Biotechnological Potential of Newly Isolated Microalga Strain in Cu and Cr Biosorption from Single and Bimetallic Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalga Strain and Cultivation Conditions
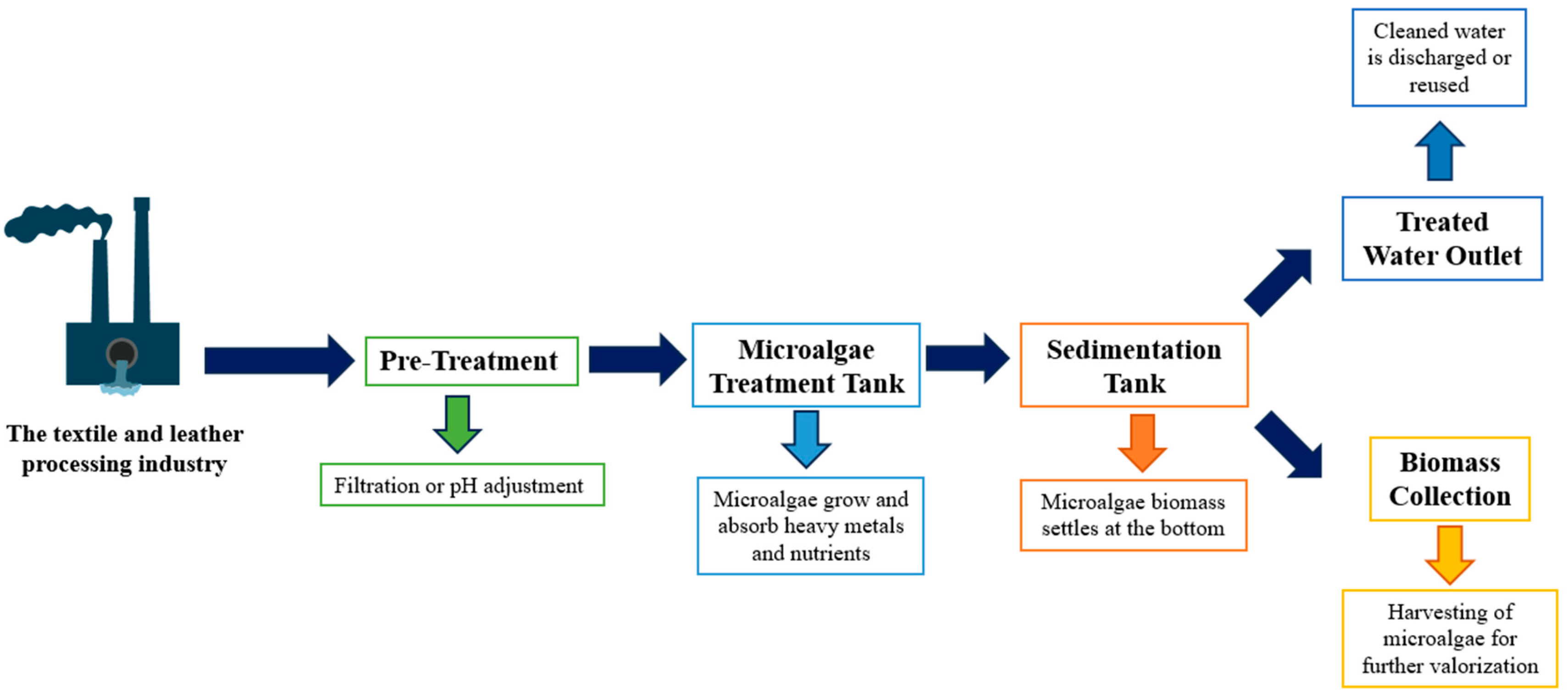
2.2. Sedimentation Efficiency
2.3. IC50 Estimation
2.4. Impact of Cu and Cr on Growth and Pigment Accumulation
2.5. Lipid Content
2.6. Fatty Acids Methyl Esters Analysis by Gas Chromatography
2.7. Carbohydrate Assays
2.8. Proteins Contents
2.9. Metal Analysis
2.10. Fourier Transform Infrared Spectroscopy (FTIR)
2.11. Statistical Analysis
3. Results and Discussion
3.1. IC50 Estimation and Sedimentation Efficiency
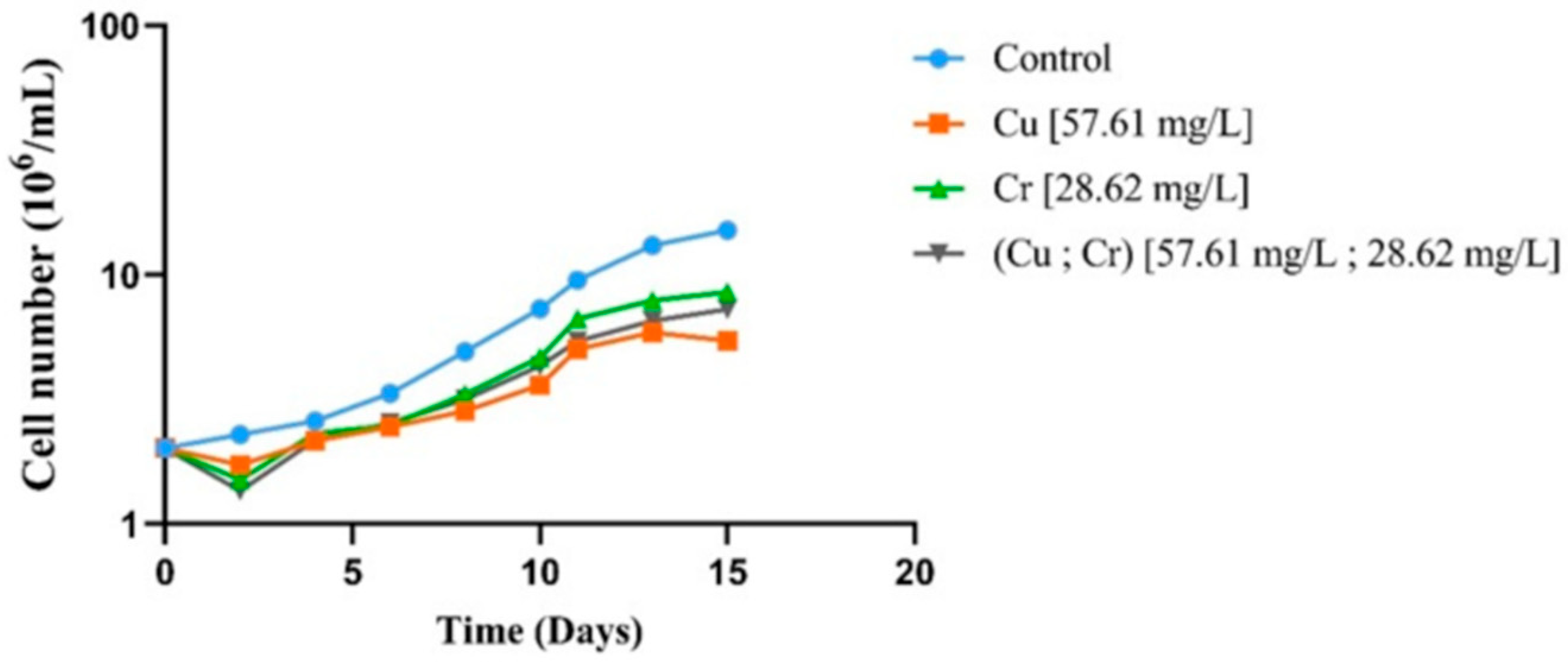
3.2. Effects of Metals Exposure on Chlamydomonas sp. Growth
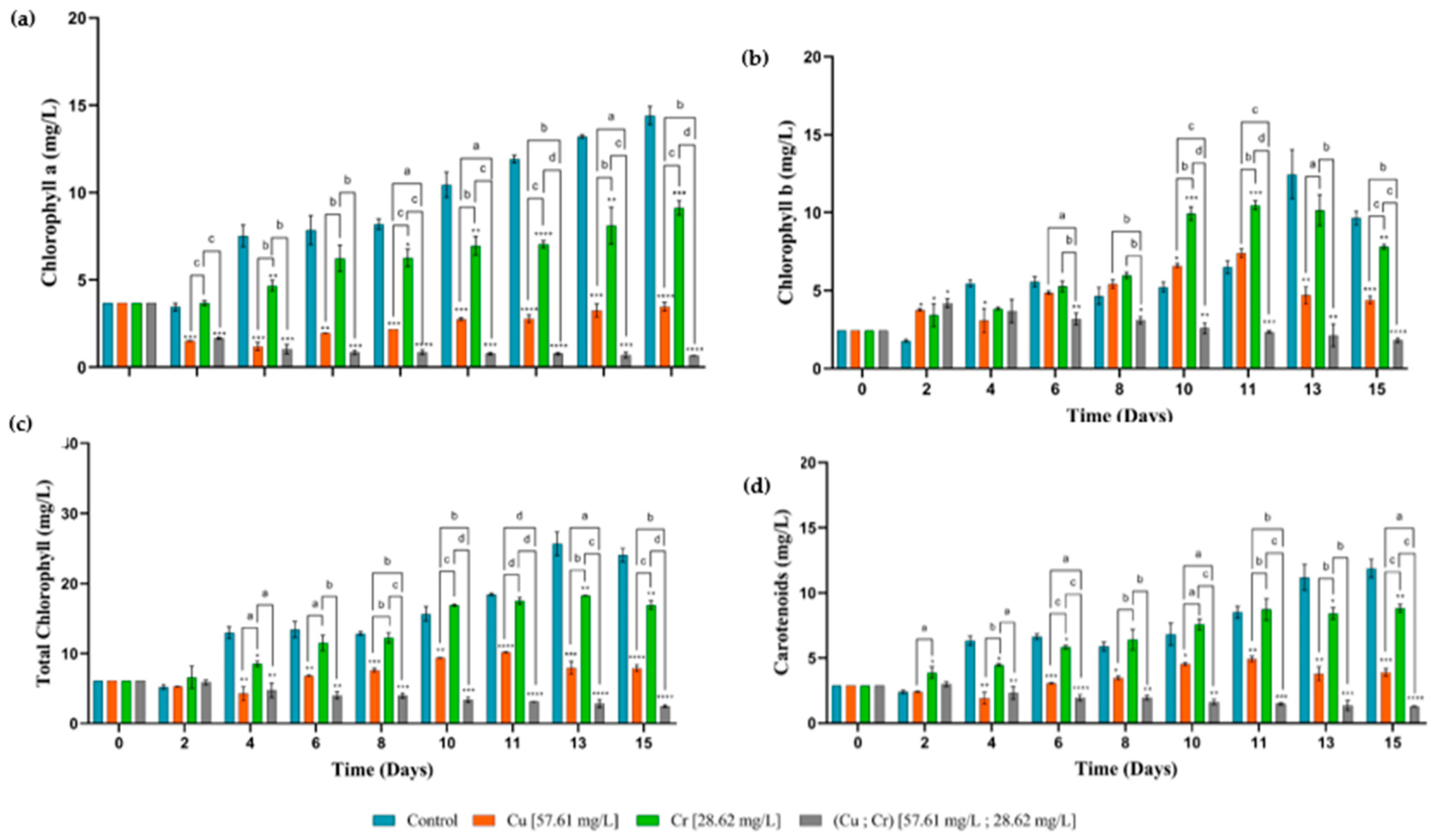
3.3. Pigment Contents
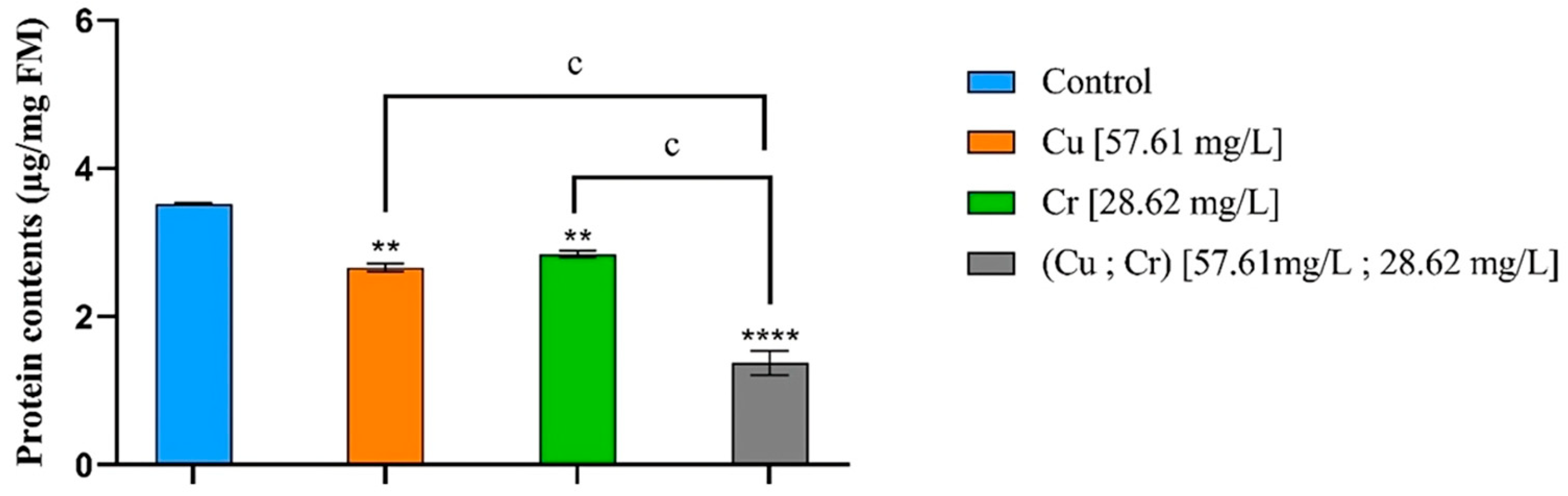
3.4. Protein Contents
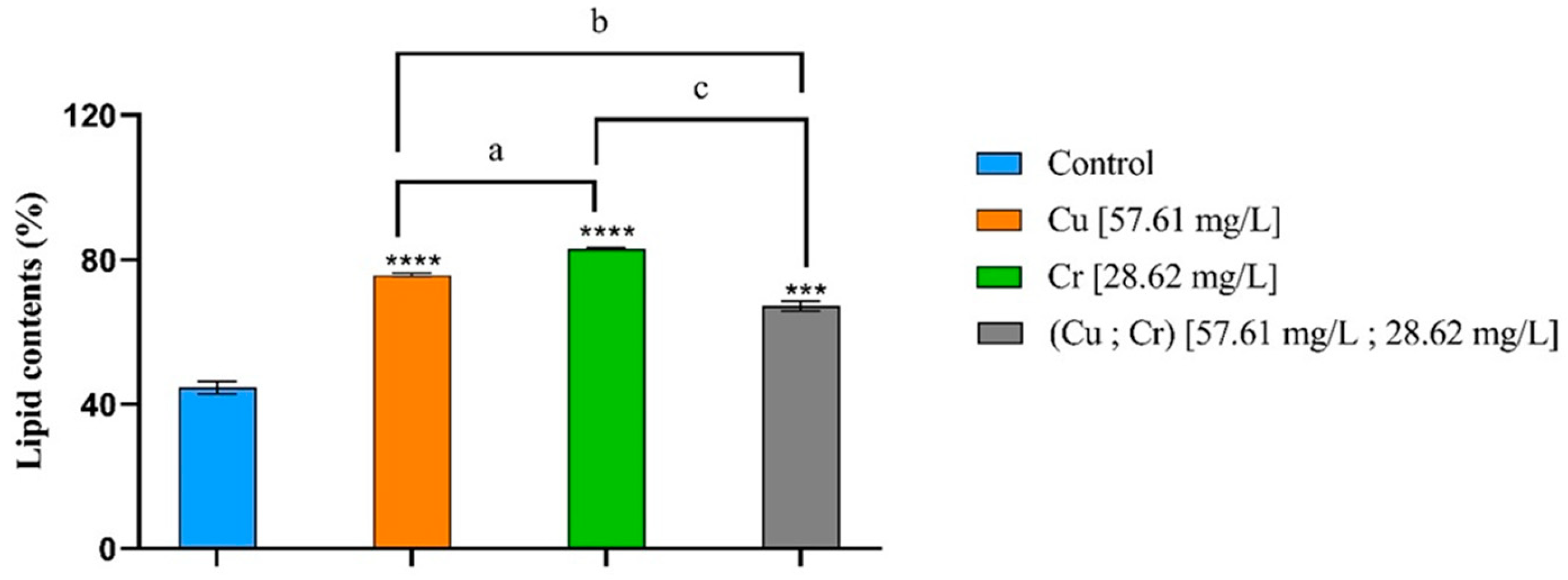
3.5. Lipid Contents and Fatty Acid Profile
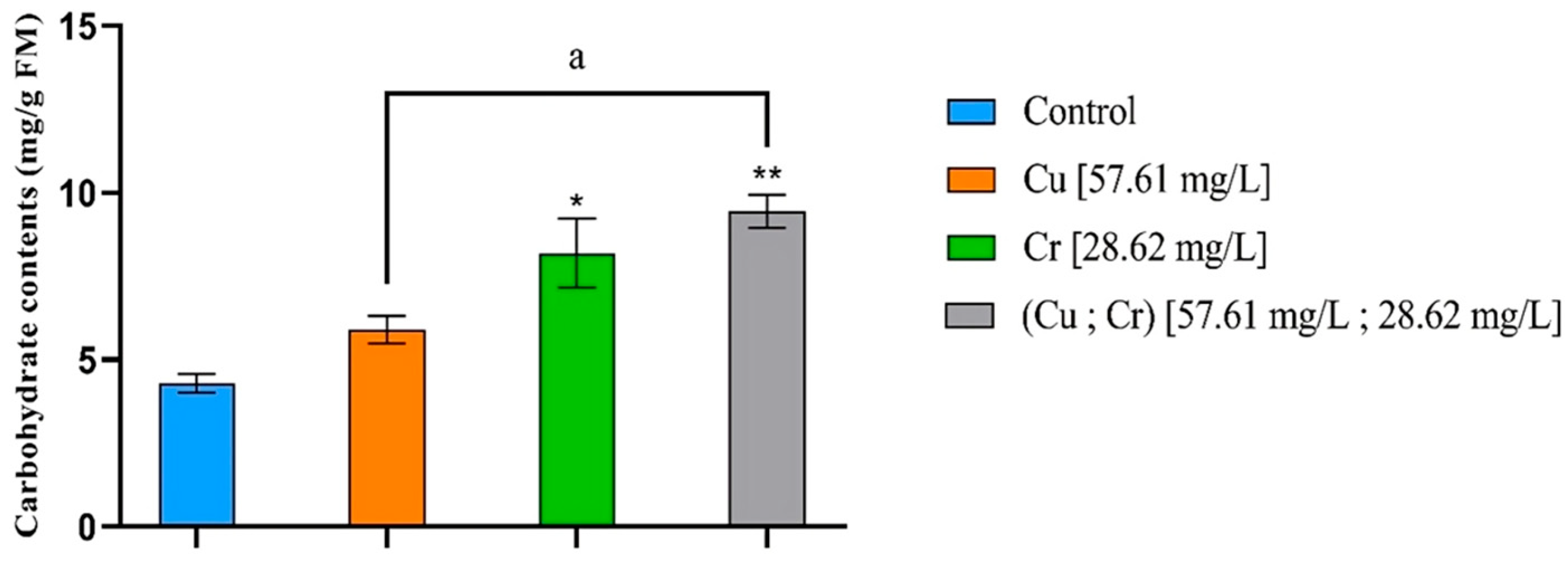
3.6. Carbohydrate Contents
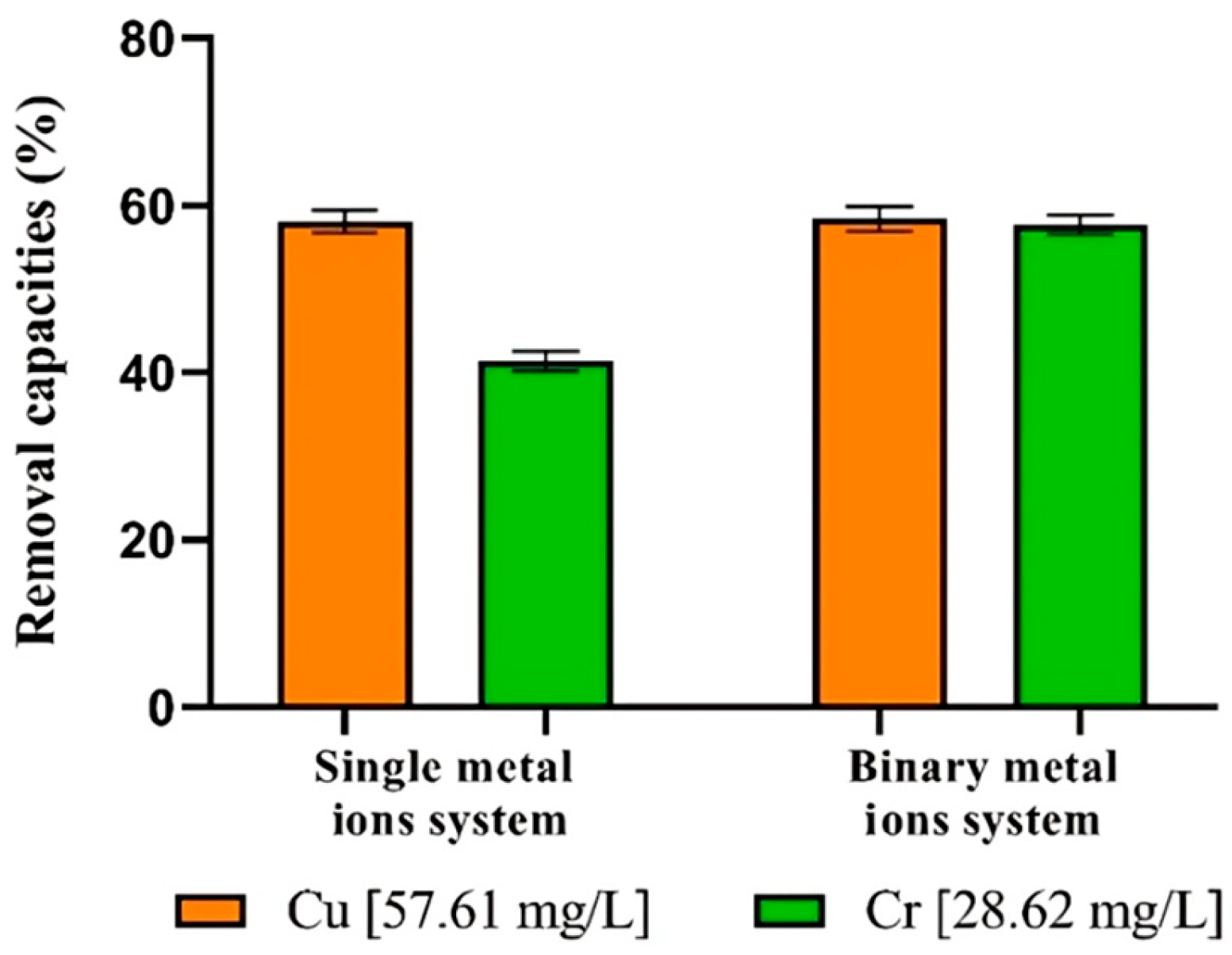
3.7. Single and Binary Metal Ions Systems Remove
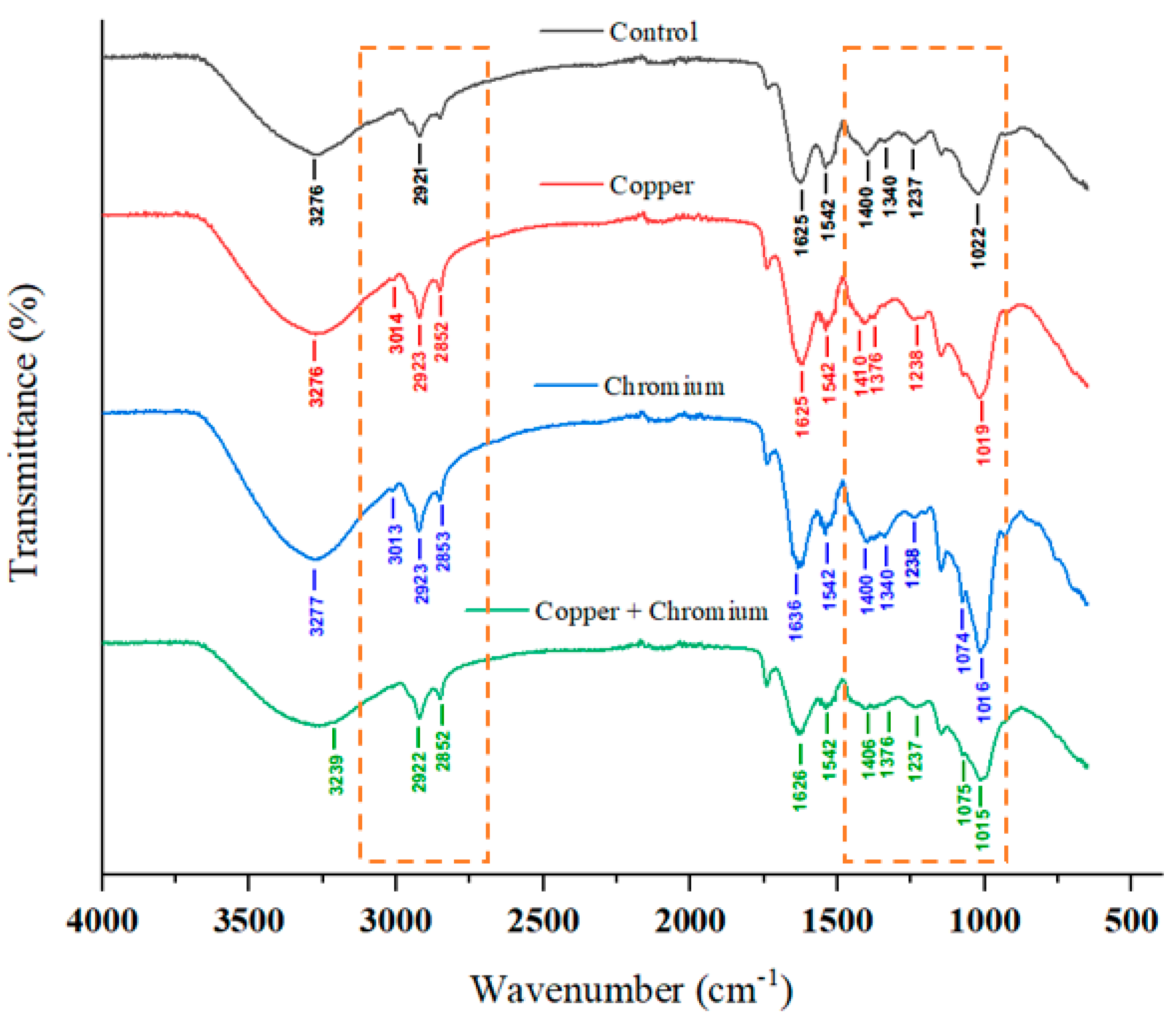
3.8. FTIR Analysis
3.9. Summary of Findings and Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hange, K.; Awofolu, O.R. Assessment of Anthropogenic Influence on the Level of Selected Heavy Metals (Cu, Zn, Cd and Pb) in Soil. J. Soil Sci. Environ. Manag. 2017, 8, 113–121. [Google Scholar] [CrossRef]
- Tumanyan, A.F.; Seliverstova, A.P.; Zaitseva, N.A. Effect of Heavy Metals on Ecosystems. Chem. Technol. Fuels Oils 2020, 56, 390–394. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.K.; Chang, J.-S. Bioremediation of Heavy Metals Using Microalgae: Recent Advances and Mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef]
- Kim, J.-J.; Kim, Y.-S.; Kumar, V. Heavy Metal Toxicity: An Update of Chelating Therapeutic Strategies. J. Trace Elem. Med. Biol. 2019, 54, 226–231. [Google Scholar] [CrossRef]
- Shabbir, Z.; Sardar, A.; Shabbir, A.; Abbas, G.; Shamshad, S.; Khalid, S.; Natasha; Murtaza, G.; Dumat, C.; Shahid, M. Copper Uptake, Essentiality, Toxicity, Detoxification and Risk Assessment in Soil-Plant Environment. Chemosphere 2020, 259, 127436. [Google Scholar] [CrossRef]
- García-Niño, W.R.; Pedraza-Chaverrí, J. Protective Effect of Curcumin against Heavy Metals-Induced Liver Damage. Food Chem. Toxicol. 2014, 69, 182–201. [Google Scholar] [CrossRef]
- Nazir, F.; Hussain, A.; Fariduddin, Q. Hydrogen Peroxide Modulates Photosynthesis and Antioxidant Systems in Tomato (Solanum lycopersicum L.) Plants under Copper Stress. Chemosphere 2019, 230, 544–558. [Google Scholar] [CrossRef]
- Rocha, G.S.; Parrish, C.C.; Espíndola, E.L.G. Effects of Copper on Photosynthetic and Physiological Parameters of a Freshwater Microalga (Chlorophyceae). Algal Res. 2021, 54, 102223. [Google Scholar] [CrossRef]
- Kumar, D.; Khan, E.A. Remediation and Detection Techniques for Heavy Metals in the Environment. In Heavy Metals in the Environment; Elsevier: Amsterdam, The Netherlands, 2021; pp. 205–222. [Google Scholar] [CrossRef]
- Tavana, M.; Pahlavanzadeh, H.; Zarei, M.J. The Novel Usage of Dead Biomass of Green Algae Schizomeris leibleinii for Biosorption of Copper(II) from Aqueous Solutions: Equilibrium, Kinetics and Thermodynamics. J. Environ. Chem. Eng. 2020, 8, 104272. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Jobby, R.; Jha, P.; Yadav, A.K.; Desai, N. Biosorption and Biotransformation of Hexavalent Chromium [Cr(VI)]: A Comprehensive Review. Chemosphere 2018, 207, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Shokri Khoubestani, R.; Mirghaffari, N.; Farhadian, O. Removal of Trivalent and Hexavalent Chromium from Aqueous Solutions Using a Microalgae Biomass-Derived Biosorbent. Environ. Prog. Sustain. Energy 2015, 34, 949–956. [Google Scholar] [CrossRef]
- Kumar, A.; Usmani, Z.; Ahirwal, J.; Tripti; Rani, P. Phytomanagement of Chromium Contaminated Brown Fields. In Phytomanagement of Polluted Sites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 447–469. [Google Scholar] [CrossRef]
- Vidyalaxmi; Kaushik, G.; Raza, K. Potential of Novel Dunaliella salina from Sambhar Salt Lake, India, for Bioremediation of Hexavalent Chromium from Aqueous Effluents: An Optimized Green Approach. Ecotoxicol. Environ. Saf. 2019, 180, 430–438. [Google Scholar] [CrossRef]
- Kannan, L.; Gokulprasath, M.; Gurusamy, R.; Selvam, R.; Palaniswamy, R.; Patel, C. Analysis of Heavy Metals Contamination in Water: A Review. SSRN Electron. J. 2021, 8, 201–213. [Google Scholar]
- Masindi, V.; Muedi, K.L. Environmental Contamination by Heavy Metals. In Heavy Metals; Saleh, H.E.-D.M., Aglan, R.F., Eds.; InTech: Houston, TX, USA, 2018. [Google Scholar] [CrossRef]
- Wei, H.; Gao, B.; Ren, J.; Li, A.; Yang, H. Coagulation/Flocculation in Dewatering of Sludge: A Review. Water Res. 2018, 143, 608–631. [Google Scholar] [CrossRef]
- Elleuch, J.; Ben Amor, F.; Chaaben, Z.; Frikha, F.; Michaud, P.; Fendri, I.; Abdelkafi, S. Zinc Biosorption by Dunaliella sp. AL-1: Mechanism and Effects on Cell Metabolism. Sci. Total Environ. 2021, 773, 145024. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Ali, S.S.; Ramadan, H.; El-Aswar, E.I.; Eltawab, R.; Ho, S.-H.; Elsamahy, T.; Li, S.; El-Sheekh, M.M.; Schagerl, M.; et al. Microalgae-Based Wastewater Treatment: Mechanisms, Challenges, Recent Advances, and Future Prospects. Environ. Sci. Ecotech. 2023, 13, 100205. [Google Scholar] [CrossRef]
- Chugh, M.; Kumar, L.; Shah, M.P.; Bharadvaja, N. Algal Bioremediation of Heavy Metals: An Insight into Removal Mechanisms, Recovery of By-Products, Challenges, and Future Opportunities. Energy Nexus 2022, 7, 100129. [Google Scholar] [CrossRef]
- Balzano, S.; Sardo, A.; Blasio, M.; Chahine, T.B.; Dell’Anno, F.; Sansone, C.; Brunet, C. Microalgal Metallothioneins and Phytochelatins and Their Potential Use in Bioremediation. Front. Microbiol. 2020, 11, 517. [Google Scholar] [CrossRef]
- Ben Mohamed, J.; Elleuch, J.; Drira, M.; Esteban, M.Á.; Michaud, P.; Abdelkafi, S.; Fendri, I. Characterization and Biotechnological Potential of Two Native Marine Microalgae Isolated from the Tunisian Coast. Appl. Sci. 2021, 11, 5295. [Google Scholar] [CrossRef]
- Ben Amor, F.; Elleuch, F.; Ben Hlima, H.; Garnier, M.; Saint-Jean, B.; Barkallah, M.; Pichon, C.; Abdelkafi, S.; Fendri, I. Proteomic Analysis of the Chlorophyta Dunaliella New Strain AL-1 Revealed Global Changes of Metabolism during High Carotenoid Production. Mar. Drugs 2017, 15, 293. [Google Scholar] [CrossRef]
- Mehra, A.; Uz Zafar, S.; Jutur, P. Optimization of Biomass Production by Chlorella saccharophila UTEX 247 Employing Response Surface Methodology. Biomass Convers. Biorefin. 2024, 14, 8549–8561. [Google Scholar] [CrossRef]
- Chtourou, H.; Dahmen, I.; Hassairi, I.; Abdelkafi, S.; Sayadi, S.; Dhouib, A. Dunaliella Sp. a Wild Algal Strain Isolated from the Sfax-Tunisia Solar Evaporating Salt-Ponds, a High Potential for Biofuel Production Purposes. J. Biobased Mat. Bioenergy 2014, 8, 27–34. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Springer: New York, NY, USA, 1975; pp. 29–60. [Google Scholar] [CrossRef]
- Cameron, H.; Mata, M.T.; Riquelme, C. The Effect of Heavy Metals on the Viability of Tetraselmis marina AC16-MESO and an Evaluation of the Potential Use of This Microalga in Bioremediation. PeerJ 2018, 6, e5295. [Google Scholar] [CrossRef]
- Rachlin, J.W.; Warkentine, B.; Jensen, T.E. The Growth Responses of Chlorella saccharophila, Navicula incerta, and Nitzschia closterium to Selected Concentrations of Cadmium. Bull. Torrey Bot. Club 1982, 109, 129. [Google Scholar] [CrossRef]
- Ponnuchamy, K.; Muthiah, R.; Kumaraguru, A. Solvent Extraction and Spectrophotometric Determination of Pigments of Some Algal Species from the Shore of Puthumadam, Southeast Coast of India. Int. J. Oceans Oceanogr. 2010, 1, 29–34. [Google Scholar]
- Elleuch, J.; Hmani, R.; Drira, M.; Michaud, P.; Fendri, I.; Abdelkafi, S. Potential of Three Local Marine Microalgae from Tunisian Coasts for Cadmium, Lead, and Chromium Removals. Sci. Total Environ. 2021, 799, 149464. [Google Scholar] [CrossRef]
- Abdelkafi, S.; Fouquet, B.; Barouh, N.; Durner, S.; Pina, M.; Scheirlinckx, F.; Villeneuve, P.; Carrière, F. In vitro comparisons between Carica papaya and pancreatic lipases during test meal lipolysis: Potential use of CPL in enzyme replacement therapy. Food Chem. 2009, 115, 488–494. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Ben Ali, Y.; Abdelkafi, S.; Leclaire, J.; Fotiadu, F.; Carrière, F.; Abousalham, A. In vitro stereoselective hydrolysis of diacylglycerols by hormone-sensitive lipase. BBA Mol. Cell Biol. Lipids 2010, 1801, 77–83. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A Colorimetric Method for the Determination of Sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef]
- Rausch, T.; Butcher, D.N.; Hilgenberg, W. Nitrilase Activity in Clubroot Diseased Plants. Physiol. Plant. 1981, 52, 467–470. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Elleuch, J.; Tounsi, S.; Belguith Ben Hassen, N.; Noël Lacoix, M.; Chandre, F.; Jaoua, S.; Zribi Zghal, R. Characterisation of Novel Bacillus thuringiensis Isolates against Aedes aegypti (Diptera: Culicidae) and Ceratitis capitata (Diptera: Tephridae). J. Invertebr. Pathol. 2015, 124, 90–97. [Google Scholar] [CrossRef]
- Chakravorty, M.; Nanda, M.; Bisht, B.; Sharma, R.; Kumar, S.; Mishra, A.; Vlaskin, M.S.; Chauhan, P.K.; Kumar, V. Heavy Metal Tolerance in Microalgae: Detoxification Mechanisms and Applications. Aquat. Toxicol. 2023, 260, 106555. [Google Scholar] [CrossRef]
- Purbonegoro, T.; Suratno; Puspitasari, R.; Husna, N.A. Toxicity of Copper on the Growth of Marine Microalgae Pavlova sp. and Its Chlorophyll-a. IOP Conf. Ser. Earth Environ. Sci. 2018, 118, 12060. [Google Scholar] [CrossRef]
- Suratno, S.; Puspitasari, R.; Purbonegoro, T.; Mansur, D. Copper and Cadmium Toxicity to Marine Phytoplankton, Chaetoceros gracilis and Isochrysis sp. Indones. J. Chem. 2015, 15, 172–178. [Google Scholar] [CrossRef]
- Sun, C.; Li, W.; Xu, Y.; Hu, N.; Ma, J.; Cao, W.; Sun, S.; Hu, C.; Zhao, Y.; Huang, Q. Effects of Carbon Nanotubes on the Toxicities of Copper, Cadmium, and Zinc Toward the Freshwater Microalgae Scenedesmus obliquus. Aquat. Toxicol. 2020, 224, 105504. [Google Scholar] [CrossRef]
- Giloni-Lima, P.C.; Delello, D.; Cremonez, M.L.M.; Éler, M.N.; Lima, V.A.; Espíndola, E.L.G. A Study of the Effects of Chromium Exposure on the Growth of Pseudokirchneriella subcapitata (Korshikov) Hindak Evaluated by Central Composite Design and Response Surface Methodology. Ecotoxicology 2010, 19, 1095–1101. [Google Scholar] [CrossRef]
- Elleuch, J.; Thabet, J.; Ghribi, I.; Jabeur, H.; Hernández, L.E.; Fendri, I.; Abdelkafi, S. Responses of Dunaliella Sp. AL-1 to Chromium and Copper: Biochemical and Physiological Studies. Chemosphere 2024, 364, 143133. [Google Scholar] [CrossRef]
- Leite, L.D.S.; Daniel, L.A. Optimization of Microalgae Harvesting by Sedimentation Induced by High pH. Water Sci. Technol. 2020, 82, 1227–1236. [Google Scholar] [CrossRef]
- Shaikh, S.M.R.; Hassan, M.K.; Nasser, M.S.; Sayadi, S.; Ayesh, A.I.; Vasagar, V. A Comprehensive Review on Harvesting of Microalgae Using Polyacrylamide-Based Flocculants: Potentials and Challenges. Sep. Purif. Technol. 2021, 277, 119508. [Google Scholar] [CrossRef]
- Nowicka, B.; Pluciński, B.; Kuczyńska, P.; Kruk, J. Physiological Characterization of Chlamydomonas reinhardtii Acclimated to Chronic Stress Induced by Ag, Cd, Cr, Cu, and Hg Ions. Ecotoxicol. Environ. Saf. 2016, 130, 133–145. [Google Scholar] [CrossRef]
- Lu, M.-M.; Gao, F.; Li, C.; Yang, H.-L. Response of Microalgae Chlorella vulgaris to Cr Stress and Continuous Cr Removal in a Membrane Photobioreactor. Chemosphere 2021, 262, 128422. [Google Scholar] [CrossRef]
- Tripathi, S.; Poluri, K.M. Heavy Metal Detoxification Mechanisms by Microalgae: Insights from Transcriptomics Analysis. Environ. Pollut. 2021, 285, 117443. [Google Scholar] [CrossRef]
- Cavalletti, E.; Romano, G.; Palma Esposito, F.; Barra, L.; Chiaiese, P.; Balzano, S.; Sardo, A. Copper Effect on Microalgae: Toxicity and Bioremediation Strategies. Toxics 2022, 10, 527. [Google Scholar] [CrossRef]
- Roca, M.; Pérez-Gálvez, A. Metabolomics of Chlorophylls and Carotenoids: Analytical Methods and Metabolome-Based Studies. Antioxidants 2021, 10, 1622. [Google Scholar] [CrossRef]
- Rodríguez, M.C.; Barsanti, L.; Passarelli, V.; Evangelista, V.; Conforti, V.; Gualtieri, P. Effects of Chromium on Photosynthetic and Photoreceptive Apparatus of the Alga Chlamydomonas reinhardtii. Environ. Res. 2007, 105, 234–239. [Google Scholar] [CrossRef]
- Zeraatkar, A.K.; Ahmadzadeh, H.; Talebi, A.F.; Moheimani, N.R.; McHenry, M.P. Potential Use of Algae for Heavy Metal Bioremediation: A Critical Review. J. Environ. Manag. 2016, 181, 817–831. [Google Scholar] [CrossRef]
- Silva, J.C.; Echeveste, P.; Lombardi, A.T. Higher Biomolecules Yield in Phytoplankton under Copper Exposure. Ecotoxicol. Environ. Saf. 2018, 161, 57–63. [Google Scholar] [CrossRef]
- El Agawany, N.; Kaamoush, M.; El-Zeiny, A.; Ahmed, M. Effect of Heavy Metals on Protein Content of Marine Unicellular Green Alga Dunaliella tertiolecta. Environ. Monit. Assess. 2021, 193, 584. [Google Scholar] [CrossRef]
- Hedayatkhah, A.; Cretoiu, M.S.; Emtiazi, G.; Stal, L.J.; Bolhuis, H. Bioremediation of chromium contaminated water by diatoms with concomitant lipid accumulation for biofuel production. J. Environ. Manag. 2018, 227, 313–320. [Google Scholar] [CrossRef]
- Mulgund, A. Increasing Lipid Accumulation in Microalgae through Environmental Manipulation, Metabolic and Genetic Engineering: A Review in the Energy NEXUS Framework. Energy Nexus 2022, 5, 100054. [Google Scholar] [CrossRef]
- He, Q.; Yang, H.; Wu, L.; Hu, C. Effect of Light Intensity on Physiological Changes, Carbon Allocation and Neutral Lipid Accumulation in Oleaginous Microalgae. Bioresour. Technol. 2015, 191, 219–228. [Google Scholar] [CrossRef]
- Yilancioglu, K.; Cokol, M.; Pastirmaci, I.; Erman, B.; Cetiner, S. Oxidative Stress Is a Mediator for Increased Lipid Accumulation in a Newly Isolated Dunaliella Salina Strain. PLoS ONE 2014, 9, e91957. [Google Scholar] [CrossRef]
- Danouche, M.; El Ghachtouli, N.; Aasfar, A.; Bennis, I.; El Arroussi, H. Pb(II)-Phycoremediation Mechanism Using Scenedesmus obliquus: Cells Physicochemical Properties and Metabolomic Profiling. Heliyon 2022, 8, e08967. [Google Scholar] [CrossRef]
- Olofsson, M.; Lamela, T.; Nilsson, E.; Bergé, J.P.; Del Pino, V.; Uronen, P.; Legrand, C. Seasonal Variation of Lipids and Fatty Acids of the Microalgae Nannochloropsis oculata Grown in Outdoor Large-Scale Photobioreactors. Energies 2012, 5, 1577–1592. [Google Scholar] [CrossRef]
- Miazek, K.; Iwanek, W.; Remacle, C.; Richel, A.; Goffin, D. Effect of Metals, Metalloids and Metallic Nanoparticles on Microalgae Growth and Industrial Product Biosynthesis: A Review. Int. J. Mol. Sci. 2015, 16, 23929–23969. [Google Scholar] [CrossRef]
- Kaur, M.; Saini, K.C.; Ojah, H.; Sahoo, R.; Gupta, K.; Kumar, A.; Bast, F. Abiotic Stress in Algae: Response, Signaling and Transgenic Approaches. J. Appl. Phycol. 2022, 34, 1843–1869. [Google Scholar] [CrossRef]
- Vieira, B.H.; Rodgher, S.; Haneda, R.N.; Lombardi, A.T.; Melão, M.d.G.G.; Daam, M.A.; Espíndola, E.L.G. Importance of Different Exposure Routes on the Toxicity of Chromium to Planktonic Organisms. Aquat. Ecol. 2023, 58, 175–189. [Google Scholar] [CrossRef]
- Kafil, M.; Berninger, F.; Koutra, E.; Kornaros, M. Utilization of the Microalga Scenedesmus quadricauda for Hexavalent Chromium Bioremediation and Biodiesel Production. Bioresour. Technol. 2022, 346, 126665. [Google Scholar] [CrossRef]
- Bauenova, M.O.; Sadvakasova, A.K.; Mustapayeva, Z.O.; Kokociński, M.; Zayadan, B.K.; Wojciechowicz, M.K.; Balouch, H.; Akmukhanova, N.R.; Alwasel, S.; Allakhverdiev, S.I. Potential of Microalgae Parachlorella kessleri Bh-2 as Bioremediation Agent of Heavy Metals Cadmium and Chromium. Algal Res. 2021, 59, 102463. [Google Scholar] [CrossRef]
- Rugnini, L.; Costa, G.; Congestri, R.; Bruno, L. Testing of Two Different Strains of Green Microalgae for Cu and Ni Removal from Aqueous Media. Sci. Total Environ. 2017, 601–602, 959–967. [Google Scholar] [CrossRef]
- Zhang, Y.; Vallin, J.R.; Sahoo, J.K.; Gao, F.; Boudouris, B.W.; Webber, M.J.; Phillip, W.A. High-Affinity Detection and Capture of Heavy Metal Contaminants Using Block Polymer Composite Membranes. ACS Cent. Sci. 2018, 4, 1697–1707. [Google Scholar] [CrossRef]
- Liu, J.; Mukherjee, J.; Hawkes, J.J.; Wilkinson, S.J. Optimization of Lipid Production for Algal Biodiesel in Nitrogen Stressed Cells of Dunaliella salina Using FTIR Analysis. J. Chem. Technol. Biotechnol. 2013, 88, 1807. [Google Scholar]
- Chandrashekharaiah, P.S.; Sanyal, D.; Dasgupta, S.; Banik, A. Cadmium Biosorption and Biomass Production by Two Freshwater Microalgae Scenedesmus acutus and Chlorella pyrenoidosa: An Integrated Approach. Chemosphere 2021, 269, 128755. [Google Scholar] [CrossRef]
- Tripathi, S.; Arora, N.; Gupta, P.; Pruthi, P.A.; Poluri, K.M.; Pruthi, V. Microalgae. In Advanced Biofuels; Elsevier: Amsterdam, The Netherlands, 2019; pp. 97–128. ISBN 978-0-08-102791-2. [Google Scholar]
- Razzak, S.A.; Lucky, R.A.; Hossain, M.M.; deLasa, H. Valorization of Microalgae Biomass to Biofuel Production: A Review. Energy Nexus 2022, 7, 100139. [Google Scholar] [CrossRef]
- Onen Cinar, S.; Chong, Z.K.; Kucuker, M.A.; Wieczorek, N.; Cengiz, U.; Kuchta, K. Bioplastic Production from Microalgae: A Review. Int. J. Environ. Res. Public Health 2020, 17, 3842. [Google Scholar] [CrossRef]
- Djamal, Z.; Henni, A. Outdoor Microalgae Cultivation for Wastewater Treatment. In Application of Microalgae in Wastewater Treatment: Volume 1: Domestic and Industrial Wastewater Treatment; Springer: Berlin/Heidelberg, Germany, 2019; pp. 81–99. ISBN 978-3-030-13912-4. [Google Scholar]
| Treatments | Growth Rate (Day−1) | Standard Deviation |
|---|---|---|
| Untreated control | 0.19 | 0.007 |
| After exposure to Cu | 0.07 | 0.009 * |
| After exposure to Cr | 0.14 | 0.003 |
| After exposure to Cu and Cr | 0.11 | 0.022 * |
| Fatty Acid Composition (% of Total Fatty Acids) | ||||
|---|---|---|---|---|
| Untreated Sample (%) | After Exposure to Cu (%) | After Exposure to Cr (%) | After Exposure to Cu and Cr (%) | |
| Myristic acid (C14:0) | 1.09 | 1.32 | 1.12 | 1.36 |
| Palmitic acid (C16:0) | 17.25 | 29.76 | 28.76 | 35.96 |
| Arachidic acid (C20:0) | 16.83 | 7.49 | 9.79 | 2.99 |
| Stearic acid (C18:0) | 3.52 | 5.6 | 4.58 | 5.6 |
| Total saturated (%) | 38.69 | 44.17 | 44.25 | 45.91 |
| Palmitoleic acid (C16:1) | 5.5 | 4.44 | 3.62 | 6.01 |
| Oleic acid (C18:1) | 33.72 | 38.03 | 34.02 | 39.54 |
| Gadoleic acid (C20:1) | 0.73 | 1.42 | 1.04 | 1.25 |
| Total monounsaturated (%) | 39.95 | 43.89 | 38.68 | 46.8 |
| Linoleic acid (C18:2) | 9.18 | 6.92 | 7.24 | 4.86 |
| Linolenic acid (C18:3) | 12.18 | 5.02 | 9.83 | 2.43 |
| Total PUFAs (%) | 21.36 | 11.94 | 17.07 | 7.29 |
| Processing Conditions | Metal Concentration (mg/L) | Metal Uptake (%) | Cells Responses | ||
|---|---|---|---|---|---|
| Cu | Cr | Cu | Cr | ||
| Cu | 57.61 | - | 58.11 | - |
|
| Cr | - | 28.62 | - | 41.4 |
|
| Cu + Cr | 57.61 | 28.62 | 58.43 | 57.71 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghribi, I.; Elleuch, J.; Dubessay, P.; Michaud, P.; Abdelkafi, S.; Fendri, I. Biotechnological Potential of Newly Isolated Microalga Strain in Cu and Cr Biosorption from Single and Bimetallic Systems. Water 2025, 17, 999. https://doi.org/10.3390/w17070999
Ghribi I, Elleuch J, Dubessay P, Michaud P, Abdelkafi S, Fendri I. Biotechnological Potential of Newly Isolated Microalga Strain in Cu and Cr Biosorption from Single and Bimetallic Systems. Water. 2025; 17(7):999. https://doi.org/10.3390/w17070999
Chicago/Turabian StyleGhribi, Imtinen, Jihen Elleuch, Pascal Dubessay, Philippe Michaud, Slim Abdelkafi, and Imen Fendri. 2025. "Biotechnological Potential of Newly Isolated Microalga Strain in Cu and Cr Biosorption from Single and Bimetallic Systems" Water 17, no. 7: 999. https://doi.org/10.3390/w17070999
APA StyleGhribi, I., Elleuch, J., Dubessay, P., Michaud, P., Abdelkafi, S., & Fendri, I. (2025). Biotechnological Potential of Newly Isolated Microalga Strain in Cu and Cr Biosorption from Single and Bimetallic Systems. Water, 17(7), 999. https://doi.org/10.3390/w17070999










