How Will the Heavy Metal Risk Change Under Continuous Changing Hydrological Regimes and Salinity?
Abstract
1. Introduction
2. Materials and Methods
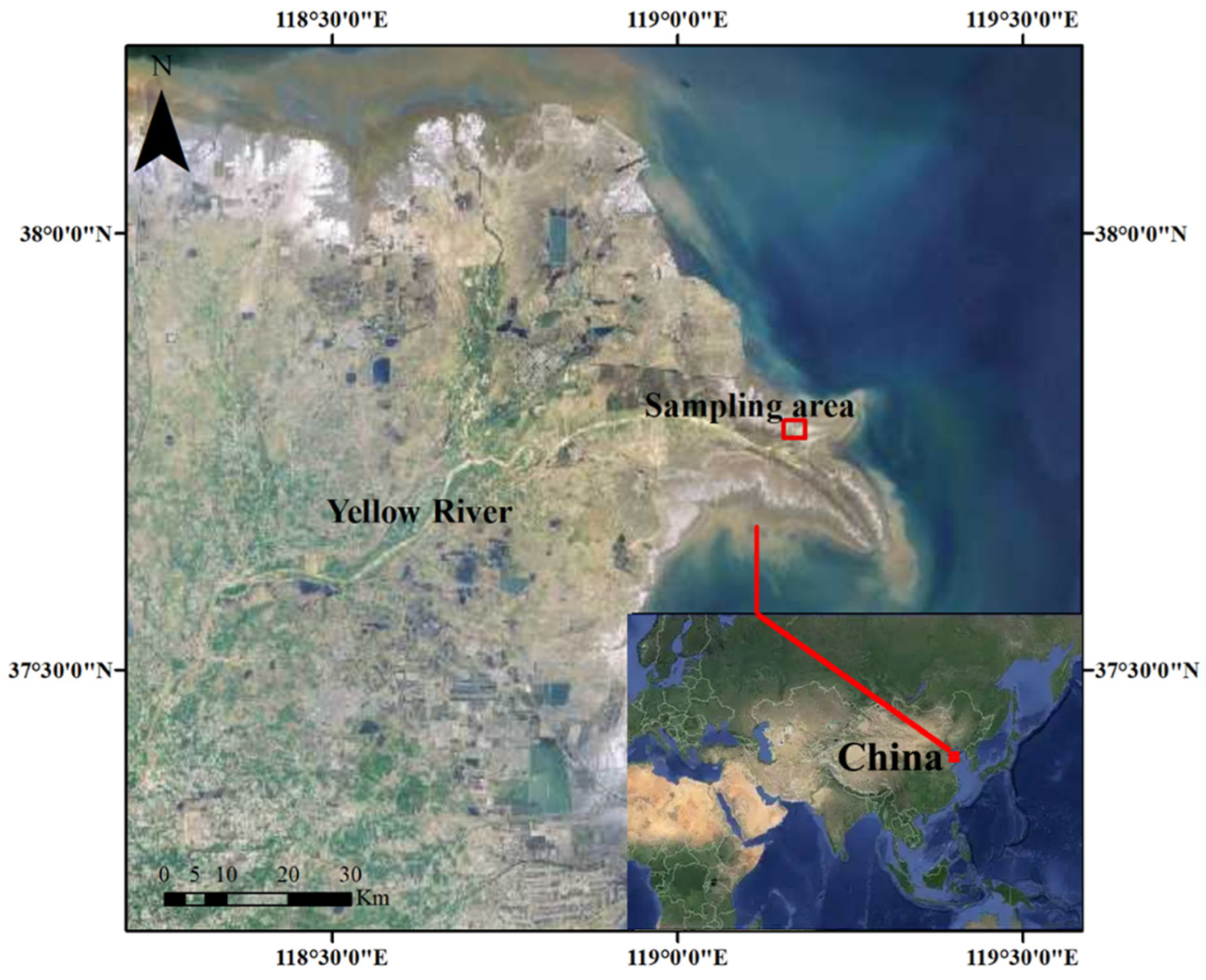
2.1. Sampling
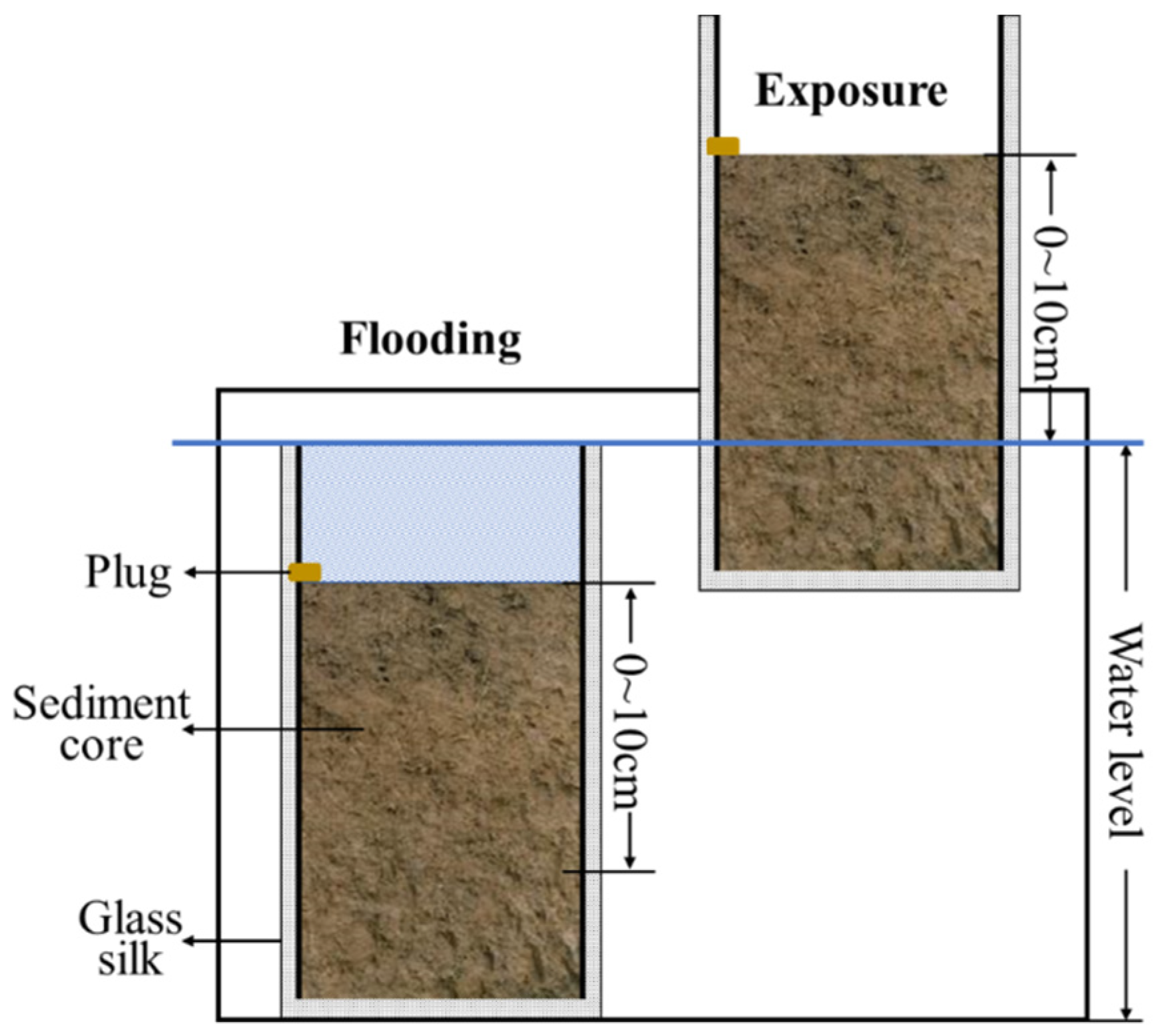
2.2. Incubation Experiment
| Hydrological Regimes | Salt | ||||
|---|---|---|---|---|---|
| 0‰ | 5‰ | 10‰ | 20‰ | 30‰ | |
| No flooding | NS0 | NS5 | NS10 | NS20 | - |
| Periodic flooding | PS0 | PS5 | PS10 | PS20 | PS30 |
| Long-term flooding | LS0 | LS5 | LS10 | LS20 | LS30 |
2.3. Analytical Method
2.4. Risk Assessment Code
2.5. Statistical Analysis of Data
3. Results
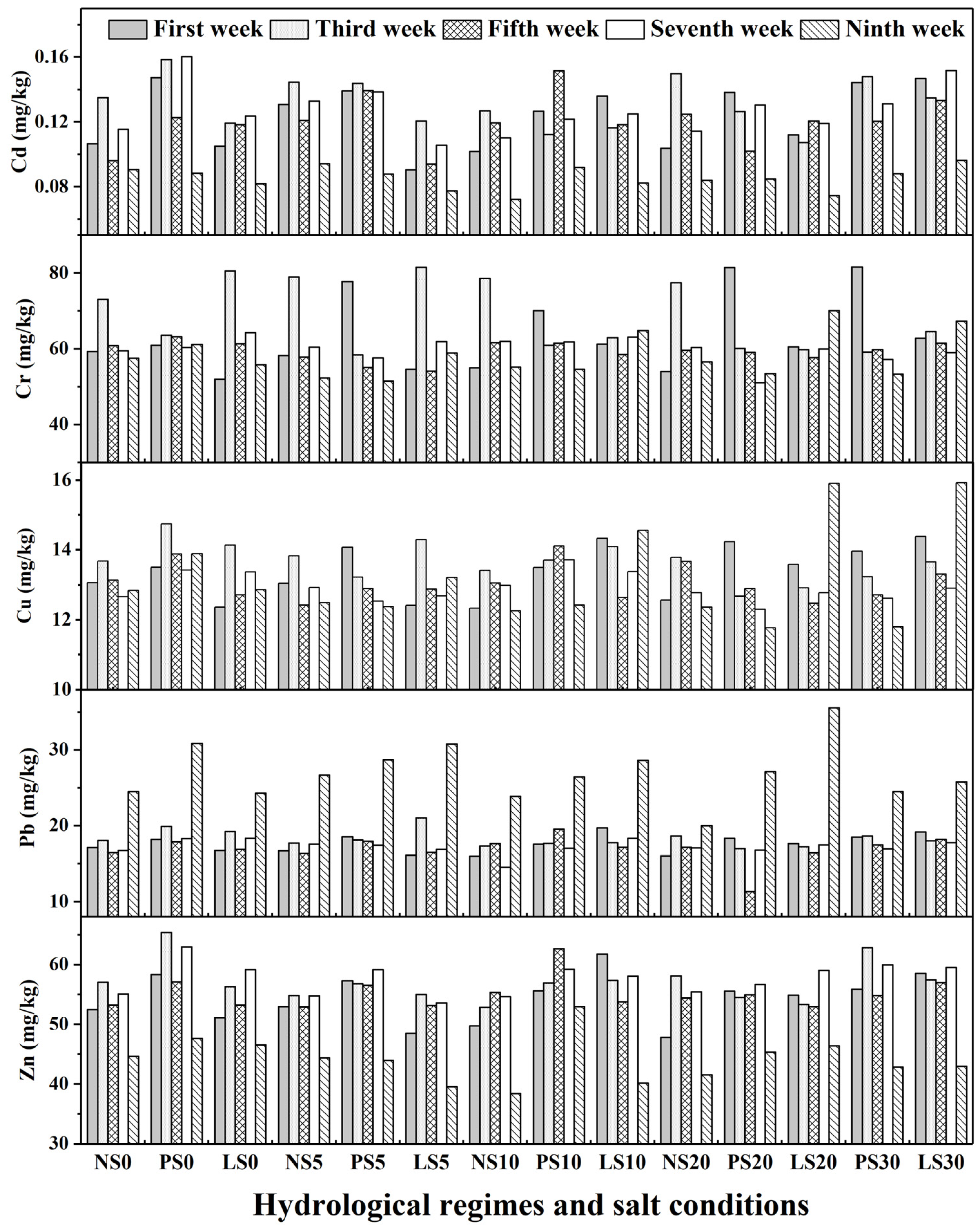
3.1. Heavy Metal Concentrations
| Mean | Cd (mg/kg) | Cr (mg/kg) | Cu (mg/kg) | Pb (mg/kg) | Zn (mg/kg) |
|---|---|---|---|---|---|
| Original samples | 0.08 | 67.36 | 16.20 | 26.30 | 47.37 |
| First week | 0.12 | 63.56 | 13.38 | 17.59 | 54.33 |
| Third week | 0.13 | 68.56 | 13.67 | 18.32 | 57.06 |
| Fifth week | 0.12 | 59.42 | 13.05 | 16.93 | 55.15 |
| Seventh week | 0.13 | 59.89 | 12.93 | 17.23 | 57.68 |
| Ninth week | 0.09 | 58.04 | 13.19 | 26.99 | 44.12 |
3.2. Heavy Metal Speciation
3.2.1. Three Hydrological Regimes
3.2.2. Five Salt Conditions
3.2.3. Different Incubation Time
3.3. GLM
3.4. RAC
| Fraction | Factor | Cd | Cr | Cu | Pb | Zn |
|---|---|---|---|---|---|---|
| Acid-soluble fraction (F1) | Hydrological regime | 0.056 | 0.391 | 0.000 ** | 0.002 ** | 0.000 ** |
| Salt | 0.505 | 0.320 | 0.000 ** | 0.032 * | 0.045 * | |
| Time | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | |
| Hydrological regime × Salt | 0.805 | 0.555 | 0.000 ** | 0.038 * | 0.621 | |
| Reducible fraction (F2) | Hydrological regime | 0.000 ** | 0.122 | 0.000 ** | 0.052 | 0.075 |
| Salt | 0.320 | 0.696 | 0.271 | 0.160 | 0.432 | |
| Time | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | |
| Hydrological regime × Salt | 0.013 * | 0.446 | 0.320 | 0.052 | 0.748 | |
| Oxidizable fraction (F3) | Hydrological regime | 0.014 * | 0.006 ** | 0.023 * | 0.101 | 0.509 |
| Salt | 0.339 | 0.465 | 0.571 | 0.608 | 0.274 | |
| Time | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | |
| Hydrological regime × Salt | 0.317 | 0.506 | 0.087 | 0.122 | 0.066 | |
| Residual fraction (F4) | Hydrological regime | 0.518 | 0.004 ** | 0.003 ** | 0.449 | 0.032 * |
| Salt | 0.874 | 0.445 | 0.598 | 0.779 | 0.120 | |
| Time | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** | |
| Hydrological regime × Salt | 0.149 | 0.519 | 0.301 | 0.286 | 0.759 |
4. Discussion
4.1. Change of Heavy Metal Concentrations During Incubation
4.2. Speciation Transformation of Heavy Metal During Incubation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, R.; Zhang, M.; Yao, X.; Ma, Z.; Yu, F.; Bai, J. Heavy Metal Distribution in Different Soil Aggregate Size Classes from Restored Brackish Marsh, Oil Exploitation Zone, and Tidal Mud Flat of the Yellow River Delta. J. Soils Sediments 2016, 16, 821–830. [Google Scholar] [CrossRef]
- Díaz-Jaramillo, M.; Islas, M.S.; Gonzalez, M. Spatial Distribution Patterns and Identification of Microplastics on Intertidal Sediments from Urban and Semi-Natural Sw Atlantic Estuaries. Environ. Pollut. 2021, 273, 116398. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Xiao, R.; Ma, Z.; Xie, Y.; Zhang, M.; Yu, F. Distribution and Contamination Assessment of Heavy Metals in Soils from Tidal Flat, Oil Exploitation Zone and Restored Wetland in the Yellow River Estuary. Wetlands 2016, 36, 153–165. [Google Scholar] [CrossRef]
- Sun, Z.; Li, J.; He, T.; Ren, P.; Zhu, H.; Gao, H.; Tian, L.; Hu, X. Spatial Variation and Toxicity Assessment for Heavy Metals in Sediments of Intertidal Zone in a Typical Subtropical Estuary (Min River) of China. Environ. Sci. Pollut. Res. 2017, 24, 23080–23095. [Google Scholar] [CrossRef]
- Lv, J.; Wang, Y. Multi-Scale Analysis of Heavy Metals Sources in Soils of Jiangsu Coast, Eastern China. Chemosphere 2018, 212, 964–973. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, L.; Yang, L.; Yu, J.; Lv, J.; Meng, M.; Li, G. Heavy Metal Pollution and the Risk from Tidal Flat Reclamation in Coastal Areas of Jiangsu, China. Mar. Pollut. Bull. 2020, 158, 111427. [Google Scholar] [CrossRef]
- Ormaza-González, F.I.; Castro-Rendón, R.D.; Maridueña-Bravo, A.; Bobadilla-Cordova, N.; Ramos-Castañeda, I.; Statham, P.J. Hg, Cd, as, and Pb in Surface Sediments from the Tropical Coastal Lagoon Estero Salado, Gulf of Guayaquil-Ecuador. Front. Mar. Sci. 2024, 11, 1457548. [Google Scholar] [CrossRef]
- Estrada, E.S.; Juhel, G.; Han, P.; Kelly, B.C.; Lee, W.K.; Bayen, S. Multi-Tool Assessment of Trace Metals in Mangroves Combining Sediment and Clam Sampling, Dgt Passive Samplers and Caged Mussels. Sci. Total Environ. 2017, 574, 847–857. [Google Scholar] [CrossRef]
- Ranjan, P.; Ramanathan, A.L.; Kumar, A.; Singhal, R.K.; Datta, D.; Venkatesh, M. Trace Metal Distribution, Assessment and Enrichment in the Surface Sediments of Sundarban Mangrove Ecosystem in India and Bangladesh. Mar. Pollut. Bull. 2018, 127, 541–547. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, E.; Xia, P.; Feng, A.; Chi, Y.; Sun, Y. Distribution and Pollution Assessment of Heavy Metals in the Intertidal Zone Environments of Typical Sea Areas in China. Mar. Pollut. Bull. 2019, 138, 397–406. [Google Scholar] [CrossRef]
- Liao, J.; Qian, X.; Liu, F.; Deng, S.; Lin, H.; Liu, X.; Wei, C. Multiphase Distribution and Migration Characteristics of Heavy Metals in Typical Sandy Intertidal Zones: Insights from Solid-Liquid Partitioning. Ecotoxicol. Environ. Saf. 2021, 208, 111674. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Hong, H.; Liang, S.; Zhao, W.; Jia, H.; Lu, H.; Li, J.; Yan, C. Coastal Reclamation Mediates Heavy Metal Fractions and Ecological Risk in Saltmarsh Sediments of Northern Jiangsu Province, China. Sci. Total Environ. 2022, 825, 154028. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Yu, J.; Yang, J.; Yu, Y.; Zhou, D.; Li, Y. Effect of Water Level and Salinity on Metal Fractionation in Sediments of the Yellow River Delta. Wetlands 2020, 40, 2765–2774. [Google Scholar] [CrossRef]
- Sungur, A.; Soylak, M.; Yilmaz, E.; Yilmaz, S.; Ozcan, H. Characterization of Heavy Metal Fractions in Agricultural Soils by Sequential Extraction Procedure: The Relationship Between Soil Properties and Heavy Metal Fractions. J. Soil Contam. 2015, 24, 1–15. [Google Scholar]
- Zhang, G.; Bai, J.; Xiao, R.; Zhao, Q.; Jia, J.; Cui, B.; Liu, X. Heavy Metal Fractions and Ecological Risk Assessment in Sediments from Urban, Rural and Reclamation-Affected Rivers of the Pearl River Estuary, China. Chemosphere 2017, 184, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Li, W.C.; Deng, H.; Wong, M.H. Metal Solubility and Speciation Under the Influence of Waterlogged Condition and the Presence of Wetland Plants. Geoderma 2016, 270, 98–108. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, X.; Zhang, Q.; Li, X.; Yan, Z. Iron Plaque Formation and Heavy Metal Uptake in Spartina Alterniflora at Different Tidal Levels and Waterlogging Conditions. Ecotoxicol. Environ. Saf. 2018, 153, 91–100. [Google Scholar] [CrossRef]
- Lu, J.; Yuan, F. The Distribution of Heavy Metals in the Sediment of Low Tidal Flat, Eastern Chongming Island, China. Iop Conf. Ser. Mater. Sci. Eng. 2019, 472, 012091. [Google Scholar] [CrossRef]
- Du Laing, G.; De Vos, R.; Vandecasteele, B.; Lesage, E.; Tack, F.M.G.; Verloo, M.G. Effect of Salinity on Heavy Metal Mobility and Availability in Intertidal Sediments of the Scheldt Estuary. Estuar. Coast. Shelf Sci. 2008, 77, 589–602. [Google Scholar] [CrossRef]
- Costa, E.S.; Sá, F.; Gomes, L.E.O.; Silva, C.A.; Lima, A.T.; Lehrback, B.D.; Neto, R.R. Can Severe Drought Periods Increase Metal Concentrations in Mangrove Sediments? A Case Study in Eastern Brazil. Sci. Total Environ. 2020, 748, 142443. [Google Scholar] [CrossRef]
- Guo, S.; Liu, Z.; Li, Q.; Yang, P.; Wang, L.; He, B.; Xu, Z.; Ye, J.; Zeng, E.Y. Leaching Heavy Metals from the Surface Soil of Reclaimed Tidal Flat by Alternating Seawater Inundation and Air Drying. Chemosphere 2016, 157, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Jia, J.; Zhang, G.; Zhao, Q.; Lu, Q.; Cui, B.; Liu, X. Spatial and Temporal Dynamics of Heavy Metal Pollution and Source Identification in Sediment Cores from the Short-Term Flooding Riparian Wetlands in a Chinese Delta. Environ. Pollut. 2016, 219, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Gao, X.; Yang, J. Influences of Hydrological Regime on Heavy Metal and Salt Ion Concentrations in Intertidal Sediment from Chongming Dongtan, Changjiang River Estuary, China. Chin. J. Oceanol. Limnol. 2017, 35, 1329–1341. [Google Scholar] [CrossRef]
- Liu, G.; Wang, J.; Zhang, E.; Hou, J.; Liu, X. Heavy Metal Speciation and Risk Assessment in Dry Land and Paddy Soils Near Mining Areas at Southern China. Environ. Sci. Pollut. Res. 2016, 23, 8709–8720. [Google Scholar] [CrossRef]
- Teuchies, J.; Singh, G.; Bervoets, L.; Meire, P. Land Use Changes and Metal Mobility: Multi-Approach Study on Tidal Marsh Restoration in a Contaminated Estuary. Sci. Total Environ. 2013, 449, 174–183. [Google Scholar] [CrossRef]
- Liang, B.; Qian, X.; Peng, S.; Liu, X.; Bai, L.; Cui, B.; Bai, J. Speciation Variation and Comprehensive Risk Assessment of Metal(Loid)S in Surface Sediments of Intertidal Zones. Int. J. Environ. Res. Public Health 2018, 15, 2125. [Google Scholar] [CrossRef]
- Huang, L.; Bai, J.; Chen, B.; Zhang, K.; Huang, C.; Liu, P. Two-Decade Wetland Cultivation and its Effects on Soil Properties in Salt Marshes in the Yellow River Delta, China. Ecol. Inform. 2012, 10, 49–55. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, J.; Zhao, Q.; Lu, Q.; Jia, J.; Wen, X. Heavy Metals in Wetland Soils Along a Wetland-Forming Chronosequence in the Yellow River Delta of China: Levels, Sources and Toxic Risks. Ecol. Indic. 2016, 69, 331–339. [Google Scholar] [CrossRef]
- Hafeez, F.; Zafar, N.; Nazir, R.; Javeed, H.M.R.; Rizwan, M.; Faridullah; Asad, S.A.; Iqbal, A. Assessment of Flood-Induced Changes in Soil Heavy Metal and Nutrient Status in Rajanpur, Pakistan. Environ. Monit. Assess. 2019, 191, 234. [Google Scholar] [CrossRef]
- Cui, B.; Yang, Q.; Zhang, K.; Zhao, X.; You, Z. Responses of Saltcedar (Tamarix chinensis) to Water Table Depth and Soil Salinity in the Yellow River Delta, China. Plant Ecol. 2010, 209, 279–290. [Google Scholar] [CrossRef]
- Zhao, Q.; Bai, J.; Gao, Y.; Zhang, G.; Lu, Q.; Jia, J. Heavy Metal Contamination in Soils from Freshwater Wetlands to Salt Marshes in the Yellow River Estuary, China. Sci. Total Environ. 2021, 774, 145072. [Google Scholar] [CrossRef]
- Khodaverdiloo, H.; Rahmanian, M.; Rezapour, S.; Dashtaki, S.G.; Hadi, H.; Han, F.X. Effect of Wetting-Drying Cycles on Redistribution of Lead in some Semi-Arid Zone Soils Spiked with a Lead Salt. Pedosphere 2012, 22, 304–313. [Google Scholar] [CrossRef]
- Feng, C.; Guo, X.; Yin, S.; Tian, C.; Li, Y.; Shen, Z. Heavy Metal Partitioning of Suspended Particulate Matter–Water and Sediment–Water in the Yangtze Estuary. Chemosphere 2017, 185, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Ibragimow, A.; Walna, B.; Siepak, M. Effects of Flooding on the Contamination of Floodplain Sediments with Available Fractions of Trace Metals (Western Poland). Pol. J. Environ. Stud. 2013, 22, 131–140. [Google Scholar]
- Balint, R.; Said-Pullicino, D.; Ajmone-Marsan, F. Copper Dynamics Under Alternating Redox Conditions is Influenced by Soil Properties and Contamination Source. J. Contam. Hydrol. 2015, 173, 83–91. [Google Scholar] [CrossRef]
- Gaulier, C.; Zhou, C.; Gao, Y.; Guo, W.; Reichstädter, M.; Ma, T.; Baeyens, W.; Billon, G. Investigation on Trace Metal Speciation and Distribution in the Scheldt Estuary. Sci. Total Environ. 2021, 757, 143827. [Google Scholar] [CrossRef]
- Yao, X.; Wang, Z.; Li, D.; Sun, H.; Ren, C.; Yu, Y.; Pei, F.; Li, Y. Distribution, Mobilization, Risk Assessment and Source Identification of Heavy Metals and Nutrients in Surface Sediments of Three Urban-Rural Rivers After Long-Term Water Pollution Treatment. Sci. Total Environ. 2024, 932, 172894. [Google Scholar] [CrossRef]
- Yan, J.; Fischel, M.; Chen, H.; Siebecker, M.G.; Wang, P.; Zhao, F.; Sparks, D.L. Cadmium Speciation and Release Kinetics in a Paddy Soil as Affected by Soil Amendments and Flooding-Draining Cycle. Environ. Pollut. 2021, 268, 115944. [Google Scholar] [CrossRef]
- Fulda, B.; Voegelin, A.; Kretzschmar, R. Redox-Controlled Changes in Cadmium Solubility and Solid-Phase Speciation in a Paddy Soil as Affected by Reducible Sulfate and Copper. Environ. Sci. Technol. 2013, 47, 12775–12783. [Google Scholar] [CrossRef]
- Song, T.; Liu, C.; Cui, G.; Tong, S. Research on the Migration and Transformation Behaviors of Soil Selenium in the Flood Irrigation Process. Arch. Agron. Soil Sci. 2020, 67, 1388–1399. [Google Scholar] [CrossRef]
- Zhao, Q.; Bai, J.; Wang, X.; Zhang, W.; Huang, Y.; Wang, L.; Gao, Y. Soil Organic Carbon Content and Stock in Wetlands with Different Hydrologic Conditions in the Yellow River Delta, China. Ecohydrol. Hydrobiol. 2020, 20, 537–547. [Google Scholar] [CrossRef]
- Wang, J.; Wang, P.; Gu, Y.; Kopittke, P.M.; Zhao, F.; Wang, P. Iron–Manganese (Oxyhydro)Oxides, Rather than Oxidation of Sulfides, Determine Mobilization of Cd During Soil Drainage in Paddy Soil Systems. Environ. Sci. Technol. 2019, 53, 2500–2508. [Google Scholar] [CrossRef] [PubMed]
- Korfali, S.I.; Karaki, H. Speciation of Metals in Soils and Water: Risk Assessment. Environ. Process. 2018, 5, 101–125. [Google Scholar] [CrossRef]
- Li, H.; Ji, H. Chemical Speciation, Vertical Profile and Human Health Risk Assessment of Heavy Metals in Soils from Coal-Mine Brownfield, Beijing, China. J. Geochem. Explor. 2017, 183, 22–32. [Google Scholar] [CrossRef]
- Laurent, C.; Bravin, M.N.; Crouzet, O.; Pelosi, C.; Tillard, E.; Lecomte, P.; Lamy, I. Increased Soil Ph and Dissolved Organic Matter after a Decade of Organic Fertilizer Application Mitigates Copper and Zinc Availability Despite Contamination. Sci. Total Environ. 2020, 709, 135927. [Google Scholar] [CrossRef]
- Emmanuel, S.; Erel, Y. Implications from Concentrations and Isotopic Data for Pb Partitioning Processes in Soils. Geochim. Cosmochim. Acta 2002, 66, 2517–2527. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Zhao, Y.; Zhong, C. The Vertical Migration and Speciation of the Pb in the Paddy Soil: A Case Study of the Yangtze River Delta, China. Environ. Res. 2019, 179, 108741. [Google Scholar] [CrossRef]
- Xiao, S.; Luo, M.; Liu, Y.; Bai, J.; Yang, Y.; Zhai, Z.; Huang, J. Rhizosphere Effect and its Associated Soil-Microbe Interactions Drive Iron Fraction Dynamics in Tidal Wetland Soils. Sci. Total Environ. 2021, 756, 144056. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Du, Y.; Cui, Z.; Shi, L.; Wang, L.; Li, H. The Behavior of Heavy Metals in Tidal Flat Sediments During Fresh Water Leaching. Chemosphere 2011, 82, 834–838. [Google Scholar] [CrossRef]

| Metal | Fraction | Original Samples | Hydrological Regimes | Salt | First Week | Third Week | Fifth Week | Seventh Week | Ninth Week | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | L | 0‰ | 5‰ | 10‰ | 20‰ | 30‰ | ||||||||
| Cd | F1 | 62.59% | 51.73% | 56.27% | 54.71% | 51.89% | 55.77% | 54.95% | 54.86% | 54.71% | 45.31% | 58.06% | 49.15% | 57.71% | 61.86% |
| F2 | 15.68% | 10.38% | 13.14% | 11.20% | 11.82% | 11.56% | 11.49% | 10.83% | 13.04% | 9.13% | 12.79% | 9.31% | 15.17% | 11.89% | |
| F3 | 5.01% | 10.91% | 6.01% | 8.65% | 10.43% | 8.07% | 6.42% | 9.35% | 7.09% | 2.77% | 3.52% | 19.52% | 8.50% | 7.47% | |
| F4 | 16.72% | 26.97% | 24.58% | 25.43% | 25.86% | 24.59% | 27.14% | 24.96% | 25.16% | 42.79% | 25.63% | 22.02% | 18.63% | 18.78% | |
| Cr | F1 | 0.13% | 0.17% | 0.17% | 0.18% | 0.16% | 0.17% | 0.18% | 0.17% | 0.20% | 0.18% | 0.14% | 0.23% | 0.14% | 0.17% |
| F2 | 0.91% | 1.22% | 1.07% | 1.13% | 1.18% | 1.18% | 1.12% | 1.11% | 1.04% | 1.36% | 0.87% | 0.95% | 1.07% | 1.41% | |
| F3 | 10.14% | 15.85% | 12.07% | 13.29% | 14.77% | 14.25% | 12.75% | 12.63% | 13.49% | 37.78% | 7.84% | 7.62% | 5.81% | 8.87% | |
| F4 | 88.81% | 82.76% | 86.70% | 85.39% | 83.89% | 84.39% | 85.95% | 86.09% | 85.26% | 60.68% | 91.15% | 91.20% | 92.97% | 89.54% | |
| Cu | F1 | 0.46% | 1.17% | 1.08% | 1.33% | 1.13% | 1.24% | 1.22% | 1.27% | 1.09% | 1.32% | 1.30% | 1.15% | 1.41% | 0.81% |
| F2 | 4.43% | 12.05% | 10.06% | 11.91% | 10.87% | 11.65% | 11.76% | 11.53% | 10.31% | 8.71% | 9.68% | 13.36% | 15.10% | 9.59% | |
| F3 | 28.72% | 31.01% | 28.10% | 27.50% | 29.35% | 29.96% | 28.66% | 27.35% | 28.04% | 37.25% | 29.21% | 19.87% | 33.36% | 23.89% | |
| F4 | 66.39% | 55.68% | 60.60% | 59.16% | 58.54% | 57.04% | 58.26% | 59.72% | 60.42% | 52.72% | 59.80% | 65.63% | 50.13% | 65.13% | |
| Pb | F1 | 0.73% | 1.72% | 1.89% | 1.88% | 1.91% | 1.79% | 1.85% | 1.74% | 1.94% | 1.74% | 2.17% | 1.94% | 2.62% | 0.73% |
| F2 | 23.47% | 32.44% | 33.49% | 31.76% | 32.49% | 31.99% | 32.10% | 32.49% | 34.41% | 31.32% | 41.05% | 31.92% | 37.50% | 21.08% | |
| F3 | 8.79% | 15.73% | 13.74% | 18.35% | 16.79% | 14.68% | 15.99% | 17.96% | 13.54% | 10.18% | 10.89% | 28.24% | 24.79% | 5.68% | |
| F4 | 67.01% | 50.11% | 50.87% | 48.01% | 48.81% | 51.55% | 50.06% | 47.80% | 50.11% | 56.76% | 45.89% | 37.91% | 35.10% | 72.51% | |
| Zn | F1 | 5.73% | 6.45% | 6.91% | 5.39% | 6.33% | 6.77% | 5.53% | 5.91% | 6.84% | 4.28% | 6.38% | 5.16% | 10.94% | 4.41% |
| F2 | 10.04% | 12.05% | 12.95% | 11.19% | 12.52% | 11.28% | 11.66% | 12.02% | 13.22% | 6.86% | 12.84% | 11.45% | 21.84% | 7.32% | |
| F3 | 17.67% | 26.92% | 27.74% | 26.28% | 28.31% | 26.14% | 25.27% | 27.20% | 28.51% | 14.28% | 18.70% | 34.44% | 48.64% | 18.86% | |
| F4 | 66.57% | 54.58% | 52.40% | 57.13% | 52.84% | 55.81% | 57.53% | 54.86% | 51.43% | 74.57% | 62.08% | 48.95% | 18.58% | 69.38% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Xu, Q.; Zhang, H.; Zhang, X.; Yang, J.; Li, Y.; Song, N.; Yu, J. How Will the Heavy Metal Risk Change Under Continuous Changing Hydrological Regimes and Salinity? Water 2025, 17, 1038. https://doi.org/10.3390/w17071038
Yu Y, Xu Q, Zhang H, Zhang X, Yang J, Li Y, Song N, Yu J. How Will the Heavy Metal Risk Change Under Continuous Changing Hydrological Regimes and Salinity? Water. 2025; 17(7):1038. https://doi.org/10.3390/w17071038
Chicago/Turabian StyleYu, Yang, Qian Xu, Hui Zhang, Xintong Zhang, Jisong Yang, Yunzhao Li, Ningning Song, and Junbao Yu. 2025. "How Will the Heavy Metal Risk Change Under Continuous Changing Hydrological Regimes and Salinity?" Water 17, no. 7: 1038. https://doi.org/10.3390/w17071038
APA StyleYu, Y., Xu, Q., Zhang, H., Zhang, X., Yang, J., Li, Y., Song, N., & Yu, J. (2025). How Will the Heavy Metal Risk Change Under Continuous Changing Hydrological Regimes and Salinity? Water, 17(7), 1038. https://doi.org/10.3390/w17071038






