Optimization of Aluminum Electrocoagulation Parameters for Nutrient Removal from Hydroponic Wastewater Using Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Hydroponic Wastewater Solution (HWS)
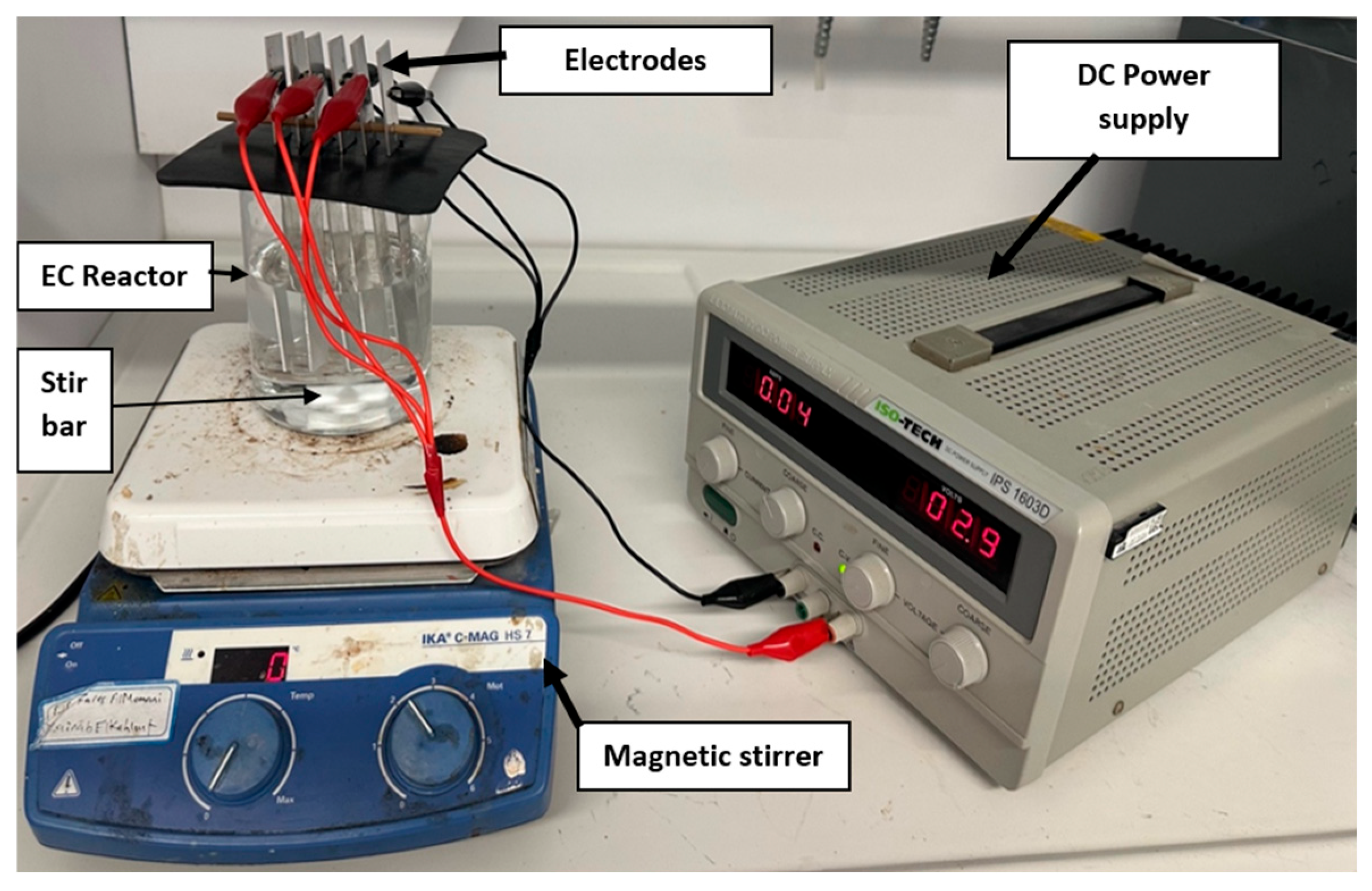
2.2. Experimental Setup and Procedure
2.3. Analytical Methods
2.4. Calculations
3. Optimization Performance Using Box–Behnken Design
4. Results and Discussion
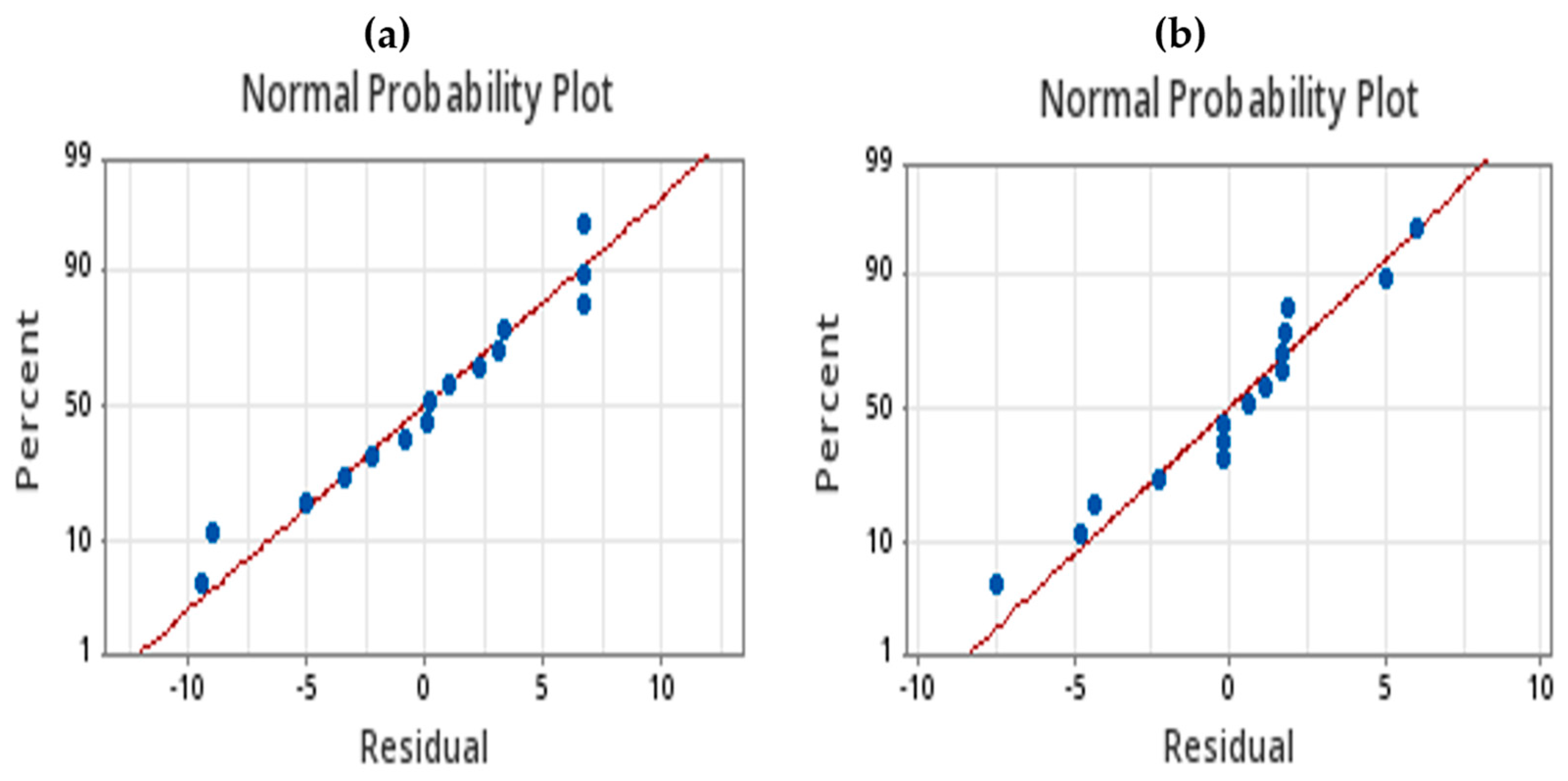
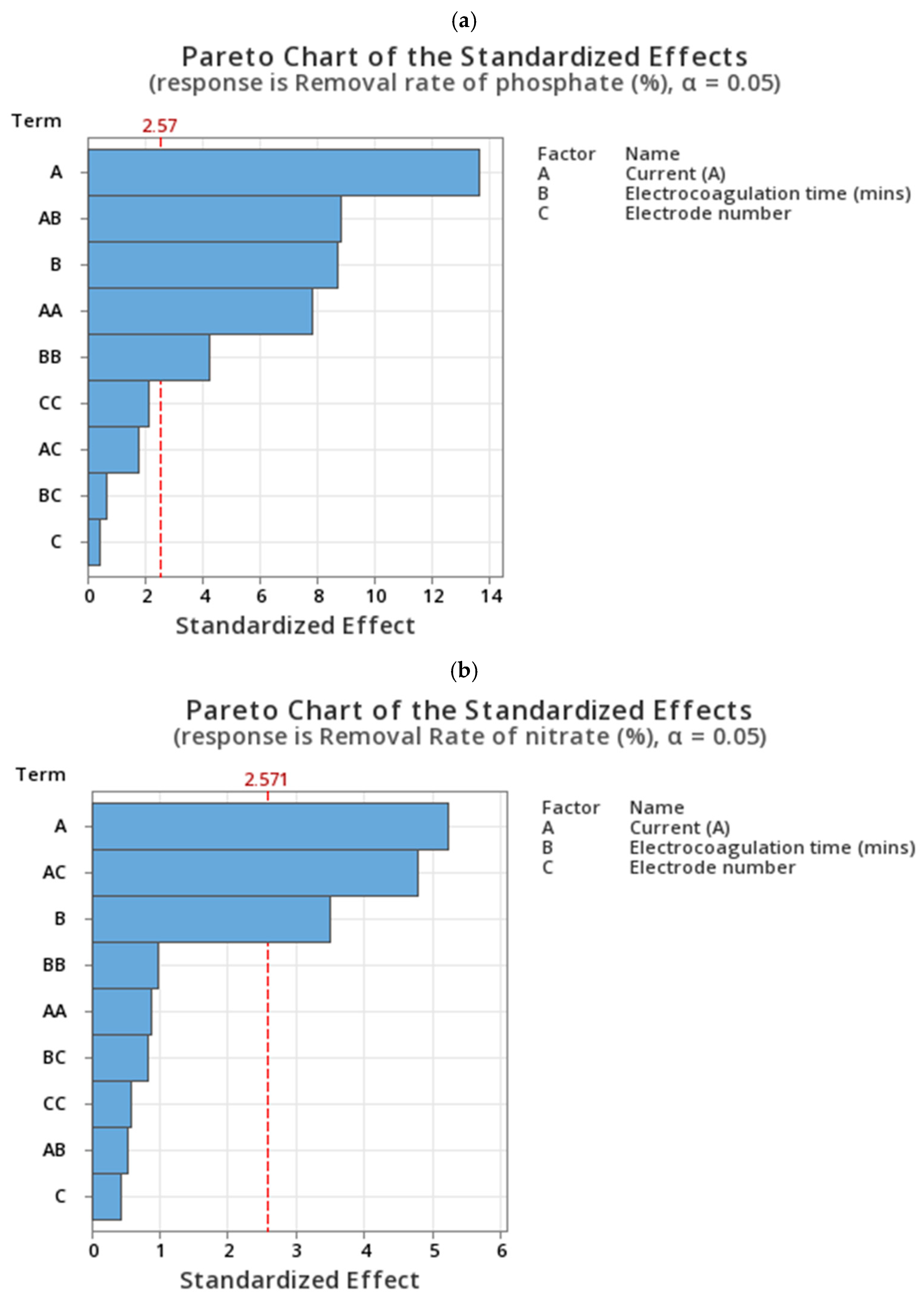
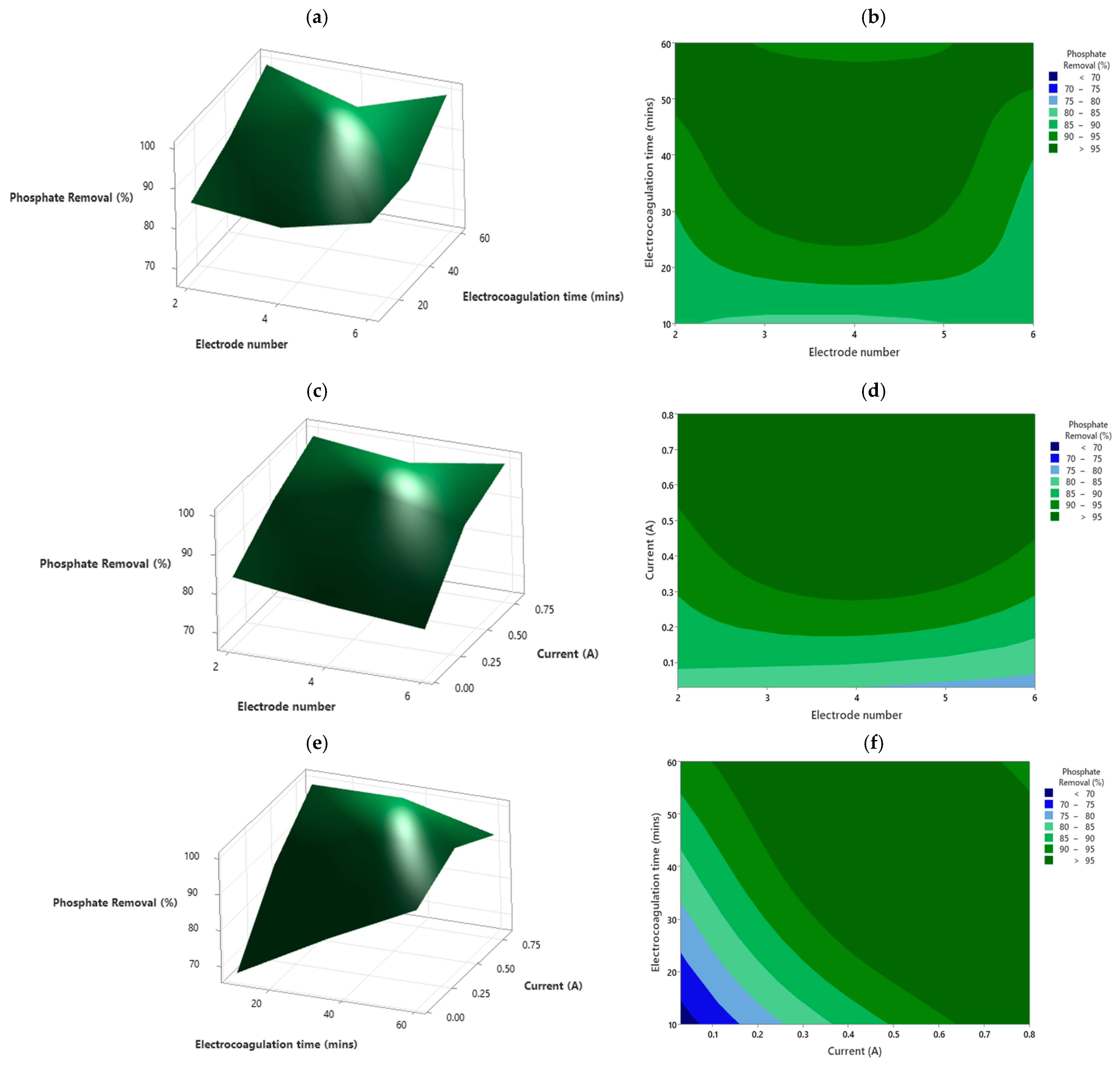
4.1. Phosphate Removal
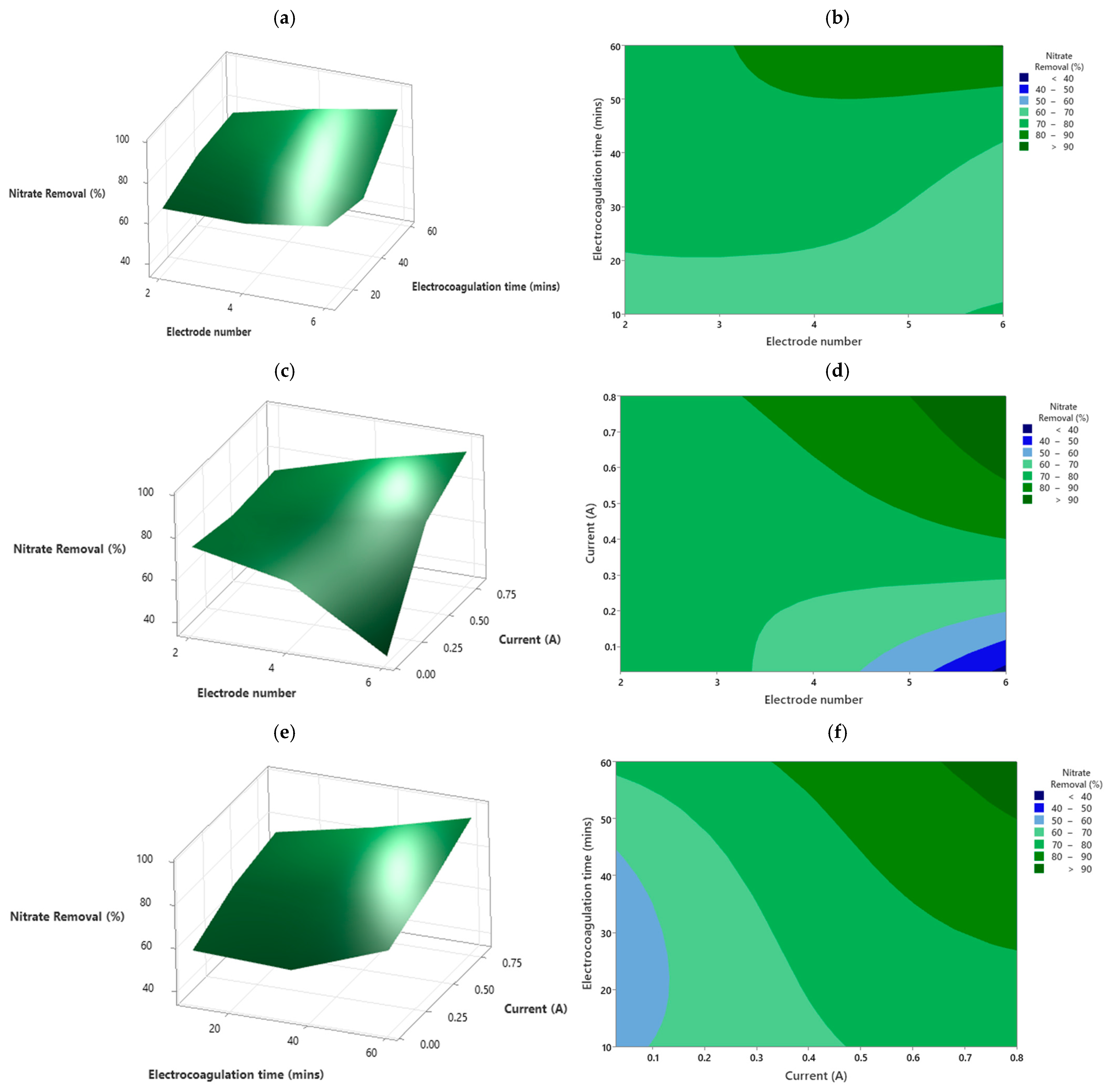
4.2. Nitrate Removal
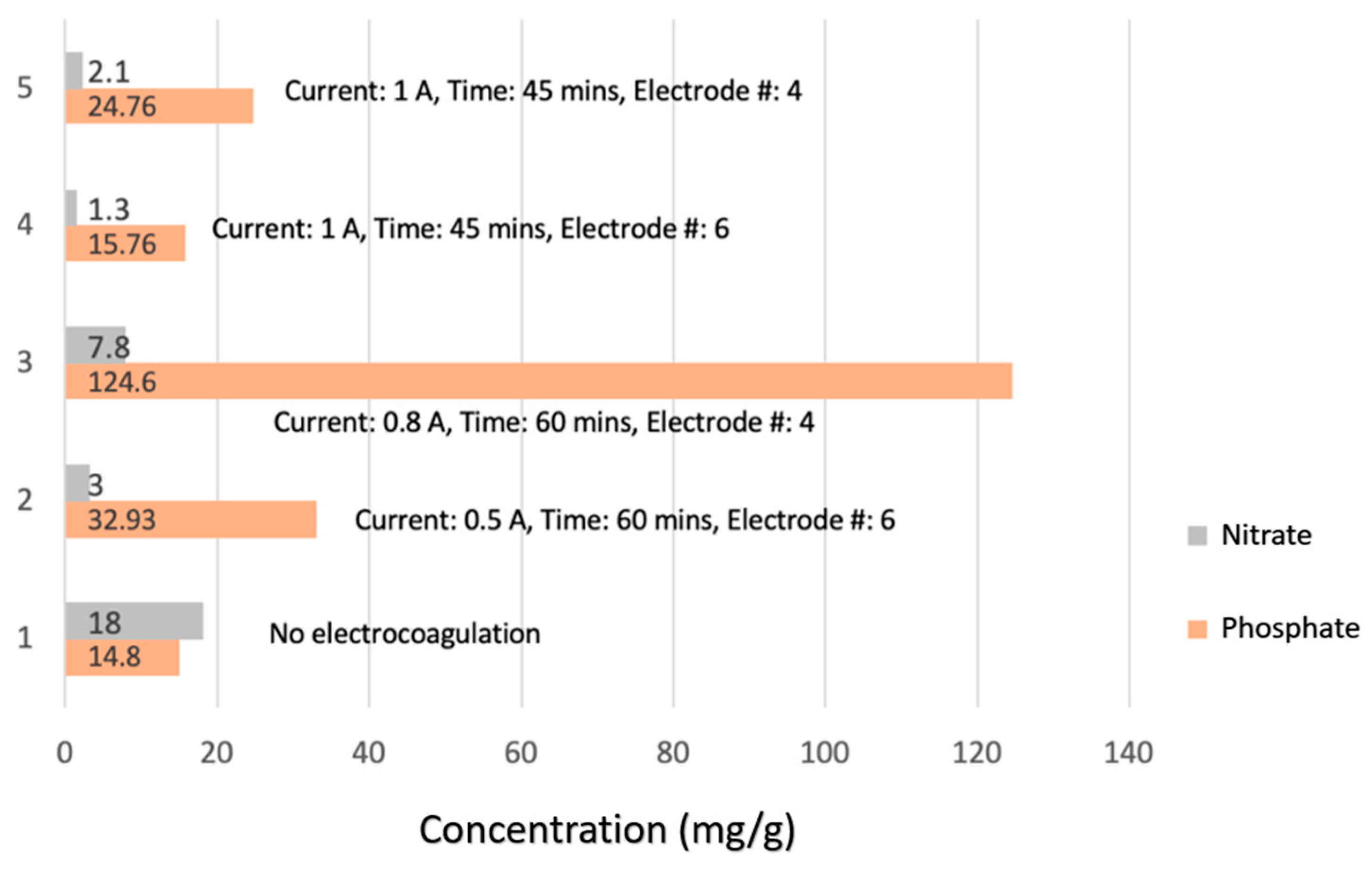
5. Sludge Characterization
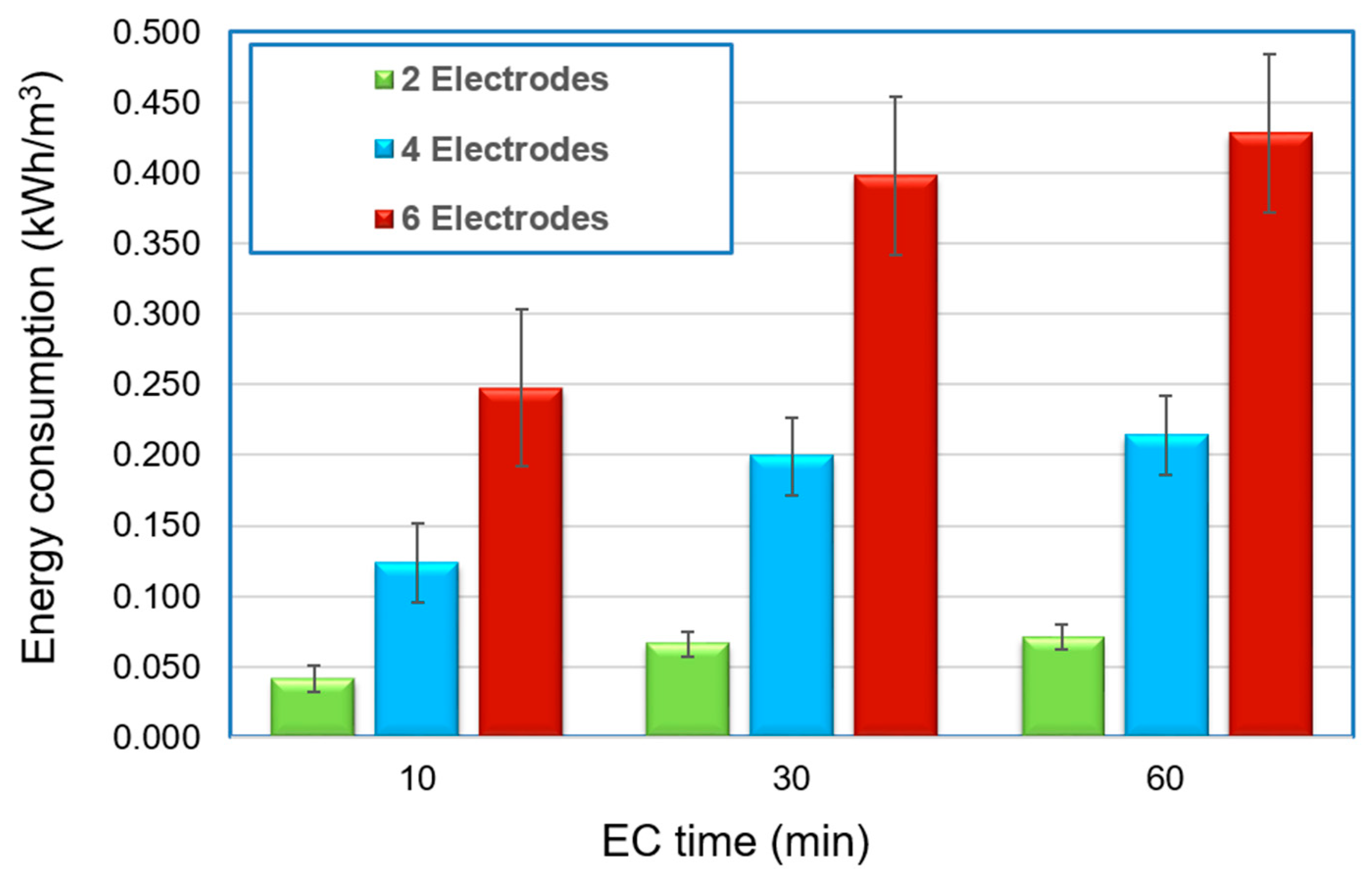
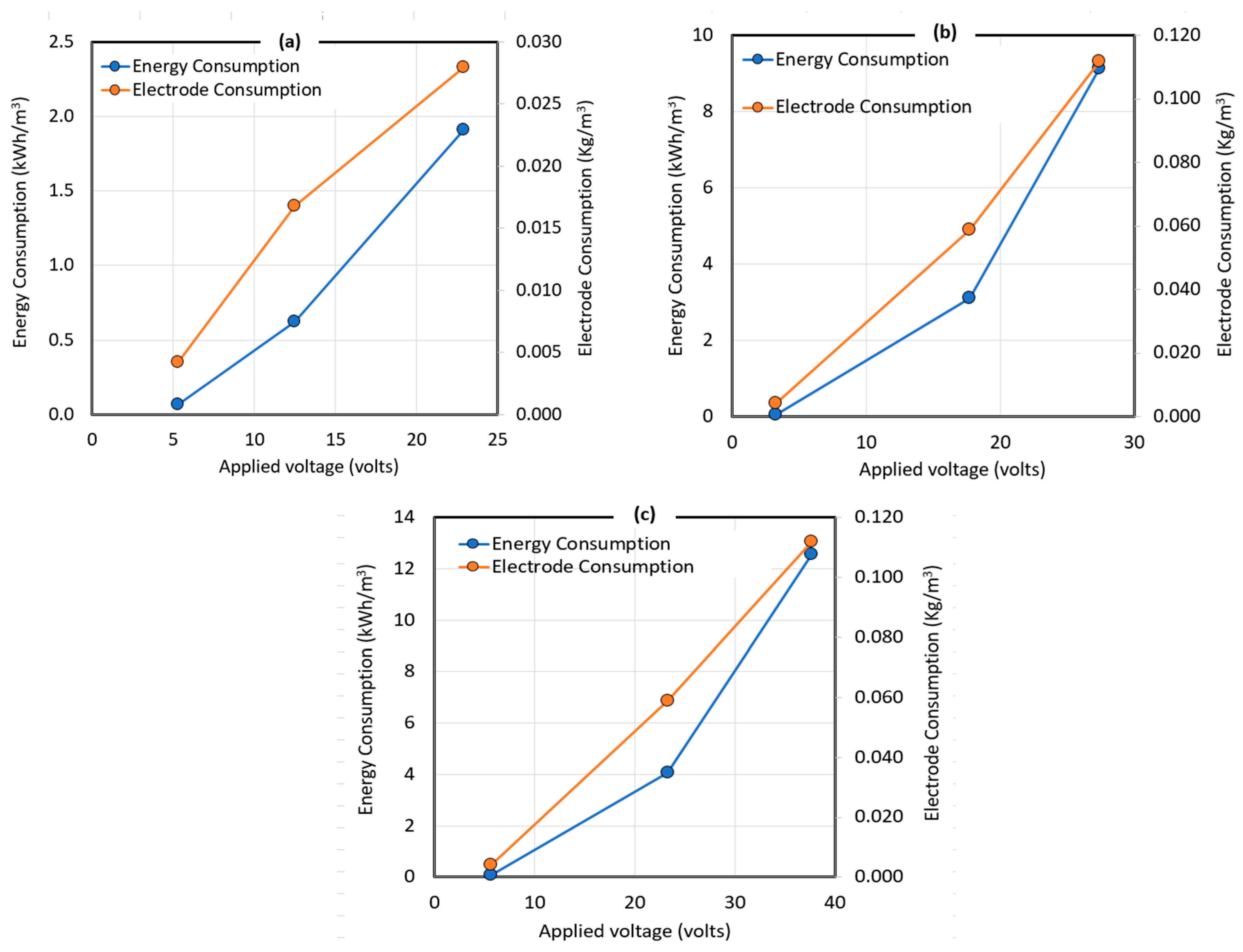
6. Energy and Electrodes Consumption
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Al | Aluminum |
| BBD | Box–Behnken design |
| COD | Chemical oxygen demand |
| DI | Deionized water |
| EC | Electrocoagulation |
| F | Faraday’s constant (96,485 C/mol) |
| HWS | Hydroponic wastewater solution |
| I | Applied current |
| M | Molar mass of aluminum |
| RSM | Response Surface Methodology |
| R2 | R-squared |
| R2 adj | R-squared adjusted |
| t | Electrocoagulation time |
| V | Volume of the treated solution |
| v | Applied voltage |
| z | Number of electrons |
References
- Rajendran, S.; Domalachenpa, T.; Arora, H.; Li, P.; Sharma, A.; Rajauria, G. Hydroponics: Exploring Innovative Sustainable Technologies and Applications across Crop Production, with Emphasis on Potato Mini-Tuber Cultivation. Heliyon 2024, 10, e26823. [Google Scholar] [CrossRef]
- Chatterjee, A.; Ghosh, P.; Winkler, B.; V, V.; Debnath, S.; Cichocki, J.; Trenkner, M.; Vanicela, B.; Riethmueller, C.; Walz, M.; et al. Demystifying the Integration of Hydroponics Cultivation System Reinforcing Bioeconomy and Sustainable Agricultural Growth. Sci. Hortic. 2025, 341, 113973. [Google Scholar] [CrossRef]
- Naresh, R.; Jadav, S.K.; Singh, M.; Patel, A.; Singh, B.; Beese, S.; Pandey, S.K. Role of Hydroponics in Improving Water-Use Efficiency and Food Security. IJECC 2024, 14, 608–633. [Google Scholar] [CrossRef]
- Santos, O.; Vaz, D.; Sebastião, F.; Sousa, H.; Vieira, J. Wastewater as a Nutrient Source for Hydroponic Production of Lettuce: Summer and Winter Growth. Agric. Water Manag. 2024, 301, 108966. [Google Scholar] [CrossRef]
- Mai, C.; Mojiri, A.; Palanisami, S.; Altaee, A.; Huang, Y.; Zhou, J.L. Wastewater Hydroponics for Pollutant Removal and Food Production: Principles, Progress and Future Outlook. Water 2023, 15, 2614. [Google Scholar] [CrossRef]
- Hofmann, A.H.; Liesegang, S.L.; Keuter, V.; Eticha, D.; Steinmetz, H.; Katayama, V.T. Nutrient Recovery from Wastewater for Hydroponic Systems: A Comparative Analysis of Fertilizer Demand, Recovery Products, and Supply Potential of WWTPs. J. Environ. Manag. 2024, 352, 119960. [Google Scholar] [CrossRef] [PubMed]
- Al-Bsoul, A.; Al-Qodah, Z.; Tawalbeh, M.; Bani-Melhem, K.; Al Bkoor Alrawashdeh, K.; Hailat, M.; Al-Taani, A.A.; Gul, E. From Waste to Solution: Modeling and Characterization of Grape Seed Bio-Waste for Phosphate Removal from Wastewater. Processes 2025, 13, 2464. [Google Scholar] [CrossRef]
- Richa, A.; Touil, S.; Fizir, M.; Martinez, V. Recent Advances and Perspectives in the Treatment of Hydroponic Wastewater: A Review. Rev. Env. Sci. Biotechnol. 2020, 19, 945–966. [Google Scholar] [CrossRef]
- Grewal, H.S.; Maheshwari, B.; Parks, S.E. Water and Nutrient Use Efficiency of a Low-Cost Hydroponic Greenhouse for a Cucumber Crop: An Australian Case Study. Agric. Water Manag. 2011, 98, 841–846. [Google Scholar] [CrossRef]
- Rufí-Salís, M.; Petit-Boix, A.; Villalba, G.; Sanjuan-Delmás, D.; Parada, F.; Ercilla-Montserrat, M.; Arcas-Pilz, V.; Muñoz-Liesa, J.; Rieradevall, J.; Gabarrell, X. Recirculating Water and Nutrients in Urban Agriculture: An Opportunity towards Environmental Sustainability and Water Use Efficiency? J. Clean. Prod. 2020, 261, 121213. [Google Scholar] [CrossRef]
- Seo, D.-C.; Park, J.-H.; Cheon, Y.-S.; Park, S.-K.; Kim, A.-R.; Lee, W.-G.; Lee, S.-W.; Lee, S.-T.; Cho, J.-S.; Heo, J.-S. Treatment Efficiency of Pollutants in Constructed Wetlands under Different Hydroponic Wastewater Injection Methods and Characteristic of Filter Media. Korean J. Environ. Agric. 2010, 29, 146–151. [Google Scholar] [CrossRef]
- Gagnon, V.; Maltais-Landry, G.; Puigagut, J.; Chazarenc, F.; Brisson, J. Treatment of Hydroponics Wastewater Using Constructed Wetlands in Winter Conditions. Water Air Soil Pollut. 2010, 212, 483–490. [Google Scholar] [CrossRef]
- Park, W.-Y.; Seo, D.-C.; Lim, J.-S.; Park, S.-K.; Cho, J.-S.; Heo, J.-S.; Yoon, H.-S. Optimum Configuration, Filter Media Depth and Wastewater Load of Small-Scale Constructed Wetlands for Treating the Hydroponic Waste Solution in Greenhouses. Korean J. Environ. Agric. 2008, 27, 217–224. [Google Scholar] [CrossRef]
- Park, J.B.K.; Craggs, R.J.; Sukias, J.P.S. Treatment of Hydroponic Wastewater by Denitrification Filters Using Plant Prunings as the Organic Carbon Source. Bioresour. Technol. 2008, 99, 2711–2716. [Google Scholar] [CrossRef]
- Castellar, J.A.C.; Formosa, J.; Fernández, A.I.; Jové, P.; Bosch, M.G.; Morató, J.; Brix, H.; Arias, C.A. Cork as a Sustainable Carbon Source for Nature-Based Solutions Treating Hydroponic Wastewaters—Preliminary Batch Studies. Sci. Total Environ. 2019, 650, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Rodziewicz, J.; Mielcarek, A.; Janczukowicz, W.; Jóźwiak, T.; Struk—Sokołowska, J.; Bryszewski, K. The Share of Electrochemical Reduction, Hydrogenotrophic and Heterotrophic Denitrification in Nitrogen Removal in Rotating Electrobiological Contactor (REBC) Treating Wastewater from Soilless Cultivation Systems. Sci. Total Environ. 2019, 683, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Hultberg, M.; Carlsson, A.S.; Gustafsson, S. Treatment of Drainage Solution from Hydroponic Greenhouse Production with Microalgae. Bioresour. Technol. 2013, 136, 401–406. [Google Scholar] [CrossRef]
- Saxena, P.; Bassi, A. Removal of Nutrients from Hydroponic Greenhouse Effluent by Alkali Precipitation and Algae Cultivation Method. J. Chem. Tech. Biotech. 2013, 88, 858–863. [Google Scholar] [CrossRef]
- Bakry, S.A.; Matta, M.E.; Noureldin, A.M.; Zaher, K. Performance Evaluation of Electrocoagulation Process for Removal of Heavy Metals from Wastewater Using Aluminum Electrodes under Variable Operating Conditions. Desalination Water Treat. 2024, 318, 100396. [Google Scholar] [CrossRef]
- Atallah, C.; Mosadeghsedghi, S.; Dashtban Kenari, S.L.; Hudder, M.; Morin, L.; Volchek, K.; Mortazavi, S.; Ben Salah, I. Removal of Heavy Metals from Mine Water Using a Hybrid Electrocoagulation-Ceramic Membrane Filtration Process. Desalination Water Treat. 2024, 320, 100730. [Google Scholar] [CrossRef]
- Garomsa, F.S.; Mehari, Y.; Desta, W.M.; Bidira, F.; Asaithambi, P. Removal of NO3-, PO3-, and Color from Brewery Wastewater by the Use of Indigenous Bio-Coagulant-Assisted Electrocoagulation. Prog. Eng. Sci. 2024, 1, 100032. [Google Scholar] [CrossRef]
- García-Colindres, M.A.; Requena-Alvarez, B.L.; Castillo-Suárez, L.A.; Linares-Hernández, I.; Martínez-Miranda, V. Removal of Nitrates in Drinking Water Polluted with Landfill Leachate by an Electrocoagulation System with Mg-Zn. Water Air Soil Pollut. 2024, 235, 269. [Google Scholar] [CrossRef]
- Gasmi, A.; Elboughdiri, N.; Ghernaout, D.; Hannachi, A.; Halim, K.S.A.; Khan, M.I. Electrocoagulation Process for Removing Dyes and Chemical Oxygen Demand from Wastewater: Operational Conditions and Economic Assessment—A Review. Desalination Water Treat. 2022, 271, 74–107. [Google Scholar] [CrossRef]
- Hasnaoui, A.; Chikhi, M.; Balaska, F.; Seraghni, W.; Boussemghoune, M.; Dizge, N. Electrocoagulation Employing Recycled Aluminum Electrodes for Methylene Blue Remediation. Desalination Water Treat. 2024, 319, 100453. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, Y.; Almatrafi, E.; Hu, T.; Zhou, C.; Song, B.; Zeng, Z.; Zeng, G. Efficient Removal of Microplastics from Wastewater by an Electrocoagulation Process. Chem. Eng. J. 2022, 428, 131161. [Google Scholar] [CrossRef]
- Elkhatib, D.; Oyanedel-Craver, V.; Carissimi, E. Electrocoagulation Applied for the Removal of Microplastics from Wastewater Treatment Facilities. Sep. Purif. Technol. 2021, 276, 118877. [Google Scholar] [CrossRef]
- Al-Kilani, M.R.; Bani-Melhem, K. The Performance of Electrocoagulation Process for Decolorization and COD Removal of Highly Colored Real Grey Water under Variable Operating Conditions. Desalination Water Treat. 2025, 321, 100924. [Google Scholar] [CrossRef]
- Bani-Melhem, K.; Alnaief, M.; Al-Qodah, Z.; Al-Shannag, M.; Elnakar, H.; AlJbour, N.; Alu’datt, M.; Alrosan, M.; Ezelden, E. On the Performance of Electrocoagulation Treatment of High-Loaded Gray Water: Kinetic Modeling and Parameters Optimization via Response Surface Methodology. Appl. Water Sci. 2025, 15, 114. [Google Scholar] [CrossRef]
- Ditta, A.; Tabish, A.N.; Farhat, I.; Razzaq, L.; Fouad, Y.; Miran, S.; Mujtaba, M.A.; Kalam, M.A. The Optimization of Operational Variables of Electrochemical Water Disinfection Using Response Surface Methodology. Sustainability 2023, 15, 4390. [Google Scholar] [CrossRef]
- Ankoliya, D.; Mudgal, A.; Sinha, M.K.; Patel, V.; Patel, J. Application of Electrocoagulation Process for the Treatment of Dairy Wastewater: A Mini Review. Mater. Today Proc. 2023, 77, 117–124. [Google Scholar] [CrossRef]
- Bani-Melhem, K.; Rasool Al-Kilani, M. A Comparison between Iron and Mild Steel Electrodes for the Treatment of Highly Loaded Grey Water Using an Electrocoagulation Technique. Arab. J. Chem. 2023, 16, 105199. [Google Scholar] [CrossRef]
- Das, P.P.; Sharma, M.; Purkait, M.K. Recent Progress on Electrocoagulation Process for Wastewater Treatment: A Review. Sep. Purif. Technol. 2022, 292, 121058. [Google Scholar] [CrossRef]
- Sadaf, S.; Roy, H.; Fariha, A.; Rahman, T.U.; Tasnim, N.; Jahan, N.; Sokan-Adeaga, A.A.; Safwat, S.M.; Islam, M.S. Electrocoagulation-Based Wastewater Treatment Process and Significance of Anode Materials for the Overall Improvement of the Process: A Critical Review. J. Water Process Eng. 2024, 62, 105409. [Google Scholar] [CrossRef]
- Bani-Melhem, K.; Al-Kilani, M.R.; Tawalbeh, M. Evaluation of Scrap Metallic Waste Electrode Materials for the Application in Electrocoagulation Treatment of Wastewater. Chemosphere 2023, 310, 136668. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; AL-Rajabi, M.M.; Da’na, E.; Al-Shannag, M.; Bani-Melhem, K.; Assirey, E. Continuous Electrocoagulation Processes for Industrial Inorganic Pollutants Removal: A Critical Review of Performance and Applications. Water 2025, 17, 2639. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; AL-Rajabi, M.M.; Al Amayreh, H.H.; Assirey, E.; Bani-Melhem, K.; Al-Shannag, M. Performance of Continuous Electrocoagulation Processes (CEPs) as an Efficient Approach for the Treatment of Industrial Organic Pollutants: A Comprehensive Review. Water 2025, 17, 2351. [Google Scholar] [CrossRef]
- Duduković, N.; Slijepčević, N.; Kulić Mandić, A.; Pešić, V.; Leovac Maćerak, A.; Kerkez, Đ. Electroprecipitation Technology for Nutrient Recovery: Unlocking Wastewater’s Potential. Environ. Technol. Innov. 2025, 40, 104402. [Google Scholar] [CrossRef]
- Yazıcı Karabulut, B. Electrochemical Coagulant Generation via Aluminum-Based Electrocoagulation for Sustainable Greywater Treatment and Reuse: Optimization Through Response Surface Methodology and Kinetic Modelling. Molecules 2025, 30, 3779. [Google Scholar] [CrossRef]
- Sahu, J.N.; Acharya, J.; Meikap, B.C. Response Surface Modeling and Optimization of Chromium(VI) Removal from Aqueous Solution Using Tamarind Wood Activated Carbon in Batch Process. J. Hazard. Mater. 2009, 172, 818–825. [Google Scholar] [CrossRef]
- Ba-Abbad, M.M.; Chai, P.V.; Benamour, A.; Ewis, D.; Mohammad, A.W.; Mahmoudi, E. Optimizing and Control of Effective Synthesize Parameters for Fe3O4 Nanoparticles Using Response Surface Methodology. Chem. Pap. 2022, 76, 6359–6370. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, N.; Yang, X.; Li, C.; He, W.; Ren, N.; Liu, G.; Yang, W. Migration Electric-Field Assisted Electrocoagulation with Sponge Biochar Capacitive Electrode for Advanced Wastewater Phosphorus Removal. Water Res. 2023, 231, 119645. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, J.; Xie, N. Study on Influencing Parameters of Total Phosphorus Degradation in Cattle Farm Wastewater by Electrocoagulation Using Magnesium, Aluminum, and Iron Electrodes. Water 2023, 15, 4134. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, N.; Yang, X.; Zhang, R.; Xu, J.; Li, C.; Yang, W.; Ren, N.; Feng, Y.; He, W. Achieved Advanced Wastewater Phosphate Removal via Improved Ionic Interference Resistance in a Migration-Electric-Field Assisted Electrocoagulation (MEAEC) System with Modified-Biochar Pseudocapacitor Electrode. Sep. Purif. Technol. 2024, 328, 124972. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Mao, R.; Shi, Y.; Lin, S.; Qiao, M.; Zhao, X. Removal of Phosphate in Secondary Effluent from Municipal Wastewater Treatment Plant by Iron and Aluminum Electrocoagulation: Efficiency and Mechanism. Sep. Purif. Technol. 2022, 286, 120439. [Google Scholar] [CrossRef]
- Zeng, J.; Ji, M.; Zhao, Y.; Helmer Pedersen, T.; Wang, H. Optimization of Electrocoagulation Process Parameters for Enhancing Phosphate Removal in a Biofilm-Electrocoagulation System. Water Sci. Technol. 2021, 83, 2560–2574. [Google Scholar] [CrossRef]
- Shirkoohi, M.G.; Tyagi, R.D.; Vanrolleghem, P.A.; Drogui, P. A Comparison of Artificial Intelligence Models for Predicting Phosphate Removal Efficiency from Wastewater Using the Electrocoagulation Process. Digit. Chem. Eng. 2022, 4, 100043. [Google Scholar] [CrossRef]
- Li, G.; Zheng, B.; Zhang, W.; Liu, Q.; Li, M.; Zhang, H. Phosphate Removal Efficiency and Life Cycle Assessment of Different Anode Materials in Electrocoagulation Treatment of Wastewater. Sustainability 2024, 16, 3836. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, Y.; Zhang, C.; Li, X. Study on Influencing Parameters and Long-Term Operation of Electrocoagulation Phosphorus Removal from Small Rural Domestic Sewage. Water Sci. Technol. 2023, 87, 1866–1878. [Google Scholar] [CrossRef] [PubMed]
- Najari, S.; Delnavaz, M.; Bahrami, D. Application of Electrocoagulation Process for the Treatment of Reactive Blue 19 Synthetic Wastewater: Evaluation of Different Operation Conditions and Financial Analysis. Chem. Phys. Lett. 2023, 832, 140897. [Google Scholar] [CrossRef]
- Benekos, A.K.; Tziora, F.E.; Tekerlekopoulou, A.G.; Pavlou, S.; Qun, Y.; Katsaounis, A.; Vayenas, D.V. Nitrate Removal from Groundwater Using a Batch and Continuous Flow Hybrid Fe-Electrocoagulation and Electrooxidation System. J. Environ. Manag. 2021, 297, 113387. [Google Scholar] [CrossRef]
- Han, X.; Qu, Y.; Dong, Y.; Chen, D.; Liang, D.; Liu, J.; Zhang, J.; Ren, N.; Feng, Y. Simultaneous Electricity Generation and Eutrophic Water Treatment Utilizing Iron Coagulation Cell with Nitrification and Denitrification Biocathodes. Sci. Total Environ. 2021, 782, 146436. [Google Scholar] [CrossRef]
- Bhatt, P.; Engel, B.A.; Shivaram, K.B.; Turco, R.F.; Zhou, Z.; Simsek, H. Treatment and Optimization of High-Strength Egg-Wash Wastewater Effluent Using Electrocoagulation and Electrooxidation Methods. Chemosphere 2024, 347, 140632. [Google Scholar] [CrossRef]
- Galvão, N.; Dutra, A.J.B.; Bassin, J.P. Understanding the Conversion of Nitrogen Compounds during Ammonia Electrooxidation: Effect of Current Density, Chloride Concentration and pH on Nitrate Formation. J. Chem. Technol. Biotechnol. 2024, 99, 2270–2277. [Google Scholar] [CrossRef]
- Dan, N.H.; Le Luu, T. Continuous Flow Sequencing Bed Biofilm Reactor Bio-Digested Landfill Leachate Treatment Using Electrocoagulation-Persulfate. J. Environ. Manag. 2021, 297, 113409. [Google Scholar] [CrossRef] [PubMed]
- Bouhaous, M.; Bengharez, Z.; Nacer, A.; Bellebia, S.; Bendaoudi, A.A.; Goosen, M.F.A.; Mahmoudi, H. Assessment of Nitrate Contamination of Domestic Wells and Remedial Treatment by Electrocoagulation. Desalination Water Treat. 2024, 317, 100010. [Google Scholar] [CrossRef]
- Bani-Melhem, K.; Elektorowicz, M.; Oleszkiewicz, J. Submerged Membrane Electro-Bioreactor (SMEBR) Reduces Membrane Fouling and Achieves Phosphorus Removal. Proc. Water Environ. Fed. 2009, 2009, 2771–2783. [Google Scholar] [CrossRef]
- Rajaniemi, K.; Tuomikoski, S.; Lassi, U. Electrocoagulation Sludge Valorization—A Review. Resources 2021, 10, 127. [Google Scholar] [CrossRef]
- Belgada, A.; Lamhar, R.; Charik, F.Z.; Ounouss, I.; Dani, A.; Ma, W.; Necibi, M.C.; Younssi, S.A. Sustainable Valorization of Electrocoagulation Sludge in Ceramic Kaolinite Membrane Fabrication and Its Application to Seawater Pretreatment for SWRO Desalination. J. Water Process Eng. 2025, 74, 107747. [Google Scholar] [CrossRef]
- Oktiawan, W.; Sarminingsih, A.; Hadiwidodo, M.; Purwono, P. Electrocoagulation Process for Phosphate Recovery of Agricultural Wastewater: Effect of Calcium Adding, Voltage, and Time. Env. Monit. Assess. 2024, 196, 842. [Google Scholar] [CrossRef]
- Emana, B.B.; Bulge, T.B. Integrated Electrodes for the Nutrient Removal from Municipal Wastewater Using Electrocoagulation Technology. Sci. Rep. 2025, 15, 28244. [Google Scholar] [CrossRef]
- Ammar, M.; Yousef, E.; Ashraf, S.; Baltrusaitis, J. Removal of Inorganic Pollutants and Recovery of Nutrients from Wastewater Using Electrocoagulation: A Review. Separations 2024, 11, 320. [Google Scholar] [CrossRef]
- Bani-Melhem, K.; Elektorowicz, M.; Tawalbeh, M.; Al Bsoul, A.; El Gendy, A.; Kamyab, H.; Yusuf, M. Integrating of Electrocoagulation Process with Submerged Membrane Bioreactor for Wastewater Treatment under Low Voltage Gradients. Chemosphere 2023, 339, 139693. [Google Scholar] [CrossRef]
- Reza, A.; Haller, S.; Mao, X. Electrocoagulation as a Remedial Approach for Phosphorus Removal from Onsite Wastewater: A Review. Water 2024, 16, 3206. [Google Scholar] [CrossRef]
- Bani-Melhem, K.; Smith, E. Grey Water Treatment by a Continuous Process of an Electrocoagulation Unit and a Submerged Membrane Bioreactor System. Chem. Eng. J. 2012, 198–199, 201–210. [Google Scholar] [CrossRef]
- Bani-Melhem, K.Q. Development of a Novel Submerged Membrane Electro-Bioreactor for Wastewater Treatment. Ph.D. Thesis, Concordia University, Montreal, QC, Canada, 2008. [Google Scholar]
- Patel, S.K.; Shukla, S.C.; Natarajan, B.R.; Asaithambi, P.; Dwivedi, H.K.; Sharma, A.; Singh, D.; Nasim, M.; Raghuvanshi, S.; Sharma, D.; et al. State of the Art Review for Industrial Wastewater Treatment by Electrocoagulation Process: Mechanism, Cost and Sludge Analysis. Desalination Water Treat. 2025, 321, 100915. [Google Scholar] [CrossRef]
- Tian, Y.; He, W.; Liang, D.; Yang, W.; Logan, B.E.; Ren, N. Effective Phosphate Removal for Advanced Water Treatment Using Low Energy, Migration Electric–Field Assisted Electrocoagulation. Water Res. 2018, 138, 129–136. [Google Scholar] [CrossRef]
- Svilović, S.; Vukojević Medvidović, N.; Vrsalović, L.; Gudić, S.; Bašić, A.; Dujmović, K. Intensification of Electrocoagulation in Compost-Derived Wastewater. Processes 2025, 13, 3207. [Google Scholar] [CrossRef]
| Parameter Index | Unit | Value |
|---|---|---|
| pH | - | 7.2 |
| Temperature | Celsius (°C) | 25.0 |
| Conductivity | (µS/cm) | 432 |
| Phosphate (PO43−) | mg/L | 14.8 |
| Nitrate (NO3−) | mg/L | 18.0 |
| Dissolved oxygen (DO) | mg/L | 6.8 |
| Independent Variables | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Current (A) | 0.03 | 0.415 | 0.8 |
| Electrocoagulation time (min) | 10 | 35 | 60 |
| Number of Electrodes | 2 | 4 | 6 |
| Run | Blk | Current (A) | Electrocoagulation Time (min) | Electrode Number |
|---|---|---|---|---|
| 1 | 1 | 0.8 | 35 | 2 |
| 2 | 1 | 0.03 | 35 | 2 |
| 3 | 1 | 0.415 | 10 | 6 |
| 4 | 1 | 0.03 | 10 | 4 |
| 5 | 1 | 0.03 | 60 | 4 |
| 6 | 1 | 0.415 | 35 | 4 |
| 7 | 1 | 0.8 | 60 | 4 |
| 8 | 1 | 0.415 | 35 | 4 |
| 9 | 1 | 0.8 | 10 | 4 |
| 10 | 1 | 0.415 | 60 | 6 |
| 11 | 1 | 0.415 | 35 | 4 |
| 12 | 1 | 0.03 | 35 | 6 |
| 13 | 1 | 0.415 | 10 | 2 |
| 14 | 1 | 0.8 | 35 | 6 |
| 15 | 1 | 0.415 | 60 | 2 |
| RunOrder | Current (A) | Electrocoagulation Time (min) | Electrode Number | Removal Rate of Nitrate (%) | Predicted Fits of Nitrate | Removal Rate of Phosphate (%) | Predicted Fits of Phosphate |
|---|---|---|---|---|---|---|---|
| 1 | 0.8 | 35 | 2 | 80.53 | 82.84 | 99.04 | 100.00 |
| 2 | 0.03 | 35 | 2 | 65.32 | 60.28 | 83.65 | 83.56 |
| 3 | 0.415 | 10 | 6 | 71.67 | 70.52 | 88.44 | 86.13 |
| 4 | 0.03 | 10 | 4 | 57.78 | 56.15 | 67.50 | 76.95 |
| 5 | 0.03 | 60 | 4 | 72.22 | 70.60 | 92.76 | 89.62 |
| 6 | 0.415 | 35 | 4 | 74.44 | 74.65 | 99.39 | 92.74 |
| 7 | 0.8 | 60 | 4 | 95.00 | 93.15 | 99.55 | 100.00 |
| 8 | 0.415 | 35 | 4 | 74.44 | 74.65 | 99.39 | 92.74 |
| 9 | 0.8 | 10 | 4 | 73.89 | 78.70 | 99.23 | 95.85 |
| 10 | 0.415 | 60 | 6 | 85.56 | 84.96 | 99.78 | 98.80 |
| 11 | 0.415 | 35 | 4 | 74.44 | 74.65 | 99.39 | 92.74 |
| 12 | 0.03 | 35 | 6 | 58.89 | 66.46 | 77.96 | 83.01 |
| 13 | 0.415 | 10 | 2 | 66.11 | 64.34 | 85.79 | 86.68 |
| 14 | 0.8 | 35 | 6 | 95.00 | 89.01 | 99.66 | 100.00 |
| 15 | 0.415 | 60 | 2 | 74.44 | 78.78 | 99.55 | 99.35 |
| Source | DF | Adj SS | Adj MS | F Value | p Value |
|---|---|---|---|---|---|
| Phosphate | |||||
| Regression | 3 | 1510.81 | 503.6 | 30.86 | <0.001 |
| Current (A) | 1 | 1017.23 | 1017.23 | 62.33 | <0.001 |
| Electrocoagulation time (min) | 1 | 417.28 | 417.28 | 25.57 | <0.001 |
| Electrode number | 1 | 76.3 | 76.3 | 4.67 | 0.054 |
| Error | 11 | 179.53 | 16.32 | ||
| Lack-of-Fit | 9 | 179.53 | 19.95 | ||
| Pure Error | 2 | 0 | 0 | ||
| Total | 14 | 1690.34 | |||
| Nitrate | |||||
| Regression | 3 | 1036.26 | 345.42 | 10.18 | 0.002 |
| Current (A) | 1 | 714.62 | 714.62 | 21.07 | 0.001 |
| Electrocoagulation time (min) | 1 | 321.04 | 321.04 | 9.46 | 0.011 |
| Electrode number | 1 | 0.6 | 0.599 | 0.02 | 0.897 |
| Error | 11 | 373.14 | 33.922 | ||
| Lack-of-Fit | 9 | 373.14 | 41.46 | ||
| Pure Error | 2 | 0 | 0 | ||
| Total | 14 | 1409.4 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soltan, Y.; Bani-Melhem, K.; Ba-Abbad, M.; Almomani, F.; Al-Muhtaseb, A. Optimization of Aluminum Electrocoagulation Parameters for Nutrient Removal from Hydroponic Wastewater Using Response Surface Methodology. Water 2025, 17, 3346. https://doi.org/10.3390/w17233346
Soltan Y, Bani-Melhem K, Ba-Abbad M, Almomani F, Al-Muhtaseb A. Optimization of Aluminum Electrocoagulation Parameters for Nutrient Removal from Hydroponic Wastewater Using Response Surface Methodology. Water. 2025; 17(23):3346. https://doi.org/10.3390/w17233346
Chicago/Turabian StyleSoltan, Yara, Khalid Bani-Melhem, Muneer Ba-Abbad, Fares Almomani, and Ala’a Al-Muhtaseb. 2025. "Optimization of Aluminum Electrocoagulation Parameters for Nutrient Removal from Hydroponic Wastewater Using Response Surface Methodology" Water 17, no. 23: 3346. https://doi.org/10.3390/w17233346
APA StyleSoltan, Y., Bani-Melhem, K., Ba-Abbad, M., Almomani, F., & Al-Muhtaseb, A. (2025). Optimization of Aluminum Electrocoagulation Parameters for Nutrient Removal from Hydroponic Wastewater Using Response Surface Methodology. Water, 17(23), 3346. https://doi.org/10.3390/w17233346








