Methodology Based on Raman Spectroscopy for Detection and Quantification of Lubricant and Diesel Oils in Saline Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Standardization of the Mass and Volume of Lubricating Oil and Diesel Oil in Saline Water
2.2. Raman Spectroscopy for Quantification of Oil Concentrations
2.3. Quantification of the Oil Added to the Saline Water via Principal Component Regression (PCR) and Partial Least Squares Regression (PLSR)
3. Results and Discussion
3.1. Raman Spectra of Synthetic Lubricating Oil (SLO) and Diesel Fuel Oil (DFO) Added to Saline Water
3.2. Quantification of Synthetic Lubricating Oil and Diesel Fuel Oil Added to Saline Water
3.3. Raman Spectroscopy as a Tool for Monitoring Oil Contamination in a Saline Environment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- United Nations. Department of Economic and Social Affairs. World Urbanization Prospects 2018. Available online: https://population.un.org/wup/ (accessed on 10 July 2025).
- Fang, C.; Cui, X.; Li, G.; Bao, C.; Wang, Z.; Ma, H.; Sun, S.; Li, H.; Luo, K.; Ren, Y. Modeling regional sustainable development scenarios using the Urbanization and Eco-environment Coupler: Case study of Beijing-Tianjin-Hebei urban agglomeration, China. Sci. Total Environ. 2019, 689, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Munir, K.; Ameer, A. Assessing nonlinear impact of urbanization, economic growth, technology, and trade on environment: Evidence from African and Asian emerging economies. GeoJournal 2022, 87, 2195–2208. [Google Scholar] [CrossRef]
- Freeman, L.A.; Reide Corbett, D.; Fitzgerald, A.M.; Lemley, D.A.; Quigg, A.; Steppe, C.N. Impacts of urbanization and development on estuarine ecosystems and water quality. Estuaries Coasts 2019, 42, 1821–1838. [Google Scholar] [CrossRef]
- Zhai, T.; Wang, J.; Fang, Y.; Qin, Y.; Huang, L.; Chen, Y. Assessing ecological risks caused by human activities in rapid urbanization coastal areas: Towards an integrated approach to determining key areas of terrestrial-oceanic ecosystems preservation and restoration. Sci. Total Environ. 2020, 708, 135153. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, L.; Yuan, L.; Guo, B.; Zhang, Q.; Huang, H. Urbanization and water quality dynamics and their spatial correlation in coastal margins of mainland China. Ecol. Indic. 2022, 138, 108812. [Google Scholar] [CrossRef]
- Silva, I.A.; Almeida, F.C.G.; Souza, T.C.; Bezerra, K.G.O.; Durval, I.J.B.; Converti, A.; Sarubbo, L.A. Oil spills: Impacts and perspectives of treatment technologies with focus on the use of green surfactants. Environ. Monit. Assess. 2022, 194, 1–29. [Google Scholar] [CrossRef]
- ITOPF—The International Tanker Owners Pollution Federation. Tanker Spill Statistics 2020. Available online: https://www.itopf.org/news/news/tanker-spill-statistics-2021/ (accessed on 10 July 2025).
- Chen, J.; Zhang, W.; Wan, Z.; Li, S.; Huang, T.; Fei, Y. Oil spills from global tankers: Status review and future governance. J. Clean. Prod. 2019, 227, 20–32. [Google Scholar] [CrossRef]
- Little, D.I.; Sheppard, S.R.J.; Hulme, D.A. Perspective on oil spills: What we should have learned about global warming. Ocean Coast Manag. 2021, 202, 105509. [Google Scholar] [CrossRef]
- Yu, L.; Han, M.; He, F. A review of treating oily wastewater. Arab. J. Chem. 2017, 10, S1913–S1922. [Google Scholar] [CrossRef]
- Andrews, N.; Bennett, N.J.; Le Billon, P.; Green, S.J.; Cisneros-Montemayor, A.M.; Amongin, S.; Gray, N.J.G.; Sumaila, U.R. Oil, fisheries and coastal communities: A review of impacts on the environment, livelihoods, space and governance. Energy Res. Soc. Sci. 2021, 75, 102009. [Google Scholar] [CrossRef]
- Câmara, S.F.; Pinto, F.R.; da Silva, F.R.; Soares, M.O.; de Paula, T.M. Socioeconomic vulnerability of communities on the Brazilian coast to the largest oil spill (2019–2020) in tropical oceans. Ocean Coast Manag. 2021, 202, 105506. [Google Scholar] [CrossRef]
- Reddy, C.M.; Quinn, J.G. GC-MS analysis of total petroleum hydrocarbons and polycyclic aromatic hydrocarbons in seawater samples after the North Cape oil spill. Mar. Pollut. Bull. 1999, 38, 126–135. [Google Scholar] [CrossRef]
- Cortes, J.E.; Suspes, A.; Roa, S.; González, C.; Castro, H.E. Total petroleum hydrocarbons by gas chromatography in Colombian waters and soils. Am. J. Environ. Sci. 2012, 8, 396–402. [Google Scholar] [CrossRef]
- Muthukumar, A.; Idayachandiran, G.; Kumaresan, S.; Kumar, T.A.; Balasubramanian, T. Petroleum hydrocarbons (PHC) in sediments of three different ecosystems from Southeast Coast of India. Int. J. Pharm. Biol. Arch. 2013, 4, 543–549. [Google Scholar]
- Okparanma, R.N.; Mouazen, A.M. Determination of total petroleum hydrocarbon (TPH) and polycyclic aromatic hydrocarbon (PAH) in soils: A review of spectroscopic and nonspectroscopic techniques. Appl. Spectrosc. Rev. 2013, 48, 458–486. [Google Scholar] [CrossRef]
- Li, T.; Xu, H.; Zhang, Y.; Zhang, H.; Hu, X.; Sun, Y.; Gu, X.; Luo, J.; Zhou, D.; Gao, B. Treatment technologies for selenium contaminated water: A critical review. Environ. Pollut. 2022, 299, 118858. [Google Scholar] [CrossRef] [PubMed]
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. N. Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Ferreiro, V.; Gómez-Motos, I.; Lombrana, J.I.; de Luis, A.; Villota, N.; Ros, O.; Etxebarria, N. Contaminants of emerging concern removal in an effluent of wastewater treatment plant under biological and continuous mode ultrafiltration treatment. Sustainability 2020, 12, 725. [Google Scholar] [CrossRef]
- Passoni, D.D.J.; Pacheco, M.T.T.; Silveira, L. Raman spectroscopy for the identification of differences in the composition of automobile lubricant oils related to SAE specifications and additives. Instrum. Sci. Technol. 2020, 49, 164–181. [Google Scholar] [CrossRef]
- de Bettignies, P. Optics/instrumentation: Micro-Raman spectroscopy: Theory and application. Phys. Sci. Rev. 2020, 5, 20190027. [Google Scholar] [CrossRef]
- Tsukada, S.; Fujii, Y. Multivariate curve resolution for angle-resolved polarized Raman spectroscopy of ferroelectric crystals. Jpn. J. Appl. Phys. 2020, 59, SKKA03. [Google Scholar] [CrossRef]
- Perraki, M.; Skliros, V.; Mecaj, P.; Vasileiou, E.; Salmas, C.; Papanikolaou, I.; Stamatis, G. Identification of microplastics using µ-Raman spectroscopy in surface and groundwater bodies of SE Attica, Greece. Water 2024, 16, 843. [Google Scholar] [CrossRef]
- Xi, Z.; Nicolas, R.; Wei, J. An edge-deployable multi-modal nano-sensor array coupled with deep learning for real-time, multi-pollutant water quality monitoring. Water 2025, 17, 2065. [Google Scholar] [CrossRef]
- Kataya, G.; Issa, M.; El Charif, Z.; Cornu, D.; Taleb, B.; Bechelany, M.; Hijazi, A. Enhanced copper adsorption with sustainable biochar derived from kitchen waste. Water 2025, 17, 1887. [Google Scholar] [CrossRef]
- Almaviva, S.; Artuso, F.; Giardina, I.; Lai, A.; Pasquo, A. Fast detection of different water contaminants by Raman spectroscopy and surface-enhanced Raman spectroscopy. Sensors 2022, 22, 8338. [Google Scholar] [CrossRef]
- Cao, S.; Zhan, G.; Wei, K.; Zhou, B.; Zhang, H.; Gao, T.; Zhang, L. Raman spectroscopic and microscopic monitoring of on-site and in-situ remediation dynamics in petroleum contaminated soil and groundwater. Water Res. 2023, 233, 119777. [Google Scholar] [CrossRef]
- Jager, M.J.; McClintic, D.P.; Tilotta, D.C. Measurement of petroleum fuel contamination in water by solid-phase microextraction with direct Raman spectroscopic detection. Appl. Spectrosc. 2000, 54, 1617–1623. [Google Scholar] [CrossRef]
- Ahmadjian, M.; Brown, C.W. Feasibility of remote detection of water pollutants and oil slicks by laser-excited Raman spectroscopy. Environ. Sci. Technol. 1973, 7, 452–453. [Google Scholar] [CrossRef]
- Nunes, C.A.; Freitas, M.P.; Pinheiro, A.C.M.; Bastos, S.C. Chemoface: A novel free user-friendly interface for chemometrics. J. Braz. Chem. Soc. 2012, 23, 2003–2010. [Google Scholar] [CrossRef]
- Fontana, M.D.; Mabrouk, K.B.; Kauffmann, Y.H. Spectroscopic Properties of Inorganic and Organometallic Compounds: Techniques, Materials and Applications; Yarwood, J., Douthwaite, R., Duckett, S., Eds.; RSC Publishing: Cambridge, UK, 2013; Volume 44, pp. 40–67. [Google Scholar] [CrossRef]
- Zhang, X.; Walz, P.M.; Kirkwood, W.J.; Hester, K.C.; Ussler, W.; Peltzer, E.T.; Brewer, P.G. Development and deployment of a deep-sea Raman probe for measurement of pore water geochemistry. Deep-Sea Res. I Oceanogr. Res. Pap. 2010, 57, 297–306. [Google Scholar] [CrossRef]
- Bezerra, A.C.M.; Coelho, N.M.A.; Bertelli, F.; Pacheco, M.T.T.; Silveira, L. Temperature-induced chemical changes in lubricant automotive oils evaluated using Raman spectroscopy. Appl. Spectrosc. 2020, 75, 145–155. [Google Scholar] [CrossRef]
- Ministério do Meio Ambiente. CONAMA—Conselho Nacional do Meio-Ambiente. Resolução No. 430 de 13 de maio de 2011. Dispõe sobre as condições e padrões de lançamento de efluentes, complementa e altera a Resolução No. 357, de 17 de março de 2005, do Conselho Nacional do Meio Ambiente—CONAMA. Available online: https://www.legisweb.com.br/legislacao/?id=114770 (accessed on 10 July 2025).
- Matos, M.A. Manual operacional para a regressão linear. Faculdade de Engenharia da Universidade do Porto. 1995. Available online: https://paginas.fe.up.pt/~mam/regressao.pdf (accessed on 20 July 2024).
- Nowak, P.; Kucharska, K.; Kamiński, M. Ecological and health effects of lubricant oils emitted into the environment. Int. J. Environ. Res. Public Health 2019, 16, 3002. [Google Scholar] [CrossRef] [PubMed]
- Deja, A.; Ulewicz, R.; Kyrychenko, Y. Analysis and assessment of environmental threats in maritime transport. Transp. Res. Procedia 2021, 55, 1073–1080. [Google Scholar] [CrossRef]
- Łapko, A.; Strulak-Wójcikiewicz, R.; Landowski, M.; Wieczorek, R. Management of waste collection from yachts and tall ships from the perspective of sustainable water tourism. Sustainability 2018, 11, 121. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Maddela, N.R.; Megharaj, M.; Venkateswarlu, K. Total Petroleum Hydrocarbons; Springer: Cham, Switzerland, 2019; Volume 1, Chapter 3; pp. 57–77. [Google Scholar] [CrossRef]
- Strother, T.; Lowry, S.; Bravo, B. Application Note 52439. Measurement of Dispersed Oil in Water Using an Infrared Analysis Method. 2013. Available online: https://analysis.rs/wp-content/uploads/2022/01/9132023968-min.pdf (accessed on 10 July 2025).
- Stevens, D.; Shi, Q.; Hsu, C.S. Novel analytical technique for petroleum biomarker analysis. Energy Fuels 2013, 27, 167–171. [Google Scholar] [CrossRef]
- Stenstrom, M.K.; Fam, S.; Silverman, G.S. Analytical methods for quantitative and qualitative determination of hydrocarbons and oil and grease in water and wastewater. Environ. Sci. Technol. Lett. 1986, 7, 625–636. [Google Scholar] [CrossRef]
- Adeniji, A.O.; Okoh, O.O.; Okoh, A.I. Analytical methods for the determination of the distribution of total petroleum hydrocarbons in the water and sediment of aquatic systems: A review. J. Chem. 2017, 2017, 5178937. [Google Scholar] [CrossRef]
- Sosnowski, K.; Loh, A.; Zubler, A.V.; Shir, H.; Ha, S.Y.; Yim, U.H.; Yoon, J.Y. Machine learning techniques for chemical and type analysis of ocean oil samples via handheld spectrophotometer device. Biosens. Bioelectron. X 2022, 10, 100128. [Google Scholar] [CrossRef]
- Shankar, R.; Jung, J.H.; Loh, A.; An, J.G.; Ha, S.Y.; Yim, U.H. Environmental significance of lubricant oil: A systematic study of photooxidation and its consequences. Water Res. 2020, 168, 115183. [Google Scholar] [CrossRef]
- Fadzil, M.F.; Yun, P.S.; Razal, A.R.; Chee, P.S.; Suratman, S.; Dagang, N.S.; Tahir, N.M. Oil and grease and total petroleum hydrocarbons in the waters of Ramsar Gazetted mangrove area, Johor. J. Sustain. Sci. Manag. 2017, 12, 30–39. [Google Scholar]
- Krupcík, J.; Oswald, P.; Oktavec, D.; Armstrong, D.W. Calibration of GC-FID and IR spectrometric methods for determination of high boiling petroleum hydrocarbons in environmental samples. Water Air Soil Pollut. 2004, 153, 329–341. [Google Scholar] [CrossRef]
- Kim, M.; Hong, S.H.; Won, J.; Yim, U.H.; Jung, J.H.; Ha, S.Y.; An, J.G.; Joo, C.; Kim, E.; Han, G.M.; et al. Petroleum hydrocarbon contaminations in the intertidal seawater after the Hebei Spirit oil spill—Effect of tidal cycle on the TPH concentrations and the chromatographic characterization of seawater extracts. Water Res. 2013, 47, 758–768. [Google Scholar] [CrossRef]
- Drozdowska, V.; Freda, W.; Baszanowska, E.; Rudź, K.; Darecki, M.; Heldt, J.; Toczek, H. Spectral properties of natural and oil-polluted Baltic seawater—Results of measurements and modelling. Eur. Phys. J. Spec. Top. 2013, 222, 2157–2170. [Google Scholar] [CrossRef]
- Drozdowska, V.; Wrobel, I.; Markuszewski, P.; Makuch, P.; Raczkowska, A.; Kowalczuk, P. Study on organic matter fractions in the surface microlayer in the Baltic Sea by spectrophotometric and spectrofluorometric methods. Ocean Sci. 2017, 13, 633–647. [Google Scholar] [CrossRef]
- Lopes, R.; Miranda, M.L.; Schuette, H.; Gassmann, S.; Zielinski, O. Microfluidic approach for controlled ultraviolet treatment of colored and fluorescent dissolved organic matter. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 239, 118435. [Google Scholar] [CrossRef] [PubMed]
- Baszanowska, E.; Otremba, Z. Fluorometric detection of oil traces in a sea water column. Sensors 2022, 22, 2039. [Google Scholar] [CrossRef] [PubMed]
- Khorshid, Z.B.; Doroodmand, M.M.; Abdollahi, S. UV-Vis. spectrophotometric method for oil and grease determination in water, soil and different mediates based on emulsion. Microchem. J. 2021, 160, 105620. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Maddela, N.R.; Megharaj, M.; Venkateswarlu, K. Total Petroleum Hydrocarbons; Springer: Cham, Switzerland, 2020; Volume 1, Chapter 2; pp. 29–55. [Google Scholar] [CrossRef]
- Yim, U.H.; Khim, J.S.; Kim, M.; Jung, J.H.; Shim, W.J. Environmental impacts and recovery after the Hebei Spirit oil spill in Korea. Arch. Environ. Contam. Toxicol. 2017, 73, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Bills, M.V.; Loh, A.; Sosnowski, K.; Nguyen, B.T.; Ha, S.Y.; Yim, U.H.; Yoon, J.Y. Handheld UV fluorescence spectrophotometer device for the classification and analysis of petroleum oil samples. Biosens. Bioelectron. 2020, 159, 112193. [Google Scholar] [CrossRef]
- Roveri, V.; Guimarães, L.L.; Correia, A.T. Spatial and temporal evaluation of the urban runoff water flowing into recreational areas of Guarujá, São Paulo State, Brazil. Int. J. River Basin Manag. 2022, 20, 93–109. [Google Scholar] [CrossRef]
- Shangguan, M.; Yang, Z.; Shangguan, M.; Lin, Z.; Liao, Z.; Guo, Y.; Liu, C. Remote sensing oil in water with an all-fiber underwater single-photon Raman lidar. Appl. Opt. 2023, 62, 5301–5305. [Google Scholar] [CrossRef] [PubMed]



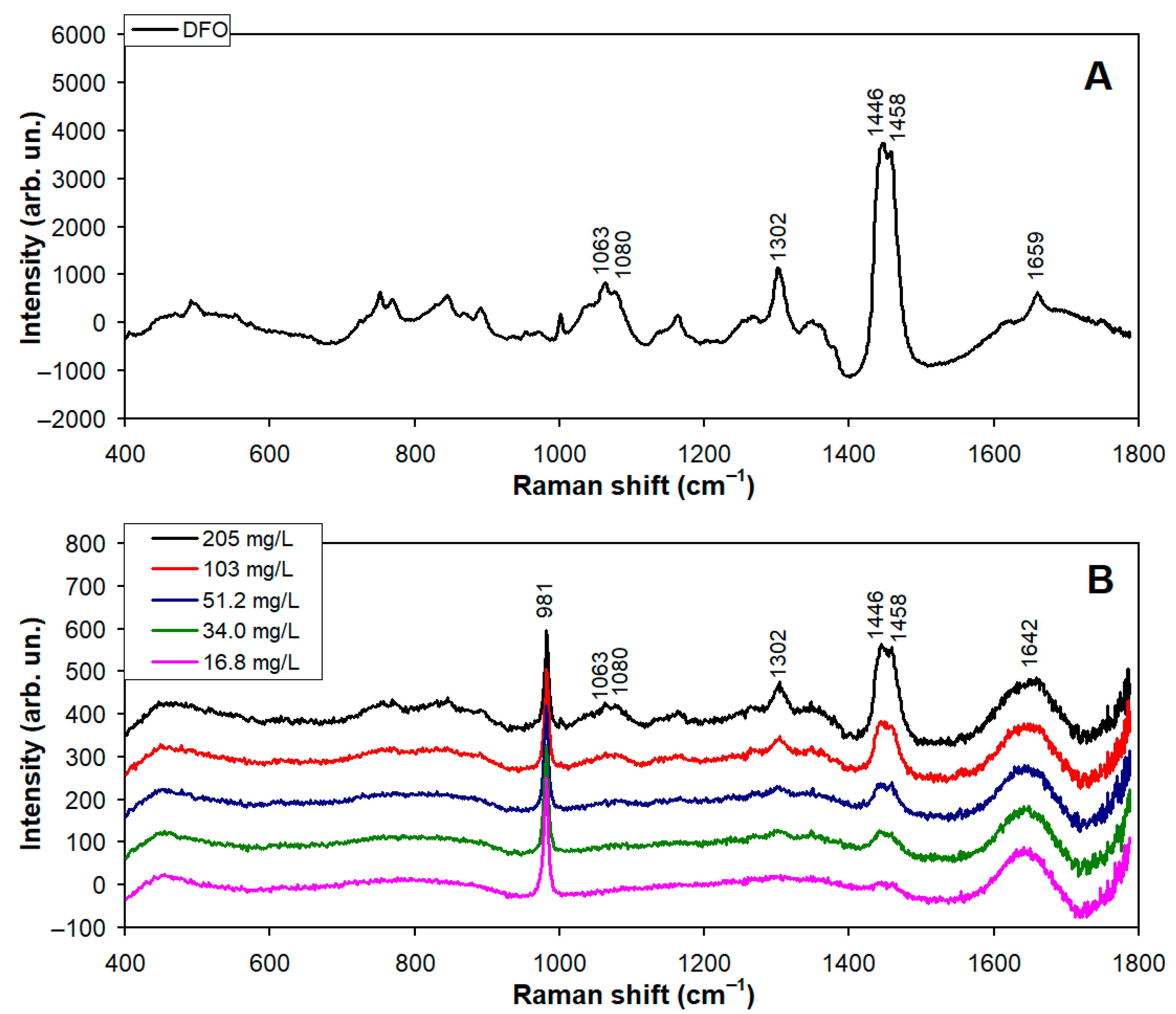
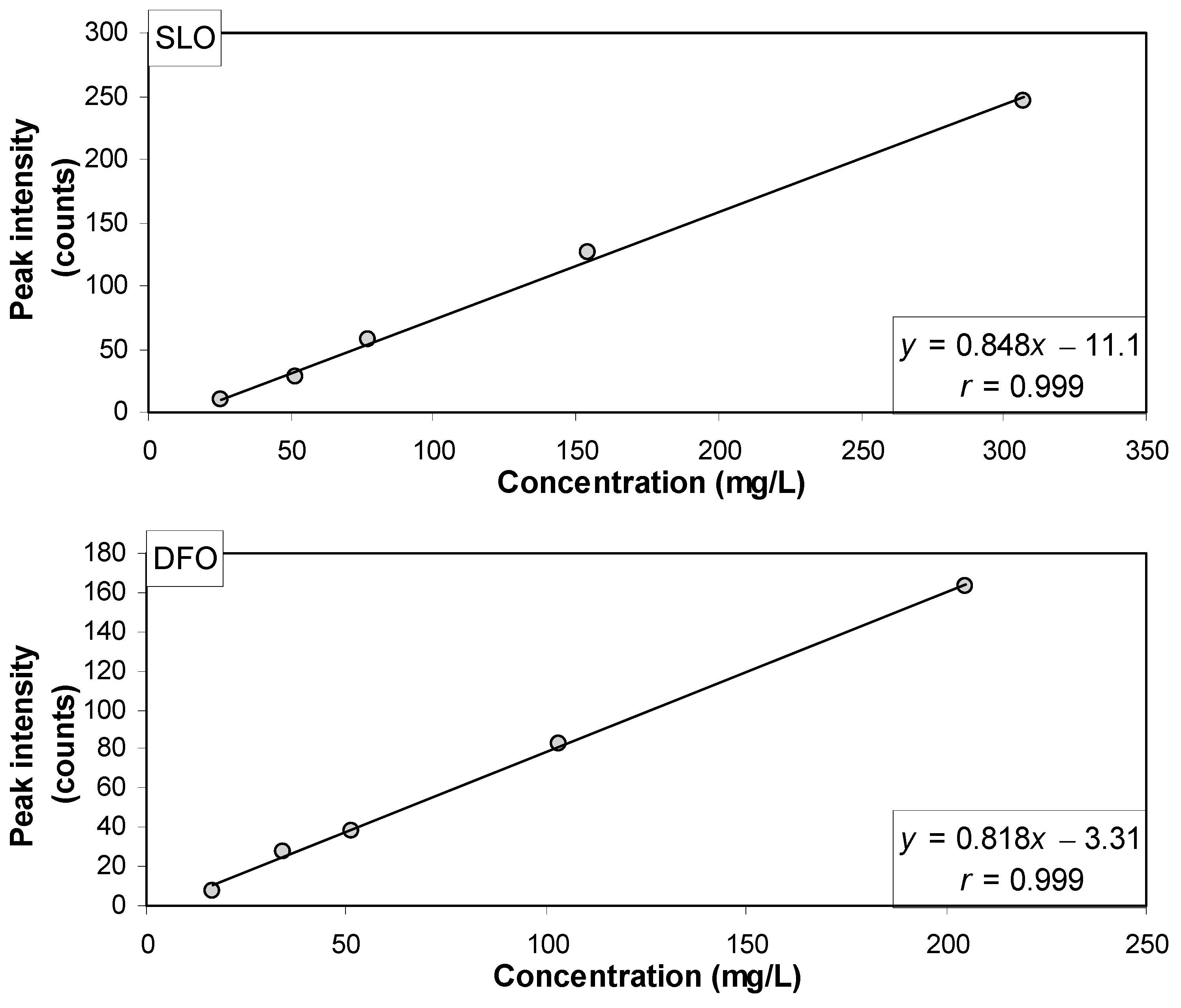

| Type of Oil | Mass Added (mg) | Concentration Estimated by Each Spectral Model (mg/L) | |||
|---|---|---|---|---|---|
| Linear * | Error (mg) | PLSR * | Error (mg) | ||
| SLO | 25.6 | 23.8 | −1.8 | 25.9 | 0.3 |
| 51.4 | 46.6 | −4.8 | 48.8 | −2.6 | |
| 77.2 | 79.9 | 2.7 | 78.8 | 1.6 | |
| 154 | 161 | 7 | 162 | 8 | |
| 307 | 304 | −3 | 294 | −13 | |
| DFO | 16.8 | 12.5 | −4.3 | 14.7 | −2.1 |
| 34.0 | 37.7 | 3.7 | 39.1 | 5.1 | |
| 51.2 | 51.2 | 0.0 | 52.4 | 1.2 | |
| 103 | 105 | 2 | 107 | 4 | |
| 205 | 204 | −1 | 194 | −11 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, G.M.d.; Guimarães, L.L.; Moreira, L.P.; Toma, W.; Roveri, V.; Pacheco, M.T.T.; Silveira, L., Jr. Methodology Based on Raman Spectroscopy for Detection and Quantification of Lubricant and Diesel Oils in Saline Water. Water 2025, 17, 3289. https://doi.org/10.3390/w17223289
Andrade GMd, Guimarães LL, Moreira LP, Toma W, Roveri V, Pacheco MTT, Silveira L Jr. Methodology Based on Raman Spectroscopy for Detection and Quantification of Lubricant and Diesel Oils in Saline Water. Water. 2025; 17(22):3289. https://doi.org/10.3390/w17223289
Chicago/Turabian StyleAndrade, Guilherme Mendes de, Luciana Lopes Guimarães, Letícia Parada Moreira, Walber Toma, Vinicius Roveri, Marcos Tadeu Tavares Pacheco, and Landulfo Silveira, Jr. 2025. "Methodology Based on Raman Spectroscopy for Detection and Quantification of Lubricant and Diesel Oils in Saline Water" Water 17, no. 22: 3289. https://doi.org/10.3390/w17223289
APA StyleAndrade, G. M. d., Guimarães, L. L., Moreira, L. P., Toma, W., Roveri, V., Pacheco, M. T. T., & Silveira, L., Jr. (2025). Methodology Based on Raman Spectroscopy for Detection and Quantification of Lubricant and Diesel Oils in Saline Water. Water, 17(22), 3289. https://doi.org/10.3390/w17223289







