Abstract
This study analyzed the relationship between zooplankton communities and water quality characteristics, with a focus on total organic carbon (TOC), in 22 reservoirs within the Geum River basin that share similar climatic conditions but exhibit varying levels of pollution. Across all reservoirs, zooplankton community structures showed the highest correlations with TOC, suspended solids (SS), chlorophyll-a (Chl-a), and Secchi depth (SD), with stronger associations observed for rotifers and cladocerans compared to copepods. The classification of zooplankton community composition patterns, followed by an analysis of their associations with TOC concentrations, revealed relatively distinct differences between high-TOC and low-TOC reservoirs, indicating that TOC functions as a key determinant of community composition. Meanwhile, network analysis based on overall water quality characteristics indicated that patterns of water quality similarity among zooplankton-based communities differed somewhat from those based solely on TOC concentrations, suggesting that TOC may exert an independent influence on zooplankton community structure. In high-TOC reservoirs, typical eutrophic characteristics—such as elevated chlorophyll-a, total phosphorus, and suspended solids, along with reduced water transparency—were observed, accompanied by higher zooplankton abundance and a greater proportion of rotifers within the community. In contrast, low-TOC reservoirs, despite exhibiting no marked differences in other water quality variables, showed higher diversity of cladocerans alongside rotifers, further supporting the independent role of TOC in shaping zooplankton community structures. These findings highlight TOC not only as a general indicator of pollution but also as an ecologically significant factor influencing zooplankton community composition and carbon dynamics in reservoir ecosystems. They suggest that TOC should be considered a key variable in future assessments and management of lentic ecosystems.
Keywords:
lentic ecosystem; TOC; zooplankton; rotifers; cladoceran; copepods; eutrophication; water quality 1. Introduction
Lakes and reservoirs are critical resources for various water supplies, particularly for agricultural purposes, with numerous reservoirs constructed to meet these demands [1]. The number of reservoirs continues to increase worldwide. While reservoirs contribute to resource security and can support the expansion of both aquatic and terrestrial habitats [2], gray infrastructure such as reservoirs may also adversely affect ecological flow by increasing water retention time, fragmenting ecosystems, and disconnecting longitudinal continuity [3,4]. Moreover, water released from reservoirs often contains elevated levels of nutrients and pollutants accumulated during stagnation, which can alter biogeochemical processes in downstream ecosystems, including rivers, estuaries, and coastal marine environments [5,6]. Therefore, understanding the physicochemical and biological processes within reservoirs and establishing tools to maintain a good ecological status are essential for the sustainable use and management of these artificial systems [7,8,9].
Total organic carbon (TOC) serves as a non-specific indicator of the total organic matter in lakes and reservoirs, encompassing both natural organic matter (NOM) and anthropogenic pollutants, and is crucial for assessing organic pollution, eutrophication, and overall ecosystem health [10]. In the European Union, TOC is designated as a key monitoring parameter for lake water quality [11], while in South Korea, water quality assessments have transitioned from Chemical Oxygen Demand (COD) to TOC to improve accuracy and reduce environmental impacts [12,13]. Unlike COD, which relies on toxic dichromate-based methods that generate hazardous waste, TOC measurement avoids harmful chemicals and provides a more precise estimate of bulk organic matter in water bodies, making it a more environmentally friendly and reliable indicator of organic pollution [14].
TOC in aquatic ecosystems, comprising dissolved organic carbon (DOC) and particulate organic carbon (POC), plays a critical role in global carbon cycling and ecosystem dynamics. TOC originates from both allochthonous sources, such as terrestrial runoff and atmospheric deposition, and autochthonous processes, including photosynthesis and microbial activity within the ecosystem [15]. In lakes, TOC undergoes complex transformations through food web interactions, microbial degradation, and sedimentation, with DOC typically constituting approximately 80% of TOC under oligotrophic conditions. However, the DOC:POC ratio varies depending on trophic status and environmental conditions [16]. In eutrophic lakes and rivers, the proportion of POC can increase significantly, reaching up to 30% of TOC due to elevated phytoplankton biomass [10,16]. Notably, in heavily eutrophic systems, such as the reservoir–river continuum of the Seonakdong River in South Korea, algal blooms can contribute up to 30% of TOC during peak bloom periods, primarily as POC from cyanobacterial biomass [17].
POC, a key component of TOC, is significantly influenced by phytoplankton production driven by photosynthesis, which enhances organic matter formation in aquatic ecosystems [18]. The composition of organic carbon is further shaped by zooplankton grazing, as zooplankton consumption of phytoplankton alters the balance between POC and DOC. Consequently, the characteristics of TOC can influence zooplankton community structure and dynamics [19,20]. Simultaneously, zooplankton feeding behavior and the transfer of carbon to higher trophic levels regulate variations in organic carbon pools, affecting carbon cycling and ecosystem functioning [21,22,23,24,25]. These interactions highlight the critical role of zooplankton in modulating TOC dynamics in aquatic systems.
Zooplankton exhibit species-specific feeding preferences that significantly influence TOC dynamics in aquatic ecosystems. Large cladocerans and copepods actively graze on phytoplankton, serving as key prey for fish and facilitating the transfer of carbon from POC to fish biomass, thereby reducing POC pools [26,27,28]. In contrast, rotifers primarily feed on bacteria and protozoans, promoting carbon cycling within the microbial loop and sustaining DOC-dominated pathways, with less influence from higher trophic predation [29,30]. Consequently, the composition of phytoplankton and zooplankton communities is a critical biological factor in understanding TOC dynamics. Furthermore, plankton serve as vital indicators in the health assessment of lakes and reservoirs, alongside water quality parameters [31,32,33]. In many countries, zooplankton indices are constructed based on functional groups defined by size, reflecting their phytoplankton grazing capacity and contribution to fish diets [34,35]. These biological interactions underscore the importance of plankton in regulating carbon fluxes and assessing ecosystem health in aquatic systems.
Given the pivotal role of zooplankton grazing in shaping TOC dynamics, elucidating the interactions between TOC indicators and plankton community composition is essential for effective lake and reservoir management and for devising strategies to mitigate organic matter pollution and ensure long-term ecosystem sustainability. Zooplankton communities exhibit strong species-specific responses to direct eutrophication drivers, such as elevated nutrient concentrations (e.g., total phosphorus and nitrogen) and chlorophyll-a, as well as indirect lake environmental factors that promote eutrophication, including shallow water depth and high densities of planktivorous fish [36]. These characteristics have positioned zooplankton as key-indicator assemblages for assessing lake eutrophication [31,32,33,37]. Numerous studies have proposed zooplankton indices based on indicator species (e.g., small rotifers like Keratella cochlearis and Polyarthra vulgaris) that show species-specific responses [38]. In contrast, while multivariate approaches, such as redundancy analysis (RDA) and canonical correspondence analysis (CCA), have been widely used to link water quality parameters to zooplankton community composition and identify influential factors [39], studies employing clustering to characterize the ecological responses of zooplankton and water quality interactions, particularly including TOC, remain scarce [20].
This study investigated the effects of TOC, along with other water quality factors, on the composition of zooplankton communities across 22 reservoirs located in the Geum River watershed (9980 km2) that share similar climatic conditions but differ in physicochemical and catchment characteristics. To analyze community patterns, we applied non-metric multidimensional scaling (NMDS) and igraph-based network clustering to classify zooplankton assemblages [40,41]. We further examined relationships between community types and water quality variables, with a focus on TOC. These approaches provide insights into the ecological drivers of TOC dynamics; highlight the importance of cluster-specific responses in understanding zooplankton–water quality interactions; and offer implications for management practices—such as nutrient reduction and algal bloom control—aimed at improving water quality and maintaining ecosystem health in eutrophic reservoirs.
2. Materials and Methods
2.1. Study Site and Data Collection
To investigate the relationships between TOC concentration and water quality parameters, as well as between TOC concentration and zooplankton community structure, 22 reservoirs located within the large watershed of the Geum River (length: 396 km, Korea’s third largest river) and its tributaries, with each reservoir situated in specific sub-tributaries, were selected for this study (Figure 1). These reservoirs share similar climatic zones and watershed environments. They are characterized by distinct seasonal variations and frequent algal blooms during the summer due to eutrophication. In addition, compared to other reservoirs within the same watershed, they exhibit higher levels of water pollution as a result of commercial fishing, angling, and tourism activities. During the farming season (April to June), the water storage levels typically decrease by approximately 40–60%. Field surveys were conducted four times annually at each reservoir. Twelve reservoirs were surveyed in May, June, September, and November of 2023, while the remaining ten reservoirs were surveyed in May, June, September, and November of 2024.

Figure 1.
Geographic distribution of the lakes in this study.
Water quality data were obtained from the Rural Agricultural Water Resource Information System (https://rawris.ekr.or.kr (accessed on 30 June 2025). The measured parameters included total organic carbon (TOC, mg/L) as the primary focus, alongside chlorophyll-a concentration (Chl-a, mg/m3), and other basic water quality and nutrient parameters, such as water temperature (WT, °C), Secchi disk depth (SD, m), suspended solids (SSs, mg/L), total nitrogen (TN, mg/L), and total phosphorus (TP, mg/L). Each water quality parameter was measured in accordance with the Standard Methods for the Examination of Water Pollution in Korea (Appendix A).
Zooplankton samples were collected using a zooplankton net (60 μm mesh, 30 cm diameter) by full-depth vertical towing at reservoirs with a water depth of 5 m or greater. For reservoirs with depths less than 5 m or where access to the central area was limited, a zooplankton net (60 μm mesh, 22 cm diameter) was used for 5 m diagonal towing. In the case of diagonal towing, the net was allowed to sink sufficiently to collect zooplankton from the lower layers of the reservoir, and then towed diagonally from the bottom to the surface to ensure effective sampling.
Collected samples were fixed in situ with formalin solution to achieve a final concentration of 4–5% and transported to the laboratory. In the laboratory, zooplankton samples were examined using an inverted microscope (CKX41; Olympus, Tokyo, Japan) at magnifications ranging from ×40 to ×400 and identified to the species or genus level [42,43,44,45]. The volume of all samples ranged between 100 and 200 mL. Depending on the volume of the sample and the number of individuals contained within it, a 1–5 mL sub-sample was taken for examination. Zooplankton abundance was quantified as individuals per liter, calculated based on the volume of water filtered through the net and the towing distance, and used to analyze population density.
2.2. Statistical Analysis
Water quality characteristics at each site were summarized as means and standard deviations using data collected from March to October. Prior to statistical analysis, both water quality and zooplankton datasets were log-transformed using the formula ln(1 + x) to normalize data distribution. The relationship between TOC and water quality, as well as the influence of TOC on zooplankton community composition, was assessed using average water quality values (March–October) and genus-level mean zooplankton densities (May, June, September, and November).
Multivariate analyses, including non-metric multidimensional scaling (NMDS) and hierarchical cluster analysis, were performed based on Euclidean distances for water quality variables and Bray–Curtis dissimilarity for zooplankton data. In the NMDS plots, TOC concentrations were represented by point size and color, while ellipses indicated groupings derived from hierarchical clustering. All ordination and clustering analyses were conducted using the “vegan” package in R (v.4.3.0).
Group-level differences in TOC and other water quality indicators among clusters based on zooplankton composition were evaluated using boxplots to visualize distributions and variability. To evaluate the concordance between zooplankton-based and water quality-based clustering, network analysis was employed [40]. Two types of networks were constructed: one derived from water quality parameters and the other from zooplankton community data. Sampling sites were visualized as nodes, and similarity-based connections were drawn as edges. Node shapes and colors were used to represent clustering results based on water quality and zooplankton composition, respectively. These network analyses were conducted using the “igraph” and “ggraph” packages in R (v.4.3.0) [41].
Lastly, Mantel correlation analysis was used to examine the association between zooplankton community composition and water quality variables [46]. This analysis, based on Bray–Curtis dissimilarity, was performed using the “microeco” package in R (v.4.3.0) [47,48].
3. Results
3.1. Physicochemical Characteristics and Zooplankton Community Composition of the Reservoirs
To understand the environmental characteristics of each reservoir, collected physicochemical data are summarized in Table 1. The physical and water quality parameters varied across the reservoirs. The catchment area ranged from a minimum of 85 ha to a maximum of 8479 ha, with a mean of 1862.2 ha. The surface area at full capacity ranged from 9 ha to 243.4 ha, with an average of 76.8 ha. The effective storage capacity varied from 241 × 103 m3 to 26,372 × 103 m3, with a mean value of 5234.3 × 103 m3.

Table 1.
Characteristics of the 22 reservoirs studied—mean water quality parameters (±standard deviation from four seasonal surveys (March–October)) (WT, water temperature; SD, Secchi dept; TOC, total organic carbon; SSs, suspended solids; Chl-a, chlorophyll-a; TN, total nitrogen; TP, total phosphorus).
Water quality parameters were averaged across monthly measurements from March to October. The TOC concentration ranged from 2.1 to 8.1 mg/L across the reservoirs, with a mean of 4.5 mg/L. Mean water temperature ranged from 15.0 °C to 22.8 °C, with a mean of 18.9 °C. Despite the fact that the reservoirs are located within the same watershed, the differences in catchment size, reservoir morphometry, and surrounding land-use types contributed to substantial variation in water quality conditions among the reservoirs.
To investigate seasonal variation in zooplankton composition across reservoirs, genus-level distribution patterns were analyzed based on data collected in May, June, September, and November. In the May survey, a total of 35 genera and 73 species of zooplankton were identified. The average zooplankton abundance across all lakes was 551.4 individuals per liter (ind./L). The minimum number of zooplankton species was recorded in L.11, with 7 species, while the maximum was observed in L.6, with 19 species. The lowest zooplankton density was also found in L.11 at 5.9 ind./L, whereas the highest density was recorded in L.1 at 6519.6 ind./L.
In the June survey, a total of 38 genera and 85 species of zooplankton were identified, the highest species richness among all survey periods. The average zooplankton abundance across all lakes was 525.4 ind./L. The minimum number of zooplankton species was recorded in L.22, with 10 species, while the maximum was observed in L.15, with 27 species. The lowest zooplankton density was found in L.14 at 73.4 ind./L, whereas the highest density was recorded in L.1 at 2599.3 ind./L.
In the September survey, a total of 36 genera and 69 species of zooplankton were identified. The average zooplankton abundance across all lakes was 307.7 ind./L, the lowest among all survey periods. The minimum number of zooplankton species was recorded in L.11, with 3 species, while the maximum was observed in L.9, with 29 species. The lowest zooplankton density was found in L.15 at 2.0 ind./L, whereas the highest density was recorded in L.5 at 1141.9 ind./L.
In the November survey, a total of 41 genera and 83 species of zooplankton were identified. The average zooplankton abundance across all lakes was 534.2 ind./L. The minimum number of zooplankton species was recorded in L.19, with 9 species, while the maximum was observed in L.17, with 27 species. The lowest zooplankton density was found in L.15 at 18.2 ind./L, whereas the highest density was recorded in L.21 at 2670.6 ind./L.
For rotifers, the highest mean density was recorded in May, with an average of 63.0 ind./L across 22 reservoirs. However, this was largely influenced by an exceptionally high density at a single reservoir (L.1), where rotifer abundance reached 6376.3 ind./L. Excluding L.1, the average density across the remaining 21 reservoirs was 23.4 ind./L, with relatively low densities and high dominance by a few taxa. In May, Keratella, Polyarthra, and Synchaeta were most frequently observed genera across multiple reservoirs (Figure 2A). In June, the mean rotifer density decreased to 43.6 ind./L, with a reduced relative abundance of Keratella and increased prevalence of Brachionus, Pompholyx, and Filinia (Figure 2B). In September, rotifer density reached its lowest level at 18.6 ind./L, but genus-level diversity increased, and dominance by a single taxon generally declined (Figure 2C). In November, rotifer density increased to an average of 50.2 ind./L, driven by higher proportions of Keratella and Polyarthra (Figure 2D).
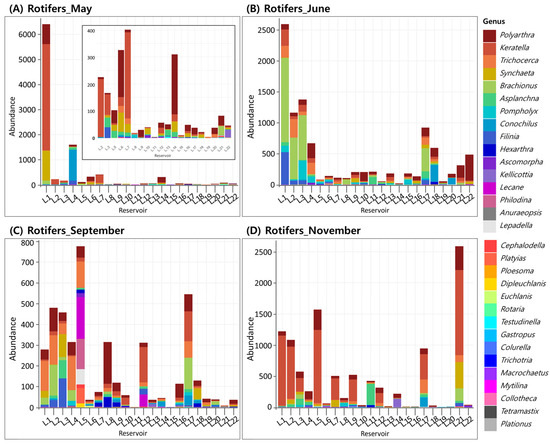
Figure 2.
Occurrence of rotifer genera across 22 reservoirs surveyed in (A) May, (B) June, (C) September, and (D) November.
For cladocerans, the highest mean density was also recorded in May (14.2 ind./L), driven primarily by an elevated density at one reservoir (L.4). Excluding L.4, the mean density across 21 reservoirs was 5.9 ind./L. During this period, Bosmina and Daphnia were the dominant genera in most reservoirs (Figure 3A). In June, the average density was 9.3 ind./L, with patterns similar to May but an increased proportion of Diaphanosoma (Figure 3B). In September, the mean density was 8.9 ind./L. Comparable to June, the proportion of Daphnia decreased, while the proportions of Ceriodaphnia and Diaphanosoma increased (Figure 3C). In November, the mean cladocerans density declined to 3.7 ind./L, the lowest among the four periods, with Daphnia becoming more dominant, followed by Bosmina and Ceriodaphnia across multiple reservoirs (Figure 3D).

Figure 3.
Occurrence of cladoceran genera across 22 reservoirs surveyed in (A) May, (B) June, (C) September, and (D) November.
For copepods, the mean density in May was 3.9 ind./L, with dominant genera varying among reservoirs, including Thermocyclops, Cyclops, and Acanthocyclops frequently detected (Figure 4A). In June, the mean density slightly decreased to 3.1 ind./L, with Thermocyclops exhibiting the highest relative abundance in most reservoirs (Figure 4B). In September, copepod density peaked at 9.8 ind./L, the highest among all sampling periods, with Thermocyclops continuing to dominate (Figure 4C). In November, the density declined to 1.8 ind./L, the lowest among the periods, and Thermocyclops, Cyclops, and Mesocyclops were commonly observed depending on the reservoir (Figure 4D). The immature stages of copepods—copepodids and nauplii—exhibited the highest mean density in September (52.6 ind./L) and the lowest in November (14.9 ind./L), showing clear seasonal variation in abundance (Figure 5A–D).
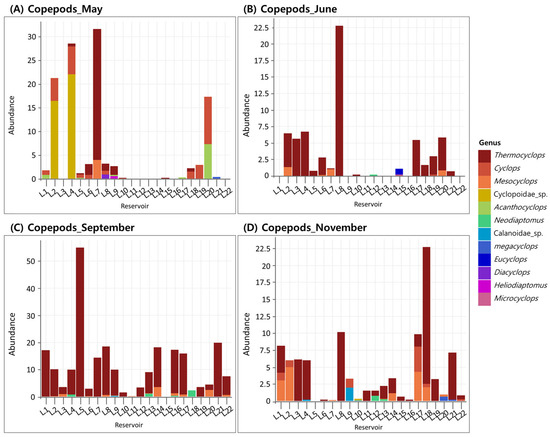
Figure 4.
Occurrence of copepod genera across 22 reservoirs surveyed in (A) May, (B) June, (C) September, and (D) November.
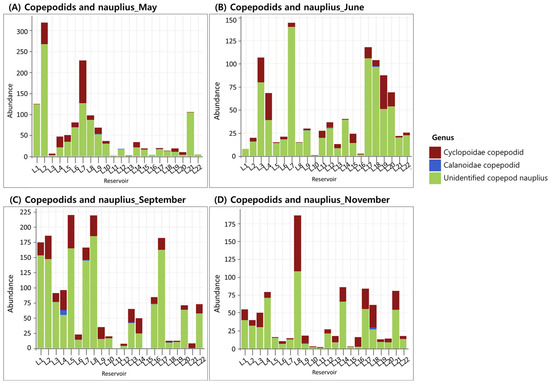
Figure 5.
Occurrence of copepodids and nauplius across 22 reservoirs surveyed in (A) May, (B) June, (C) September, and (D) November.
A Mantel test was conducted to assess the relationship between water quality variables and zooplankton community composition. The analysis revealed a significant correlation between the zooplankton community structure and water quality variables (Mantel r = 0.50, p = 0.001; Figure 6). Correlation analysis among environmental variables indicated strong positive correlations between TOC and SS, Chl-a, and TP (r > 0.7, p < 0.001), while SD was negatively correlated with WT, TOC, SS, Chl-a, and TP (r < −0.55, p < 0.001). Additional positive correlations were observed between SS and both Chl-a and TP, as well as between TP and TN and Chl-a (p < 0.05) (Figure 6).
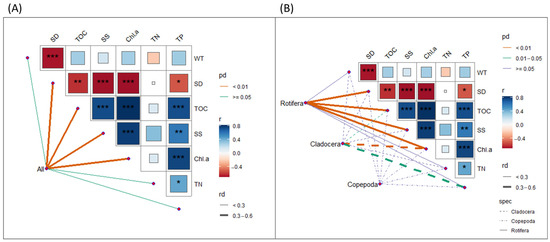
Figure 6.
Mantel’s correlation between environmental variables and zooplankton composition: (A) total zooplankton community and (B) taxonomic groups (Rotifera, Cladocera, and Copepoda). Line thickness reflects correlation strength (r), and color indicates significance. Asterisk (*) indicates statistically significant correlations (* p < 0.05, ** p < 0.01, and *** p < 0.001).
Mantel test results for the overall zooplankton community confirmed significant correlations with TOC (r = 0.417, p = 0.003), SS (r = 0.358, p = 0.002), Chl-a (r = 0.386, p = 0.001), and SD (r = 0.435, p = 0.001) (Figure 6A). Taxon-specific analyses revealed that Rotifera exhibited significant correlations with TOC (r = 0.472, p = 0.0035), SS (r = 0.364, p = 0.0035), Chl-a (r = 0.376, p = 0.0035), and SD (r = 0.451, p = 0.0035). Cladocerans showed strong associations with TP (r = 0.594, p = 0.032) and Chl-a (r = 0.467, p = 0.007), and a weaker correlation with TOC (r = 0.263, p = 0.040). In contrast, Copepods exhibited weak or non-significant correlations with all environmental variables (r < 0.22, p > 0.12, Figure 6B).
3.2. Influence of Total Organic Carbon (TOC) on Water Quality Index and Zooplankton Community Composition
Clustering of the 22 reservoirs based on zooplankton community composition resulted in four broad groups (Figure 7A). To evaluate the relationship between TOC and zooplankton community composition, an NMDS analysis was performed, yielding a stress value of 0.139, which indicated a reasonably good fit. The mean TOC concentrations for Groups 1 through 4 were 4.16, 3.19, 6.36, and 4.31 mg/L, respectively, with Group 3 exhibiting the highest TOC levels, and Group 2 the lowest. In the NMDS ordination space, reservoirs with higher TOC concentrations tended to be positioned toward the positive end of the NMDS1 axis, whereas those with lower TOC concentrations were distributed toward the negative end (Figure 7B). This spatial pattern produced a comparatively clear separation among clusters, indicating that high and low TOC levels are strongly reflected in zooplankton community composition.
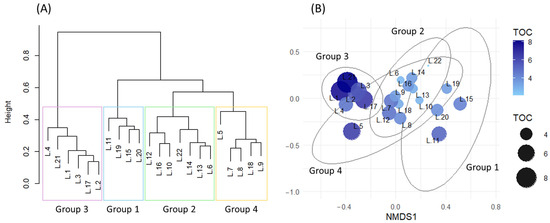
Figure 7.
(A) Clustering of reservoirs based on zooplankton community composition using Bray–Curtis distance. (B) NMDS ordination based on water quality variables, with visualization of TOC concentrations by point size and color. Ellipses on the NMDS plot represent reservoir groupings based on the clustering results shown in (A).
Network analysis was performed to evaluate the degree of water quality similarity within and between zooplankton-based clusters. The network was constructed based on Euclidean distances of water quality variables among the 22 reservoirs, retaining only the top 30% of similarity values to define edges. The zooplankton-based clustering results were then mapped onto this network to visually assess the degree of alignment between the two classification approaches (Figure 8). The average edge weight in the water quality-based network was 0.64. Within zooplankton-based clusters, the mean edge weights ranged from 0.52 to 0.64, with Group 1 exhibiting the highest internal similarity. Among inter-cluster connections, the lowest similarity was observed between Groups 1 and 3 (NaN), indicating a clear separation in water quality characteristics. Conversely, other group pairs—such as Groups 1 and 2 (0.79), 1 and 4 (0.76), and 2 and 3 (0.65)—exhibited relatively high edge weights, suggesting more moderate water quality dissimilarities. This pattern implies that Groups 1 and 3 are the most distinct from each other, while the remaining clusters share more similar water quality profiles. Additionally, in the water quality-based network, zooplankton-based Groups 2 and 4 were diffusely distributed, indicating poorly defined boundaries.
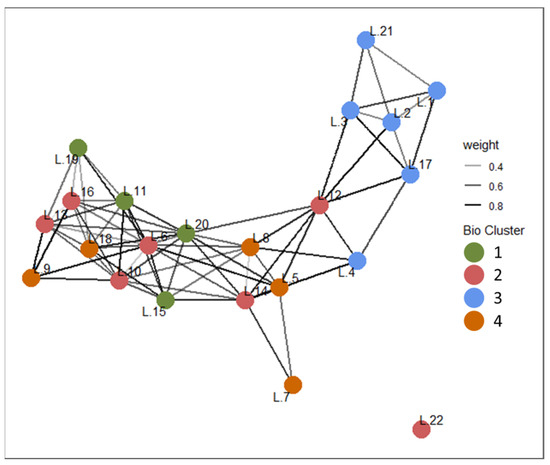
Figure 8.
Site-level network analysis based on water quality indicators (Euclidean distance). In this network, node color represents the zooplankton-based cluster classification.
Statistical comparisons among the four reservoir groups clustered by zooplankton composition revealed significant differences in TOC and other water quality variables. TOC concentration varied significantly among groups (F = 8.683, p < 0.001), with Group 3 showing significantly higher concentrations than Group 2 (post hoc Tukey HSD, p < 0.001) (Figure 9A). Reservoirs in Group 3 differed not only in TOC concentrations but also in several other water quality variables, including SD, SS, Chl-a, and TP, compared to the other groups. Specifically, Group 3 exhibited distinctly higher concentrations of SS (mean: 12.35 mg/L), Chl-a (mean: 38.84 µg/L), and TP (mean: 0.12 mg/L), while the other groups—Groups 1, 2, and 4—showed relatively similar and lower values (mean SS: 4.48–5.36 mg/L; mean Chl-a: 10.9–12.5 µg/L; mean TP: 0.03–0.04 mg/L) (p < 0.05 for all; Figure 9D,E,G).

Figure 9.
Distribution of major water quality variables across zooplankton-based lake clusters: (A) TOC, (B) SD, (C) WT, (D) SS (E) Chl-a, (F) TN, and (G) TP. Asterisk (*) indicates statistically significant differences among clusters (* p < 0.05, ** p < 0.01, and *** p < 0.001). Outliers are shown as individual points beyond the whiskers in each boxplot.
Group 3 also had the lowest SD values (mean: 0.56 m), in stark contrast to Group 1, which showed the highest SD values (mean: 2.45 m) (p < 0.05; Figure 9B). No significant differences in water temperature or total nitrogen were observed among the groups (WT: p = 0.1136; TN: p = 0.179), suggesting a limited role of these factors in shaping zooplankton community structure.
Group 1 (L.11, L.15, L.19, and L.20) had the lowest zooplankton abundances (max: 178.3 ind./L; mean: 109.4 ind./L), the highest average transparency, and the lowest temperature. While community composition varied among sites—some dominated by Asplanchna spp., Pompholyx, or Conochilus—these reservoirs generally supported higher proportions of large-bodied cladocerans and diverse copepods compared to other groups (Figure 10A).
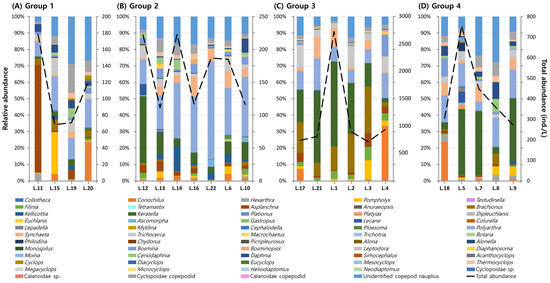
Figure 10.
Relative abundance patterns of zooplankton genera across zooplankton-based clusters.
Group 2 (L.6, L.10, L.12, L.13, L.14, L.16, and L.22), including seven reservoirs, showed relatively low zooplankton abundances (max: 222.6 ind./L; mean: 165.8 ind./L) with intermediate SD and WT values. The group had moderate rotifer dominance (62.2–82.7%) and consistent presence of Asplanchna spp., along with greater diversity of both rotifers and cladocerans, including Daphnia and Ceriodaphnia (Figure 10B).
Group 3 (L.1, L.2, L.3, L.4, L.17, and L.21) exhibited the highest zooplankton densities (max: 2724.1 ind./L; mean: 1136.8 ind./L), with rotifers accounting for 75.9–96.2% of the community. This group was dominated by typical eutrophic taxa, such as Keratella, Polyarthra, Brachionus, Synchaeta, and Conochilus, with minimal presence of large-bodied cladocerans and copepods. Environmental indicators such as TOC, SS, Chl-a, and TP were all markedly elevated, clearly reflecting eutrophic conditions (Figure 10C).
Group 4 (L.5, L.7, L.8, L.9, and L.18), comprising five reservoirs, had intermediate zooplankton abundances (max: 753.5 ind./L; mean: 426.8 ind./L), with relatively high proportions of large-bodied cladocerans and copepods. This group was characterized by the highest mean water temperature and low SD, with considerable intra-group environmental variability suggesting more heterogeneous habitats (Figure 10D).
4. Discussion
Despite their location within the same watershed and geographic proximity, the 22 reservoirs investigated in this study exhibited significant variation in water quality parameters, including TOC, and zooplankton community composition. These differences are attributed to variations in physical features such as catchment area, full water surface area, and effective storage capacity, as well as disparities in surrounding land-use conditions. Zooplankton communities showed notable temporal variation, with substantial changes in both abundance and dominant taxa across sampling periods. Excluding a few outliers (e.g., reservoirs L.1 and L.4), rotifers and cladocerans exhibited the highest densities in June, whereas copepods peaked in September.
Mantel test results revealed that zooplankton community composition was significantly associated with several key water quality variables, including total organic carbon (TOC), Secchi depth (SD), suspended solids (SSs), and chlorophyll-a (Chl-a). Among the three major taxonomic groups—Rotifera, Cladocera, and Copepoda—these associations were most pronounced for rotifers, while copepods showed little to no association with environmental gradients. In the case of rotifers, although certain species can be classified as indicators of eutrophic or oligotrophic conditions, eutrophic species tend to exhibit a much greater increase under eutrophic conditions compared to the decrease in oligotrophic species. As a result, in environmental correlation analyses, the role of oligotrophic indicator species is greatly diminished, and rotifers are often interpreted as eutrophication indicators at the overall taxonomic-group level, showing a general increase in abundance as eutrophication-related environmental variables increase [38,49].
In contrast, within temperate freshwater ecosystems, copepods typically consist of two predominant orders: Cyclopoida and Calanoida. While species-specific responses may vary, Cyclopoida are generally considered indicators of eutrophic conditions, whereas Calanoida are associated with oligotrophic or mesotrophic environments, showing opposing ecological tendencies [50,51]. Notably, a previous study investigating copepod distribution patterns across 49 lakes in Korea—including 8 lakes within the Geum River basin, similar to this study area—reported that Calanoida exhibited consistent distribution patterns in relation to water quality factors such as TOC, Chl-a, SS, COD, and TN at the order level, regardless of genus. However, Cyclopoida displayed highly variable distribution patterns depending on the genus, making it difficult to identify clear environmental associations at the order level [35]. Thus, when examining the relationship between rotifers, cladocerans, and copepods with environmental variables at the taxonomic-group level, the contrasting ecological responses of Cyclopoida and Calanoida within the copepod group may obscure overall trends. This likely explains the lack of a clear association between copepods as a whole and environmental gradients in the present analysis.
Clustering results based on zooplankton composition revealed distinct patterns in community structure, water quality parameters, and physical features across the groups. Group 1 (L.11, L.15, L.19, and L.20) showed the lowest zooplankton abundance among all groups, although the species composition of zooplankton varied within the reservoir. This group was characterized by a higher proportion of large cladocerans and the presence of diverse copepod species. Reservoirs in Group 1 were also distinguished by high transparency and low water temperature. Their surface areas ranged from 13.7 ha (L.11) to 101 ha (L.15), with an average of 85.1 ha, making it the second smallest among the four groups. Water depths ranged from 9 m (L.11) to 18 m (L.15, L.19), with an average of 14.3 m, the second deepest among the groups.
Group 2 (L.6, L.10, L.12, L.13, L.14, L.16, and L.22) showed relatively low zooplankton abundance, with rotifers accounting for a moderate proportion (62.2–82.7%), including large rotifer Asplanchna spp., large cladocerans such as Daphnia, and Ceriodaphnia, forming functionally diverse communities. Water temperature and transparency were at moderate levels compared to the other groups, and TOC concentrations were the lowest. This group had the largest average surface area, ranging from 50.4 ha (L.12) to 243.4 ha (L.13), with a mean of 118.3 ha. Water depth ranged from 10 m (L.6) to 30 m (L.22), with an average of 17.9 m, the deepest among the four groups, indicating large and deep reservoirs overall.
Group 3 (L.1, L.2, L.3, L.4, L.17, and L.21) exhibited the highest zooplankton abundance among the four groups. Eutrophic rotifers such as Keratella, Polyarthra, Brachionus, Synchaeta, and Conochilus were highly dominant, accounting for 75.9–96.2%. This group had the lowest transparency and the highest levels of water quality indicators, such as TOC, SS, Chl-a, and TP. The reservoirs in this group had the smallest surface areas, ranging from 9 ha (L.17) to 36 ha (L.4), with an average of 18.8 ha. Water depths ranged from less than 1 m (L.21) to 4 m (L.1, L.3), with an average of 3.9 m, indicating overall shallow and small-sized reservoirs.
Group 4 (L.5, L.7, L.8, L.9, and L.18) showed relatively high zooplankton abundance, with high proportions of large cladocerans and copepods. This group had the highest water temperatures and relatively low transparency. Unlike the other groups, reservoirs in Group 4 exhibited significant variation in water quality parameters among reservoirs within the group. Surface areas ranged from 55.6 ha (L.8) to 192 ha (L.7), with an average of 80.9 ha, the second largest among the groups. Water depths ranged from 2.5 m (L.5) to 20 m (L.18), with an average of 9.6 m, the second shallowest among the four groups.
Further analysis using NMDS ordination confirmed that TOC concentration was closely aligned with the zooplankton-based clustering pattern. In particular, reservoirs with either very high or very low TOC levels exhibited more similar community structures, with Group 3 (high TOC) and Group 2 (low TOC) showing the greatest separation.
However, this biological pattern did not align with the clustering derived from water quality-based network analysis. While the NMDS indicated a clearer distinction between Groups 2 and 3, the network analysis showed that Group 3 was most dissimilar from Group 1 in terms of water quality. This mismatch suggests that TOC may act as an independent driver of zooplankton community structure, separate from other water quality indicators (e.g., TP, Chl-a, and SS). To further examine whether these group differences were statistically supported, a boxplot analysis was conducted. TOC concentrations differed significantly between Group 2 and Group 3. In contrast, eutrophication-related variables such as TP, SS, and Chl-a showed significant elevation only in Group 3, with minimal differences among Groups 1, 2, and 4. These results suggest that TOC may represent a biologically relevant gradient, particularly in conditions where conventional eutrophication metrics are not markedly elevated.
A closer comparison between Group 2 and Group 3 reveals meaningful ecological contrasts, particularly in how trophic conditions and organic carbon levels shape zooplankton community structures. While both groups showed rotifer dominance, the composition and ecological characteristics of their communities were markedly different. Group 3, with significantly higher TOC concentrations (6.36 ± 1.23 mg/L) and elevated Chl-a, SS, and TP levels, exhibited a typical eutrophic pattern: overwhelming dominance by small-bodied indicator rotifers and virtual exclusion of larger zooplankton taxa. The uniformity of these communities across reservoirs reflected strong eutrophication pressure, limiting niche space for more sensitive or larger-bodied species. In contrast, Group 2 reservoirs had significantly lower TOC levels (3.19 ± 0.80 mg/L) and supported a more functionally diverse assemblage, including consistent appearances of large-bodied cladocerans (e.g., Daphnia and Ceriodaphnia) and predatory rotifers (Asplanchna spp.). Notably, although nutrient-related variables (e.g., Chl-a and TP) were not dramatically different from those in Groups 1 and 4, the distinctiveness of Group 2′s zooplankton composition suggests that factors beyond nutrient load—such as TOC, light availability (SD), and temperature (WT)—may play a central role under moderate eutrophic conditions. Moreover, Group 2′s community structure was less homogenized compared to that of Group 3. The presence of multiple functional groups implies more balanced energy flow and trophic interactions, which could be indicative of transitional or less degraded ecosystem states. These differences emphasize that TOC is not merely a background variable, but potentially an important ecological filter influencing zooplankton dynamics alongside traditional eutrophication metrics.
According to previous studies conducted across various geographic regions, zooplankton species’ richness tends to increase with increasing lake area and depth [52,53]. In this study, the average number of zooplankton species observed in Groups 1, 2, 3, and 4 was 21.3, 25.3, 22.5, and 24.8, respectively. Group 2, which encompassed the largest and deepest reservoirs, exhibited the highest species richness. While this pattern may suggest a positive influence of lake size and depth on species diversity, it should be noted that Group 2 also showed distinct differences in several water quality parameters, including TOC, SD, WT, and TN. Therefore, it is unlikely that lake morphometry alone accounts for the observed richness; however, lake area and depth may have contributed to shaping the zooplankton community structure in conjunction with other environmental factors. Additionally, Goździejewska et al. (2021) demonstrated that lake depth, trophic status, and ion concentrations significantly influence zooplankton community composition [54]. Their study reported that shallow lakes (< 6 m) subject to anthropogenic pressures such as fishing and recreation—similar to the reservoirs in Group 3 of the present study—tended to exhibit elevated levels of eutrophic indicators such as Chl-a, trophic state index (TSI), and TP. These systems were characterized by high zooplankton biomass and the dominance of eutrophic indicator species, including Keratella quadrata, Brachionus angularis, Trichocerca similis, Bosmina longirostris, Chydorus sphaericus, Diaphanosoma brachyurum, and Thermocyclops sp. Such dominance patterns reflect functionally degraded communities, with reduced ecosystem integrity and biodiversity.
In general, elevated levels of nutrients such as phosphorus, nitrogen, and chlorophyll-a, which represent a lake’s trophic state, significantly increase the abundance and biomass of eutrophic indicator species, including Polyarthra vulgaris, Keratella cochlearis, and Synchaeta spp., leading to their classification as indicators of eutrophication [38]. On the other hand, although the genus Asplanchna belongs to rotifers, it is known to consume a broad range of food items depending on the species—ranging from colonial algae and cyanobacteria to protists and various rotifers, such as Anuraeopsis, Brachionus, and Keratella [55]. The genus Asplanchna was consistently observed, particularly in Group 2, which spanned mesotrophic to eutrophic conditions, suggesting a random distribution across habitats with moderate nutrient levels. This dietary flexibility likely explains their presence in varied trophic states, contrasting with the more nutrient-specific responses of eutrophic-indicator rotifers.
Large-bodied cladocerans such as Daphnia are known to decrease in average biomass as Chl-a and TP concentrations increase [36,56,57]. In our clustering analysis, Daphnia and large cladocerans were associated with the group exhibiting the lowest Chl-a and TP levels and highest transparency, serving as indicator species for less eutrophicated lakes. However, TOC concentrations in this group were intermediate, differing from typical eutrophication indicator species, suggesting that Daphnia requires moderate levels of autochthonous organic matter for feeding. In contrast, large Cyclopoida such as Eucyclops and Cyclops genera have shown conflicting results in the literature, with some studies reporting their prevalence in eutrophic conditions and others noting increased abundance in oligotrophic systems [31,58,59,60]. In this study, these genera exhibited grouping patterns similar to Daphnia, suggesting a preference for meso- and oligotrophic conditions over eutrophic ones. Additionally, their predatory feeding behavior likely contributes to reducing populations of small rotifers and cladocerans, which are typical eutrophication indicator species.
Unfortunately, our study did not differentiate TOC into POC and DOC, restricting the capacity to provide detailed insights into their specific contributions. Previous studies indicate that TOC concentrations increase predictably in eutrophic lakes, exhibiting strong positive correlations with chlorophyll-a (Chl-a) concentrations, with increases in TOC typically mirroring rises in both POC and DOC [15,17,20,61]. However, in mesotrophic systems, unlike eutrophic lakes, the correlations between TOC and these components weaken, reflecting greater lake-specific variability in organic carbon dynamics [20]. Consistent with these findings, our results suggest that as trophic levels decrease (e.g., in Group 2 reservoirs with TOC: 3.19 ± 0.42 mg/L), zooplankton communities exhibit more lake-specific responses, diverging from the eutrophication-driven convergence observed in Group 3 (TOC: 6.36 ± 0.84 mg/L). Thus, TOC increases in parallel with eutrophication indicators during periods of high productivity. At the same time, it serves as a representative measure of organic matter, suggesting its potential as an indicator not only of organic pollution but also of biological responses in less eutrophic reservoirs. Therefore, in the context of lake and reservoir management, TOC may be valuable as both a chemical and biological indicator of ecosystem condition.
Author Contributions
Conceptualization, Y.C. and K.-H.C.; methodology, Y.C., H.-J.O. and K.-H.C.; validation, H.-J.O. and K.-H.C.; formal analysis, Y.C. and G.-H.H.; data curation, Y.C., G.-H.H., D.-H.L., S.-H.P., J.-H.K. and K.-H.C.; writing—original draft preparation, Y.C. and K.-H.C.; writing—review, H.-J.O.; writing—editing, Y.C., H.-J.O., J.-H.Y. and K.-H.C.; visualization, Y.C.; supervision, H.-J.O., J.-H.Y. and K.-H.C.; project administration, S.-H.P. and J.-H.K.; funding acquisition, J.-H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant from Environmental Basic Research program funded by Committee for Management of Geum River Basin.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
Authors Jeong-Hui Kim and Sang-Hyeon Park were employed by the company Eco Research Incorporated. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Appendix A

Table A1.
Summary of standard analytical methods for water quality parameters according to the Korean Ministry of Environment Guidelines.
Table A1.
Summary of standard analytical methods for water quality parameters according to the Korean Ministry of Environment Guidelines.
| Parameter | Measurement Method |
|---|---|
| Water temperature | WT was measured in situ using a portable multi-parameter water quality meter. |
| Secchi depth (SD) | SD was measured using a Secchi disk with a diameter of 30 cm. |
| Total organic carbon (TOC) | TOC was determined by the high-temperature catalytic oxidation (HTCO) method. An appropriate volume of sample was introduced into a high-temperature combustion tube filled with an oxidizing catalyst. The sample was combusted at elevated temperature, converting the organic carbon in water to carbon dioxide (CO2) for quantification. Inorganic carbon was removed prior to analysis by acidification and purging with inert gas. |
| Chlorophyll-a (Chl-a) | Chl-a was extracted from water samples filtered through glass fiber filters (Whatman GF/F, 47 mm diameter), using acetone solution. The chlorophyll pigments were extracted from the filters, and the absorbance of the extract was measured at 630 nm, 645 nm, 663 nm, and 750 nm, using a spectrophotometer. Chl-a concentration was calculated based on the measured absorbances according to standard equations. |
| Suspended solids (SS) | SS concentration was determined by filtering a known volume of water sample through a pre-weighed glass fiber filter (Whatman GF/C, 47 mm diameter), using a filtration apparatus. The filter was dried to constant weight and reweighed. The difference in weight before and after filtration was used to calculate the SS concentration. |
| Total nitrogen (TN) | Total nitrogen (TN) was measured using ultraviolet/visible (UV/VIS) spectrophotometry (alkaline persulfate oxidation method). All nitrogen compounds were decomposed and oxidized to nitrate (NO3−) by heating with alkaline potassium persulfate (K2S2O8) at approximately 120 °C. After oxidation, the sample was acidified, and the absorbance was measured at 220 nm to quantify total nitrogen. |
| Total phosphorus (TP) | Total phosphorus (TP) was determined by oxidizing all phosphorus compounds to phosphate (PO43−), using persulfate digestion at high temperature. The resulting phosphate (PO43−) was reacted with ammonium molybdate ((NH4)2MoO4) to form ammonium phosphomolybdate, which was reduced by ascorbic acid (C6H8O6). The absorbance of the resultant phosphomolybdenum blue complex was measured at 880 nm to quantify total phosphorus. |
References
- Tundisi, J.G. Reservoirs: New challenges for ecosystem studies and environmental management. Water Secur. 2018, 4–5, 1–7. [Google Scholar]
- Oertli, B. Editorial: Freshwater biodiversity conservation: The role of artificial ponds in the 21st century. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 264–269. [Google Scholar]
- Eekhout, J.P.; Boix-Fayos, C.; Pérez-Cutillas, P.; de Vente, J. The impact of reservoir construction and changes in land use and climate on ecosystem services in a large Mediterranean catchment. J. Hydrol. 2020, 590, 125208. [Google Scholar]
- Lehner, B.; Liermann, C.R.; Revenga, C.; Vörömsmarty, C.; Fekete, B.; Crouzet, P.; Döll, P.; Endejan, M.; Frenken, K.; Magome, J.; et al. High-resolution mapping of the world’s reservoirs and dams for sustainable river-flow management. Front. Ecol. Environ. 2011, 9, 494–502. [Google Scholar]
- Li, B.; Chen, N.; Wang, W.; Wang, C.; Schmitt, R.J.P.; Lin, A.; Daily, G.C. Eco-environmental impacts of dams in the Yangtze River Basin, China. Sci. Total Environ. 2021, 774, 145743. [Google Scholar] [PubMed]
- Kondolf, G.M.; Rubin, Z.K.; Minear, J.T. Dams on the Mekong: Cumulative sediment starvation. Water Resour. Res. 2014, 50, 5158–5169. [Google Scholar]
- Brainwood, M.; Burgin, S. Hotspots of biodiversity or homogeneous landscapes? Farm dams as biodiversity reserves in Australia. Biodivers. Conserv. 2009, 18, 3043–3052. [Google Scholar]
- Suen, J.P.; Eheart, J.W. Reservoir management to balance ecosystem and human needs: Incorporating the paradigm of the ecological flow regime. Water Resour. Res. 2006, 42, W03417. [Google Scholar]
- Daus, M.; Koberger, K.; Koca, K.; Beckers, F.; Fernandez, J.E.; Weisbrod, B.; Dietrich, D.; Gerbersdorf, S.U.; Glaser, R.; Haun, S.; et al. Interdisciplinary reservoir management—A tool for sustainable water resources management. Sustainability 2021, 13, 4498. [Google Scholar] [CrossRef]
- Jiang, Y.; Ha, K.; Li, Y.; Qin, M.; Cui, Z.; Zhang, Y.; Yao, Y.; Chen, X.; Deng, M.; Gray, A.; et al. Driving factors of total organic carbon in Danjiangkou Reservoir using generalized additive model. Water 2022, 14, 891. [Google Scholar] [CrossRef]
- Sepp, M.; Koiv, T.; Noges, P.; Noges, T. Do organic matter metrics included in lake surveillance monitoring in Europe provide a broad picture of brownification and enrichment with oxygen consuming substances? Sci. Total Environ. 2018, 610–611, 1288–1297. [Google Scholar]
- Kim, B.; Kong, D. Examination of the applicability of TOC to Korean trophic state index (TSIko). J. Korean Soc. Water Environ. 2019, 35, 271–277, (In Korean with English abstract). [Google Scholar]
- Lee, Y.; Kim, J.K.; Jung, S.; Eum, J.; Kim, C.; Kim, B. Development of a water quality index model for lakes and reservoirs. Paddy Water Environ. 2014, 12, 19–28. [Google Scholar]
- Park, J.W.; Kim, S.Y.; Noh, J.H.; Bae, Y.H.; Lee, J.W.; Maeng, S.K. A shift from chemical oxygen demand to total organic carbon for stringent industrial wastewater regulations: Utilization of organic matter characteristics. J. Environ. Manag. 2022, 305, 114412. [Google Scholar]
- Tranvik, L.J.; Downing, J.A.; Cotner, J.B.; Loiselle, S.A.; Striegl, R.G.; Balltore, T.J.; Dillon, P.; Finlay, K.; Fortino, K.; Knoll, L.B.; et al. Lakes and reservoirs as regulators of carbon cycling and climate. Limnol. Oceanogr. 2009, 54, 2298–2314. [Google Scholar]
- Silva, L.H.S.; Huszar, V.L.M.; Marinho, M.M.; Rangel, L.M.; Brasil, J.; Domingues, C.D.; Branco, C.C.; Roland, F. Drivers of phytoplankton, bacterioplankton, and zooplankton carbon biomass in tropical hydroelectric reservoirs. Limnologica 2014, 48, 1–10. [Google Scholar]
- Lee, Y.J. Contribution of phytoplankton and zooplankton to total organic carbon (TOC) in the Reservoir-river-Seonakdong River, Busan. J. Environ. Sci. Int. 2022, 29, 691–702, (In Korean with English abstract). [Google Scholar]
- Brett, M.T.; Kainz, M.J.; Taipale, S.J.; Seshan, H. Phytoplankton, not allochthonous carbon, sustains herbivorous zooplankton production. Proc. Natl. Acad. Sci. USA 2009, 106, 21197–21201. [Google Scholar]
- Schram, M.D.; Schmitz, E.H. Correlation of total organic carbon and dry weight data as indices of fresh-water zooplankton biomass. Hydrobiologia 1983, 106, 283–284. [Google Scholar]
- Bowszys, M.; Dunalska, J.A.; Jaworska, B. Zooplankton response to organic carbon level in lakes of differing trophic states. Knowl. Manag. Aquat. Ecosyst. 2014, 412, 10. [Google Scholar]
- Grey, J.; Jones, R.; Sleep, D. Stable isotope analysis of the origins of zooplankton carbon in lakes of differing trophic state. Oecologia 2000, 123, 232–240. [Google Scholar]
- Attayde, J.L.; Menezes, R.F. Effects of fish biomass and planktivore type on plankton communities. J. Plankton Res. 2008, 30, 885–892. [Google Scholar]
- Kelly, P.T.; Craig, N.; Solomon, C.T.; Weidel, B.C.; Zwart, J.A.; Jones, S.E. Experimental whole-lake increase of dissolved organic carbon concentration produces unexpected increase in crustacean zooplankton density. Glob. Change Biol. 2016, 22, 2766–2775. [Google Scholar]
- Meyjes, S.A.; Petrik, C.M.; Rohr, T.; Cael, B.B.; Mashayek, A. Impact of spatial variability in zooplankton grazing rates on carbon export flux. Glob. Biogeochem. Cycles 2024, 38, e2023GB008085. [Google Scholar]
- Schenone, L.; Modenutti, B.; Martyniuk, N.; Navarro, M.B. Modelling key variables for understanding the effects of grazing and nutrient recycling by zooplankton on the freshwater microbial loop. Freshw. Biol. 2021, 66, 2322–2337. [Google Scholar]
- Hrbácek, J.; Brandl, Z.; Straškraba, M. Do the long-term changes in zooplankton biomass indicate changes in fish stock? Hydrobiologia 2003, 504, 203–213. [Google Scholar]
- Prater, C.; Wagner, N.D.; Frost, P.C. Seasonal effects of food quality and temperature on body stoichiometry, biochemistry, and biomass production in Daphnia populations. Limnol. Oceanogr. 2018, 63, 1727–1740. [Google Scholar]
- Hyman, M.; Wang, Q.; Wilson, A.E.; Adhikari, S.; Higgins, B.T. Production of Daphnia zooplankton on wastewater-grown algae for sustainable conversion of waste nutrients to fish feed. J. Clean. Prod. 2021, 310, 127501. [Google Scholar]
- Matveev, V.; Robson, B.J. Aquatic food webs structure and the flow of carbon. Freshw. Rev. 2014, 7, 1–24. [Google Scholar]
- Steinberg, D.K.; Landry, M.R. Zooplankton and the ocean carbon cycle. Annu. Rev. Mar. Sci. 2017, 9, 413–444. [Google Scholar]
- Munoz-Colmenares, M.E.; Sendra, M.D.; Soria-Perpinya, X.; Soria, J.M.; Vicente, E. The use of zooplankton metrics to determine the trophic status and ecological potential: An approach in a large Mediterranean watershed. Water 2021, 13, 2382. [Google Scholar] [CrossRef]
- Stamou, G.; Mazaris, A.D.; Moustaka-Gouni, M.; Spoljar, M.; Ternjej, I.; Dranzina, T.; Dorak, Z.; Michaloudi, E. Introducing a zooplanktonic index for assessing water quality of natural lakes in the Mediterranean region. Ecol. Inform. 2022, 69, 101616. [Google Scholar]
- Choi, Y.; Oh, H.J.; Lee, D.H.; Jang, M.H.; Lee, K.L.; Chang, K.H.; Kim, H.Y. Current utilization and further application of zooplankton indices for ecosystem health assessment of lake ecosystems. Sustainability 2023, 15, 10950. [Google Scholar] [CrossRef]
- Jeppesen, E.; Peder Jensen, J.; Søndergaard, M.; Lauridsen, T.; Landkildehus, F. Trophic structure, species richness and biodiversity in Danish lakes: Changes along a phosphorus gradient. Freshw. Biol. 2000, 45, 201–218. [Google Scholar]
- Choi, Y.; Hong, G.H.; La, G.H.; Kim, H.W.; Kim, M.S.; Jang, M.H.; Chang, K.H.; Oh, H.J. Spatial distribution of Calanoida in freshwater ecosystems and their application as a food web assessment index. Water 2024, 16, 3414. [Google Scholar] [CrossRef]
- Oh, H.J.; Oda, Y.; Ha, J.Y.; Nagata, T.; Hanazato, T.; Miyabara, Y.; Sakamoto, M.; Chang, K.H. Responses of daphnids and other zooplankton populations to massive fish kill in Lake Suwa. Ecol. Res. 2019, 34, 856–863. [Google Scholar]
- Ochocka, A. ZIPLAs: Zooplankton index for Polish laeks’ assessment: A new method to assess the ecological status of stratified lakes. Environ. Monit. Assess. 2021, 193, 664. [Google Scholar]
- Ejsmont-Karabin, J. The usefulness of zooplankton as lake ecosystem indicators: Rotifer trophic state index. Pol. J. Ecol. 2012, 60, 339–350. [Google Scholar]
- Hong, G.H.; Chang, K.H.; Oh, H.J.; Choi, Y.; Han, S.; Jeong, H.G. Multivariate analysis of rotifer community and environmental factors using the decomposed components extracted from a time series. Water 2022, 14, 4113. [Google Scholar] [CrossRef]
- Proulx, S.R.; Promislow, D.E.L.; Phillips, P.C. Network thinking in ecology and evolution. Trends Ecol. Evol. 2005, 20, 345–353. [Google Scholar]
- Csárdi, G.; Nepusz, T. The igraph software package for complex network research. Complex Syst. 2006, 1695. Available online: https://igraph.org (accessed on 30 May 2025).
- Cho, K.S. Illustration of the Fresh Water Zooplankton of Korea; Academy books: Seoul, Republic of Korea, 1993. [Google Scholar]
- Jeong, H.G. A Practical Guide to Common Freshwater Zooplankton: Cladocera; Han River Environment Research Center, National Institute of Environmental Research: Yangpyeong, Republic of Korea, 2016. [Google Scholar]
- Mizuno, T.; Takahashi, E. An Illustrated Guide to Freshwater Zooplankton in Japan; Tokai University Press: Tokyo, Japan, 1991. [Google Scholar]
- NIER. Guidelines for Survey and Health Assessment Methods of Aquatic Ecosystems; National Institute of Environmental Research: Incheon, Republic of Korea, 2017. [Google Scholar]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar]
- Layeghifard, M.; Hwang, D.M.; Guttman, D.S. Constructing and analysing microbiome networks in R. In Microbiome Analysis. Methods in Molecular Biology; Beiko, R., Hsiao, W., Parkinson, J., Eds.; Humana Press: New York, NY, USA, 2018; Volume 1849. [Google Scholar]
- Liu, C.; Cui, Y.; Li, X.; Yao, M. microeco: An R package for data mining in microbial community ecology. FEMS Microbiol. Ecol. 2021, 97, fiaa255. [Google Scholar]
- Virro, T.; Haberman, J.; Haldna, M.; Blank, K. Diversity and structure of the winter rotifer assemblage in a shallow eutrophic northern temperate Lake Võrtsjärv. Aquat. Ecol. 2009, 43, 755–764. [Google Scholar]
- Min, C.; Johansson, L.S.; Søndergaard, M.; Lauridsen, T.L.; Chen, F.; Sh, T.; Jeppesen, E. Copepods as environmental indicators in lakes: Special focus on changes in the proportion of calanoids along nutrient and pH gradients. Aquat. Ecol. 2021, 55, 1241–1252. [Google Scholar]
- Haberman, J.; Haldna, M. Indices of zooplankton community as valuable tools in assessing the trophic state and water quality of eutrophic lakes: Long term study of Lake Võrtsjärv. J. Limnol. 2014, 73, 2. [Google Scholar]
- Pennak, R.W. Regional lake typology in northern Colorado, U.S.A. Verh. Der Int. Ver. Theor. Und Angew. Limnol. 1958, 13, 264–283. [Google Scholar]
- Fryer, G. Crustacean diversity in relation to the size of water bodies: Some facts and problems. Freshw. Biol. 1985, 15, 347–361. [Google Scholar]
- Goździejewska, A.M.; Koszałka, J.; Tandyrak, R.; Grochowska, J.; Parszuto, K. Functional responses of zooplankton communities to depth, trophic status, and ion content in mine pit lakes. Hydrobiologia 2021, 848, 2699–2719. [Google Scholar]
- Chang, K.H.; Doi, H.; Nishibe, Y.; Nakano, S. Feeding habitats of omnivorous Asplanchna: Comparison of diet composition among Asplanchna herricki, A. priodonata and A. girodi in pond ecosystems. J. Limnol. 2010, 69, 209–216. [Google Scholar]
- Yufeng, Y.; Xiangfei, H.; Jiankang, L. Long-term changes in crustacean zooplankton and water quality in a shallow, eutrophic Chinese lake densely stocked with fish. Hydrobiologia 1999, 391, 195–203. [Google Scholar]
- Stemberger, R.S.; Lazorchak, J.M. Zooplankton assemblage responses to disturbance gradients. Can. J. Fish. Aquat. Sci. 1994, 51, 2435–2447. [Google Scholar]
- Kobari, T.; Ban, S. Life cycles of two limnetic cyclopoid copepods, Cyclops vicinus and Thermocyclops crassus, in two different habitats. J. Plankton Res. 1998, 20, 1073–1086. [Google Scholar]
- Makino, W.; Ban, S. Response of life history traits to food conditions in a cyclopoid copepod from an oligotrophic environment. Limnol. Oceanogr. 2000, 45, 396–407. [Google Scholar]
- Adamczuk, M.; Mieczan, T.; Tarkowska-Kukuryk, M.; Demetraki-Paleolog, A. Rotatoria-Cladocera-Copepoda relations in the long-term monitoring of water quality in lakes with trophic variation (E. Poland). Environ. Earth Sci. 2015, 73, 8189–8196. [Google Scholar]
- Parszuto, K.; Teodorowicz, M.; Grochowska, J. Relationship between organic carbon and other measures of organic matter in the waters of Lake Isag. Limnol. Rev. 2006, 6, 233–238. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
