1. Introduction
Effluents generated from aquaculture production are well known to have potential detrimental effects on water quality and the biodiversity of local fauna [
1,
2]. This is because aquaculture effluent is rich in nutrient substances like phosphorus (P) and nitrogen (N) and can therefore pose a risk of eutrophication in natural waterbodies when released in excessive amounts [
1,
2,
3]. Due to the potential negative environmental impact, N and P are regulated in the global aquaculture industry, especially for countries in the European Union (EU), where farmers are subject to strict guidelines in terms of effluent regulations on what and how much can be emitted [
4,
5,
6,
7]. While N originates from protein digestion in fish [
8,
9], P, among other things, is a key bone-forming mineral, along with calcium (Ca) and magnesium (Mg), and is thus an essential part of the diet for several aquaculture species [
8,
10]. The strict regulations on N and P also motivate salmon farmers to invest in technology-intensive farming solutions as a way of reducing and controlling their emissions. One such solution is the recirculating aquaculture system (RAS), which has improved the operational sustainability of fish production by reducing the environmental impact compared to traditional flow-through systems (FTSs) and has generally increased biomass productivity in fish farming [
11,
12,
13]. The multiple benefits of RASs can be credited to the system’s capability of temperature control, reduced water consumption, and its potential for effluent discharge treatment [
14,
15,
16].
The high reuse capability of RASs can cause the production water within the system itself to contain higher levels of total suspended solids (TSS), minerals, metals, nitrogenous compounds, and total gas pressure (TGP) compared to a FTS [
17,
18,
19,
20]. This can, in a worst-case scenario, pose a risk to the operation of the RAS and the safety of the fish. In most aquaculture systems, nitrate-N (NO
3−) concentrations are below 50 mg L
−1, but in RASs with low water exchange rates, the concentrations can exceed 400 mg L
−1 [
21,
22]. For the production of salmonids, elevated levels of NO
3− in the production water can reduce growth, health, and general performance or increase the mortality of the fish group [
23,
24,
25]. Conversely, the concentration of P in the production water exceeding the recommended levels of 3+ mg L
−1 [
18] does not necessarily affect fish performance in a negative manner [
26]. However, in terms of waste production, elevated P levels increase the risk of eutrophication when combined with elevated N concentrations [
1,
2,
3]. It is important to remember that even in a RAS with a high degree of water reuse, the nutrients that are not removed by the drum filter or foam fractionator, will accumulate in the production water and ultimately be released from the facility in a highly concentrated manner.
While RASs offer operational benefits, the impact of temperature manipulation on fish performance remains understudied. For the production of Atlantic salmon (
Salmo salar), the technical system itself will not necessarily affect the performance of the fish post-transfer as long as environmental parameters are kept equal [
27]. However, manipulating the temperature can significantly affect the performance of the fish post-transfer to seawater due to its effect on the general biology of the fish, such as heart weight and intestinal fat accumulation [
28,
29]. Growth rate, feed intake, FCR, and stomach evacuation rate are all affected by temperature [
30,
31,
32]. These parameters are also directly linked to the waste production of fish and, thus, the nutrient release to the production water [
33,
34,
35]. While optimal temperature for freshwater smolts up to 300 g ranges around 14 °C, the optimal temperature for the feed conversion ratio (FCR) is 2 °C lower [
31,
36]. Since post-transfer seawater performance studies have primarily been conducted by comparing FTSs to RASs, it could be useful for the industry to understand how temperature manipulation within these ranges relates to a pure RAS comparison.
The effect of temperature in RASs has also been documented to strongly correlate with total ammonia nitrogen (TAN) and nitrite (NO
2−) removal rates in the biofilters, where nitrification rates decrease with decreasing temperature [
37,
38,
39]. Moreover, the biophysical characteristics of feces, such as settling velocities, from channel catfish (
Ictalurus punctatus) and bighead carp (
Aristichthys nobilis) vary with temperature, affecting how waste disperses and accumulates [
40]. Increased particle size and improved fecal stability can be crucial to better encapsulate the particle fraction of N and P compounds until they can be removed by drum filtration [
41,
42], thus reducing the total emission.
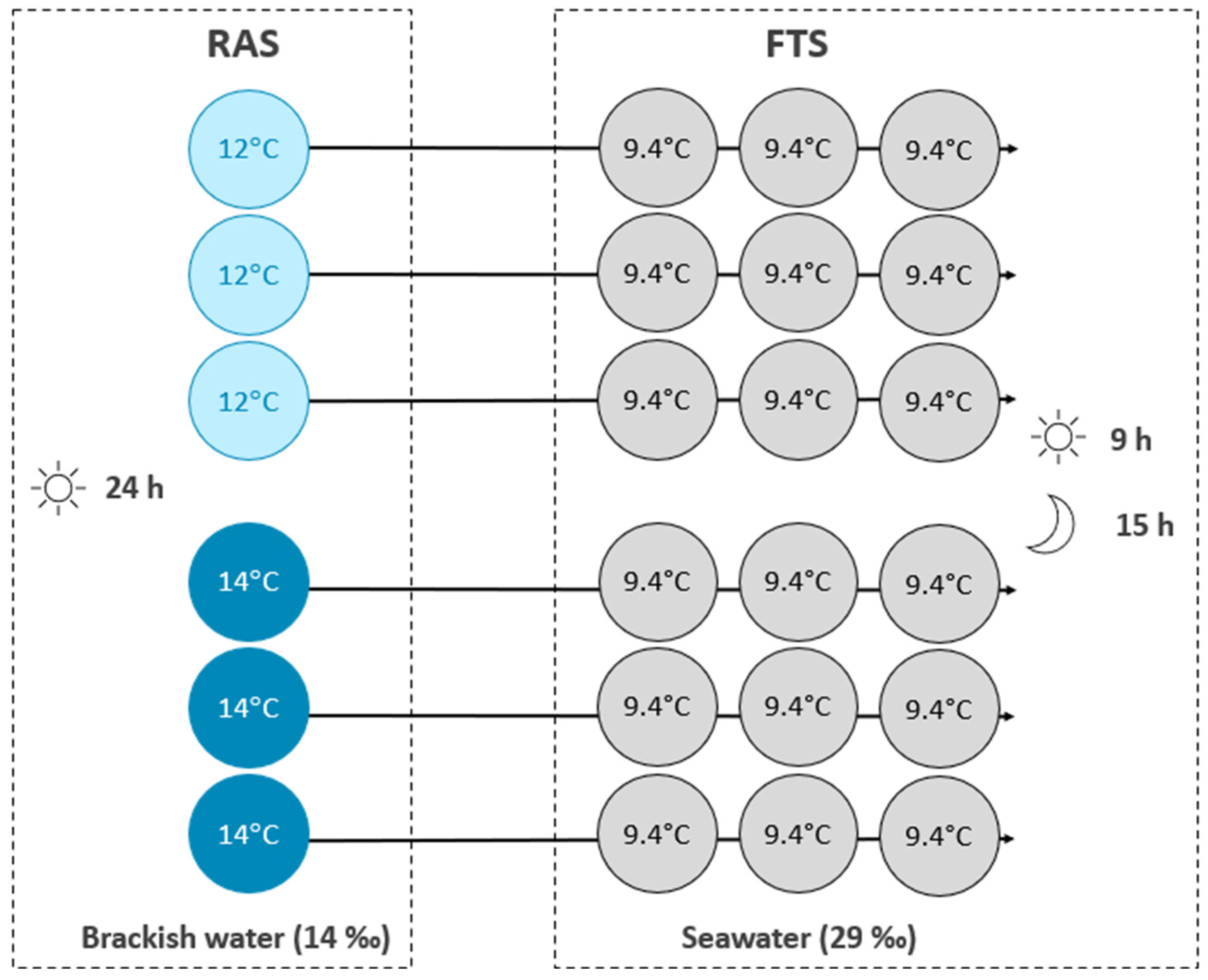
The primary objective of this experiment was, therefore, to compare fish performance of Atlantic salmon reared at a water temperature of 14 °C and a cooler temperature of 12 °C over a 9-week RAS period and a subsequent 10-week post-transfer seawater period in FTSs. A secondary objective was to determine the accumulation pattern of minerals in the production water of the RAS operated at the two water temperatures.
4. Discussion
Fish reared at 14 °C showed higher growth during the RAS period compared to those at 12 °C, aligning with previous studies on Atlantic salmon [
31,
36,
55,
56,
57] and findings from the Freshwater Institute in West Virginia [
58]. Although the TGC was higher for fish reared at 12 °C, it remained within the target proposed for Atlantic salmon in RASs [
59]. The K factor was similar between the two temperature regimes, indicating a comparable slaughter yield [
50,
51]. Although the FCR was not significantly different between 12 and 14 °C, there was a tendency for higher FCR at lower temperatures (
p = 0.08), which was opposite to what was expected [
31,
36]. Interestingly, the tendency shifted post-transfer to seawater, and the fish with a rearing history of 12 °C ended up with a tendency of lower FCR (
p = 0.07) compared to those reared at 14 °C. For the post-transfer period, fish with a rearing history of 12 °C maintained a higher TGC and SGR. This is in line with results showing that lower freshwater temperatures (4–6 °C) before transfer can enhance post-transfer growth in different Atlantic salmon strains, and the pattern in the current study seems to be consistent with studies on compensatory growth [
60,
61,
62]. Additionally, reducing temperatures from 14 to 12 °C during the freshwater period can reduce sexual maturation [
58] and improve physiological adaptation to seawater [
28,
31,
61]. Acclimation of fish to post-transfer temperatures is also highlighted as beneficial for growth parameters [
31,
63,
64]. In the current trial, the post-transfer water temperature of 9.4 ± 0.6 °C, being closer to 12 °C, likely gave an advantage to the fish with a freshwater rearing history of 12 over those reared at 14 °C.
Post-transfer, both the HSI and VSI were significantly lower in fish reared at 12 compared to 14 °C. These observations in the RAS, along with the significantly lower HSI post-transfer for the fish reared at a lower temperature, are consistent with a previous study examining fish raised at lower temperatures in a FTS compared to a RAS [
28]. Although the current study did not show significant differences in the CSI, it is believed that the small difference in water temperature of 2 °C was not sufficient to inflict a different CSI compared to the previous study, which had a larger difference in water temperature (approximately 5 °C) [
28]. The similar post-transfer K factor, combined with a lower VSI for fish with a rearing history of 12 °C, suggests that their weight gain was primarily due to muscle growth. Fish grow faster early in life, with new muscle fibers forming until they reach 40 to 50% of their maximum body length [
65]. The growth period where they reach up to approximately 1 kg is thus of high importance due to the recruitment of new muscle fibers [
66,
67], regardless of smolt type [
68]. However, further research is needed to determine if temperature reduction in RASs affects muscle fiber development and fish robustness.
Although the energy requirements for cooling water differ between different RAS setups [
69], the RAS units in the current trial generated water temperatures of 20 °C, which needed to be cooled to 12 and 14 °C. Based on the heat capacity of the water, the energy required to cool water an additional 2 °C, from 14 to 12 °C, can be calculated. In the current trial, the total consumption of new water did not differ between the groups (148–145 L day
−1). The following equation can be used to determine the energy required to cool 146.5 L day
−1, representing the average new water consumption of the tanks in the current study [
70]:
where Q is the heat energy (in joules), m is the mass of the water (in kg), c is the specific heat capacity of water (approximately 4.18 J g°C
−1 or 4180 J kg°C
−1 [
70]), and Δt is the change in temperature (in °C).
To cool the water by an additional 2 °C, 1 224 740 J is required. Using the average price of electricity in Norway for Q3 24 at NOK 1.123 kWh
−1, the cost can be calculated as follows [
71]:
An extra NOK 0.382 day−1 per RAS unit is needed to cool the water by an additional 2 °C. In relation to the accumulated biomass production of 44.3 kg, this translates to an additional cost of NOK 0.00058 per kg fish produced per day (NOK 0.058 ton fish produced day−1). These calculations are based purely on the effect of the cooling of water, and to relate this to specific RAS productions, energy efficiency, cooling equipment, and additional production time must be considered to adapt the estimate. For the current trial, which only included differences in the water temperature, the extra cost of NOK 0.058 ton fish produced day−1 achieved an approximately 8% increase in body weight gain after 66 days in seawater.
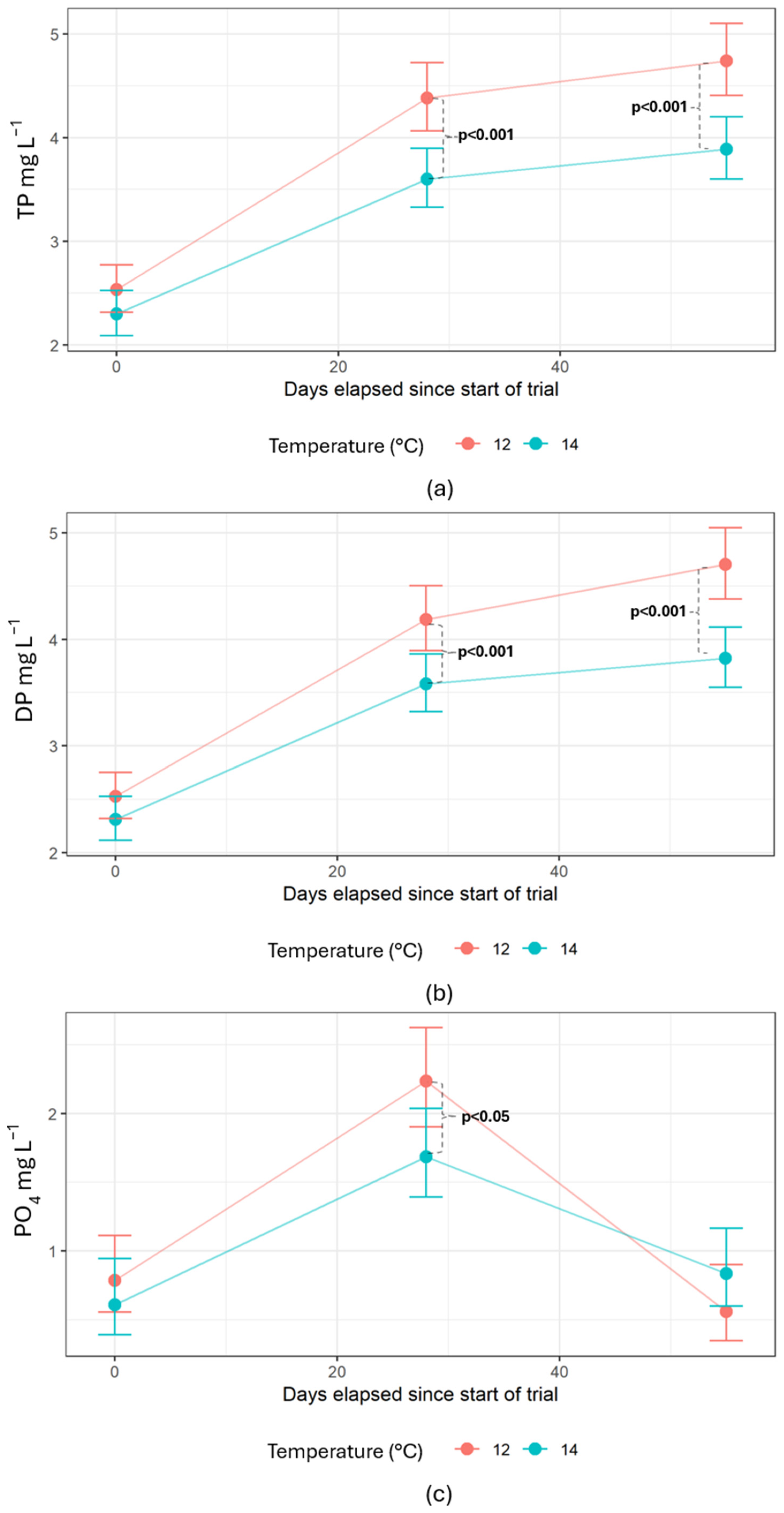
Water samples taken in the RAS showed no differences in compound concentrations between different water treatment steps. Crouse et al. (2022) experienced slightly elevated TSS and nitrate for the salmon reared at 14 compared to 12 °C due to higher feed consumption driven by the elevated temperature, as also shown by previous studies [
58,
72,
73,
74]. Similar results were found in the current study, with significantly elevated nitrate at 14 °C. However, the upper recommended limits for nitrate-N (443 mg L
−1) and TSS (15 mg L
−1) were not exceeded [
75,
76,
77]. Both at 12 and 14 °C, the concentrations of nitrite-N (2.4 and 2.6 mg L
−1, respectively) were within the recommended levels reported for salmonids reared in freshwater and seawater (0.1 and 0.5 mg L
−1, respectively) [
22,
78,
79]. The concentration of unionized ammonia nitrogen, which has sublethal effect on Atlantic salmon at water concentrations of 10 μg L
−1 [
80], was also below the lowest recommended level of 12.5 μg L
−1 [
79]. Since intermediate salinities reduce ammonia toxicity, it is concluded that the concentrations in the current study were well within acceptable levels for salmon [
81]. Phosphates play a crucial role in bacterial development, and PO
4 can act as a supporting or limiting factor for bacterial growth and activity [
3,
82,
83,
84]. The biofilter bacteria, including
Nitrosomonas and
Nitrobacter, consume PO
4 to grow [
85]. In the current experiment, a spike in PO
4 was observed mid-trial for both temperatures before the levels were reduced again, unlike TP, DP, and dissolved Fe. This could indicate periodic drops in bacterial culture due to the moving bed itself, where biochips regularly collide and detach bacterial pieces that ultimately exit through the recirculation loop [
86], potentially also causing variations in TP and DP concentrations. Since the spikes were observed for both temperature regimes and only for PO
4, they are more likely to represent the natural development of bacterial cultures [
87]. When the biofilter bacteria die, it will take some time to build up the new culture, and in this period, PO
4 could increase, as observed in the current trial. Thus, PO
4 had the highest fluctuations between the sampling dates of the P components measured in this experiment.
A solution with settling columns was used for fecal removal in the RAS, which is not normal in commercial systems, but the wastewater discharge rates were similar to those of other studies [
17,
18]. Both the Guelph system and total urine and feces columns are confirmed as suitable methods for fecal collection, ensuring minimal nutrient leakage and damage to the fecal particle [
88]. The current experimental system successfully removed larger particles, leaving mainly microparticles to accumulate within the system. This was indicated by analyses that showed no significant difference between TP and DP (NS-EN ISO 15681-2) [
47]. Higher TP and DP accumulation at 12 °C were also observed, suggesting that the dissolved fractions will control the accumulation rate in the RAS. This study therefore highlights the challenge of capturing DP in a mechanical filter and supports the suggestions that precipitation or enhanced biological phosphorus removal must be applied to remove DP from the production water [
89,
90]. Although not proven in the current study, the removal of settleable particles (> 100 μm) [
91] may have generated a lack of cake filtration in the drum filter. Accumulated material on the filter (cake) has the potential to provide greater filtration efficiencies than the filter screen alone [
92]; subsequently, this lack of cake could have caused a lower removal efficiency of P and particles in general. Drum filters can easily remove particles larger than 30 μm, but smaller microparticles tend to accumulate [
77,
93,
94,
95,
96]. The material of the drum filter may also affect the removal rates or general accumulation [
97]; for example, in the current experiment, polyester was used, but filter media consisting of glass or metal components could generate a different effect within the system. However, this remains unclear and needs to be examined in further studies.
By using an equation to determine concentrations in the outlet of a reuse system [
89], it is possible to predict the accumulated concentration measured in RASs compared to what would be expected in a FTS:
where C is the concentration measured in the outlet or, in our case, the tank, R equals the degree of reuse, and RE equals the removal efficiency.
The removal efficiency is set to zero since the current study suggests that DP will accumulate. With 90% reuse, the concentration of P in the RAS is estimated to be ten times higher than in a FTS. If the current experiment had been conducted in a FTS, the concentrations in the fish tank would have been approximately (4.7 and 3.8 mg L
−1)/10 = 0.47 and 0.38 mg L
−1 for the 12 and 14 °C regimes, respectively. Cooling the water by an additional 2 °C resulted in a 23.7% increase in DP, which, from an environmental perspective, means that temperature affects how concentrated or dilute the emitted P effluent will be. To illustrate this effect in a practical example, where it is assumed that an average of 18% dietary P is emitted as DP and 52% is emitted as particles in feces [
35,
98,
99,
100], reducing the water temperature from 14 to 12 °C could therefore change the emission characteristic to 22.3% DP and 47.7% particle-emitted P, making it harder to collect in sludge treatment. These levels are used as an example, and the availability of P in different raw materials is still the most important factor affecting the composition of the excreted P waste [
26]. The elevated DP at 12 °C, compared to 14 °C, may most likely be attributable to a combination of reduced feed consumption and altered P excretion rates, both driven by temperature changes.
In rainbow trout (
Oncorhynchus mykiss), the Mg requirement of 330–600 mg kg
−1 [
43] can be met through the diet or water if water-borne Mg concentrations are at least 46 mg L
−1 [
101], as seen in low water exchange in brackish RASs [
26], similar to that in the current trial. While the Mg requirements can be sufficiently met, the observed 20.5% increase in DP in the current trial is unlikely to cover the total requirement of P for the fish. Estimating water concentrations of P against drinking rates for seawater-adapted salmon (4 mL kg h
−1–6.4 mL kg h
−1 [
102,
103]) and assuming, using a simplified example, that a salmon needs approximately 70 days to reach 1 kg, it drinks approximately 6.72–10.75 L of the production water. If the average production water in a RAS contains 5 mg P L
−1 (reflecting the 12 °C regime in the current trial), the salmon can only have 33.6–53.7 mg kg
−1 growth, which is insignificant compared to the dietary requirement of 6000–10,000 mg kg
−1 [
43]. To cover the P requirement, a water concentration of 893–930 mg P L
−1 would be needed, nearly 200 times higher than that measured in the current trial.
The Ca/P ratio in the salmon was around 1, which was expected and has been confirmed in several studies [
104,
105,
106]. The whole-body composition thus provided an estimated average P retention of 59%, implying a P excretion of 41%. Fecal stability was similar for both temperature treatments, meaning that the amount of feces removed from the system was equal. The feces composition only showed significantly lower Mg for fish reared at 12 °C. The unaffected Zn accumulation in water and in the feces was a positive observation as it indicated that reducing the temperature from 14 to 12 °C did not alter sludge composition. This would be beneficial since the sludge can be graded similarly when used as a fertilizer [
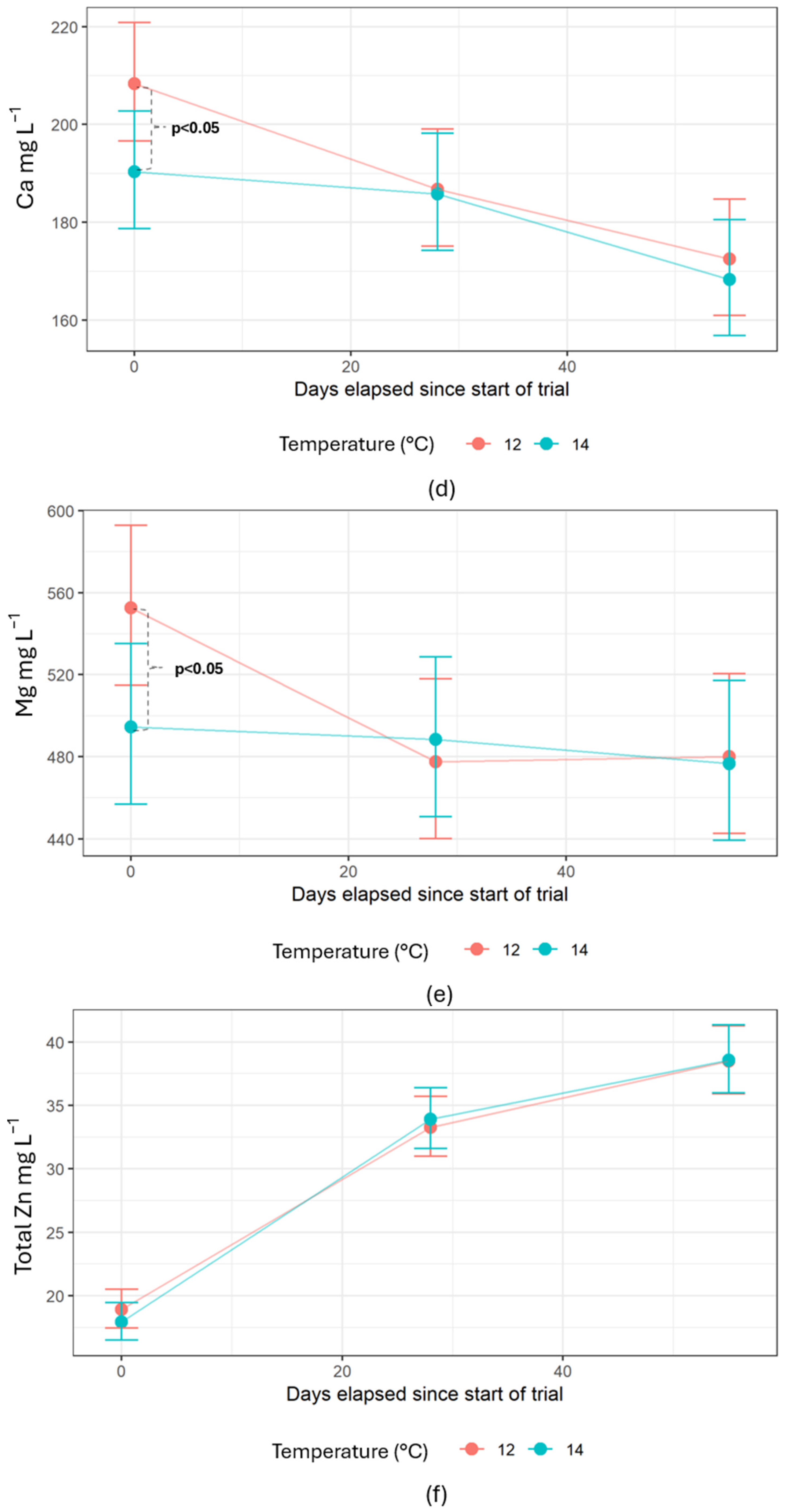
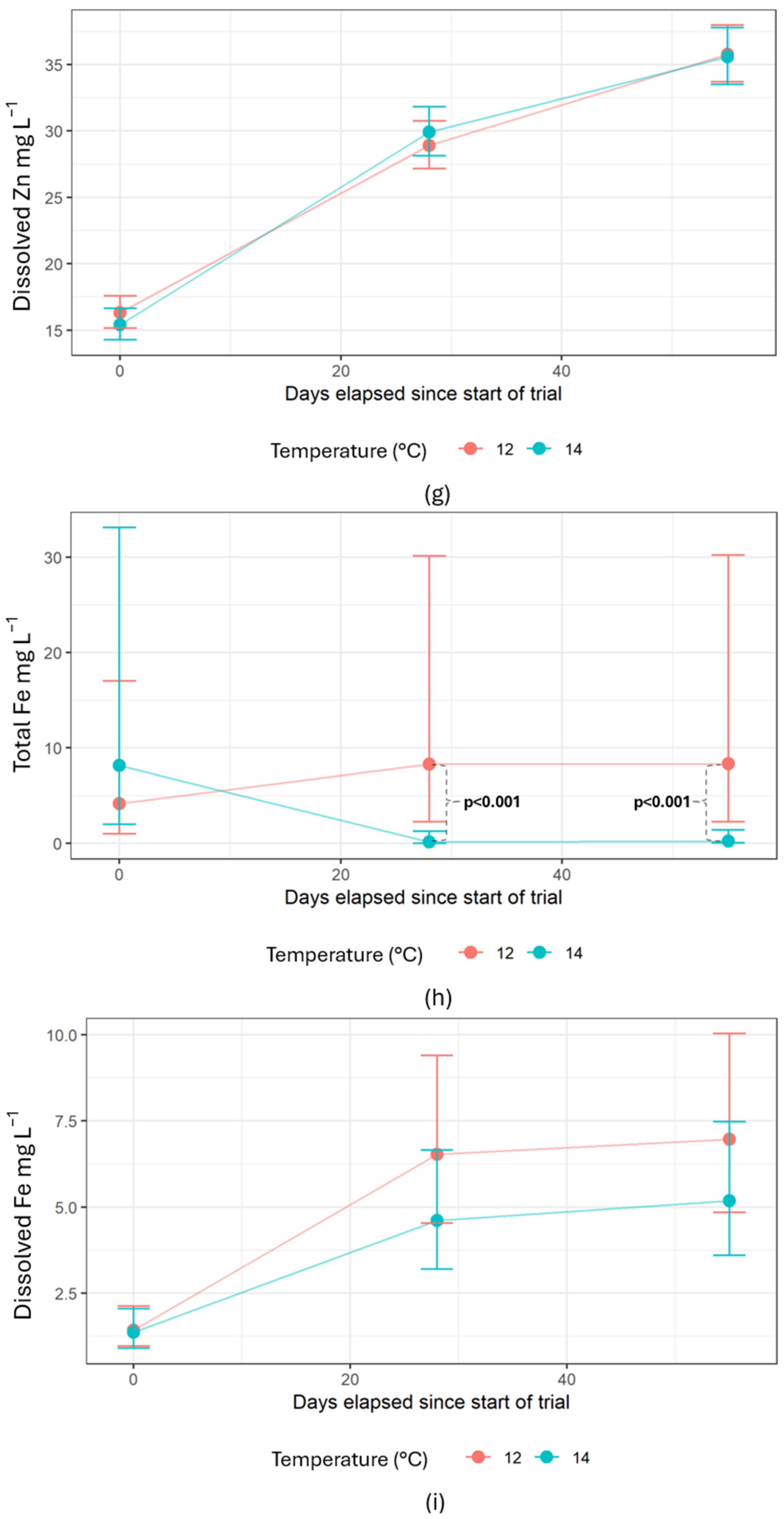
107]. However, the 23.7% higher DP concentration in the production water at 12 °C suggests a difference in the nutritional retention of P. Although not significant, P in the feces showed a tendency to be lower (
p = 0.1) for fish reared at 12 °C, along with Ca, another bone-forming mineral. It has been proven that a faster growth rate initiated by temperature will promote a more rapid bone development [
43,
74], meaning the fish will have a higher dietary P requirement at higher temperatures. Exceeding this dietary requirement leads to higher urinary P excretion [
108], measurable as DP. While the current trial cannot determine this, future studies with a nutritional trial design and longer RAS period could investigate it. The stable conditions of a RAS can prevent drops in appetite initiated by sudden fluctuations in temperature, thus securing a stable growth rate throughout a production period [
109,
110,
111]. Lowering dietary P to counteract the increased excretion of P when reducing the temperature from 14 to 12 °C while still maintaining growth and health would be beneficial for the industry and environment.