Response of Phytoplankton to Nutrient Limitation in the Ecological Restoration of a Subtropical Shallow Lake
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling and Analysis
2.3. Nutrient Limitation Bioassay Experiments
2.4. Data Analysis
3. Results
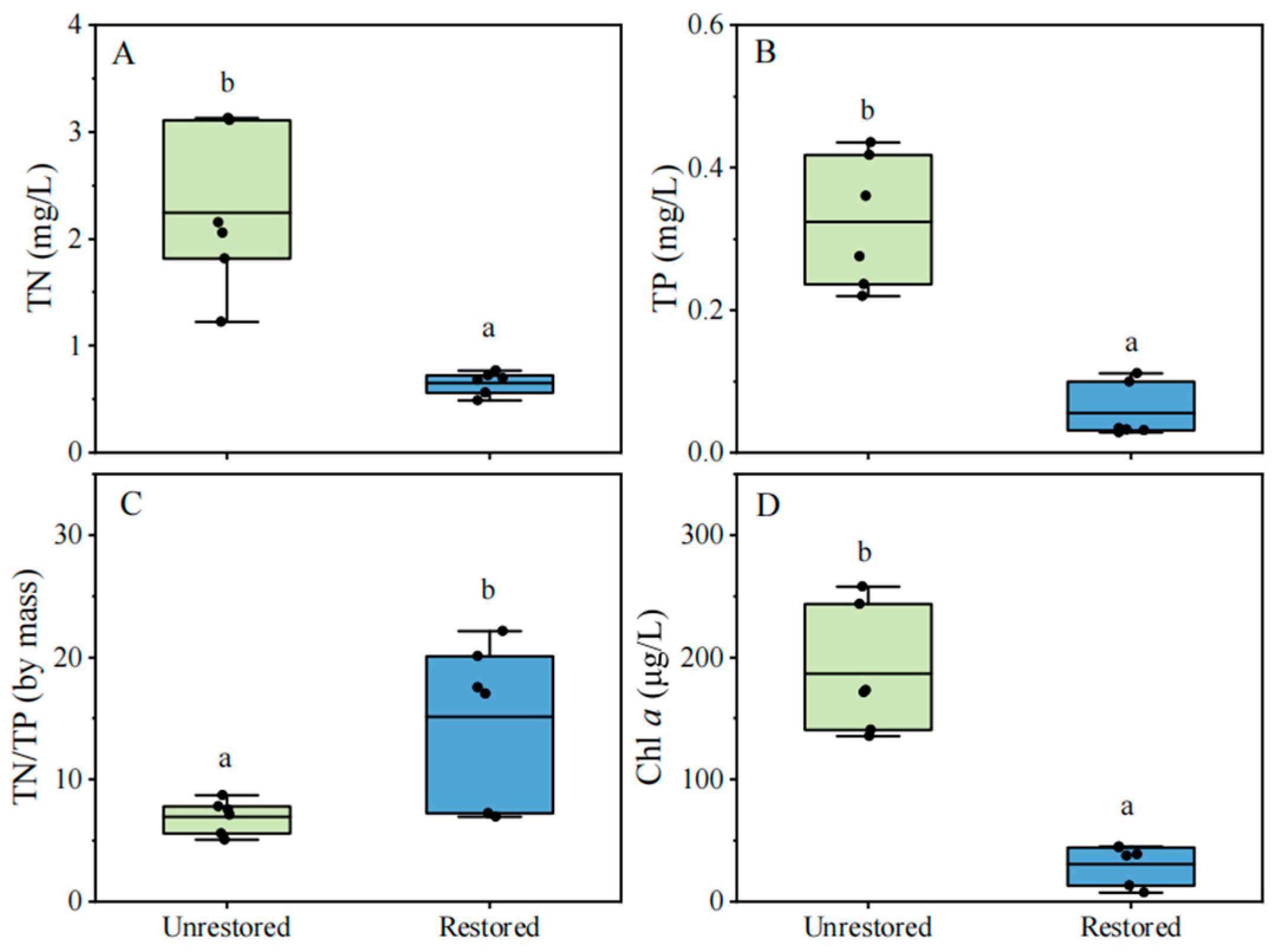
3.1. Nutrient and Chl a Concentrations in the Restored and Unrestored Lake Bay
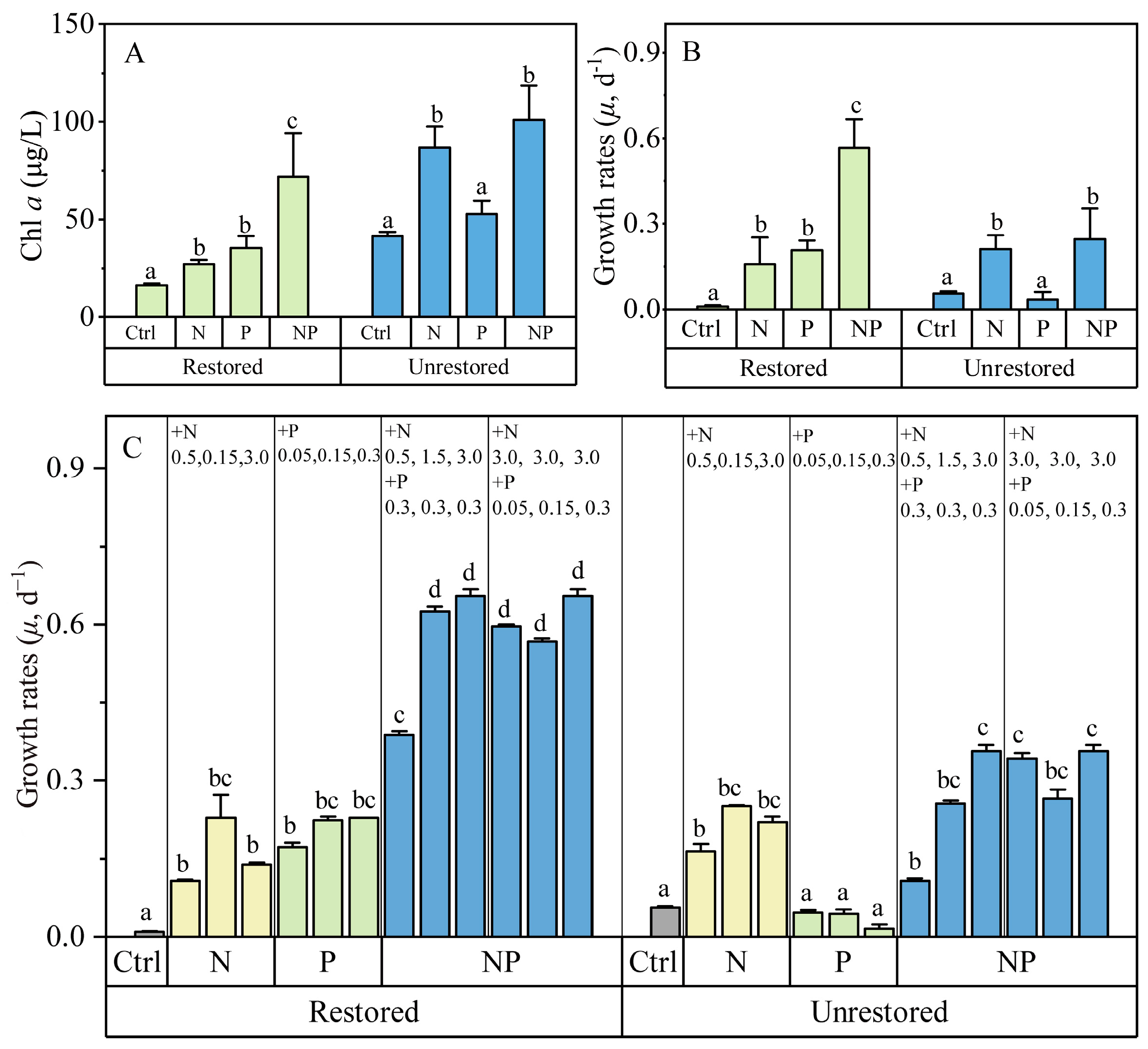
3.2. Field Nutrient Limitation Experiments
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variables | Control (mg/L) | N Addition (mg/L) | P Addition (mg/L) | NP Co-Addition (mg/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Restored | ||||||||||||
| Total nitrogen | 1.62 | 2.12 | 3.12 | 4.62 | 1.62 | 1.62 | 1.62 | 2.12 | 3.12 | 4.62 | 4.62 | 4.62 |
| Dissolved inorganic nitrogen | 1.01 | 1.51 | 2.51 | 4.01 | 1.01 | 1.01 | 1.01 | 1.51 | 2.51 | 4.01 | 4.01 | 4.01 |
| Total phosphorus | 0.12 | 0.12 | 0.12 | 0.12 | 0.17 | 0.27 | 0.42 | 0.42 | 0.42 | 0.42 | 0.27 | 0.17 |
| Soluble reactive phosphorus | 0.07 | 0.07 | 0.07 | 0.07 | 0.12 | 0.22 | 0.37 | 0.37 | 0.37 | 0.37 | 0.22 | 0.12 |
| TN:TP | 13.20 | 17.27 | 25.40 | 37.59 | 9.39 | 5.95 | 3.84 | 5.02 | 7.39 | 10.93 | 16.94 | 26.73 |
| Unrestored | ||||||||||||
| Total nitrogen | 2.75 | 3.25 | 4.25 | 5.75 | 2.75 | 2.75 | 2.75 | 3.25 | 4.25 | 5.75 | 5.75 | 5.75 |
| Dissolved inorganic nitrogen | 1.66 | 2.16 | 3.16 | 4.66 | 1.66 | 1.66 | 1.66 | 2.16 | 3.16 | 4.66 | 4.66 | 4.66 |
| Total phosphorus | 0.31 | 0.31 | 0.31 | 0.31 | 0.36 | 0.46 | 0.61 | 0.61 | 0.61 | 0.61 | 0.46 | 0.36 |
| Soluble reactive phosphorus | 0.16 | 0.16 | 0.16 | 0.16 | 0.21 | 0.31 | 0.46 | 0.46 | 0.46 | 0.46 | 0.31 | 0.21 |
| TN:TP | 8.88 | 10.49 | 13.72 | 18.56 | 7.65 | 5.98 | 4.51 | 5.33 | 6.97 | 9.43 | 12.51 | 15.98 |
| Sig (Shapiro–Wilk Test) | ||
|---|---|---|
| Unrestored | Restored | |
| TP | 0.354 | 0.100 |
| TN | 0.443 | 0.440 |
| Chl a | 0.191 | 0.070 |
| TN/TP | 0.647 | 0.152 |
| Test of Homogeneity of Variances | t-Test | |||
|---|---|---|---|---|
| F | sig | t | sig | |
| TP | 11.82 | 0.006 | 6.51 | <0.0001 |
| TN | 9.26 | 0.012 | 5.16 | <0.0001 |
| Chl a | 8.70 | 0.015 | 7.02 | <0.0001 |
| TN/TP | 13.34 | 0.004 | 3.01 | <0.0001 |
References
- Scheffer, M.; Hosper, S.H.; Meijer, M.-L.; Moss, B.; Jeppesen, E. Alternative Equilibria in Shallow Lakes. Trends Ecol. Evol. 1993, 8, 275–279. [Google Scholar] [CrossRef]
- Scheffer, M.; Jeppesen, E. Regime Shifts in Shallow Lakes. Ecosystems 2007, 10, 1–3. [Google Scholar] [CrossRef]
- Su, H.; Wu, Y.; Xia, W.; Yang, L.; Chen, J.; Han, W.; Fang, J.; Xie, P. Stoichiometric Mechanisms of Regime Shifts in Freshwater Ecosystem. Water Res. 2019, 149, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Hansson, L.; Annadotter, H.; Bergman, E.; Hamrin, S.; Jeppesen, E.; Kairesalo, T.; Luokkanen, E.; Nilsson, P.; Sondergaard, M.; Strand, J. Biomanipulation as an Application of Food-Chain Theory: Constraints, Synthesis, and Recommendations for Temperate Lakes. Ecosystems 1998, 1, 558–574. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, J.; Zhong, P.; Zhang, X.; Ning, J.; Larsen, S.E.; Chen, D.; Gao, Y.; He, H.; Jeppesen, E. Successful Restoration of a Tropical Shallow Eutrophic Lake: Strong Bottom-up but Weak Top-down Effects Recorded. Water Res. 2018, 146, 88–97. [Google Scholar] [CrossRef]
- Li, B.; Chen, D.; Lu, J.; Liu, S.; Wu, J.; Gan, L.; Yang, X.; He, X.; He, H.; Yu, J.; et al. Restoring Turbid Eutrophic Shallow Lakes to a Clear-Water State by Combined Biomanipulation and Chemical Treatment: A 4-Hectare in-Situ Experiment in Subtropical China. J. Environ. Manag. 2025, 380, 125061. [Google Scholar] [CrossRef]
- Hilt, S.; Koehler, J.; Adrian, R.; Monaghan, M.T.; Sayer, C.D. Clear, Crashing, Turbid and Back—Long-Term Changes in Macrophyte Assemblages in a Shallow Lake. Freshw. Biol. 2013, 58, 2027–2036. [Google Scholar] [CrossRef]
- Zeng, L.; He, F.; Dai, Z.; Xu, D.; Liu, B.; Zhou, Q.; Wu, Z. Effect of Submerged Macrophyte Restoration on Improving Aquatic Ecosystem in a Subtropical, Shallow Lake. Ecol. Eng. 2017, 106, 578–587. [Google Scholar] [CrossRef]
- Schriver, P.; Bogestrand, J.; Jeppesen, E.; Sondergaard, M. Impact of submerged macrophytes on fish-zooplankton-phytoplankton interactions—Large-scale enclosure experiments in a shallow eutrophic lake. Freshw. Biol. 1995, 33, 255–270. [Google Scholar] [CrossRef]
- He, H.; Liu, X.; Liu, X.; Yu, J.; Li, K.; Guan, B.; Jeppesen, E.; Liu, Z. Effects of Cyanobacterial Blooms on Submerged Macrophytes Alleviated by the Native Chinese Bivalve Hyriopsis Cumingii: A Mesocosm Experiment Study. Ecol. Eng. 2014, 71, 363–367. [Google Scholar] [CrossRef]
- Hilt, S. Regime Shifts between Macrophytes and Phytoplankton—Concepts beyond Shallow Lakes, Unravelling Stabilizing Mechanisms and Practical Consequences. Limnetica 2015, 34, 467–479. [Google Scholar]
- Liu, Z.; Bai, G.; Liu, Y.; Zou, Y.; Ding, Z.; Wang, R.; Chen, D.; Kong, L.; Wang, C.; Liu, L.; et al. Long-Term Study of Ecological Restoration in a Typical Shallow Urban Lake. Sci. Total Environ. 2022, 846, 157505. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global Analysis of Nitrogen and Phosphorus Limitation of Primary Producers in Freshwater, Marine and Terrestrial Ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef]
- Moss, B.; Jeppesen, E.; Søndergaard, M.; Lauridsen, T.L.; Liu, Z. Nitrogen, Macrophytes, Shallow Lakes and Nutrient Limitation: Resolution of a Current Controversy? Hydrobiologia 2013, 710, 3–21. [Google Scholar] [CrossRef]
- Søndergaard, M.; Lauridsen, T.L.; Johansson, L.S.; Jeppesen, E. Nitrogen or Phosphorus Limitation in Lakes and Its Impact on Phytoplankton Biomass and Submerged Macrophyte Cover. Hydrobiologia 2017, 795, 35–48. [Google Scholar] [CrossRef]
- Jeppesen, E.; Søndergaard, M.; Jensen, J.P.; Havens, K.E.; Anneville, O.; Carvalho, L.; Coveney, M.F.; Deneke, R.; Dokulil, M.T.; Foy, B.; et al. Lake Responses to Reduced Nutrient Loading—An Analysis of Contemporary Long-term Data from 35 Case Studies. Freshw. Biol. 2005, 50, 1747–1771. [Google Scholar] [CrossRef]
- Noges, T.; Järvet, A.; Kisand, A.; Laugaste, R.; Loigu, E.; Skakalski, B.; Noges, P. Reaction of Large and Shallow Lakes Peipsi and Vortsjarv to the Changes of Nutrient Loading. Hydrobiologia 2007, 584, 253–264. [Google Scholar] [CrossRef]
- Xu, H.; Paerl, H.W.; Qin, B.; Zhu, G.; Hall, N.S.; Wu, Y. Determining Critical Nutrient Thresholds Needed to Control Harmful Cyanobacterial Blooms in Eutrophic Lake Taihu, China. Environ. Sci. Technol. 2015, 49, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Paerl, H.W.; Zhu, G.; Qin, B.; Hall, N.S.; Zhu, M. Long-Term Nutrient Trends and Harmful Cyanobacterial Bloom Potential in Hypertrophic Lake Taihu, China. Hydrobiologia 2017, 787, 229–242. [Google Scholar] [CrossRef]
- Wang, M.; Xu, X.; Wu, Z.; Zhang, X.; Sun, P.; Wen, Y.; Wang, Z.; Lu, X.; Zhang, W.; Wang, X.; et al. Seasonal Pattern of Nutrient Limitation in a Eutrophic Lake and Quantitative Analysis of the Impacts from Internal Nutrient Cycling. Environ. Sci. Technol. 2019, 53, 13675–13686. [Google Scholar] [CrossRef]
- Ebina, J.; Tsutsui, T.; Shirai, T. Simultaneous Determination of Total Nitrogen and Total Phosphorus in Water Using Peroxodisulfate Oxidation. Water Res. 1983, 17, 1721–1726. [Google Scholar] [CrossRef]
- Pápista, É.; Ács, É.; Böddi, B. Chlorophyll-a Determination with Ethanol—A Critical Test. Hydrobiologia 2002, 485, 191–198. [Google Scholar] [CrossRef]
- Paerl, H.W.; Dyble, J.; Pinckney, J.L.; Valdes, L.M.; Millie, D.F.; Moisander, P.H.; Morris, J.T.; Bendis, B.; Piehler, M.F. Using Microalgal Indicators to Assess Human- and Climate-Induced Ecological Change in Estuaries. In Estuarine Indicators; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Rudek, J.; Paerl, H.; Mallin, M.; Bates, P. Seasonal and Hydrological Control of Phytoplankton Nutrient Limitation in the Lower Neuse River Estuary, North Carolina. Mar. Ecol. Prog. Ser. 1991, 75, 133–142. [Google Scholar] [CrossRef]
- Sommer, U. A Comparison of the Droop and the Monod Models of Nutrient Limited Growth Applied to Natural Populations of Phytoplankton. Funct. Ecol. 1991, 5, 535. [Google Scholar] [CrossRef]
- Lv, C.; Shan, H.; Tian, Y.; Zhao, X.; Wen, Z.; Yin, C.; Li, Z.; Su, H.; Wang, W.; Chou, Q.; et al. The Dual Role of Benthic Fish: Effects on Water Quality in the Presence and Absence of Submerged Macrophytes. Water Res. 2024, 267, 122466. [Google Scholar] [CrossRef]
- Jeppesen, E.; Sørensen, P.B.; Johansson, L.S.; Søndergaard, M.; Lauridsen, T.L.; Nielsen, A.; Mejlhede, P. Recovery of Lakes from Eutrophication: Changes in Nitrogen Retention Capacity and the Role of Nitrogen Legacy in 10 Danish Lakes Studied over 30 Years. Hydrobiologia 2025, 852, 377–387. [Google Scholar] [CrossRef]
- Hilt, S.; Alirangues Nuñez, M.M.; Bakker, E.S.; Blindow, I.; Davidson, T.A.; Gillefalk, M.; Hansson, L.-A.; Janse, J.H.; Janssen, A.B.G.; Jeppesen, E.; et al. Response of Submerged Macrophyte Communities to External and Internal Restoration Measures in North Temperate Shallow Lakes. Front. Plant Sci. 2018, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Zhou, Y.; Jeppesen, E.; Shi, K.; Qin, B. Response of Community Composition and Biomass of Submerged Macrophytes to Variation in Underwater Light, Wind and Trophic Status in a Large Eutrophic Shallow Lake. J. Environ. Sci. 2021, 103, 298–310. [Google Scholar] [CrossRef]
- Li, C.; Sheng, H.; Tan, M.; Dai, H.; Wang, X.; Xu, H.; Ding, S.; Zhao, G. Rhythmic Radial Oxygen Loss Enhances Soil Phosphorus Bioavailability. Nat. Commun. 2025, 16, 4413. [Google Scholar] [CrossRef]
- Luong, H.A.; Rohlfs, A.-M.; Facey, J.A.; Colville, A.; Davie, A.W.; Pera, J.B.; Mitrovic, S.M. Influence of Macronutrient and Iron Enrichment on Phytoplankton Productivity and Community Dynamics: An in situ Microcosm Study in a Drinking Water Supply Reservoir. Inland Waters 2025, 15, 2475662. [Google Scholar] [CrossRef]
- Guildford, S.J.; Hecky, R.E. Total Nitrogen, Total Phosphorus, and Nutrient Limitation in Lakes and Oceans: Is There a Common Relationship? Limnol. Oceanogr. 2000, 45, 1213–1223. [Google Scholar] [CrossRef]
- Xu, H.; McCarthy, M.J.; Paerl, H.W.; Brookes, J.D.; Zhu, G.; Hall, N.S.; Qin, B.; Zhang, Y.; Zhu, M.; Hampel, J.J.; et al. Contributions of External Nutrient Loading and Internal Cycling to Cyanobacterial Bloom Dynamics in Lake Taihu, China: Implications for Nutrient Management. Limnol. Oceanogr. 2021, 66, 1492–1509. [Google Scholar] [CrossRef]
- Reckhow, K.H.; Arhonditsis, G.B.; Kenney, M.A.; Hauser, L.; Tribo, J.; Wu, C.; Elcock, K.J.; Steinberg, L.J.; Stow, C.A.; McBride, S.J. A Predictive Approach to Nutrient Criteria. Environ. Sci. Technol. 2005, 39, 2913–2919. [Google Scholar] [CrossRef]
- Reynolds, C.S. Eutrophication and the Management of Planktonic Algae—What Vollenweider Couldn’t Tell Us; Freshwater Biological Association: Milnthorpe, UK, 1992. [Google Scholar]
- Redoglio, A.; Sperfeld, E. What Drives Growth Responses of Nitrogen and Phosphorus (Co-)Limited Primary Producer Communities? Front. Ecol. Evol. 2024, 12, 1368445. [Google Scholar] [CrossRef]
- Andersen, I.M.; Williamson, T.J.; González, M.J.; Vanni, M.J. Nitrate, Ammonium, and Phosphorus Drive Seasonal Nutrient Limitation of Chlorophytes, Cyanobacteria, and Diatoms in a Hyper-eutrophic Reservoir. Limnol. Oceanogr. 2020, 65, 962–978. [Google Scholar] [CrossRef]
- Swarbrick, V.J.; Quinones-Rivera, Z.J.; Leavitt, P.R. Seasonal Variation in Effects of Urea and Phosphorus on Phytoplankton Abundance and Community Composition in a Hypereutrophic Hardwater Lake. Freshw. Biol. 2020, 65, 1765–1781. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, S.; Lin, Z.; He, H.; Li, K.; Gao, J.; Liu, Z.; Yu, J. Response of Phytoplankton to Nutrient Limitation in the Ecological Restoration of a Subtropical Shallow Lake. Water 2025, 17, 3371. https://doi.org/10.3390/w17233371
Fu S, Lin Z, He H, Li K, Gao J, Liu Z, Yu J. Response of Phytoplankton to Nutrient Limitation in the Ecological Restoration of a Subtropical Shallow Lake. Water. 2025; 17(23):3371. https://doi.org/10.3390/w17233371
Chicago/Turabian StyleFu, Shi, Zhenmei Lin, Hu He, Kuanyi Li, Jian Gao, Zhengwen Liu, and Jinlei Yu. 2025. "Response of Phytoplankton to Nutrient Limitation in the Ecological Restoration of a Subtropical Shallow Lake" Water 17, no. 23: 3371. https://doi.org/10.3390/w17233371
APA StyleFu, S., Lin, Z., He, H., Li, K., Gao, J., Liu, Z., & Yu, J. (2025). Response of Phytoplankton to Nutrient Limitation in the Ecological Restoration of a Subtropical Shallow Lake. Water, 17(23), 3371. https://doi.org/10.3390/w17233371









