2024, 410, 125513. [Google Scholar] [CrossRef]
- Amrhar, O.; Lee, H.-S.; Lgaz, H.; Berisha, A.; Ebenso, E.E.; Cho, Y. Computational Insights into the Adsorption Mechanisms of Anionic Dyes on the Rutile TiO2 (1 1 0) Surface: Combining SCC-DFT Tight Binding with Quantum Chemical and Molecular Dynamics Simulations. J. Mol. Liq. 2023, 377, 121554. [Google Scholar] [CrossRef]
- Khnifira, M.; Mahsoune, A.; Belghiti, M.E.; Khamar, L.; Sadiq, M.; Abdennouri, M.; Barka, N. Combined DFT and MD Simulation Approach for the Study of SO2 and CO2 Adsorption on Graphite (111) Surface in Aqueous Medium. Curr. Res. Green Sustain. Chem. 2021, 4, 100085. [Google Scholar] [CrossRef]
- Zhou, F.; Ye, G.; Gao, Y.; Wang, H.; Zhou, S.; Liu, Y.; Yan, C. Cadmium Adsorption by Thermal-Activated Sepiolite: Application to in-Situ Remediation of Artificially Contaminated Soil. J. Hazard. Mater. 2022, 423, 127104. [Google Scholar] [CrossRef] [PubMed]
- Parr, R.G. Density Functional Theory of Atoms and Molecules. In Proceedings of the Horizons of Quantum Chemistry, Kyoto, Japan, 29 October–3 November 1979; Fukui, K., Pullman, B., Eds.; Springer: Dordrecht, The Netherlands, 1980; pp. 5–15. [Google Scholar]
- Kohn, W.; Sham, L.J. Quantum Density Oscillations in an Inhomogeneous Electron Gas. Phys. Rev. 1965, 137, A1697–A1705. [Google Scholar] [CrossRef]
- Lu, L.; Hu, H.; Hou, H.; Wang, B. An Improved B3LYP Method in the Calculation of Organic Thermochemistry and Reactivity. Comput. Theor. Chem. 2013, 1015, 64–71. [Google Scholar] [CrossRef]
- Tirado-Rives, J.; Jorgensen, W.L. Performance of B3LYP Density Functional Methods for a Large Set of Organic Molecules. J. Chem. Theory Comput. 2008, 4, 297–306. [Google Scholar] [CrossRef]
- North, S.C.; Jorgensen, K.R.; Pricetolstoy, J.; Wilson, A.K. Population Analysis and the Effects of Gaussian Basis Set Quality and Quantum Mechanical Approach: Main Group through Heavy Element Species. Front. Chem. 2023, 11, 121554. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpály, L.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Islam, N.; Kaya, S. (Eds.) Conceptual Density Functional Theory and Its Application in the Chemical Domain; Apple Academic Press: New York, NY, USA, 2018; ISBN 978-0-203-71139-2. [Google Scholar]
- Chattaraj, P.K. (Ed.) Chemical Reactivity Theory: A Density Functional View; CRC Press: Boca Raton, FL, USA, 2009; ISBN 978-0-429-13722-8. [Google Scholar]
- Kaya, S.; Kaya, C. A New Method for Calculation of Molecular Hardness: A Theoretical Study. Comput. Theor. Chem. 2015, 1060, 66–70. [Google Scholar] [CrossRef]
- Vismara, R.; Terruzzi, S.; Maspero, A.; Grell, T.; Bossola, F.; Sironi, A.; Galli, S.; Navarro, J.A.R.; Colombo, V. CO2 Adsorption in a Robust Iron(III) Pyrazolate-Based MOF: Molecular-Level Details and Frameworks Dynamics From Powder X-Ray Diffraction Adsorption Isotherms. Adv. Mater. 2024, 36, 2209907. [Google Scholar] [CrossRef] [PubMed]
- Sun, H. COMPASS: An Ab Initio Force-Field Optimized for Condensed-Phase ApplicationsOverview with Details on Alkane and Benzene Compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Vlugt, T.J.H.; Krishna, R.; Smit, B. Molecular Simulations of Adsorption Isotherms for Linear and Branched Alkanes and Their Mixtures in Silicalite. J. Phys. Chem. B 1999, 103, 1102–1118. Available online: https://pubs.acs.org/doi/pdf/10.1021/jp982736c (accessed on 26 March 2025). [CrossRef]
- Ouallal, H.; Dehmani, Y.; Moussout, H.; Messaoudi, L.; Azrour, M. Kinetic, Isotherm and Mechanism Investigations of the Removal of Phenols from Water by Raw and Calcined Clays. Heliyon 2019, 5, e01616. [Google Scholar] [CrossRef]
- Li, Y.; Hu, X.; Liu, X.; Zhang, Y.; Zhao, Q.; Ning, P.; Tian, S. Adsorption Behavior of Phenol by Reversible Surfactant-Modified Montmorillonite: Mechanism, Thermodynamics, and Regeneration. Chem. Eng. J. 2018, 334, 1214–1221. [Google Scholar] [CrossRef]
- Becker, H.G.O. Jan Fleming, Frontier Orbitals and Organic Chemical Reactions. 249 S., John Wiley u. Sons LTD., London/New York/Syndney/Toronto 1976. Clothed £ 8,95, Paperb. £ 3,95. J. Für Prakt. Chem. 1978, 320, 879–880. [Google Scholar] [CrossRef]
- Eddy, N.O.; Ita, B.I. QSAR, DFT and Quantum Chemical Studies on the Inhibition Potentials of Some Carbozones for the Corrosion of Mild Steel in HCl. J. Mol. Model. 2011, 17, 359–376. [Google Scholar] [CrossRef]
- Bahrani-Pour, M.; Beheshti, A.; Sedaghat, T.; Samiee, S.; Hosen, M.A.; Kawsar, S.M.A. A Comparison between the Anti-Thyroid Properties of Methimazole and Its Derivatives with Two S Donor Atoms Endorsed by Iodine Adsorption Capacity, Docking and DFT Calculations. J. Mol. Struct. 2024, 1296, 136819. [Google Scholar] [CrossRef]
- Chen, M.; Gu, Q.; Shao, H.; Liu, H.; Luan, J.; Yan, Z.; Liu, W.; Ke, X. How PPY/CMC Aerogels Possess Selective Adsorption Capacity for Norfloxacin: Coupling Molecular Scale Interpretation with Experiments. Chem. Eng. J. 2023, 464, 142485. [Google Scholar] [CrossRef]
- Ourhzif, E.-M.; Ketatni, E.M.; Akssira, M.; Troin, Y.; Khouili, M. Crystal Structure, Hirshfeld Surface Analysis and DFT Studies of Euphorbioside Monohydrate a Major Bisnorsesquiterpene Isolated from Euphorbia Resinifera Latex. J. Mol. Struct. 2021, 1241, 130511. [Google Scholar] [CrossRef]
- Hasanov, R.; Sadıkoğlu, M.; Bilgiç, S. Electrochemical and Quantum Chemical Studies of Some Schiff Bases on the Corrosion of Steel in H2SO4 Solution. Appl. Surf. Sci. 2007, 253, 3913–3921. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density Functional Approach to the Frontier-Electron Theory of Chemical Reactivity. J. Am. Chem. Soc. 1984, 106, 4049–4050. [Google Scholar] [CrossRef]
- Obot, I.B.; Onyeachu, I.B.; Wazzan, N.; Al-Amri, A.H. Theoretical and Experimental Investigation of Two Alkyl Carboxylates as Corrosion Inhibitors for Steel in Acidic Medium. J. Mol. Liq. 2019, 279, 190–207. [Google Scholar] [CrossRef]
- Bahamon, D.; Khalil, M.; Belabbes, A.; Alwahedi, Y.; Vega, L.F.; Polychronopoulou, K. A DFT Study of the Adsorption Energy and Electronic Interactions of the SO2 Molecule on a CoP Hydrotreating Catalyst. RSC Adv. 2021, 11, 2947–2957. [Google Scholar] [CrossRef]
- Boumya, W.; Khnifira, M.; Machrouhi, A.; Abdennouri, M.; Sadiq, M.; Achak, M.; Serdaroğlu, G.; Kaya, S.; Şimşek, S.; Barka, N.; et al. Adsorption of Eriochrome Black T on the Chitin Surface: Experimental Study, DFT Calculations and Molecular Dynamics Simulation. J. Mol. Liq. 2021, 331, 115706. [Google Scholar] [CrossRef]
- Bourzi, H.; Oukhrib, R.; El Ibrahimi, B.; Oualid, H.A.; Abdellaoui, Y.; Balkard, B.; El Issami, S.; Hilali, M.; Bazzi, L.; Len, C. Furfural Analogs as Sustainable Corrosion Inhibitors—Predictive Efficiency Using DFT and Monte Carlo Simulations on the Cu(111), Fe(110), Al(111) and Sn(111) Surfaces in Acid Media. Sustainability 2020, 12, 3304. Available online: https://www.mdpi.com/2071-1050/12/8/3304 (accessed on 26 March 2025). [CrossRef]
- Zhao, Z.; Fu, D.; Ma, Q. Adsorption Characteristics of Bisphenol A from Aqueous Solution onto HDTMAB-Modified Palygorskite. Sep. Sci. Technol. 2014, 49, 81–89. Available online: https://www.tandfonline.com/doi/full/10.1080/01496395.2013.818693 (accessed on 25 March 2025). [CrossRef]
- Zheng, S.; Sun, Z.; Park, Y.; Ayoko, G.A.; Frost, R.L. Removal of Bisphenol A from Wastewater by Ca-Montmorillonite Modified with Selected Surfactants. Chem. Eng. J. 2013, 234, 416–422. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Dey, D.G. Adsorption of 2-Chlorophenol on Kaolin. SSRN. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=2979013 (accessed on 25 March 2025).
- Moghazy, M.A. High-Efficiency Adsorptive Removal of Phenol from Aqueous Solution Using Natural Red Clay and ZnO Nanoparticles. ChemistrySelect 2022, 7, e202104074. [Google Scholar] [CrossRef]
- Messaid, B.; Djemai, I.; Boudouh, I.; Robustillo, M.D. Assessment of the Performance of an Acid-Activated Composite Made of Na-Montmorillonite Algerian Clay to Remove Phenol and 4-Chlorophenol from Water. Desalination Water Treat. 2025, 322, 101088. [Google Scholar] [CrossRef]
- Ge, M.; Ge, M.; Du, M.; Liang, G.; Hu, G.; Jahangir Alam, S.M. Adsorption Analyses of Phenol from Aqueous Solutions Using Magadiite Modified with Organo-Functional Groups: Kinetic and Equilibrium Studies. Materials 2019, 12, 96. Available online: https://www.mdpi.com/1996-1944/12/1/96 (accessed on 25 March 2025). [CrossRef] [PubMed]
- Ghogomu, J.N.; Noufame, D.T.; Tamungang, E.B.N.; Ajifack, D.L.; Ndi, J.N.; Ketcha, J.M. Adsorption of Phenol from Aqueous Solutions onto Natural and Thermallymodified Kaolinitic Materials. Int. J. Biol. Chem. Sci. 2015, 8, 2325. Available online: https://www.researchgate.net/publication/281689674_Adsorption_of_phenol_from_aqueous_solutions_onto_natural_and_thermallymodified_kaolinitic_materials (accessed on 25 March 2025).
- Richards, S.; Bouazza, A. Phenol Adsorption in Organo-Modified Basaltic Clay and Bentonite. Appl. Clay Sci. 2007, 37, 133–142. [Google Scholar] [CrossRef]
- Yildiz, A.; Gür, A. Adsorption of Phenol and Chlorophenols on Pure and Modified Sepiolite. J. Serbian Chem. Soc. 2024, 72, 467–474. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).