Biological Production and Nitrogen Use Efficiency in a Water-Sharing and Water-Saving System Combining Aquaculture and Vegetable Hydroponic Cultivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Treatments, Measurements, and Calculations
2.2. Measurement of Nitrogen Content
2.3. Statistical Analysis
3. Results
3.1. Loach Growth and FCR
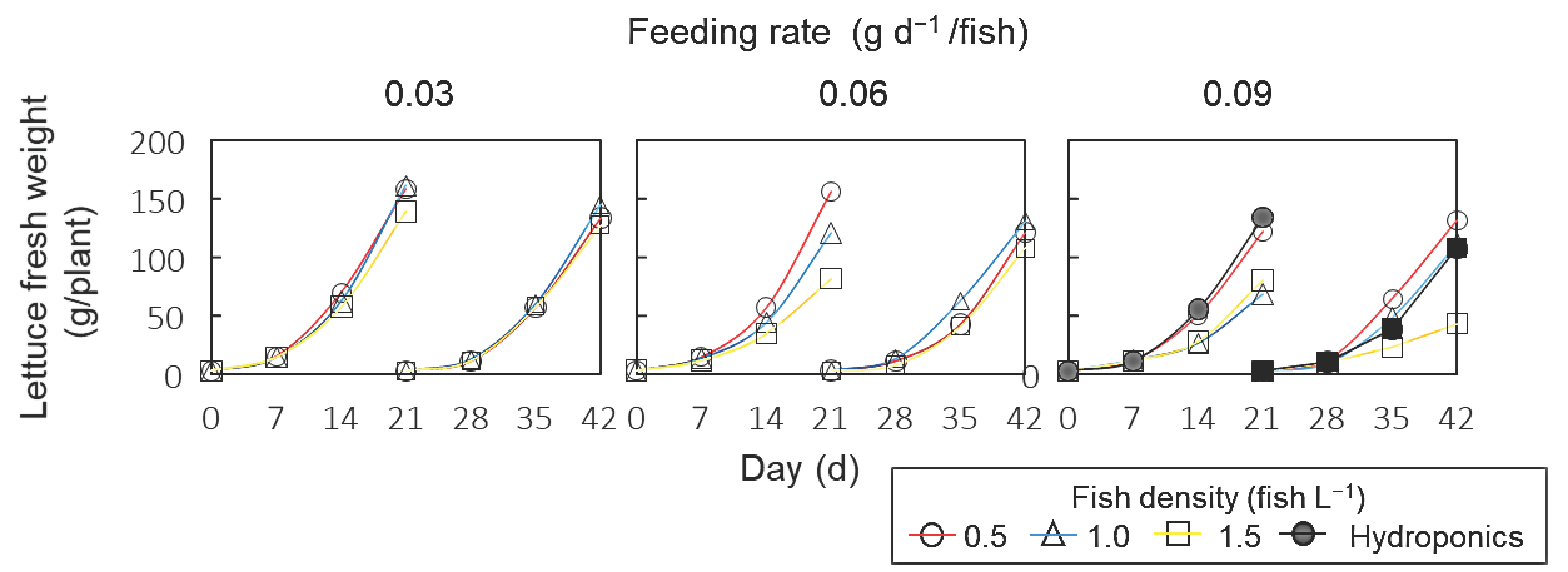
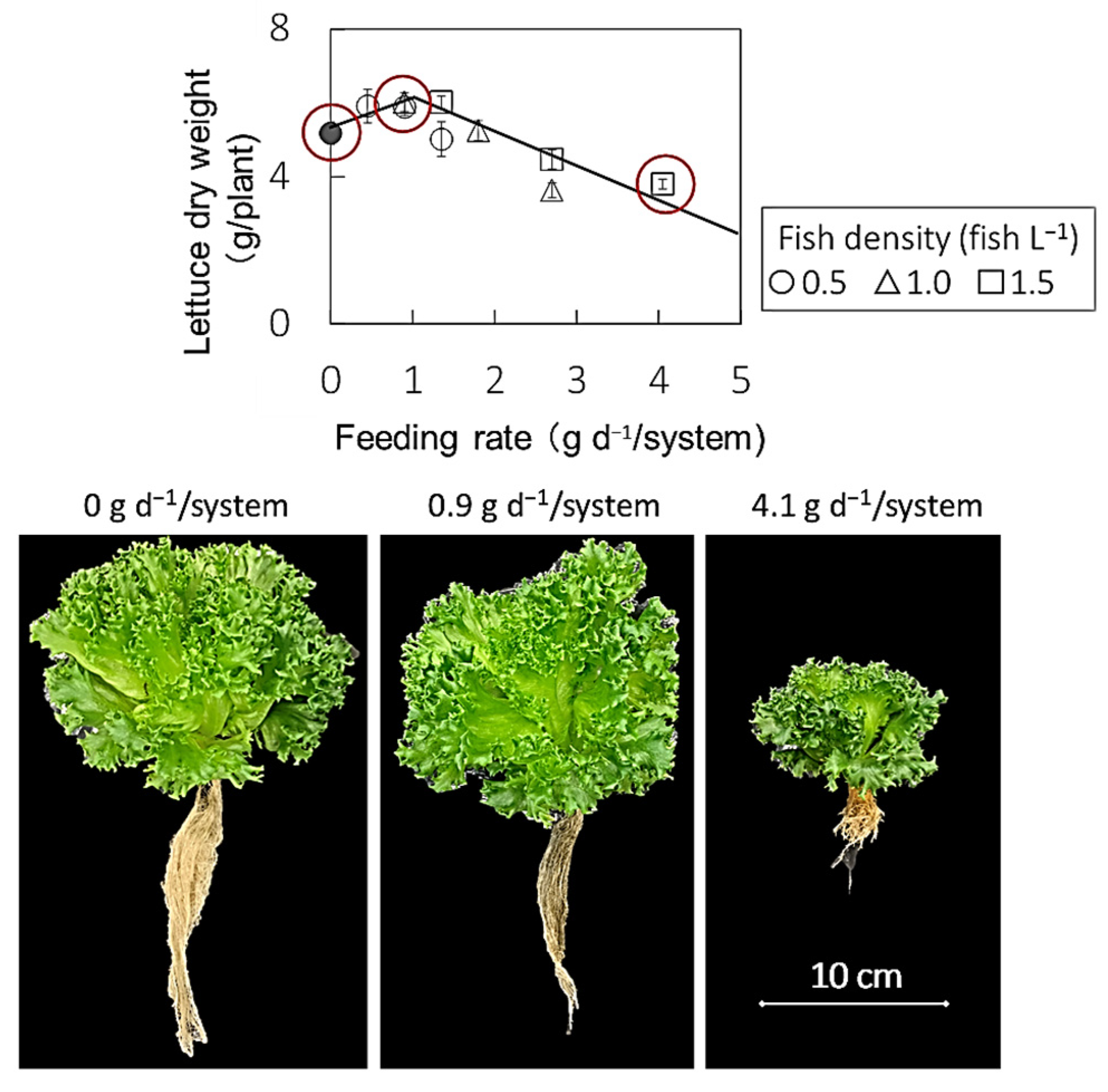
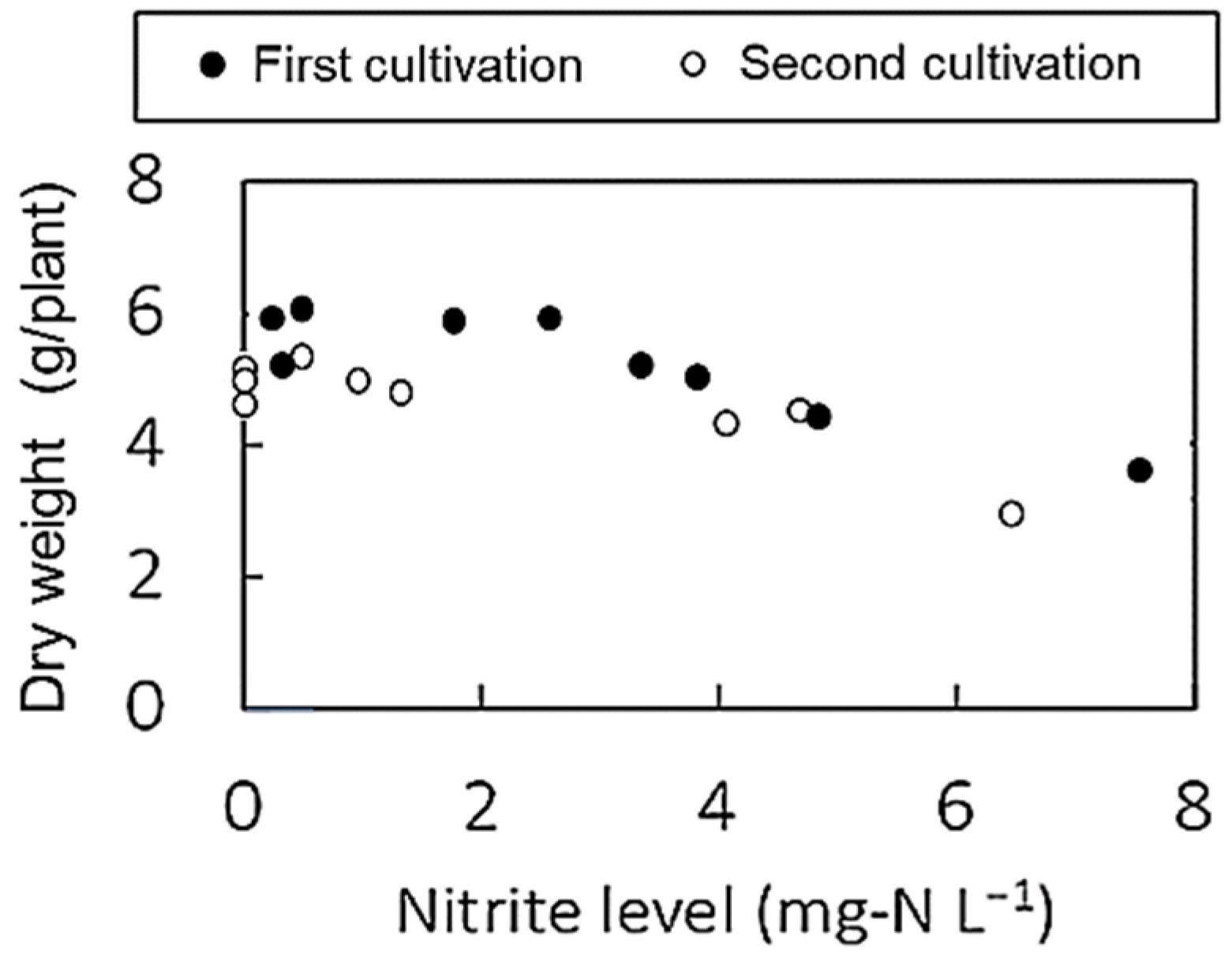
3.2. Lettuce Growth
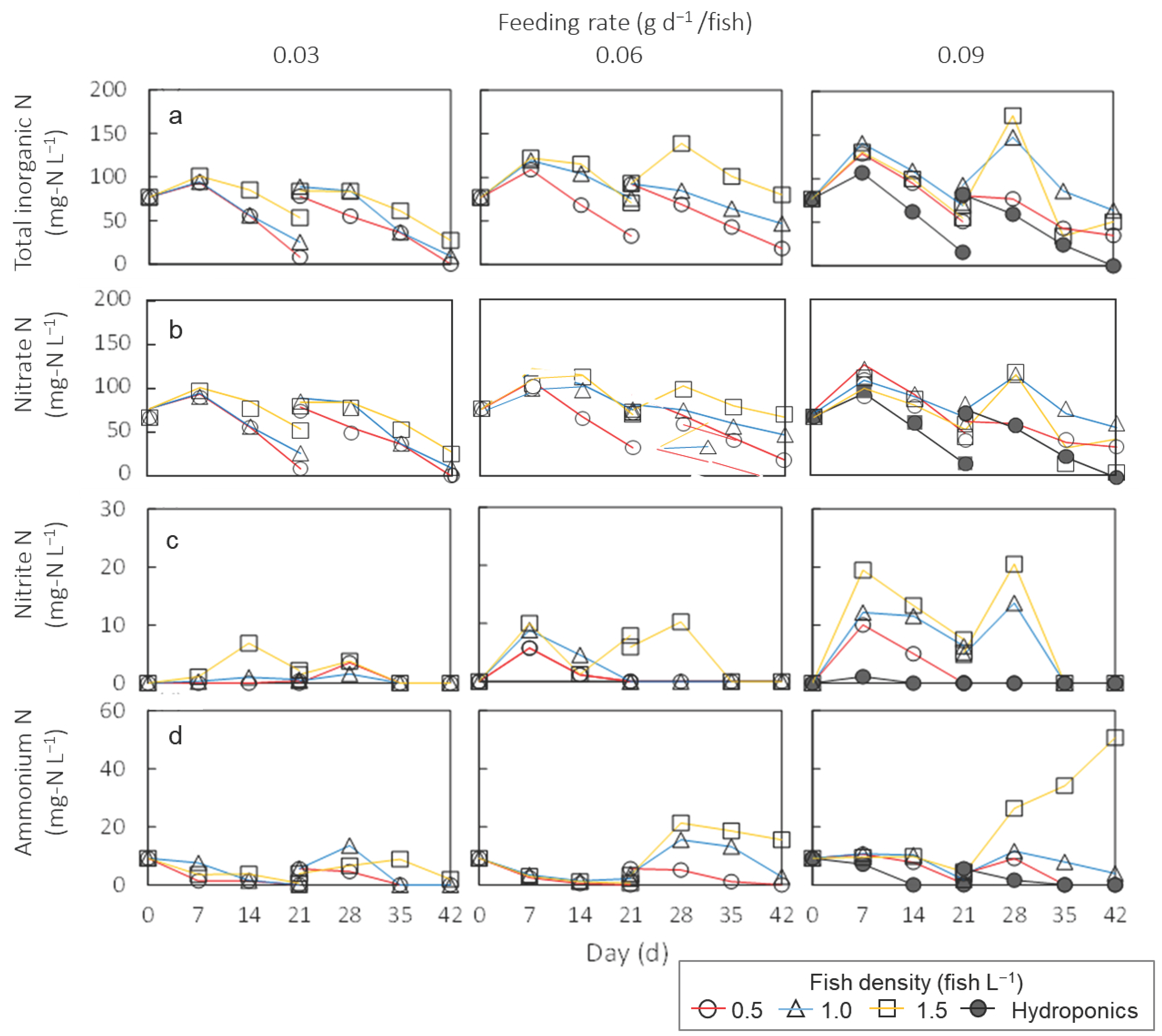
3.3. Nitrogen Dynamics
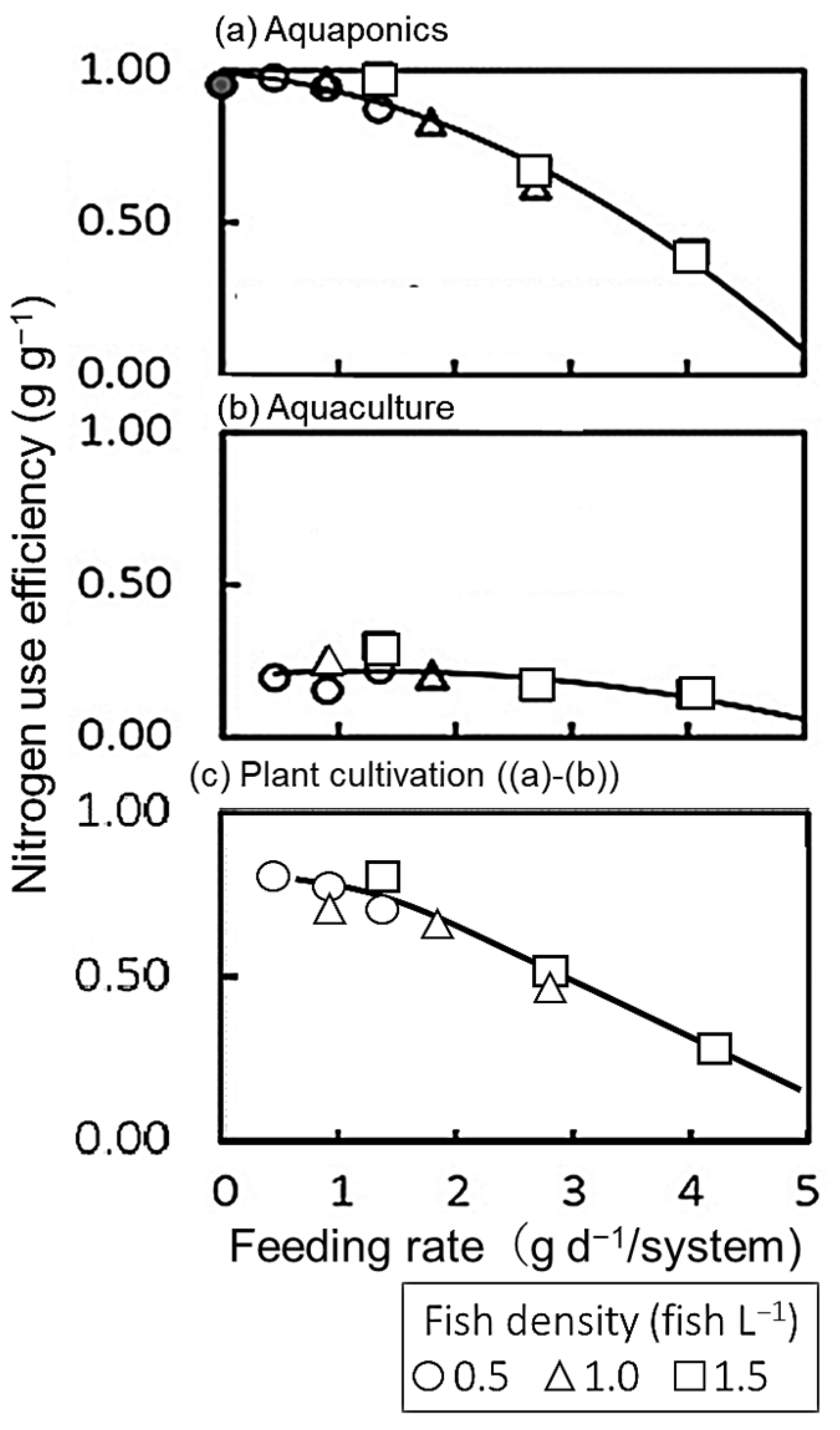
3.4. Nitrogen Use Efficiency (NUE)
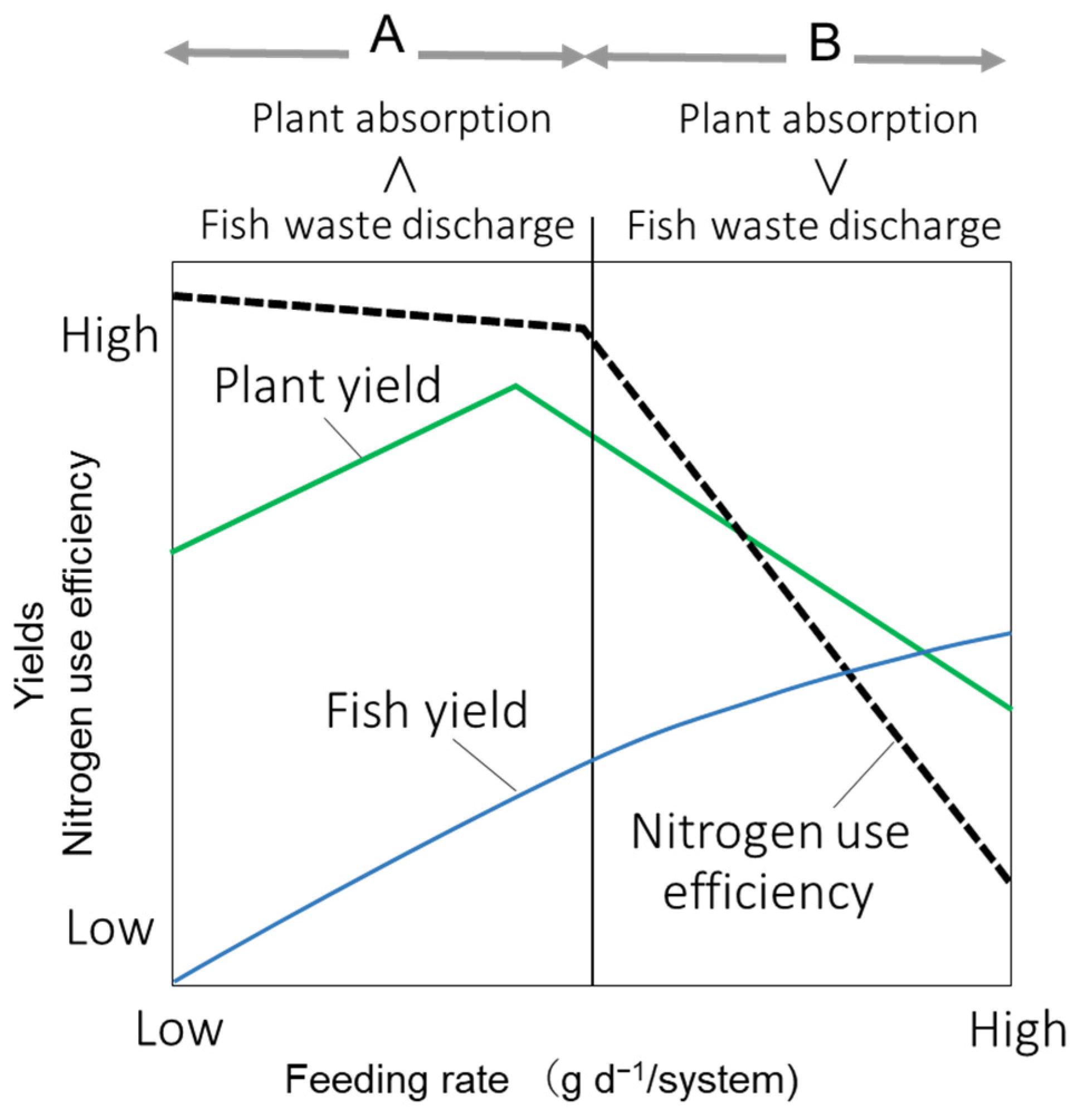
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| NUE | Nitrogen use efficiency |
| FCR | Feed conversion ratio |
References
- Yamada, S. Development of Aquaponics Combined with Open Culture Adapting to Arid Regions for Sustainable Food Production. Science and Technology Research Partnership for Sustainable Development. 2015, pp. 1–20. Available online: https://www.macs-g20.org/fileadmin/macs/Annual_Meetings/2020_SaudiArabia/Documents/Activites/water__Energy__Food_Nexus/1.Session_1/1.4_Session_Mexico_Acuaponia_Tottori_CIBNOR_G20workshop_Final_26_ago_2020.pdf (accessed on 1 March 2022).
- Goddek, S.; Delaide, B.; Mankasingh, U.; Ragnarsdottir, K.V.; Jijakli, H.; Thorarinsdottir, R. Challenges of sustainable and commercial aquaponics. Sustainability 2015, 7, 4199–4224. [Google Scholar] [CrossRef]
- Wongkiew, S.; Hu, Z.; Chandran, K.; Lee, J.W.; Khanal, S.K. Nitrogen transformations in aquaponic systems: A review. Aquac. Eng. 2017, 76, 9–19. [Google Scholar]
- Endut, A.; Jusoh, A.; Ali, N.; Wan Nik, W.B. Nutrient removal from aquaculture wastewater by vegetable production in aquaponics recirculation system. Desalin. Water Treat. 2011, 32, 422–430. [Google Scholar]
- Neto, R.M.; Ostrensky, A. Nutrient load estimation in the waste of Nile tilapia Oreochromis niloticus (L.) reared in cages in tropical climate conditions. Aquac. Res. 2013, 46, 1309–1322. [Google Scholar]
- Petrea, S.M.; Cristea, V.; Dediu, I.; Contoman, M.; Ion, S.P.; Mocanu, M.C.; Antache, A. A Study of Nitrogen Cycle in an Integrated Aquaponic System with Different Plant Densities. Bull. UASVM Anim. Sci. Biotechnol. 2013, 70, 55–64. [Google Scholar]
- Shimakawa, Y.; Kitaya, Y.; Shibuya, T.; Endo, R. Plant Growth and Nitrogen Flow in an Aquaponics System Integrating Lettuce Hydroponics and Loach Aquaculture. Discov. Agric. Food Sci. 2023, 11, 89–95. [Google Scholar]
- Kitaya, Y.; Kawamoto, T.; Endo, R.; Shibuya, T. Effect of fish density on biological production in aquaponics combining lettuce hydroponics and loach aquaculture for controlled ecological life support systems in space. Front. Astron. Space Sci. 2023, 10, 1197402. [Google Scholar]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef]
- Yang, T.; Kim, H.J. Characterizing nutrient composition and concentration in tomato-, basil-, and lettuce-based aquaponic and hydroponic systems. Water 2020, 12, 1259. [Google Scholar] [CrossRef]
- Tyson, R.V.; Simonne, E.H.; White, J.M.; Lamb, E.M. Reconciling water quality parameters impacting nitrification in aquaponics: The pH levels. Proc. Fla. State Hortic. Soc. 2004, 117, 79–83. [Google Scholar]
- Yildiz, H.Y.; Robaina, L.; Pirhonen, J.; Mente, E.; Domínguez, D.; Parisi, G. Fish welfare in aquaponic systems: Its relation to water quality with an emphasis on feed and feces—A review. Water 2017, 9, 13. [Google Scholar] [CrossRef]
- Kasozi, N.; Tandlich, R.; Fick, M.; Kaiser, H.; Wilhelmi, B. Iron supplementation and management in aquaponic systems: A review. Aquac. Rep. 2019, 15, 100221. [Google Scholar]
- Vandam, D.A.; Anderson, T.S.; De Villiers, D.; Timmons, M.B. Growth and tissue elemental composition response of spinach (Spinacia oleracea) to hydroponic and aquaponic water quality conditions. Horticulturae 2017, 3, 32. [Google Scholar] [CrossRef]
- Raun, W.R.; Solie, J.B.; Johnson, G.V.; Stone, M.L.; Mutten, R.W.; Freeman, K.W.; Thomason, W.E.; Lukina, E.V. Improving nitrogen use efficiency in cereal grain production with optical sensing and variable rate application. Agron. J. 2002, 94, 815–820. [Google Scholar]
- Rathke, G.W.; Behrens, T.; Diepenbrock, W. Integrated nitrogen management strategies to improve seed yield, oil content and nitrogen efficiency of winter oilseed rape (Brassica napus L.): A review. Agric. Ecosyst. Environ. 2006, 117, 80–108. [Google Scholar]
- Fang, Y.; Hu, Z.; Zou, Y.; Fan, J.; Wang, Q.; Zhu, Z. Increasing economic and environmental benefits of media-based aquaponics through optimizing aeration pattern. J. Clean. Prod. 2017, 162, 1111–1117. [Google Scholar]
- Groenveld, T.; Kohn, Y.Y.; Gross, A.; Lazarovitch, N. Optimization of nitrogen use efficiency by means of fertigation management in an integrated aquaculture-agriculture system. J. Clean. Prod. 2019, 212, 401–408. [Google Scholar]
- Love, D.C.; Fry, J.P.; Li, X.; Hill, E.S.; Genello, L.; Semmens, K.; Thompson, R.E. Commercial aquaponics production and profitability: Findings from an international survey. Aquaculture 2015, 435, 67–74. [Google Scholar]
- Kitaya, Y.; Hirai, H.; Islam, A.F.M. An aquaponic system with hydroponic culture of sweet potato and tilapia culture. J. Agric. Ecol. Res. Int. 2022, 23, 62–72. [Google Scholar]
- Benlì, A.Ç.K.; Köksal, G. The acute toxicity of ammonia on tilapia (Oreochromis niloticus L.) larvae and fingerlings. Turk. J. Vet. Anim. Sci. 2005, 29, 339–344. [Google Scholar]
- Miao, W.; Wang, W. Trends of aquaculture production and trade: Carp, tilapia, and shrimp. Asian Fish. Sci. 2020, 33, 1–10. [Google Scholar]
- Maucieri, C.; Nicoletto, C.; Zanin, G.; Birolo, M.; Trocino, A.; Sambo, P.; Borin, M.; Xiccato, G. Effect of stocking density of fish on water quality and growth performance of European Carp and leafy vegetables in a low-tech aquaponic system. PLoS ONE 2019, 14, e0217561. [Google Scholar] [CrossRef] [PubMed]
- Oladimeji, S.A.; Okomoda, V.T.; Olufeagba, S.O.; Solomon, S.G.; Abol-Munafi, A.B.; Alabi, K.I.; Hassan, A. Aquaponics production of catfish and pumpkin: Comparison with conventional production systems. Food Sci. Nutr. 2020, 8, 2307–2315. [Google Scholar] [PubMed]
- Petrea, Ş.M.; Cristea, V.; Dediu, L.; Contoman, M.; Stroe, M.D.; Antache, A.; Coadă, T.; Placinta, S. Vegetable production in an integrated aquaponic system with stellate sturgeon and spinach–Matador variety. Sci. Pap. Anim. Sci. Biotechnol. 2014, 47, 235. [Google Scholar]
- Fadhilah, N.; Estim, A.; Fatihah, S.N. Water quality and growth performance of giant freshwater prawn, Macrobrachium rosenbergii and green bean, Phaseolus vulgaris in aquaponics system at different flow rates. Trans. Sci. Technol. 2019, 6, 1–8. [Google Scholar]
- Chu, Y.T.; Brown, P.B. Evaluation of Pacific whiteleg shrimp and three halophytic plants in marine aquaponic systems under three salinities. Sustainability 2020, 13, 269. [Google Scholar] [CrossRef]
- Ministry of Education. Standard Tables of Food Composition in Japan. 2020. Available online: https://www.mext.go.jp/a_menu/syokuhinseibun/mext_01110.html (accessed on 10 January 2022).
- Anderson, T.S.; De Villiers, D.; Timmons, M.B. Growth and tissue elemental composition response of butterhead lettuce (Lactuca sativa, cv. flandria) to hydroponic and aquaponic conditions. Horticulturae 2017, 3, 43. [Google Scholar] [CrossRef]
- Delaide, B.; Goddek, S.; Gott, J.; Soyeurt, H.; Jijakli, M.H. Lettuce (Lactuca sativa L. var. Sucrine) growth performance in complemented aquaponic solution outperforms hydroponics. Water 2016, 8, 467. [Google Scholar] [CrossRef]
- Goddek, S.; Espinal, C.A.; Delaide, B.; Jijakli, M.H.; Schmautz, Z.; Wuertz, S.; Keesman, K.J. Navigating toward decoupled aquaponic systems: A system dynamics design approach. Water 2016, 8, 303. [Google Scholar] [CrossRef]
- Hoque, M.M.; Ajwa, H.A.; Smith, R. Nitrite and Ammonium Toxicity on Lettuce Grown under Hydroponics. Commun. Soil Sci. Plant Anal. 2007, 39, 207–216. [Google Scholar] [CrossRef]
- Farnesellia, M.; Benincasaa, P.; Tostia, G.; Simonneb, E.; Guiduccia, M.; Tei, F. High fertigation frequency improves nitrogen uptake and crop performance in processing tomato grown with high nitrogen and water supply. Agric. Water Manag. 2015, 154, 52–58. [Google Scholar] [CrossRef]
- Gichana, Z.M.; Liti, D.; Waidbacher, H.; Zollitsch, W.; Drexler, S.; Waikibia, J. Waste management in recirculating aquaculture system through bacteria dissimilation and plant assimilation. Aquac. Int. 2018, 26, 1541–1572. [Google Scholar] [CrossRef]
- Jones, R.D.; Hood, M.A. Effects of temperature, pH, salinity, and inorganic nitrogen on the rate of ammonium oxidation by nitrifiers isolated from wetland environments. Microb. Ecol. 1980, 6, 339–347. [Google Scholar] [CrossRef]
- Antoniou, P.; Hamilton, J.; Koopman, B.; Jain, R.; Holloway, B.; Lyberatos, G.; Svoronos, S.A. Effect of temperature and pH on the effective maximum specific growth rate of nitrifying bacteria. Water Res. 1990, 24, 97–101. [Google Scholar] [CrossRef]
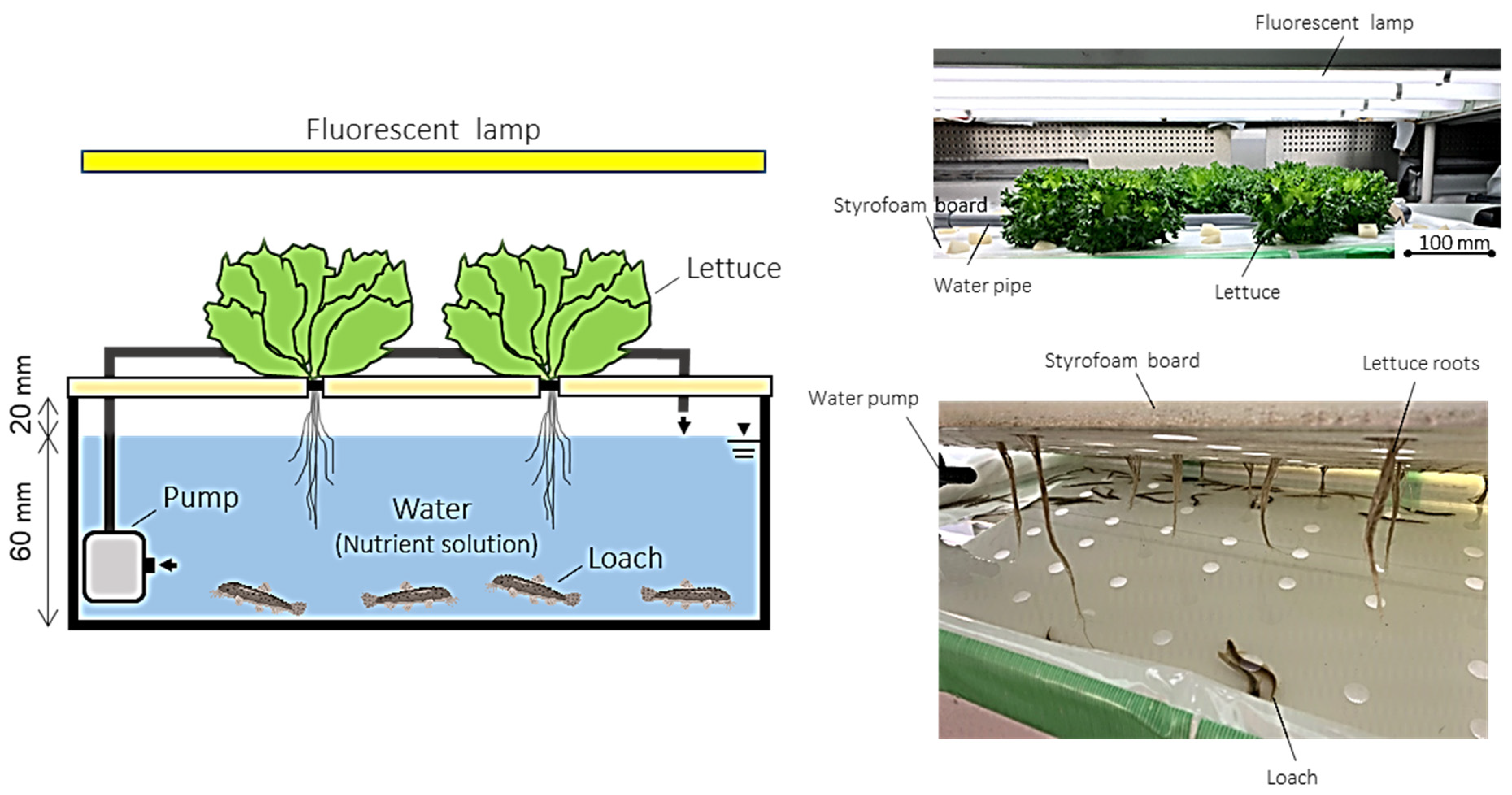
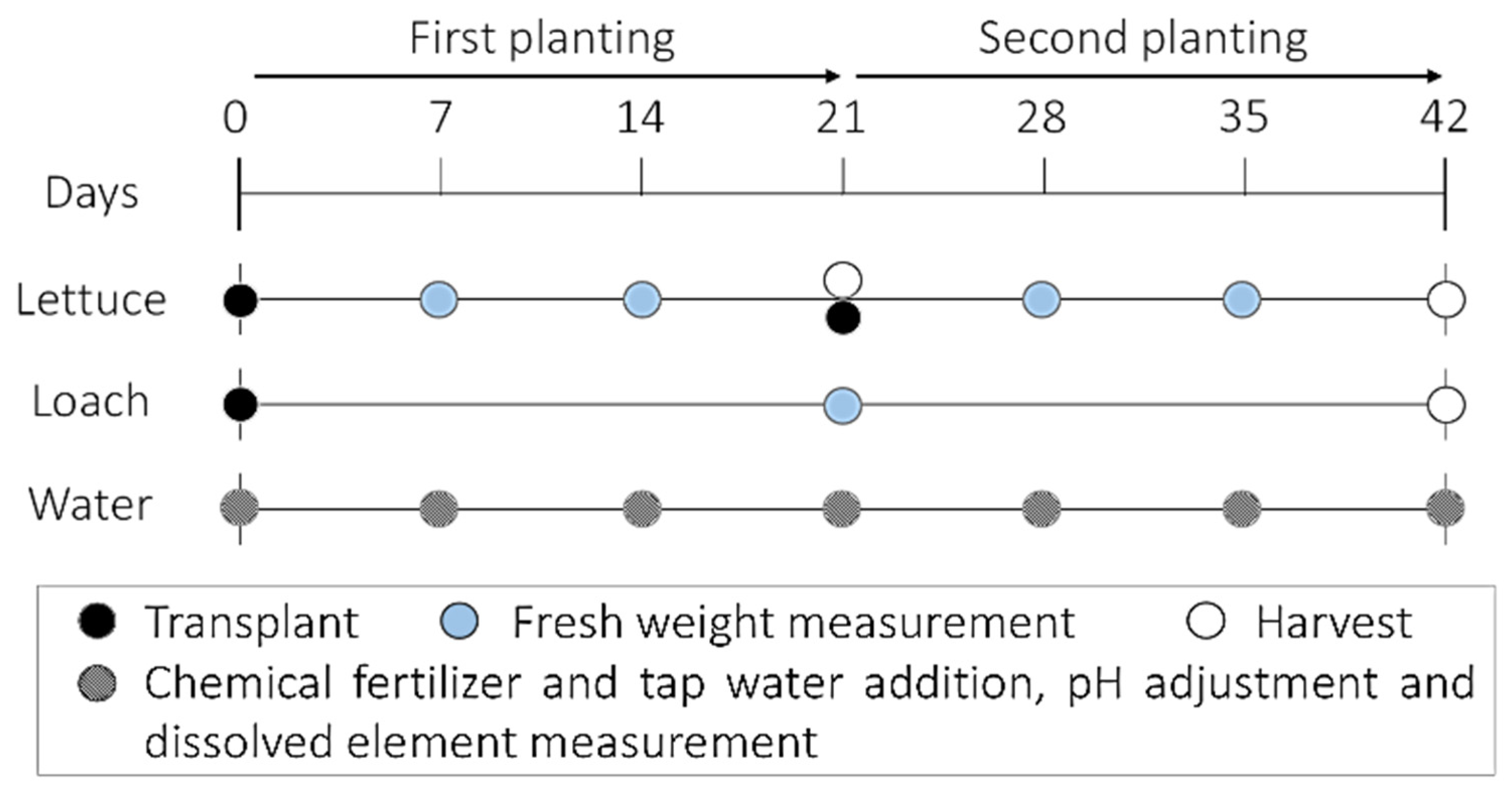


| Component | Content (%) |
|---|---|
| Protein | 43 |
| Lipid | 7 |
| Crude fiber | 3 |
| Ash | 20 |
| Phosphorus | 1.7 |
| Moisture | 10 |
| Elements | Concentration (mg L−1) |
|---|---|
| Anmonium-N | 12 |
| Nitrate-N | 117 |
| P2O5 | 60 |
| K2O | 203 |
| CaO | 115 |
| MgO | 30 |
| MnO | 0.75 |
| B2O3 | 0.75 |
| Fe | 1.35 |
| Cu | 0.015 |
| Zn | 0.045 |
| Mo | 0.015 |
| Fish Density (fish L−1) | No. of Fish (fish) | No. of Lettuce (plant) | Feeding Rate | Nitrogen Supply by Feeding (mg-N d−1/plant) | |
|---|---|---|---|---|---|
| (g d−1/fish) | (g d−1/system) | ||||
| 0.5 | 15 | 15 | 0.03 | 0.45 | 2.4 |
| 1.0 | 30 | 0.90 | 4.7 | ||
| 1.5 | 45 | 1.35 | 7.1 | ||
| 0.5 | 15 | 15 | 0.06 | 0.90 | 4.7 |
| 1.0 | 30 | 1.80 | 9.4 | ||
| 1.5 | 45 | 2.70 | 14.1 | ||
| 0.5 | 15 | 15 | 0.09 | 1.35 | 7.1 |
| 1.0 | 30 | 2.70 | 14.1 | ||
| 1.5 | 45 | 4.05 | 21.2 | ||
| 0 (Hydroponics) | - | 15 | - | - | - |
| FCR | |||
|---|---|---|---|
| Feeding rate (g d−1/fish) | 0.03 | 2.0 ± 0.5 | a |
| 0.06 | 1.3 ± 0.1 | b | |
| 0.09 | 1.4 ± 0.2 | b | |
| Fish density (fish L−1) | 0.5 | 1.7 ± 0.4 | a |
| 1.0 | 1.5 ± 0.2 | ab | |
| 1.5 | 1.5 ± 0.1 | ab | |
| Fish Density (fish L−1) | Feeding Rate | Fresh Weight (g/plant) | Dry Weight (g/plant) | Water Contents (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (g d−1/fish) | (g d−1/system) | First | Second | First | Second | |||||||
| 0.5 | 0.03 | 0.45 | 159 ± 10 | a | 134 ± 4 | ab | 5.9 ± 0.5 | ab | 5.0 ± 0.2 | a | 96.3 ± 0.3 | a |
| 1.0 | 0.90 | 162 ± 7 | a | 146 ± 5 | ab | 6.0 ± 0.3 | ab | 5.3 ± 0.2 | a | 96.3 ± 0.3 | a | |
| 1.5 | 1.35 | 139 ± 4 | abc | 128 ± 4 | abc | 6.0 ± 0.2 | ab | 4.8 ± 0.2 | a | 95.6 ± 0.3 | a | |
| 0.5 | 0.06 | 0.90 | 157 ± 5 | ab | 121 ± 4 | b | 5.9 ± 0.2 | ab | 4.8 ± 0.1 | a | 96.2 ± 0.3 | a |
| 1.0 | 1.80 | 121 ± 6 | c | 131 ± 5 | ab | 5.2 ± 0.3 | abc | 5.0 ± 0.2 | a | 95.7 ± 0.3 | ab | |
| 1.5 | 2.70 | 82 ± 5 | d | 108 ± 3 | c | 4.5 ± 0.3 | bcd | 4.3 ± 0.1 | a | 94.5 ± 0.2 | c | |
| 0.5 | 0.09 | 1.35 | 123 ± 11 | bc | 132 ± 4 | ab | 5.0 ± 0.5 | abcd | 5.2 ± 0.1 | a | 95.9 ± 0.3 | ab |
| 1.0 | 2.70 | 69 ± 4 | d | 112 ± 7 | b | 3.6 ± 0.2 | d | 4.6 ± 0.3 | a | 94.8 ± 0.2 | c | |
| 1.5 | 4.05 | 80 ± 4 | d | 43 ± 2 | d | 3.8 ± 0.1 | cd | 2.9 ± 0.1 | b | 95.3 ± 0.2 | b | |
| 0 (Hydroponics) | 134 ± 9 | abc | 108 ± 5 | c | 5.2 ± 0.3 | abc | 4.7 ± 0.2 | a | 96.1 ± 0.3 | a | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitaya, Y.; Shimakawa, Y.; Wada, T.; Nakamura, K.; Endo, R.; Shibuya, T. Biological Production and Nitrogen Use Efficiency in a Water-Sharing and Water-Saving System Combining Aquaculture and Vegetable Hydroponic Cultivation. Water 2025, 17, 963. https://doi.org/10.3390/w17070963
Kitaya Y, Shimakawa Y, Wada T, Nakamura K, Endo R, Shibuya T. Biological Production and Nitrogen Use Efficiency in a Water-Sharing and Water-Saving System Combining Aquaculture and Vegetable Hydroponic Cultivation. Water. 2025; 17(7):963. https://doi.org/10.3390/w17070963
Chicago/Turabian StyleKitaya, Yoshiaki, Yotsuba Shimakawa, Teruo Wada, Kenji Nakamura, Ryosuke Endo, and Toshio Shibuya. 2025. "Biological Production and Nitrogen Use Efficiency in a Water-Sharing and Water-Saving System Combining Aquaculture and Vegetable Hydroponic Cultivation" Water 17, no. 7: 963. https://doi.org/10.3390/w17070963
APA StyleKitaya, Y., Shimakawa, Y., Wada, T., Nakamura, K., Endo, R., & Shibuya, T. (2025). Biological Production and Nitrogen Use Efficiency in a Water-Sharing and Water-Saving System Combining Aquaculture and Vegetable Hydroponic Cultivation. Water, 17(7), 963. https://doi.org/10.3390/w17070963






