Abstract
The production of volatile fatty acids (VFAs) through the acidogenic fermentation of wastewater is an emerging technology that requires further research to optimize operational variables for specific substrates. Cassava wastewater, which is a byproduct of the cassava sour starch extraction process, has been minimally studied regarding its potential for VFA production through acidogenic fermentation. Batch reactors were used to evaluate the effects of the substrate-to-microorganism (S/M) ratio and temperature on VFA production from cassava wastewater. The results showed no statistically significant differences between the evaluated S/M ratios. The maximum total VFA concentration observed was 2214.64 mg of acetic acid (HAc)/L (0.32 gCODVFA/gCOD), which was achieved at a S/M ratio of 4 gCOD/gVS. This concentration was predominantly composed of acetic acid (42.7%), followed by butyric acid (30.1%) and propionic acid (24.6%), with a minor quantity of isovaleric acid (2.6%). The statistical analysis for the temperature variable showed significant differences between the evaluated conditions. The maximum concentration of total VFAs was 2650.19 mgHAc/L (0.45 gCODVFA/gCOD) at 34 ± 1 °C, with acetic (40.9%), butyric (29.8%), and propionic (29.3%) acids as primary metabolites. Cassava wastewater shows promise as a potential substrate for VFA production, warranting evaluation in continuous reactors.
1. Introduction
Volatile fatty acids (VFAs) are organic acids that are primarily derived from petroleum refining, meeting approximately 90% of market demand [1]. However, these acids are also intermediates in natural microbial metabolic pathways and have the potential to be produced through the acidogenic fermentation (AF) of organic biomass by mixed microbial cultures [2,3]. AF is a stage derived from the initial phase of conventional anaerobic digestion, in which fermentative bacteria metabolize simple organic compounds—such as monosaccharides, long-chain fatty acids, glycerol, and amino acids—generated through the hydrolysis of complex particulate material to produce VFAs, alcohols, ketones, carbon dioxide, hydrogen, ammonia, sulfur, and new bacterial cells [4,5]. VFAs have wide applications in biological nutrient removal, pharmaceuticals, and the food and chemical industries. Moreover, they serve as feedstock for various products, including biogas, biodiesel, bioplastic, biohydrogen, biofertilizer, and biosurfactant [6].
In recent years, there has been increasing interest in researching the production of VFAs from wastewater [3,7,8,9,10]. Laboratory and pilot-scale studies, employing both continuous and batch reactors, have explored VFA production from various substrates. These investigations aimed to assess the impact of operational factors, such as hydraulic retention time (HRT), pH, temperature (T), the substrate-to-microorganism (S/M) ratio, substrate characteristics and concentrations, and nutrient composition and availability, as well as headspace partial pressure on the process. Operating parameters play a significant role in determining thermodynamically viable reactions and the growth of the most efficient microorganisms [11].
Cassava wastewater (CWW) is a byproduct of the cassava sour starch extraction process. By 2020, global starch production ranged from 88.1 to 97.7 million tons, with 14% of it derived from cassava [12]. In Colombia, sour starch is produced in small- and medium-sized processing plants through a fermentation process and is primarily used in baked goods [13]. Sweet or native starch is produced by large industries, is not fermented, and has no odor or flavor. It is primarily used as a binder in the food industry—such as in cookies, meat products, pasta, and seasoning sauces—but also finds applications in other sectors including paper and cardboard, adhesives, and textiles [14].
Treating CWW through AF not only produces VFAs but also helps mitigate environmental impacts on receiving water sources. These impacts are mainly due to the addition of substances (such as organic matter, cyanide, and acids), heat, and microorganisms to the water, which cause temperature fluctuations, increased populations of microorganisms, and the eutrophication of the receiving body [15].
AF holds promise as a technology; however, there is limited information on yields and an insufficient understanding of the mechanisms involved in metabolic changes (changes in the type of products), especially concerning CWW. These metabolic changes are primarily influenced by operational factors. For CWW, previous studies evaluated the effect of fermentation time, pH, alkalinity, biomass adaptation, and methanogenesis inhibition techniques on VFA production [16,17,18]. Nevertheless, research on the impact of S/M ratio and temperature on AF of CWW is lacking. To our knowledge, the scientific literature reveals no studies evaluating the effect of the S/M ratio on VFA production from the substrate of interest, and temperature analysis has been conducted in only one study [16].
On the one hand, the S/M ratio can influence the oxidative and reductive pathways involved in VFA production [19]. Proper adjustment of the amount of inoculum is also crucial in fermentation processes. According to Arevalo Ortiz and Arias Arroyo [20], large inoculum concentrations can lead to substrate loss due to intense competition among microorganisms, resulting in cell death and excess heat. Conversely, low inoculum concentrations increase the risk of contamination by undesirable microorganisms and prolong fermentation time, consequently reducing productivity. De Sousa e Silva et al. [19] evaluated the impact of the S/M ratio (0.8 to 1.9 gCOD/gVSS) on the profile and kinetics of VFA production from the AF of dairy wastewater. The authors concluded that although increasing the S/M ratio did not affect the percentage conversion of dairy wastewater to carboxylic acids (42 to 44%), it positively influenced productivity. Pérez-Morales et al. [21] analyzed the effects of pH (5.04 to 7.41) and the S/M ratio (25.86 to 71.99 gCOD/gVSS) on VFA production from raw cheese whey. They found that decreasing the S/M ratio at a neutral pH enhanced VFA production, achieving the highest degree of acidification (45.37%) at an S/M ratio of 32.63 gCOD/gVSS and a pH of 7.0. Vergine et al. [22] tested three S/M ratios (1.6, 4.0, and 6.4 gCOD/gVSS) and different initial alkalinity values (1.0 to 2.5 gCaCO3/L) for synthetic soft drink wastewater. The authors achieved the maximum degree of acidification (70.3 ± 0.4%) at an S/M ratio of 4.0 gCOD/gVSS and an initial alkalinity of 2.0 gCaCO3/L. Similarly, Silva et al. [23] maximized VFA production (up to 0.63 gVFA/gCOD) from cheese whey with an initial alkalinity of 5 to 7 gCaCO3/L and an S/M ratio of 2 to 4 gCOD/gVSS. Finally, in a study focused on hydrogen production from CWW, Mañunga [24] evaluated the effect of the S/M ratio and observed that VFA concentration increased as the S/M ratio rose from 2 to 6 gCOD/gVS.
On the other hand, temperature plays a crucial role in microbial growth and metabolism, as most fermentative bacteria are unable to survive in extreme temperature conditions. Increasing temperature reduces the activation energy of enzymes [25]. Furthermore, each type of microorganism has an optimal temperature range, and variations in bioreactor temperature can modify the structure of the microbial consortium involved in AF [25,26].
Hasan et al. [16] investigated the effect of temperature (30 to 50 °C) and alkalinity (2 to 4 g/L sodium bicarbonate) on VFA production from CWW using a central composite design and found the highest yield (3400 mgTVFA/L) at 30 °C and 3 g/L sodium bicarbonate. The authors recommended a mesophilic temperature due to its lower energy demand and the more stable operation of the fermentation process. Furthermore, in their study on hydrogen production from glucose in batch reactors, Wang and Wan [27] analyzed VFA production across a temperature range of 20 to 55 °C. They observed that the production of ethanol and acetic acid—accounting for more than 86% of the total soluble metabolites—increased as the temperature rose from 20 to 35 °C but began to decline when the temperature was raised further from 35 to 55 °C. In a separate study, Yu and Fang [28] investigated the influence of pH (4.0 to 7.0) and temperature (20 to 55 °C) on the acidification of gelatin-rich wastewater using an up-flow anaerobic reactor. The authors observed that both the degree of acidification and the rate of VFA and alcohol formation increased slightly with rising temperature. Most studies evaluating the impact of temperature on VFA production utilized biological waste or food waste as a substrate [11,29,30].
Since the exploration of CWW for VFA production is still in its early stages, this study aimed to assess the individual effects of the S/M ratio and temperature on VFA production and composition through AF, employing a completely randomized design. Understanding the acidogenic potential of an organic waste stream, which refers to the quantity of VFAs produced through the fermentation of organic constituents and knowledge of their profiles, is crucial for developing locally based biorefinery concepts aimed at producing value-added compounds [23]. This study stands out as one of the few to assess the potential for recovering soluble metabolites from CWW, offering valuable insights to orient future research on continuous acidogenic reactors.
2. Materials and Methods
2.1. Substrate and Inoculum
CWW was used as a substrate for the AF process. The substrate underwent physico-chemical characterization and was stored at 4 °C for less than 24 h to preserve its properties.
Sludge from an up-flow anaerobic sludge blanket (UASB) reactor at a pig slaughterhouse wastewater treatment plant served as the inoculum. The inoculum was gradually adapted to the substrate to enrich it with acidogenic microorganisms capable of converting the carbohydrates present in the CWW into VFAs. After adaptation, the inoculum was heat-pretreated at 85 °C for 30 min [24] to inhibit methanogenic archaea activity and promote the accumulation of VFAs, thereby preventing their conversion to methane.
2.2. Acidogenic Fermentation Assays
Two experiments (E1 and E2) evaluated the individual effects of the S/M ratio and temperature on VFA production from CWW by AF. Batch flow amber glass flasks with a total volume of 400 mL (200 mL for reaction and 200 mL for headspace) served as acidogenic reactors. Each reactor was loaded with the substrate and inoculum at the specified S/M ratio. The initial pH of the mixture was adjusted to 5.4 using Na2HPO4 (Thermo Fisher Scientific Inc., Waltham, MA, USA) or HCl (Merck KGaA, Darmstadt, Germany) solutions. The pH value was chosen based on the results of previous studies [3,24]. A magnetic bar to promote complete mixing and a cap with two NaOH (Merck KGaA, Darmstadt, Germany) pellets for CO2 absorption were inserted into each reactor. The reactors were then sealed with rubber septa and aluminum seals and incubated at the designated temperature for 6 d [18]. A control was established for each experiment.
At the end of the experiment, a sample of the liquid phase was collected and analyzed for pH, total solids (TS), volatile solids (VS), soluble chemical oxygen demand (SCOD), total VFAs, total alkalinity, bicarbonate alkalinity, total carbohydrates, and VFA composition. Figure 1 illustrates the unit and procedure adopted in experiments E1 and E2.
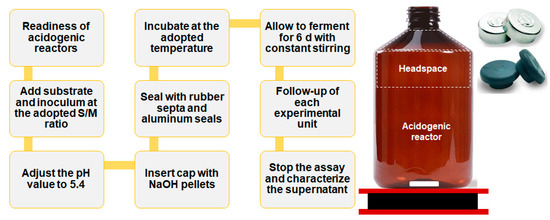
Figure 1.
Scheme of the unit and experimental procedure adopted in the AF of CWW.
The yield for each evaluated experimental condition was calculated using Equation (1), as follows [31]:
where CODVFA(final) represents the COD of VFAs produced in the reactors, and TCODinitial and CODVFA(initial) are the total COD and the COD of the VFAs present in the feed, respectively.
2.3. Experimental Design and Statistical Analyses
A completely randomized design was used to evaluate the individual effects of the S/M ratio and temperature variables. Five S/M ratios and four temperatures were tested in experiments E1 and E2, respectively, with each condition performed in triplicate. The evaluated ranges were adopted based on results reported in the scientific literature. Table 1 displays the conditions of E1 and E2.

Table 1.
Characteristics of the experiments assessing the effects of S/M ratio and temperature on VFA production through the AF of CWW.
A one-way analysis of variance (ANOVA) with a 95% confidence level (ρ < 0.05) was used to investigate significant differences among the S/M ratios and temperatures evaluated. When ANOVA indicated significant differences, a multiple comparison test was employed. All analyses were performed using R Studio software version 2023.06.1+524.
2.4. Analytical Methods
The pH, total and bicarbonate alkalinity, TS, and VS were determined following standard methods [32]. An iris HI801 spectrophotometer (Hanna Instruments, Woonsocket, RI, USA) was used to measure TCOD, SCOD, ammonia nitrogen, and orthophosphates by adapting USEPA method 410.4, the Nessler method, and the ascorbic acid method, respectively. Total carbohydrates were analyzed using the phenol-sulfuric acid method described by Dubois et al. [33]. Total VFAs were analyzed by adapting the potentiometric titration method described by DiLallo and Albertson [34].
A Perkin Elmer gas chromatograph model Clarus 590 equipped with a flame ionization detector (FID) (T = 250 °C) was used to analyze VFA composition. Nitrogen served as the carrier gas, and filtered liquid samples were injected into an Elite-FFAP capillary column (30 m length, 0.25 mm ID, 0.25 μm DF) in split mode with a 20:1 split ratio. A mixture of VFAs (acetic, propionic, butyric, isobutyric, valeric, isovaleric, 4-methylvaleric, hexanoic, and heptanoic acids) at a concentration of 10 mM was used to prepare the standards for the calibration curve. From this concentration, six standards of 1, 2, 4, 6, 8, and 10 mM were prepared. The weight of the mixture and solvent (0.12 M HCl) was measured to convert the concentration to parts per million (ppm) and to correct the concentration readings from the gas chromatograph. Using the corrected weights of each standard and the areas provided by the gas chromatograph, calibration curves for each acid were constructed, along with calculations of the method’s precision. Finally, the precision and accuracy of the method were evaluated by measuring a standard five times using the gas chromatograph and assessing the variability of the results. A 5 mM standard was prepared, and the coefficient of variation for each acid was calculated, with good precision defined as a value below 5%.
3. Results and Discussion
3.1. Physicochemical Characterization of CWW and Inoculum Solids Concentration
The physicochemical characteristics of the substrate and the solids concentration of the inoculum used to evaluate the effect of the S/M ratio and temperature on VFA production are shown in Table 2 and Table 3, respectively. These values are consistent with those found in studies using the same or similar substrates [18,24].

Table 2.
Physicochemical characterization of CWW in the set of experiments.

Table 3.
Concentration of total and volatile solids in the inoculum utilized in the set of experiments.
3.2. VFA Production, Yields, and Substrate Uptake from CWW
Figure 2 shows the production and yield of VFAs in the AF of CWW at different S/M ratios and temperatures. The results for each condition evaluated in experiments E1 and E2 are presented as the average of three replicates, which is a decision informed by a thorough cost and time analysis to ensure the feasibility and efficiency of this study.
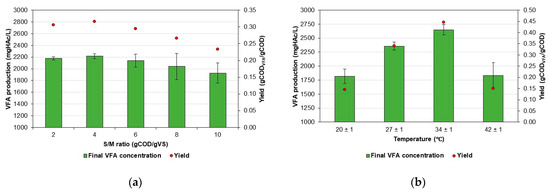
Figure 2.
Production and yield of VFAs by AF of CWW. (a) Effect of the S/M ratio and (b) effect of the temperature.
Figure 2a shows an increase in VFA production when the S/M ratio changed from 2 to 4 gCOD/gVS. Nevertheless, S/M ratios greater than 4 gCOD/gVS resulted in decreased VFA production in the bioreactors. A maximum VFA concentration of 2214.64 mgHAc/L, representing an increase of 98.4% compared to the initial VFA concentration and a yield of 0.32 gCODVFA/gCOD, was measured at an S/M ratio of 4 gCOD/gVS. The lowest VFA concentration was measured at an S/M ratio of 10 gCOD/gVS, which was equivalent to 1928.33 mgHAc/L and a yield of 0.23 gCODVFA/gCOD.
A statistical analysis showed no significant differences (ρ = 0.1514) in VFA production between the S/M ratios tested. Table 4 summarizes the ANOVA results for the S/M ratio variable.

Table 4.
Summary of one-way ANOVA results for the S/M ratio variable.
Few studies in the scientific literature evaluate the impact of the S/M ratio on VFA production from wastewater [19,21,23]. However, the production trend observed in our study and the S/M ratio that favored VFA production are consistent with findings from previous studies. Silva et al. [23] evaluated the effect of S/M ratio (2, 4, 7, and 10 gCOD/gVSS) and alkalinity (1, 2, 5, and 7 gCaCO3/L) on VFA production from cheese whey in 320 mL batch reactors, and the experiments were conducted in triplicate. Using response surface analysis, they confirmed that at lower alkalinity levels, raising the S/M ratio from 2 to 10 gCOD/gVSS significantly reduced the degree of acidification, from 50% to less than 29%. Moreover, they achieved maximum VFA production (up to 0.63 gVFA/gCODfed) with initial alkalinity levels between 5 and 7 gCaCO3/L and S/M ratios between 3 and 4 gCOD/gVSS. De Sousa e Silva et al. [19] investigated the influence of the S/M ratio (0.8, 1.2, 1.6, and 1.9 gCOD/gVSS) on the profile and kinetics of VFA production from the AF of dairy wastewater, using 300 mL batch reactors and conducting three replicates for each experimental condition. The authors concluded that increasing the S/M ratio did not affect the percentage conversion of dairy wastewater to VFAs (42–44%), but it did significantly enhance productivity (100–200%). Based on their kinetic parameter estimates, an S/M ratio of 1.6 was deemed most suitable for VFA production from the AF of dairy wastewater under the evaluated conditions. However, the study by Vergine et al. [22] found that an S/M ratio of 1.6 gCOD/gVSS was unstable, as acidogenic conditions were not maintained until the end of the experiment. The authors evaluated the AF of synthetic soft drink wastewater in triplicate using 320 mL batch reactors with a mixed microbial culture. They varied the S/M ratio (1.6, 4.0, and 6.4 gCOD/gVSS) and the initial alkalinity (1.0 to 2.5 gCaCO3/L). After 21 d of fermentation, the highest acidification rate achieved was 70.3 ± 0.4%, which was observed at an S/M ratio of 4.0 gCOD/gVSS and an initial alkalinity of 2.0 gCaCO3/L. In this study, the S/M ratio proved to be the key factor in sustaining acidogenic conditions by suppressing methanogenic archaea and other microorganisms that consume VFAs. Also, Wang et al. [35] evaluated the influence of the S/M ratio (0.2 to 4.09 gTScassava/gVSSsludge) on the AF of cassava powder using triplicate 1000 mL CSTR reactors operated at a pH of 6 and a temperature of 35 °C. During the first 22 h of fermentation, the authors observed that the VFA concentration increased with the S/M ratio, except at 4.09 gTScassava/gVSSsludge, at which the VFA production rate was lower than at S/M ratios of 2.05 and 3.07 gTScassava/gVSSsludge. The authors attributed this behavior to inhibition by high substrate concentrations and the need for a longer acclimatization period for the acidogenic microorganisms. However, after 22 h and by the end of the fermentation period, the S/M ratio of 4.09 gTScassava/gVSSsludge outperformed the others, achieving a VFA concentration of 6.79 gCOD/L (VFA/COD of 72%).
Nevertheless, other studies reported differing findings. Karaca et al. [36] investigated the optimization of polyhydroxyalkanoate (PHA) production using acidified dairy wastewater as a substrate. In batch reactors at 37 °C, they evaluated the initial S/M ratios of 1, 2.5, 5, and 10 gCOD/gVSS on VFA production. The authors noted that the acidification efficiency increased with the S/M ratio, achieving a maximum yield of 5.2 gCOD/gVSS (51.7%) at 10 gCOD/gVSS on the fourth day of fermentation. Also, Pérez-Morales et al. [21], evaluating the same substrate (cheese whey) used by Silva et al. [23], arrived at different conclusions. The authors evaluated the influence of pH (5.04 to 7.41), the S/M ratio (25.86 to 71.99 gCOD/gVSS), and their interaction in wide ranges within the limits of process feasibility. The authors concluded that both pH and the S/M ratio significantly affected VFA production, while their interaction was insignificant. Using response surface analysis, they observed that at a constant pH, a decrease in the S/M ratio favored the yield. The maximum yield (45.37%) was achieved with an S/M ratio of 32.63 gCOD/gVSS and a pH of 7.0. The significant impact of the S/M ratio in this study may be attributed to the broader range evaluated compared to the one tested in our study, as well as differences in the operational conditions used (e.g., pH, temperature, alkalinity, fermentation time). Consistent with our findings, Casero-Díaz et al. [37] concluded that the tested S/M ratios (2 and 4 gCOD/gVS) had no significant effect on VFA yield during the AF of three fish canning wastewaters using duplicate 500 mL batch reactors.
Based on the findings of our study and previous reports, an S/M ratio of 4 gCOD/gVS could be used to produce VFA from CWW.
Figure 2b shows that VFA production increases with temperature up to 34 ± 1 °C, after which it declines. At this temperature, a VFA concentration of 2650.19 mgHAc/L was measured, corresponding to a yield of 0.45 gCODVFA/gCOD. Increasing the temperature to 42 ± 1 °C resulted in a drop in yield to 0.15 gCODVFA/gCOD.
Statistical analysis revealed significant differences in VFA production between the temperatures tested (ρ = 0.0002271). Table 5 summarizes the ANOVA results for the temperature variable.

Table 5.
Summary of one-way ANOVA results for the temperature variable.
Fisher’s test was applied to identify the treatments that generated the difference, clustering the results into three groups: Group A (temperatures of 20 and 42 ± 1 °C), Group B (temperature of 27 ± 1 °C), and Group C (temperature of 34 ± 1 °C).
Studies emphasized the significant influence of temperature on the AF process for VFA production. However, there is a scarcity of research examining the specific impact of temperature on VFA production from wastewater. Most studies have focused on biological sludge or food waste as substrates [11,29,38]. Furthermore, it is observed that the optimal temperature depends on the type of substrate used. Fernández-Domínguez et al. [29] analyzed the impact of temperature (20, 35, 45, 55, 70 °C) on the production of VFAs in triplicate 250 mL batch reactors using biowaste collected in a mechanical–biological treatment plant. The highest VFA yields (0.49 to 0.59 gCODVFA/gVS) were observed at 35 °C. However, the differences in the tests performed at 20, 45, and 55 °C were minimal (maximum difference of 10%). Valentino et al. [30] evaluated the effect of temperatures ranging from 20 to 70 °C on the degree of acidification and production yields in batch reactors with thermally pretreated sewage sludge. They found that 55 °C was the optimal temperature for AF, achieving a degree of acidification of 0.81 ± 0.03 CODVFA/CODSOL and a yield of 0.29 ± 0.04 gCODVFA/gVS0. In addition, Garcia-Aguirre et al. [11] investigated the impact of the pH level (5.5 and 10) and temperature (35 and 55 °C) on VFA production potential in duplicate 500 mL batch reactors using seven urban and agro-industrial waste streams. The authors found that VFA production kinetics were more favorable under acidic conditions and mesophilic temperatures, leading to a quicker achievement of maximum VFA concentration. Similarly, Song et al. [39] evaluated the effects of inoculum amount, initial pH, and temperature (25 to 55 °C) on VFA production from banana waste juice in triplicate, obtaining the highest yield (223.34 mgCOD/gVS) at 35 °C due to the higher activity of VFA-producing microorganisms at that temperature. Eng et al. [40] evaluated the impact of temperature (30, 40, 50, and 60 °C) and initial pH (6, 7, and 8) in triplicate 500 mL batch reactors to recover soluble metabolites using sugarcane vinasse as substrate. Maximum yields of 0.319 and 0.337 gCOD/gCODtinitial were observed under slightly basic conditions (pH 8) for both mesophilic (40 °C) and thermophilic (60 °C) temperatures, respectively. For wastewater, Koottatep et al. [8] evaluated temperatures between 30 and 60 °C using mixed wastewater from toilets and a cafeteria (ratio of 90:10% v/v) and determined the highest production of total VFAs at 40 °C.
Additionally, in a mainly mesophilic temperature range, the VFA production trend observed in our study is consistent with that reported by other researchers. Pittmann and Steinmetz [41] evaluated the generation of VFAs as the initial stage of the polyhydroxyalkanoate production cycle using different sludges from a WWTP (primary sludge, excess sludge, a mixture of primary and digested sludge, and a mixture of excess and digested sludge). The sludges were tested under controlled pH conditions (pH 6) and without pH control at temperatures of approximately 20 and 30 °C. In six out of eight combinations, raising the temperature from 20 to 30 °C resulted in increased VFA production. Specifically, when using primary sludge as a substrate under pH-controlled conditions, the temperature change increased the degree of acidification from 14 to 31%. Eng et al. [42] assessed VFA production from sugarcane vinasse in triplicate 500 mL batch reactors using a response surface methodology, with temperatures ranging from 33 to 47 °C and the initial pH ranging from 7.1 to 9.9. The authors observed a gradual increase in soluble metabolite production with rising temperature and initial pH, reaching peak levels near optimal conditions (39.6 °C and initial pH of 8.8). For CWW, Hasan et al. [16] investigated the impact of alkalinity and temperature (between 30 and 50 °C) on VFA production and observed a negative influence for certain temperature values, measuring the highest production yield at 30 °C.
The particularities of the AF process, such as the source of inoculum and substrate complexity, undoubtedly influence fermentation performance to varying degrees. Therefore, selecting well-balanced microbial consortia helps minimize limitations associated with temperature and pH [40].
According to our results, a temperature of 34 ± 1 °C, using the selected inoculum source, is deemed suitable for VFA production from CWW. This temperature can facilitate the growth of acid-forming bacteria and promote acid-acetogenesis reactions within the system [8]. Perez-Esteban et al. [43] demonstrated higher microorganism growth (51%) at 35 °C through biomass mass balance analysis. However, they also observed acetic acid consumption at this temperature, suggesting an increased risk of such consumption likely due to the proliferation of methanogenic archaea introduced from the waste-activated sludge used as part of the substrate. In our study, the inoculum pretreatment aimed to decrease the activity of acid-consuming microorganisms.
According to Jung et al. [44], temperatures exceeding 40 °C may delay acidogenic activity due to decreased microbial growth rates, potentially resulting in reduced VFA production. Infantes et al. [45] conducted experiments with glucose at temperatures of 26, 33, and 40 °C and concluded that the energy required for cell maintenance increased at low pH and high temperatures. The authors noted that at a pH of 6, biomass increased to 1.4 and 0.9 g/L at 26 and 40 °C, respectively, confirming reduced biomass growth with increasing temperature. At elevated temperatures, the heightened permeability of the cell membrane would facilitate the passage of undissociated acids more readily, thereby increasing the energy requirements for cell maintenance.
Figure 3 shows the carbohydrate concentration in the reactor supernatant at the end of the AF trials and its consumption when the S/M ratio and temperature were evaluated.
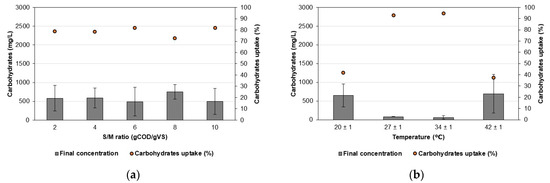
Figure 3.
Final carbohydrate concentration and uptake in AF of CWW. (a) Effect of the S/M ratio and (b) effect of the temperature.
In all evaluated S/M ratios, carbohydrate concentrations in the supernatant remained below 750 mg/L, indicating consumptions exceeding 72% (Figure 3a). Our findings partly corroborate those of Pérez-Morales et al. [21], who reported carbohydrate consumption ranging from 50.34 to 97.24% during AF of cheese whey for VFA production. Nevertheless, they observed the highest consumption at a pH of 7.01 and an S/M ratio of 32.62 gCOD/gVSS. When evaluating the temperature effect (Figure 3b), the extracted supernatant from the reactors exhibited concentrations below 520 mg/L, indicating carbohydrate consumption exceeding 90% at temperatures of 27 and 34 ± 1 °C. Infantes et al. [45] demonstrated that both biomass growth and substrate consumption are influenced by pH and temperature. The authors observed complete glucose consumption at pH levels of 5 and 6 across all evaluated temperatures (26, 33, and 40 °C). However, at a pH of 4, complete glucose consumption occurred only at 26 °C, with consumption decreasing to 42 and 23 mM as the temperature increased to 33 and 40 °C, respectively. Similar to our results, Song et al. [39] observed a decreasing trend in soluble sugar concentration during the AF of banana waste juice at temperatures of 25, 35, 40, and 45 °C, with a rapid decrease from 19.47 to 0.96, 0.50, 0.71, and 1.37 g/L, respectively, by day 4. However, at 55 °C, the soluble sugar concentration decreased more slowly, reaching a minimum value of 6.68 g/L.
The high carbohydrate consumption observed in E1 and E2 confirms the high affinity of the microorganisms for the substrate, which was also reported in previous studies [46,47].
3.3. Distribution of VFAs Produced from CWW
Figure 4 shows the composition of VFAs produced at the end of the AF trials, which investigated the influence of the S/M ratio and temperature variables.
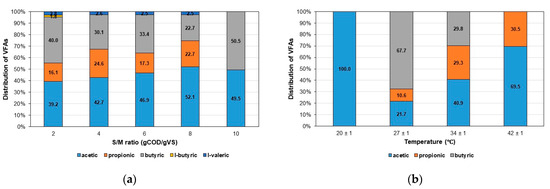
Figure 4.
Distribution of VFAs produced in AF of CWW. (a) Effect of the S/M ratio and (b) effect of the temperature.
At the beginning of the experiments, only acetic acid was detected in the CWW used as substrate. Figure 4a shows for the S/M ratio variable that at the end of the experiment, acetic and butyric acids were quantified in the greater proportion for all S/M ratios tested, followed by propionic and isovaleric acids for ratios of 2 to 8 gCOD/gVS. Also, a discrete formation of isobutyric acid was measured at 2 gCOD/gVS, suggesting a greater variety of VFAs at lower S/M ratios. Additionally, the proportion of acetic acid (ranging from 39.2 to 52.1%) tended to increase as the S/M ratio rose, except for the ratio of 10 gCOD/gVS. However, this trend contrasts with the findings of de Sousa e Silva et al. [19], who examined the impact of the S/M ratio within a range of 0.8 to 1.9 gCOD/gVSS for dairy wastewater and concluded that the percentage of COD as acetic acid decreased as the S/M ratio increased.
In our study, the percentages of butyric acid (ranging from 22.7 to 50.5%) and propionic acid (0 to 24.6%) exhibited an oscillatory behavior, resembling a zigzag pattern, as the S/M ratio increased. Interestingly, this behavior was the opposite for the two acids. Additionally, the proportion of isovaleric acid (ranging from 2.5 to 2.8%) showed minimal variation across the different S/M ratios where it was detected.
Our findings are consistent with those reported by Silva et al. [23] from AF experiments using cheese whey. The authors observed that acetic and n-butyric acids accounted for 70–90% of the total VFAs produced in all their trials. Additionally, they demonstrated that a low S/M ratio (ranging from 2 to 10 gCOD/gVSS) combined with a high alkalinity supply (ranging from 1 to 7 gCaCO3/L) could modify the VFA profile by increasing the production of propionic and n-valeric acids (up to 500 mgCOD/L). For the same substrate (cheese whey), Pérez-Morales et al. [21] observed a similar predominance of acetic, butyric, and propionic acids. However, the authors noted that the proportion of propionic acid was higher than that of butyric acid. The maximum VFA production (7.91 gCOD/L) was achieved at a pH of 7 and an S/M ratio of 32.62 gCOD/gVSS, which was composed of 32.35, 41.58, 19.45, and 6.62% of acetic, propionic, butyric (normal and iso), and valeric (normal and iso) acids, respectively. Vergine et al. [22] observed that at pH levels near 7, synthetic soft drink wastewater produced butyric and acetic acids across all tested S/M ratios (1.6, 4.0, and 6.4 gCOD/gVSS) and alkalinity conditions (ranging from 1.0 to 2.5 gCaCO3/L). Propionic acid was also produced, albeit at lower levels (approximately 10%). The study concluded that with constant initial alkalinity, increasing the S/M ratio resulted in higher percentages of butyric acid, rising from 5 to 44%. Wang et al. [35] observed a mixture of acetic, propionic, and butyric acids at the end of AF of cassava powder (55 h) for S/M ratios of 0.2 and 0.61 gTScassava/gVSSsludge. However, for higher S/M ratios (1.02, 2.05, 3.07, and 4.09 gTScassava/gVSSsludge), the mixture also included lactic acid. Acetic acid was the predominant metabolite in all S/M ratios in this study, accounting for 39 to 56% of total VFAs.
Figure 4b presents the composition of VFAs produced at the conclusion of the AF test for the four temperatures being evaluated. At 20 ± 1 °C, only acetic acid was produced in the bioreactors, whereas at 27 and 34 ± 1 °C, a mixture of acetic (21.7 and 40.9%), butyric (67.7 and 29.8%), and propionic (10.6 and 29.3%) acids was observed. At 42 ± 1 °C, butyric acid was absent from the bioreactor mixture. Moreover, there was an upward trend in the proportion of acetic and propionic acids with increasing temperature from 27 to 42 ± 1 °C, while conversely, a declining trend was noted for butyric acid. The findings at higher temperatures (34 and 42 ± 1 °C) are consistent with those reported by Jiang et al. [48] for food waste. The authors observed that acetic and propionic acids were the predominant VFAs generated at 35 and 45 °C, collectively constituting approximately 70% of the total VFAs. Additionally, Wang et al. [49] used sucrose-rich wastewater as a substrate and observed that AF was individually and interactively affected by pH, temperature, and substrate concentration, which were evaluated in ranges of 4.7 to 6.3, 25 to 45 °C, and 15 to 35 g/L, respectively. The optimal conditions for total VFA yield (1769 mmol/mol-sucrose) were determined to be a pH of 5.6, a temperature of 33.5 °C, and a sucrose concentration of 24.2 g/L. The major metabolites observed were acetate, propionate, and butyrate, along with minor quantities of isobutyrate, valerate, and caproate. Moreover, ethanol was the sole alcohol detected. Fernández-Domínguez et al. [29] analyzed the influence of temperature (20, 35, 45, 55, 70 °C) on VFA production from biowaste collected in a mechanical–biological treatment plant and concluded that the VFA profile, which was dominated by acetic, propionic, and butyric acids (75 to 86% CODVFA), remained consistent regardless of fermentation temperature or seasonality. In contrast, Perez-Esteban et al. [43] found that temperature (25, 35, 45, and 55 °C) notably influenced the VFA profile in a continuous co-fermentation process of waste-activated sludge and food waste. At 25 and 35 °C, the VFA profiles were mainly composed of acetic (34 and 37%), butyric (32 and 31%), and propionic (18 and 17%) acids, aligning qualitatively with our results at 27 and 34 ± 1 °C. However, at 45 °C, the authors observed an accumulation of caproic acid, which was not observed in our study at any evaluated temperature. At 55 °C, acetic and butyric acids predominated (40% each) in Perez-Esteban et al.’s study.
In our study, acetic, butyric, and propionic acids were evidently the primary metabolites for CWW and the variables evaluated, which is consistent with previous findings in studies using the same substrate [16,18]. Acetic acid typically forms from pyruvate and via the branching of acetyl-CoA, accompanied by the simultaneous generation of H2 and CO2. Conversely, the butyrate metabolic pathway involves pyruvate and various intermediates like acetyl-CoA, acetoacetyl-CoA, and butyryl-CoA, resulting in the generation of CO2 and ATP, alongside the utilization of NADH to reduce the intermediate [50,51]. Propionate synthesis can occur through the reduction of pyruvate, utilizing lactate as an intermediate and involving the conversion of NADH to NAD+. Alternatively, it can proceed via a pathway that includes various intermediary compounds, including oxaloacetic, malic, fumaric, and succinic acids, along with succinyl-CoA, methylmalonyl-CoA, and propionyl-CoA [50,52].
The production of VFAs in this study did not result in a decrease in pH, contrary to observations reported in previous studies [53]. In all bioreactors that evaluated the S/M ratio and temperature, the final pH of the test was higher than that initially adjusted (pH of 5.40 ± 0.01). This behavior may result from the increased bicarbonate alkalinity and buffering capacity of the medium. Although the bicarbonate alkalinity of the CWW used in the experiments was 0 mg CaCO3/L (Table 2), much of it was initially established in the system through the alkalinizer used to adjust the initial pH in the bioreactors, as Na2HPO4 can influence the acid-base equilibrium. According to Ceron et al. [54], substances with buffering capacity can prevent a drop in pH for a certain period. However, only when the alkalinity of the medium is insufficient to neutralize VFAs, a drop in pH may occur. Figure 5 illustrates the final pH for each condition evaluated in E1 and E2, along with the observed bicarbonate alkalinity at the end of the test.
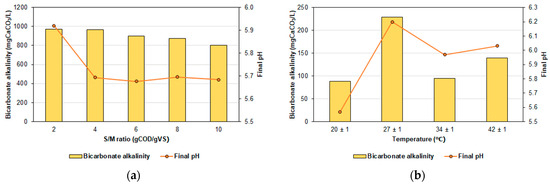
Figure 5.
Variation of pH and bicarbonate alkalinity in AF of CWW. (a) Effect of the S/M ratio and (b) effect of the temperature.
When evaluating the S/M ratio, Figure 5a indicates that pH values ranged between 5.68 and 5.92. Although the final pH was higher than the initial in all bioreactors, the lowest values were observed for S/M ratios greater than or equal to 4 gCOD/gVS. In terms of bicarbonate alkalinity, the initial value in the bioreactors was 410.56 ± 16.84 mgCaCO3/L, increasing to between 803.20 and 969.97 mgCaCO3/L by the end of the experiment (6 d). The final bicarbonate alkalinity decreased as the S/M ratio increased. Additionally, in Figure 5b, the final pH ranged between 5.57 and 6.20 when the temperature was evaluated. The higher pH was recorded at 27 ± 1 °C. Furthermore, the initial bicarbonate alkalinity was 0 mgCaCO3/L, increasing to a range of 88.69 to 229.37 mgCaCO3/L by the end of the test. Alkalinity was notably higher at temperatures of 27 °C (229.37 mgCaCO3/L) and 42 °C (139.71 mgCaCO3/L). In both experiments (E1 and E2), the conditions with the highest alkalinity also exhibited the highest pH values at the conclusion of the AF test.
The increase in pH value can be attributed to several factors mentioned in our previous study [18]. These factors include the capture of CO2 from the gas phase through NaOH pellets [55], the buffering capacity generated by the reaction of water-soluble CO2 with hydroxide ions to form bicarbonate ions [56], and the increase in bicarbonate alkalinity due to the production of ammonia nitrogen. Additionally, the increase in system pH at the end could also be due to the breakdown of fatty acids by obligate proton-reducing bacteria, methanogenic archaea, or heterotrophic acetogens [57].
Bicarbonate alkalinity can influence VFA production and composition. Our results indicate a trend in VFA production (Figure 2) that mirrors the patterns observed for bicarbonate alkalinity (Figure 5). Dahiya et al. [57] evaluated the AF of food waste with biohydrogen cogeneration and concluded that the buffering capacity of the system correlated well with total VFA production. Additionally, Hasan et al. [16] reported that the alkalinity of the medium enhanced VFA production in experiments assessing the effects of temperature and alkalinity (2 to 4 g NaHCO3/L) on CWW fermentation. Therefore, we recommend that future research evaluate the impact of alkalinity on VFA production and composition in CWW.
Finally, we would like to emphasize that significant challenges must be addressed in producing VFA from wastewater using mixed cultures in continuous reactors, both at the laboratory and pilot scale, as well as during the transition to the industrial scale.
Batch reactor studies are valuable for assessing the potential of AF for specific substrates. However, they provide limited insights into industrial processes, particularly regarding organic loading rate, recirculation, methanogen washing, inoculum adaptation, and product removal [58]. Therefore, it is essential to evaluate VFA production in continuous reactors. In continuous reactor operations, variability in wastewater composition and the inability to control optimized operational parameters can negatively affect fermentation efficiency, VFA production yield, and reproducibility. According to Ramos-Suarez et al. [58], lower productivity compared to batch fermentations is a significant disadvantage of continuous processes, making it challenging to optimize quickly and understand parameter interactions.
Optimized values of biological process operating parameters at small scales may change during scale-up, potentially leading to lower yields [59]. For the transition to a sustainable and economically viable industrial scale, AF faces several challenges, including insufficient control over the reactions occurring during fermentation (acidogenesis and acetogenesis) and the need to direct the formation of specific end products at an appropriate rate [60,61]; inhibition caused by the accumulation of fermentation products, which hinders further substrate conversion; the economic inhibition of methanogens [62]; and the separation and purification of produced VFAs, as a variety of products are generated at low concentrations and with similar physicochemical properties. This necessitates the evaluation and selection of cost-effective recovery methods to maximize VFA recovery [6].
The production of VFAs from wastewater is a promising technology, but it faces significant technical and economic challenges. Therefore, interdisciplinary collaboration, along with continuous research and innovation, is essential to overcome these obstacles, optimize processes, and ensure sustainability.
4. Conclusions
VFA production from the AF of CWW is feasible and can be enhanced by controlling the S/M ratio and temperature. While the S/M ratio had a non-significant influence, temperature significantly impacted VFA production. An S/M ratio of 4 gCOD/gVS and a temperature of 34 ± 1 °C are considered adequate to produce VFAs from CWW using a thermally pretreated inoculum. Under these conditions, yields of approximately 0.45 gCODVFA/gCOD were achieved, with variability observed in the VFAs produced, primarily consisting of acetic, butyric, and propionic acids. Overall, CWW is a potential substrate for VFA production. Future research should pay special attention to butyric acid production, given its relatively high market price and the natural predominance of butyric-type fermentation in acidogenic systems fed with CWW.
Author Contributions
Conceptualization, L.M.S.-L., J.A.R.-V. and H.R.-M.; methodology, L.M.S.-L., J.A.R.-V. and H.R.-M.; validation, L.M.S.-L.; formal analysis, L.M.S.-L.; investigation, L.M.S.-L.; resources, L.M.S.-L.; data curation, L.M.S.-L.; writing—original draft preparation, L.M.S.-L.; writing—review and editing, J.A.R.-V. and H.R.-M.; visualization, L.M.S.-L.; supervision, J.A.R.-V. and H.R.-M.; project administration, H.R.-M.; funding acquisition, H.R.-M. and L.M.S.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Universidad del Valle (project CI-21237) and the Ministerio de Ciencia, Tecnología e Innovación de Colombia—MinCiencias (convocatoria No. 933—2023).
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- de Sousa e Silva, A.; Morais, N.W.S.; Coelho, M.M.H.; Pereira, E.L.; dos Santos, A.B. Potentialities of Biotechnological Recovery of Methane, Hydrogen and Carboxylic Acids from Agro-Industrial Wastewaters. Bioresour. Technol. Rep. 2020, 10, 100406. [Google Scholar] [CrossRef]
- Dahiya, S.; Lingam, Y.; Venkata Mohan, S. Understanding Acidogenesis towards Green Hydrogen and Volatile Fatty Acid Production—Critical Analysis and Circular Economy Perspective. Chem. Eng. J. 2023, 464, 141550. [Google Scholar] [CrossRef]
- Sanchez-Ledesma, L.M.; Ramírez-Malule, H.; Rodríguez-Victoria, J.A. Volatile Fatty Acids Production by Acidogenic Fermentation of Wastewater: A Bibliometric Analysis. Sustainability 2023, 15, 2370. [Google Scholar] [CrossRef]
- de Lemos Chernicharo, C.A. Anaerobic Reactors; IWA Publishing: Minas Gerais, Brazil, 2007; Volume 4, ISBN 9781843391647. [Google Scholar]
- Pavlostathis, S.G. Kinetics and Modeling of Anaerobic Treatment and Biotransformation Processes. In Comprehensive Biotechnology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 6, pp. 385–397. ISBN 9780080885049. [Google Scholar]
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-Based Volatile Fatty Acid Production and Recovery from Waste Streams: Current Status and Future Challenges. Bioresour. Technol. 2018, 268, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.M.H.; Morais, N.W.S.; Pereira, E.L.; Leitão, R.C.; dos Santos, A.B. Potential Assessment and Kinetic Modeling of Carboxylic Acids Production Using Dairy Wastewater as Substrate. Biochem. Eng. J. 2020, 156, 107502. [Google Scholar] [CrossRef]
- Koottatep, T.; Khamyai, S.; Pussayanavin, T.; Kunsit, U.; Prapasriket, P.; Polprasert, C. Meso-Thermophilic Acidogenic Biotreatment of Mixed Wastewater from Toilets and Coffeeshop: Effect of Temperature on the Efficiency of Organic Removal and VFA Productions. Biomass Convers. Biorefinery 2022, 13, 12431–12436. [Google Scholar] [CrossRef]
- Morais, N.W.S.; Coelho, M.M.H.; de Sousa e Silva, A.; Pereira, E.L.; Leitão, R.C.; dos Santos, A.B. Kinetic Modeling of Anaerobic Carboxylic Acid Production from Swine Wastewater. Bioresour. Technol. 2020, 297, 122520. [Google Scholar] [CrossRef]
- Morais, N.W.S.; Coelho, M.M.H.; Ferreira, T.J.T.; Pereira, E.L.; Leitão, R.C.; dos Santos, A.B. A Kinetic Study on Carboxylic Acids Production Using Bovine Slaughterhouse Wastewater: A Promising Substrate for Resource Recovery in Biotechnological Processes. Bioprocess Biosyst. Eng. 2021, 44, 271–282. [Google Scholar] [CrossRef]
- Garcia-Aguirre, J.; Aymerich, E.; de Goñi, J.G.-M.; Esteban-Gutiérrez, M. Selective VFA Production Potential from Organic Waste Streams: Assessing Temperature and pH Influence. Bioresour. Technol. 2017, 244, 1081–1088. [Google Scholar] [CrossRef]
- Vilpoux, O.F.; Santos Silveira Junior, J.F. Global Production and Use of Starch. In Starchy Crops Morphology, Extraction, Properties and Applications. Vol 1: Underground Starchy Crops of South American Origin: Production, Processing, Utilization and Economic Perspectives; Elsevier: Amsterdam, The Netherlands, 2022; Volume 1, pp. 43–66. [Google Scholar] [CrossRef]
- Taborda-Andrade, L.A. Determinación y Análisis Integral de Impactos de La Agroindustria Rural de Almidón de Yuca En Cauca Colombia. Ph.D. Thesis, Universidad Nacional de Colombia, Palmira, Colombia, 2018. [Google Scholar]
- Canales, N.; Trujillo, M. La Red de Valor de La Yuca y Su Potencial En La Bioeconomía de Colombia; Stockholm Environment Institute: Estocolmo, Suecia, 2021. [Google Scholar]
- Howeler, R.H.; Oates, C.G.; Costa Allem, A. Strategic Environmental Assessment. An Assessment of the Impact of Cassava Production and Processing on the Environment and Biodiversity; Food and Agriculture Organization of the United Nations International Fund for Agricultural Development: Rome, Italy, 2001; Volume 5. [Google Scholar]
- Hasan, S.D.M.; Giongo, C.; Fiorese, M.L.; Gomes, S.D.; Ferrari, T.C.; Savoldi, T.E. Volatile Fatty Acids Production from Anaerobic Treatment of Cassava Waste Water: Effect of Temperature and Alkalinity. Environ. Technol. 2015, 36, 2637–2646. [Google Scholar] [CrossRef]
- Niz, M.Y.K.; Formagini, E.L.; Boncz, M.À.; Paulo, P.L. Acidogenic Fermentation of Cassava Wastewater for Volatile Fatty Acids Production. Int. J. Environ. Waste Manag. 2020, 25, 245–261. [Google Scholar] [CrossRef]
- Sanchez-Ledesma, L.M.; Rodríguez-Victoria, J.A.; Ramírez-Malule, H. Effect of Fermentation Time, PH, and Their Interaction on the Production of Volatile Fatty Acids from Cassava Wastewater. Water 2024, 16, 1514. [Google Scholar] [CrossRef]
- de Sousa e Silva, A.; Tavares Ferreira, T.J.; Sales Morais, N.W.; Lopes Pereira, E.; Bezerra dos Santos, A. S/X Ratio Impacts the Profile and Kinetics of Carboxylic Acids Production from the Acidogenic Fermentation of Dairy Wastewater. Environ. Pollut. 2021, 287, 117605. [Google Scholar] [CrossRef] [PubMed]
- Arevalo Ortiz, H.F.; Arias Arroyo, G.C. Determinación de La Concentración de Inoculo y Tiempo de Fermentacion, Utilizando Microbiota de Los Granos de Kefir Como Agente Biológico y Suero de Leche Como Sustrato. Cienc. Investig. 2008, 11, 16–22. [Google Scholar] [CrossRef]
- Pérez-Morales, J.; B.-Arroyo, C.; Morales-Zarate, E.; Hernández-García, H.; Méndez-Acosta, H.O.; Hernández-Martínez, E. Mathematical Modeling of Volatile Fatty Acids Production from Cheese Whey: Evaluation of PH and Substrate-Inoculum Ratio Effects. Fuel 2021, 287, 119510. [Google Scholar] [CrossRef]
- Vergine, P.; Sousa, F.; Lopes, M.; Silva, F.; Gameiro, T.; Nadais, H.; Capela, I. Synthetic Soft Drink Wastewater Suitability for the Production of Volatile Fatty Acids. Process Biochem. 2015, 50, 1308–1312. [Google Scholar] [CrossRef]
- Silva, F.C.; Serafim, L.S.; Nadais, H.; Arroja, L.; Capela, I. Acidogenic Fermentation towards Valorisation of Organic Waste Streams into Volatile Fatty Acids. Chem. Biochem. Eng. Q. 2013, 27, 467–476. [Google Scholar]
- Mañunga, T. Acople Entre Un Reactor Anaerobio de Medio Suspendido y Un Reactor Anaerobio de Crecimiento Adherido Para La Producción de Hidrógeno y Metano a Partir de Agua Residual Del Proceso de Extracción de Almidón de Yuca. Ph.D. Thesis, Universidad del Valle, Cali, Colombia, 2019. [Google Scholar]
- Bastidas-Oyanedel, J.-R.; Bonk, F.; Thomsen, M.H.; Schmidt, J.E. Dark Fermentation Biorefinery in the Present and Future (Bio)Chemical Industry. Rev. Environ. Sci. Biotechnol. 2015, 14, 473–498. [Google Scholar] [CrossRef]
- Wainaina, S.; Lukitawesa; Kumar Awasthi, M.; Taherzadeh, M.J. Bioengineering of Anaerobic Digestion for Volatile Fatty Acids, Hydrogen or Methane Production: A Critical Review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef]
- Wang, J.; Wan, W. Effect of Temperature on Fermentative Hydrogen Production by Mixed Cultures. Int. J. Hydrogen Energy 2008, 33, 5392–5397. [Google Scholar] [CrossRef]
- Yu, H.Q.; Fang, H.H.P. Acidogenesis of Gelatin-Rich Wastewater in an Upflow Anaerobic Reactor: Influence of PH and Temperature. Water Res. 2003, 37, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Domínguez, D.; Astals, S.; Peces, M.; Frison, N.; Bolzonella, D.; Mata-Alvarez, J.; Dosta, J. Volatile Fatty Acids Production from Biowaste at Mechanical-Biological Treatment Plants: Focusing on Fermentation Temperature. Bioresour. Technol. 2020, 314, 123729. [Google Scholar] [CrossRef] [PubMed]
- Valentino, F.; Chitharanjan, A.; Micolucci, F.; Pavan, P.; Gottardo, M. Valuable Routes for Sewage Sludge Utilization: Effect of Temperature and Hydraulic Retention Time in the Acidogenic Fermentation Process. Chem. Eng. Trans. 2022, 93, 193–198. [Google Scholar] [CrossRef]
- Bolaji, I.O.; Dionisi, D. Acidogenic Fermentation of Vegetable and Salad Waste for Chemicals Production: Effect of PH Buffer and Retention Time. J. Environ. Chem. Eng. 2017, 5, 5933–5943. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater; Amer Public Health Assn: Washington, DC, USA, 2005. [Google Scholar]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A Colorimetric Method for the Determination of Sugars. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- DiLallo, R.; Albertson, O. Volatile Acids by Direct Titration. Water Pollut. Control Fed. 1961, 33, 356–365. [Google Scholar]
- Wang, X.; Zhang, S.; Wang, J.; Yu, X.; Lu, X. Exploring Optimal Feed to Microbes Ratio for Anaerobic Acidogenic Fermentation of Cassava Residue from Brewery. Bioresources 2012, 7, 1111–1122. [Google Scholar] [CrossRef]
- Karaca, S.; Yagci, N.; Randall, C.W. Polyhydroxyalkanoate Production Using Enriched Biomass and Acidogenic Fermentation Products of Dairy Wastewater and Organic Food Waste. Desalin. Water Treat. 2021, 215, 388–397. [Google Scholar] [CrossRef]
- Casero-Díaz, T.; Castro-Barros, C.; Taboada-Santos, A.; Rodríguez-Hernández, L.; Mauricio-Iglesias, M.; Carballa, M. Turning Fish Canning Wastewater into Resources: Effluents and Operational Conditions Selection for Volatile Fatty Acids Production. J. Water Process Eng. 2024, 64, 2–9. [Google Scholar] [CrossRef]
- Liu, H.; Wang, F.; Wang, Z.; Wu, D.; Xing, T.; Kong, X.; Sun, Y. Impact of PH, Temperature, and Hydraulic Residence Time on the Acidogenic Fermentation of Fruit and Vegetable Waste and Microbial Community Analysis. J. Chem. Technol. Biotechnol. 2023, 98, 819–828. [Google Scholar] [CrossRef]
- Song, X.; Chen, G.; Wang, F.; Zhang, J.; Liu, Y.; Zhao, J. Potential of Volatile Fatty Acids Production from Banana Waste Juice: Impacts of Storage, Substrate Phase, Operation Conditions, and Microbial Community Analysis. Fuel 2024, 366, 131294. [Google Scholar] [CrossRef]
- Eng Sánchez, F.; Tadeu Fuess, L.; Soares Cavalcante, G.; Ângela Talarico Adorno, M.; Zaiat, M. Value-Added Soluble Metabolite Production from Sugarcane Vinasse within the Carboxylate Platform: An Application of the Anaerobic Biorefinery beyond Biogas Production. Fuel 2021, 286, 119378. [Google Scholar] [CrossRef]
- Pittmann, T.; Steinmetz, H. Influence of Operating Conditions for Volatile Fatty Acids Enrichment as a First Step for Polyhydroxyalkanoate Production on a Municipal Waste Water Treatment Plant. Bioresour. Technol. 2013, 148, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Eng, F.; Fuess, L.T.; Bovio-Winkler, P.; Etchebehere, C.; Sakamoto, I.K.; Zaiat, M. Optimization of Volatile Fatty Acid Production by Sugarcane Vinasse Dark Fermentation Using a Response Surface Methodology. Links between Performance and Microbial Community Composition. Sustain. Energy Technol. Assess. 2022, 53, 102764. [Google Scholar] [CrossRef]
- Perez-Esteban, N.; Vives-Egea, J.; Peces, M.; Dosta, J.; Astals, S. Temperature-Driven Carboxylic Acid Production from Waste Activated Sludge and Food Waste: Co-Fermentation Performance and Microbial Dynamics. Waste Manag. 2024, 178, 176–185. [Google Scholar] [CrossRef]
- Jung, K.; Kim, W.; Park, G.W.; Seo, C.; Chang, H.N.; Kim, Y.C. Optimization of Volatile Fatty Acids and Hydrogen Production from Saccharina Japonica: Acidogenesis and Molecular Analysis of the Resulting Microbial Communities. Appl. Microbiol. Biotechnol. 2015, 99, 3327–3337. [Google Scholar] [CrossRef]
- Infantes, D.; Gonzáles del Campo, A.; Villaseñor, J.; Fernández, F.J. Kinetic Model and Study of the Influence of PH, Temperature and Undissociated Acids on Acidogenic Fermentation. Biochem. Eng. J. 2012, 66, 66–72. [Google Scholar] [CrossRef]
- Alibardi, L.; Cossu, R. Effects of Carbohydrate, Protein and Lipid Content of Organic Waste on Hydrogen Production and Fermentation Products. Waste Manag. 2016, 47, 69–77. [Google Scholar] [CrossRef]
- Castilla-Archilla, J.; Papirio, S.; Lens, P.N.L. Two Step Process for Volatile Fatty Acid Production from Brewery Spent Grain: Hydrolysis and Direct Acidogenic Fermentation Using Anaerobic Granular Sludge. Process Biochem. 2021, 100, 272–283. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Y.; Li, K.; Wang, Q.; Gong, C.; Li, M. Volatile Fatty Acids Production from Food Waste: Effects of PH, Temperature, and Organic Loading Rate. Bioresour. Technol. 2013, 143, 525–530. [Google Scholar] [CrossRef]
- Wang, G.; Mu, Y.; Yu, H.Q. Response Surface Analysis to Evaluate the Influence of PH, Temperature and Substrate Concentration on the Acidogenesis of Sucrose-Rich Wastewater. Biochem. Eng. J. 2005, 23, 175–184. [Google Scholar] [CrossRef]
- Lee, H.S.; Salerno, M.B.; Rittmann, B.E. Thermodynamic Evaluation on H2 Production in Glucose Fermentation. Environ. Sci. Technol. 2008, 42, 2401–2407. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhou, J.; Tan, M.; Du, J.; Yan, B.; Wong, J.W.C.; Zhang, Y. Enhanced Carboxylic Acids Production by Decreasing Hydrogen Partial Pressure during Acidogenic Fermentation of Glucose. Bioresour. Technol. 2017, 245, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Feng, L.; Zhang, C.; Wisniewski, C.; Zhou, Q. Ultrasonic Enhancement of Waste Activated Sludge Hydrolysis and Volatile Fatty Acids Accumulation at pH 10.0. Water Res. 2010, 44, 3329–3336. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Burgos, W.J.; Sydney, E.B.; de Paula, D.R.; Medeiros, A.B.P.; de Carvalho, J.C.; Soccol, V.T.; de Souza Vandenberghe, L.P.; Woiciechowski, A.L.; Soccol, C.R. Biohydrogen Production in Cassava Processing Wastewater Using Microbial Consortia: Process Optimization and Kinetic Analysis of the Microbial Community. Bioresour. Technol. 2020, 309, 123331. [Google Scholar] [CrossRef]
- Ceron, C.; Andres, A.; Vidal, P.; Lozada, T.; Del, I.; La, Y.; En, A.; Tratamiento, E.L.; Las, A.D.E. Importancia Del PH y La Alcalinidad En El Tratamiento Anaerobio de Las Aguas Residuales Del Proceso de Extracción de Almidón de Yuca. Sci. Tech. 2005, 11, 243–248. [Google Scholar]
- Pabón Pereira, C.P.; Castañares, G.; Van Lier, J.B. An OxiTop® Protocol for Screening Plant Material for Its Biochemical Methane Potential (BMP). Water Sci. Technol. 2012, 66, 1416–1423. [Google Scholar] [CrossRef]
- Hilkiah Igoni, A.; Ayotamuno, M.J.; Eze, C.L.; Ogaji, S.O.T.; Probert, S.D. Designs of Anaerobic Digesters for Producing Biogas from Municipal Solid-Waste. Appl. Energy 2008, 85, 430–438. [Google Scholar] [CrossRef]
- Dahiya, S.; Sarkar, O.; Swamy, Y.V.; Venkata Mohan, S. Acidogenic Fermentation of Food Waste for Volatile Fatty Acid Production with Co-Generation of Biohydrogen. Bioresour. Technol. 2015, 182, 103–113. [Google Scholar] [CrossRef]
- Ramos-Suarez, M.; Zhang, Y.; Outram, V. Current Perspectives on Acidogenic Fermentation to Produce Volatile Fatty Acids from Waste. Rev. Environ. Sci. Bio/Technol. 2021, 20, 439–478. [Google Scholar] [CrossRef]
- Wu, H.; Scheve, T.; Dalke, R.; Holtzapple, M.; Urgun-Demirtas, M. Scaling up Carboxylic Acid Production from Cheese Whey and Brewery Wastewater via Methane-Arrested Anaerobic Digestion. Chem. Eng. J. 2023, 459, 140080. [Google Scholar] [CrossRef]
- Arslan, D.; Steinbusch, K.J.J.; Diels, L.; Hamelers, H.V.M.; Strik, D.P.B.T.B.; Buisman, C.J.N.; De Wever, H. Selective Short-Chain Carboxylates Production: A Review of Control Mechanisms to Direct Mixed Culture Fermentations. Crit. Rev. Environ. Sci. Technol. 2016, 46, 592–634. [Google Scholar] [CrossRef]
- Rafay, R.; Allegue, T.; Fowler, S.J.; Rodríguez, J. Exploring the Limits of Carbohydrate Conversion and Product Formation in Open Mixed Culture Fermentation. J. Environ. Chem. Eng. 2022, 10, 107513. [Google Scholar] [CrossRef]
- Agler, M.T.; Wrenn, B.A.; Zinder, S.H.; Angenent, L.T. Waste to Bioproduct Conversion with Undefined Mixed Cultures: The Carboxylate Platform. Trends Biotechnol. 2011, 29, 70–78. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).