Abstract
The provision of potable water for armed forces at their operational sites necessitates a robust treatment chain to ensure the production of safe drinking water from potentially contaminated local water sources. Relying on single-use water bottles is not considered an eco-friendly option and on-site production may exhibit limited efficiency depending on the water contamination. This study therefore aimed to define a mobile processing chain that could efficiently produce drinking water on-site while offering a multi-barrier level of protection. To evaluate the system, contaminated water was prepared from different water sources and then spiked with various inorganic contaminants (metals, anions: Cl−, F−, I−, NO2−, NO3−, SO42−, CN−), organic contaminants (e.g., pesticides, petroleum hydrocarbons, polycyclic aromatic hydrocarbons, chlorinated solvents), and energetic compound (perchlorate) at levels ranging from 5 to 50 times the standard water quality criteria. A specific treatment process was defined optimized and evaluated at flow rates reaching 500 L/h. This treatment chain includes the following: a sediment filter, a greensand filter, a cation exchange resin, an anion exchange resin, an activated carbon adsorption filter, ultrafiltration, a UV lamp, and a reverse osmosis (RO) unit. This treatment system successfully met all water quality criteria, providing a reliable and effective alternative to an RO-only treatment regime.
1. Introduction
Armed forces in operation require a large supply of quality water for drinking purposes. Current methods for meeting drinking water needs include transporting bottled water from a bottling plant or producing drinking water from local water sources such as rivers or ponds. Although considered a flexible option [], single-use water bottles are significantly more harmful to the environment compared to on-site water production using reverse osmosis (RO) separation technology, specifically a reverse osmosis water purification unit (ROWPU) []. Furthermore, the quality of the water produced in bottling facilities can vary and face microbial contamination issues [,].
When considering on-site water production, the quality of local natural water sources can vary and may be affected by concerning levels of organic, inorganic, and microbial pollutants. Specifically, many cases of illness have been recorded due to microbial contamination during training operations or military missions [,,]. Water sources used by military forces can of course also be contaminated with explosives (RDX, HMX, TNT, and perchlorate) [,] and ammunition residues (Cu, Pb, As, Sb, and Zn) [,]. Heavy vehicle traffic also impacts soil quality and consequently the quality of nearby water sources [,,]. The presence of large troops in an environment not suitable for this purpose can also result in the discharge of contaminated wastewater []. It is also important to consider that military operations can take place in degraded environments resulting from industrial, mining, urban, or agricultural activities. Consequently, the types of contaminants that may be encountered are therefore very diverse (pesticides, fertilizers, metals, combustion residues, petroleum hydrocarbons, solvents, etc.) [,].
Currently, mobile treatment systems using ROWPU have been adopted in several army corps to produce drinking water in operations []. For instance, the use of a treatment process combining the use of RO and ultraviolet (UV) radiation has been described in a pilot-scale drinking water production unit for the armed forces in Germany [].
RO is one of the most effective types of water treatment and is a widely used water purification process globally []. It is typically used to remove salts [], metals [], pesticides [], pharmaceuticals and personal care products [], perfluoroalkyl compounds (PFAS) [], dyes [], energetic compounds [], and microbes [,].
However, this type of technology, despite its excellent removal performance for most contaminants, requires expertise and maintenance to function properly, in addition to being energy demanding [,]. ROWPUs can face relatively rapid membrane clogging issues that can significantly affect the unit efficiency and reliability, leading to increased operational cost and the necessity to supplement the water production with bottled water to fulfill drinking water needs [].
Among available alternative water treatment technologies, activated carbon is one of the first adsorbents that was used in the field of water treatment. This material, which has a large specific surface area for the adsorption of contaminants, continues to be widely used today [] and is also known for its ability to eliminate taste and odor compounds present in drinking water []. Activated carbon has also shown efficacy for the removal of metals [,], hydrocarbons [,], anions and oxyanions [,], dissolved organic carbon (DOC) [], polycyclic aromatic hydrocarbons (PAHs) [], solvents [], pesticides [], dyes [], perfluoroalkyl substances (PFASs) [], algal toxins [], and energetic compounds [,,]. Activated carbon filters can also help remove pharmaceuticals and personal care products (PPSPs) [], and disinfection by-products (DBPs) like trihalomethanes (THMs) or haloacetic acids (HAAs) [].
Cationic exchange resins are used in water treatment facilities to reduce the hardness of the water (elimination of calcium and magnesium ions) and make it possible to remove part of the other metal ions, like iron, manganese, barium, and others [,].
In potable water treatment, anion exchange is the best available technology for removing a range of negatively charged, regulated contaminants, including nitrite (NO2−), nitrate (NO3−), perchlorate (ClO4−), fluoride (F−), and sulfate (SO42−). More specifically, when resins are made of polymer matrix with quaternary ammonium functionality, the quaternary ammonium groups (R-N+(CH3)3+) are positively charged and initially saturated with monovalent chloride anions Cl−. Anions listed previously show greater affinity than Cl− towards quaternary ammonium groups. When those anions are in contact with the resin, an anion swap occurs and Cl− anions are released into the water. In toxic groundwater, trace metals are commonly present as oxyanions, including arsenate (H2AsO4− or HAsO42−), chromate (CrO42−), and uranyl carbonate complexes (UO2(CO3)22− or UO2(CO3)34−) which can also be removed by these resins []. Anionic resins can also be used to retain selenate (SeO42−), borate (BO33−), and molybdate (MoO42−) ions.
Sand filtration technologies aim at removing suspended solids (TSS) and turbidity caused by the presence of clays, silts, sand, organic matter, algae, leaf particles, and similar materials. The turbidity may shield bacteria, preventing disinfection chemicals from attacking and destroying the cells. For instance, manganese-coated greensand filling media acts as a fine filter that can physically trap suspended solids, decreasing TSS concentration and turbidity. The greensand is coated with a layer of manganese dioxide (MnO2) acting as an oxidizing agent. Soluble iron ions Fe2+ react with MnO2 and are oxidized into an insoluble form, Fe3+, that precipitates and is filtered by the media. Soluble manganese ions (Mn2+) found in water, or generated by the iron oxidation reaction, are oxidized in insoluble MnO2 that is then filtered. Moreover, the MnO2 coating is negatively charged under typical water drinking pH and can adsorb positively charged copper soluble ions (Cu2+), trapping them onto the filter media.
UV disinfection units are very efficient in eliminating any microbiological threads in drinking water production systems. As the water passes the UV unit, the biological contaminants are exposed to UV light, which damages the genetic components of the microbes. The exposed microorganisms are unable to reproduce (inactivated), preventing them from causing infection []. The use of UV-LED lamps represents a significant advance compared to conventional UV lamps since they are less energy-consuming, more resistant, and efficient [,].
Finally, ultrafiltration technologies allow the retention of particles with a diameter greater than 0.2 µm. Its primary purpose is to produce water free of particular or colloidal materials to protect the reverse osmosis membranes placed downstream [] or to ensure optimal action of the UV lamps for subsequent treatment. Ultrafiltration can also help remove high molecular weight organic molecules [] and retain microorganisms such as viruses and macromolecules such as proteins.
This study was carried out with the aim of defining a complete water treatment system as a sequence of different technologies capable of eliminating all the contaminants that may be encountered simultaneously in water sources used in the context of military operations. The system is composed of eight distinctive treatment steps, including the following: pre-filtration, sand filtration, anion exchange and cation exchange resins, ultrafiltration, UV disinfection, and RO. This treatment process is designed with a view to both reducing operational costs and increasing treatment efficiency by limiting the load of contaminants reaching the RO treatment. This approach limits rapid clogging issues and extends membranes lifetime. Therefore, the specific arrangement of the treatment steps constitutes a multi-barrier type protection system capable of producing quality drinking water, even if one of the treatment steps becomes ineffective.
2. Materials and Methods
2.1. Description of the Pilot-Scale Drinking Water Production Unit
A pilot unit was designed to test the performance of the complete water treatment process. The initial pilot configuration was composed of eight distinct treatment steps, ensuring the elimination of suspended and dissolved pollution of inorganic, organic, and microbial nature. This setup was able to produce clean water at a flow ranging from 100 to 500 L/h. The final pilot configuration was composed of the same treatment steps but set in a different order. The ultrafiltration component was placed at the sixth treatment step instead of the fourth, as in the initial configuration. This optimized sequence helped reduce the contaminant load on the ultrafiltration membrane, thus decreasing the backwash frequency and improving the water treatment capacity and removal efficiency regarding turbidity.
After modification, the different components were installed in a mobile trailer. This mobile pilot unit was confined in a 2.13 m × 4.26 m × 2.18 m height Ideal Cargo brand trailer (model IDAV714TA2 sportzone, Ideal Cargo Inc., Saint-Valère, QC, Canada). The pilot was powered, from an energy point, by a 7000 watts Honda generator (model EU7000ISCT, Honda Power Equipment, Markham, ON, Canada), providing 120 V and 240 V current, and was able to produce a consumption water flow of 500 L/h. Figure 1 illustrates the different stages for the initial and final configurations of this process. Figures S1 and S2 show the configuration of the initial and final configuration of the treatment chain, respectively. Figure S3 shows a detailed technical scheme of the final treatment chain.
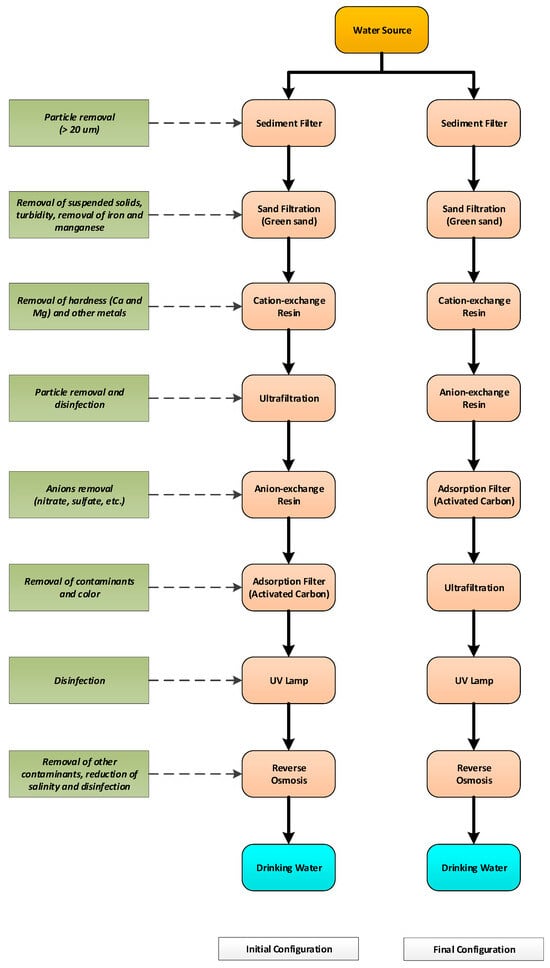
Figure 1.
Pilot scale treatment systems tested to produce drinking water from contaminated water source.
The different treatment stages involved in the initial and final pilot configuration are listed below, respecting the sequence of the initial configuration.
2.1.1. Sediment Filter
The first treatment component of the unit consists of a Pentair propylene sediment pre-filter 50.8 cm tall (Pentair, Suzhou, China). The cartridge used had a porosity of 20 microns, acting as a physical filter. This step easily eliminates larger particles, such as sand and macroscopic algae that can be found in the pumped water. This type of cartridge filter is easily and quickly replaced once the cartridges are clogged.
2.1.2. Sand Filter
The next component of the pilot water production unit is a 25.4 × 111.8 cm sand filter tank (Poly GlassTM Vessels, Pentair, Suzhou, China) with a head controller. This vessel is filled with Manganese Greensand Plus media (0.30–0.35 mm effective diameter, Inversand Company, Clayton, NJ, USA).
The sand filter unit also comes with a reservoir filled with KMnO4 solution that is periodically injected into the sand filter. In contact with KMnO4, Mn2+ ions are oxidized into MnO2, allowing the regeneration of the sand coating.
2.1.3. Cation Exchange Resin
This treatment step consists of a 25.4 × 111.8 cm cationic resin tank with a head controller. It is filled with Aldex C-800 resin (Aldex Chemical Company, Ltd., Granby, QC, Canada), a high capacity, high quality, gel-type (sulfonated styrene/divinylbenzene copolymer) cation resin in its sodium form that can remove calcium, magnesium, and certain other metal cations in hard water via ion exchange. Sulfonic functional groups (R-SO3−) present in gel-type sulfonated resins are negatively charged and initially saturated with sodium ions Na+. Divalent cations such as Mg2+, Ca2+ and other metals (ex: Fe2+, Mn2+, Zn2+) show a greater affinity than monovalent cation Na+ toward the sulfonic group. A cations swap occurs when the water passes through the resin leading to the release of Na+ ions.
This resin has an ion exchange capacity of 1.9 meq/mL and a particle size between 0.30 and 1.19 mm. The tank also comes with a NaCl reservoir for resin regeneration. When the active sites of the resin are saturated with divalent cations, the highly concentrated NaCl solution is injected into the water softener tank in order to replace the divalent cations by Na+.
2.1.4. Ultrafiltration
The following unit consisted in a Kleen Water ultrafiltration device (model 130-KW4520-PES, Pavel Companies Inc., Richmond Hill, ON, Canada) with a 50.2 cm MicroVantage™ MAS0.2-G series membrane (Shelco Filters, Middletown, CT, USA), a general grade polyethersulfone (PES) membrane filter cartridge.
The device comes with a bladder tank for re-pressurization. Backwash can also be manually operated when differential pressure reaches 30 psi in the unit.
2.1.5. Anion Exchange Resin
The next treatment step in the pilot is a 25.4 × 111.8 cm anion exchange resin tank with a head controller. It is filled with Aldex CRA resin (Aldex Chemical Company, Ltd.), a strongly basic, type 1 anion built on a macro-porous, cross-linked acrylic polymer matrix with quaternary ammonium functionality.
This resin has an ion exchange capacity of 0.8 meq/mL and a particle size between 0.30 and 1.19 mm. The tank also comes with a reservoir containing NaCl at a high concentration. When the resin is saturated with anions, the NaCl solution is circulated through the media and the anions bonded to the functional groups are swapped with Cl− anions.
2.1.6. Adsorption Filter
Next is a 25.4 × 137.2 cm adsorption filter tank with a head controller. It is filled with ResinTech AGC-40 D (ResinTech Inc., Camden, NJ, USA), a semi-moist, acid-washed, bituminous-coal-based, premium grade, granular-activated carbon with a size range of 0.40 to 1.68 mm. AGC-40-D offers a large surface area and a balanced distribution of pore sizes. It shows a high physical adsorption capacity for dissolved organic contaminant in general and for large organic molecules in particular.
2.1.7. UV Disinfection
The following component in the water treatment chain is an Arrow 5 Acuva UV-LED disinfection unit (dose delivery: >16 mJ/cm2; flow rate: 5 L/min) (Acuva Technologies Inc., Burnaby, BC, Canada). Acuva’s patented IntenseBeam™ Technology inactivates harmful microorganisms present in water with powerful UV-LED.
The UV disinfected water is stored in blue salvage drums purchased at ULINE (Pleasant Prairie, WI, USA) while waiting to be treated by reverse osmosis.
2.1.8. Reverse Osmosis
The last component of the mobile water drinking production unit for freshwater consists of a 45STDRO reverse osmosis unit (NGMP, East Syracuse, NY, USA), having a maximum operating pressure of 190 psi (1310 kPa) and a maximal flow rate of 170 L/h. A Dupont Filmtec BW30 (6.1 × 101.6 cm), (Dupont, Edina, MN, USA), membrane for reverse osmosis has been used for the tests.
The BW30 membrane is very effective for the removal of cations, anions, and metals. It is able to physically reject compounds larger than the size of water molecules such as most dissolved organic compounds, suspended solids, and microorganisms. The polyamide layer of the membrane also provides a charge-repulsion mechanism able to reject dissolved ions. The negatively charged polyamide repels anions and contributes to the formation of a layer of cations at the surface of the membrane. This positively charged layer, in turn, repels additional cations.
2.2. Test Water Preparation and Feed for the Initial System Configuration
The basic test water used for the treatment trials consists of a mixture of 20% wastewater sampled from the Québec City wastewater treatment plant (effluent after screening and de-sanding pre-treatment) and 80% water from the St. Charles River (Québec City, QC, Canada). This basic water was then artificially contaminated by the addition of inorganic and organic pollutants. The nature and concentration of the contaminants have been selected, with the help of the armed forces, to be representative of contamination typically found in operational sites.
To introduce metallic contaminants (As, B, Ba, Be, Cd, Cr, Cs, Cu, Mg, Mo, Hg, Ni, Pb, Sb, Se, Sr, and Zn), salts of the target substances were added to the water. The addition of sulfate and chloride salts was prioritized. When no solid form was available, the required volume of a ICP (inductively coupled plasma) standard solution 1000 mg/L (PlasmaCal) was added (Sb, As, Hg, Se). Soluble sodium or potassium salts were also added as sources of anionic pollutants (CN−, F−, I−, NO3−, NO2−). These added volumes are small and thus considered negligible when compared to the total test water volume.
For organic contaminants, three main groups of contaminants have been added to the synthetic organic water for assays: volatile organic compounds (1,2-dichlorobenzene, 1,2-dichloroethane, ethylbenzene, tetrachloroethene (PERC), toluene, trichloroethene, xylenes), phenolic compounds (pentachlorophenol (PCP)), and herbicides (atrazine). A stock solution of 50 mL containing all the required contaminants was prepared in methanol in an amber glass bottle. This stock solution is 6500 times more concentrated than the targeted concentration. Before assays, 0.15 mL of stock solution was added for each liter of test water.
For the pilot tests, a 1040 L tote tank was used to store the initial contaminated water to be treated. The inorganic and organic contaminants described above were added to the basic water (20% wastewater + 80% river water). Contaminants added to this water include SbCl3, As2O3, H3BO3, BaSO4, BeSO4.4H2O, CdSO4, K2CrO4, CuSO4·5H2O, NaCN, CaF2, Pb(NO3)2, MnSO4·H2O, (NH4)6Mo7O2·4H2O, HgSO4, NiSO4·6H2O, Ca(NO3)2·4H2O, KNO3, NaNO2, SeO2, SrCl2·6H2O, ZnSO4·7H2O, 1,2-dichlorobenzene, 1-2-dichloroethane, ethylbenzene, tetraethylene, toluene, trichloroethylene, xylenes (o-xylene and m,p-xylene), pentachlorophenol, and atrazine. Inorganic and organic contaminants were added to achieve an initial concentration approximately 10 times higher than drinking water quality standards (DWC).
The water reservoir was agitated by a head stirrer and a shaft with two propellers at 550 rpm at least 30 min before the start of the experiment to assure that all added contaminants were well dissolved and to prevent suspended solids from setting at the bottom of the tote. Throughout the experiment, stirring is reduced to 400 rpm and maintained. For the experiments in the laboratory, a MQ 3-45 Grundfos “smart” pump (1 HP (0.746 kW), 115 V, 83.2 L/min, Groundfos, Brookshire, TX, USA) and its accessories were used to adjust the water flow (ball valve and rotameter (3.78–37.8 L/min)).
2.3. Test Water Preparation and Feed for the Final System Configuration
The water used for these tests came from the Environmental Hydraulics Laboratory at INRS, used to simulate swells, tides, and high-flow currents. The water was in contact with various inert materials (sand, rock, and concrete) and different types of vegetation for around 6 months (from April to September 2023), then confined in an underground concrete reservoir (3000 m3 in total) for around four months. The water was decanted and kept at a temperature of between 10 °C and 18 °C for the duration of the project.
When required, raw water was transferred from the main underground concrete reservoir to two stainless steel storage bulk tanks, each with a capacity of 2200 L, using a submersible sump pump (Myers ME150 2”, cast iron, 208 V, Pentair, Ashland, OH, USA). To avoid the transfer of settled material, the pump was installed between 0.5 and 1.0 m below the water level, using a mobile crane. The chemicals used to artificially contaminate the water were added directly to the 2200 L storage tanks. Contaminants added to this water include NaAsO2, CsCl, HgCl2, PbCl2, NaI, NaF, NaNO3, NaCl, NaClO4, MTBE, CCl4, CHCl3, and benzene. Inorganic and organic contaminants were added to achieve an initial concentration approximately 5 to 10 times higher than drinking water quality standards. For energetic material (perchlorate), the targeted test water concentration was 50 times the drinking water criteria (4 µg/L). A concentrated stock solution (20,000×) of 10 mL containing all the required contaminants in 50% methanol and 50% acetonitrile (v/v) was prepared in an amber glass bottle. Before assays, 0.1 mL of stock solution was added for each liter of the test water. The nature and concentration of the contaminants have been selected, with the help of the armed forces, to be representative of contamination typically found in operational sites.
2.4. Analytical Methods
Table S1 details the methods and instruments used for the analytical measurements of the required parameters, along with their respective detection limits and the drinking water criteria (DWC).
3. Results and Discussion
3.1. Evaluation of the Performance of the Initial System Configuration
Following various preliminary tests carried out at the laboratory scale (cf. Supplementary Material), a complete water treatment system was defined to produce drinking water for National Defense personnel operating in the field. It should be emphasized that the treatment chain to produce drinking water is designed to provide multi-barrier type protection. Thus, if one of the treatment steps becomes inefficient, the removal of the contaminants targeted by this treatment step could be carried out in whole or in part by another step. For example, the elimination of toxic metals and anions will be carried out at the stages of ion exchange, adsorption on activated carbon, and by membrane filtration. Organic compounds are usually well removed by activated carbon adsorption and reverse osmosis. Using the same approach, the elimination of pathogens will be ensured by ultrafiltration, reverse osmosis, and UV radiation treatment steps.
The cation exchange and anion exchange steps have not been tested as such on a laboratory scale. However, these are well-established processes which, once integrated into the water treatment chain, bring significant advantages. Firstly, these processes make it possible to eliminate a significant part of the positively (e.g., metal ions and ammonium) and negatively (e.g., sulfate, chloride, nitrate, nitrite, fluoride, and cyanide) charged pollutants present in water. They also constitute a protective barrier to reduce the clogging of the membranes by deposits of metal precipitates (e.g., metal hydroxides or limestone) or organic matter. Similarly, these are treatment steps representing, with the activated carbon adsorption step, a multi-layered protection if the membranes become defective. Finally, in many cases of freshwater treatment, the proposed process could be operated without the final stage of filtration by reverse osmosis, making it possible to considerably reduce the energy consumption of the drinking water production sector.
The complete treatment chain was first tested during a 400 L test with water containing both inorganic and organic contaminants. The physico-chemical characteristics and contaminant concentrations before and after each treatment stage are shown in Table 1. The overall contaminant removal efficiencies are also shown. The values shown in the table are the averages of four or five analyses performed during treatment.

Table 1.
Water quality parameters monitored during operation of the initial complete water treatment process.
3.1.1. Sediment Filter
The sediment filter allowed for a reduction in turbidity from 54.9 NTU to 46.5 NTU, which corresponds to a 15.3% removal percentage, and suspended solids reduction from 85 mg/L to 72 mg/L, which corresponds to a 15.6% removal percentage (Table 1). The other parameters are only slightly modified by this water pre-treatment step.
3.1.2. Sand Filter
The filtered water is then filtered through a rapid sand filter. During treatment using the greensand filter, there was a considerable reduction in turbidity from 46.5 NTU to 5.4 NTU, which corresponds to a removal percentage of 88.3%, and in suspended solids from 72 mg/L to 5 mg/L, which corresponds to a removal percentage of 93.1%. Some elements such as Fe, Ba, Be, Cd, Cu, Mn, and Mo were also eliminated with high efficiency (more than 85% of removal), especially copper, which had a concentration of 19.6 mg/L before treatment, while the concentration after treatment was 0.11 mg/L, corresponding to a removal efficiency of 99.4%.
Generally, surface water sources have higher turbidity compared to groundwater sources. The turbidity of a surface water source can vary greatly from 1 to 200 NTU (nephelometric turbidity unit) []. According to the legislative authorities and the technologies used, the turbidity limit value allowed to produce drinking water is ≤1 NTU. It is thus set at 1 NTU according to the NATO protocol AMedP-4.9, whereas it is 0.3 NTU for Health Canada [] and 0.2 NTU for WHO [].
3.1.3. Cation Exchange Resin
The water that had undergone the sand filtration stage was then passed through a cationic exchanger unit. After passing through the cation exchange unit, the Ca concentrations were lower than the detection limit, with Ca concentration decreasing from 111 mg/L to less than 0.03 mg/L, corresponding to a removal of >99.9%. For Mg, the concentration decreased from 16 mg/L to 0.1 mg/L and corresponded to a removal efficiency of 99.4%. In this stage, some metals (Cu, Fe, Mn, Mo, Ni, Sr, and Zn) were below the detection limits. However, since the cation exchange unit uses Na+ as a cation to exchange, the concentration of Na increased after this stage (from 158 mg/L to 456 mg/L).
3.1.4. Ultrafiltration
The water then underwent ultrafiltration, which enabled a decrease in the turbidity from 2.45 to 0.36 mg/L, which corresponds to an 85.5% reduction. Other monitored parameters, such as cations and metals, remain almost unchanged after this treatment stage.
3.1.5. Anion Exchange Resin
Since the anion exchange unit uses Cl− as an anion to exchange, an increase in chloride concentration from 96 mg/L to 506 mg/L was observed after passing through the anion exchange resin. In contrast to Cl−, the concentration of the other anions decreased. SO42− concentration decreased from 437 mg/L to 276 mg/L, corresponding to a removal percentage of 37%. Nitrite concentration decreased from 29 mg/L to 0.2 mg/L, nitrate from 466 mg/L to 15.5 mg/L, and cyanide decreased from 0.91 mg/L to 0.08 mg/L. All three anions reached a removal efficiency higher than 90%.
Anionic resins can also help reduce the concentrations of natural organic matter, such as humic and fulvic acids, which are largely responsible for the color of water withdrawn from the natural environment []. The use of anionic resin in combination or not with a carbon adsorption step has been shown to be effective for the removal of various organic micropollutants, such as pharmaceuticals, pesticides, and PFAS []. It is also known that the combined use of cation and anion exchange resins makes it possible to reduce the hardness, which contributes to preserving the good operating condition of the membrane processes placed downstream of the ion exchange units [].
3.1.6. Adsorption Filter
After activated carbon adsorption, an increase in turbidity (from 0.35 to 0.94 mg/L) and nitrite concentration (from 0.20 to 0.85 mg/L) was observed. An increase in turbidity after the adsorption filter shows that the process of backwash needs more time to avoid fine particles to contaminate the treated water. However, the nitrate concentration decreased from 15.5 to 4.2 mg/L, corresponding to a removal of 72.9%, and the cyanide concentration decreased from 0.08 mg/L to below the detection limit (0.005 mg/L), corresponding to a removal percentage higher than 93.6%. Boron concentration was also decreased by 85.3%.
The activated carbon adsorption step was particularly effective for removing organic compounds. The dissolved organic carbon content (DOC), which consists of the sum of all organic compounds present in addition to the organic matter, was decreased by 51% during this stage. Concentration of PCP decreased from 3.63 to 1.31 µg/L after this stage, which corresponds to a reduction of 63.9%. However, an important removal of this compound was performed before the activated carbon adsorption stage, as the initial concentration measured in raw water was 591 µg/L (removal percentage of 99.4%). For atrazine and other volatile compounds, post-treatment concentrations are consistently below the analytical detection limits of 0.5 µg/L for atrazine and 0.4 µg/L for volatile compounds, regardless of the before-treatment concentrations (45.8 µg/L for atrazine, 5.6 µg/L for 1,2-dichlorobenzene, or 106 µg/L for ethylbenzene). The concentrations measured before adsorption filter were lower than the concentrations in raw water for these parameters which suggest that significant removal was performed in the treatment chain before the carbon adsorption step. As an example, total removal percentages before adsorption step were already 44.6% for atrazine, 99.7% for 1,2-dichlorobenzene, and 94.6% for ethylbenzene.
3.1.7. UV Disinfection
In the ultraviolet treatment, the water to be treated passes through germicidal ultraviolet (UV) light configured inside a low-pressure lamp. This step provides an additional step for the elimination of pathogenic germs if the filtration step is deficient. This multi-barrier strategy represents increased security to prevent the spread of waterborne diseases. In the initial configuration of the treatment system, the UV disinfection step did not complete its objective and the treated water did not respect the DWC for Total coliforms, AAHB (Aerobic and Anaerobic Heterotrophic Bacteria), and atypical bacteria concentrations.
3.1.8. Reverse Osmosis
After the RO step, 96% of the residual conductivity was removed as well as 99.9% of the total dissolved solids, decreasing from 1.95 mS/cm to 0.08 mS/cm for conductivity and from 973 mg/L to <1 mg/L for total dissolved solids (Table 1). For the various monitored parameters, the concentrations increased in the concentrate when they decrease in the permeate. For the anions, removal percentage higher than 97.5% were achieved for Cl−, CN−, F−, NO3−, NO2− and SO42−. Concentrations decreased from 458 mg/L to 1.90 mg/L for Cl−, from 2.88 mg/L to <0.003 mg/L for NO2−, and from 231 mg/L to 1.15 mg/L for SO42−. For NO3−, concentrations decreased from 5.19 mg/L to 0.75 mg/L and from 0.20 mg/L to <0.002 mg/L for F−. Reverse osmosis is therefore very effective in removing anions; the previous steps of the treatment chain did not allow them to reach such low concentrations. For Ca, Mg, and Na, the removal percentages were ≥99.9%. For metals still present in water before RO, concentrations were also below the detection limits after this stage. For DOC, a removal percentage of 62.3% was achieved during this stage with a final concentration in the permeate of 2.30 mg/L. For PCP, concentration decreased from 1.23 µg/L to 0.98 µg/L with a global removing percentage of 99.8%. Other monitored parameters were already lower than analytical detection limits before the RO stage.
Table 1 presents the results of the global chain of the drinking water production unit for freshwater. The overall process allows efficient removals of the conductivity, COD, and TSS. The initial turbidity was also decreased to 0.82 mg/L, but it still does not respect the DWC (≤0.3 mg/L). The TDS were entirely removed (>99.9%) after the RO stage. Moreover, the final pH (7.87) was within the recommended range. A global removal percentage of 84.7% was achieved for DOC during the water treatment process.
Anions such as fluoride, chloride, nitrite, nitrate, sulfate, and cyanide were efficiently removed during the water treatment process. They respect the DWC concentrations. After the whole process, all cations were efficiently decreased below the DWC with high removal efficiencies (all >97.1%, except for aluminum with 87.7%). The organic contaminants were also adequately withdrawn (>99.4%) from the contaminated water which allows the final concentrations of the produced water to respect the DWC.
The designed pilot-scale water treatment unit allows the efficient removal of inorganic and organic contaminants from freshwater (20% wastewater taken from the Québec City wastewater combined with 80% drinking water and contaminated with inorganic and organic contaminants). However, the system does not meet the DWC for turbidity. It would therefore be advisable to place the ultrafiltration stage upstream of the UV lamp disinfection stage and downstream of the carbon adsorption stage to ensure the compliance of turbidity with drinking water standard.
3.2. Evaluation of the Performance of the Final System Configuration
Following these trials, the treatment process was modified, as shown in Figure 1. The aim of this process modification was to ensure that the treatment process met drinking water quality criteria for turbidity (≤0.3 NTU). In addition, this research also aimed to evaluate the performance of the process for the removal of other pollutants (iodide, perchlorate, cesium, benzene, and chloroform). This new process was tested by treating 20,000 L of water. The parameters monitored during treatment are shown in Table 2, together with overall contaminant removal performance. The values shown in the table are generally the averages of five analyses performed after processing 4000, 8000, 12,000, 16,000, and 20,000 L.

Table 2.
Water quality parameters monitored during operation of the modified water treatment process.
The results obtained show that the final configuration of the water treatment process satisfies the turbidity criterion, as well as the other quality criteria for the contaminants under study. The DEL-UV disinfection unit achieved its objectives, and the water respected the DWC for bacteriological parameters AAHB, total coliforms, and E. coli.
The new contaminants studied, i.e., iodide, perchlorate, cesium, benzene, and chloroform, are also well eliminated by this complete treatment process, with removal yields of 99.8%, >99.8%, >99.3%, >94.3%, and >99.8%, respectively.
Of note is the excellent removal efficiency of perchlorate (>99.8%), which is a toxic explosion by-product that is harmful to the environment [,]. The use of adsorption, ion exchange, and filtration steps constitutes a highly effective multi-layer process for removing this pollutant.
Finally, the authors recommend performing additional trials in real operating conditions while armed forced are in operations. The future tests should focus on evaluating the scalability and resilience of the system when facing fluctuating field conditions. Among other parameters, data regarding long-term media exhaustion and regeneration frequency should be collected.
The performance of the treatment process also relies on its economic efficiency. Therefore, the authors recommend passing through a complete technico-economic study before any scaling up of the process. The study will provide estimated costs regarding initial capital costs (CAPEX) necessary to construct a full-size mobile unit, and operational costs (OPEX) when the unit is used in the field. The study should also consider the economic feasibility of the unit relative to actual water purifications methods used by armed forces.
4. Conclusions
A treatment chain is proposed to produce drinking water for the armed forces operating in the field. The treatment line that was mounted on a mobile unit includes the following successive treatment steps, namely a sediment filter (20 µm porosity), a sand filter (greensand: sand covered with manganese oxide) (for the removal of suspended solids, turbidity, iron, sulfides, and odors), a cation exchange resin (for the reduction in hardness of water and other metals), an anion exchange resin (for the removal of anions such as nitrates, sulfates, and cyanide), an activated carbon adsorption filter (for the removal of organic contaminants), an ultrafiltration (MAS0.2-G series membrane) unit (for the reduction in turbidity and disinfection), a UV lamp (disinfection stage) and, finally, a reverse osmosis (Filmtech BW30 membrane) stage (for the elimination of all contaminants and the reduction in salinity and disinfection). This treatment process ensures the production of good quality water from water sources heavily polluted with organic and inorganic contaminants. The arrangement of the treatment steps also constitutes a multi-barrier type protection capable of operating, even if one of the treatment steps becomes ineffective.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/w17223246/s1, Figure S1: Photographs of the different components of the initial configuration of the pilot water production unit; Figure S2: Photographs of the different components of the final configuration of the pilot water production unit; Figure S3: Detailed technical scheme of the final treatment chain; Table S1: Physico-chemical parameters analyzed in test waters (References [,,] are cited in the Supplementary Materials); Table S2: Solid chemicals for preparation of the 2200 liters of challenge water in Res 1 or Res 2; Table S3: Liquid chemicals for preparation of the 2200 liters of challenge water in Res 3; File S1: Test water preparation protocol, final system configuration; File S2: Simplified operator guide, final system configuration.
Author Contributions
J.-F.B.: conceptualization, Supervision, writing—original draft. V.T.: methodology, investigation, formal analysis, writing—review and editing. G.R.: methodology, investigation, writing—review and editing. J.D.: methodology, investigation, writing—review and editing. R.L.: methodology, investigation, writing—review and editing. P.A.: methodology, investigation, writing—review and editing. L.H.T.: supervision, writing—review and editing. R.M.: funding acquisition, project administration, supervision, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Canadian National Defence (Project Advanced Water Supply System—AWSS).
Data Availability Statement
The data are available from the authors upon reasonable request due to legal reasons.
Acknowledgments
We thank M. David Brochu from Defence Research and Development Canada—Valcartier Research Centre and Janick Lalonde from Canada Department of National Defence for administrative and technical support.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
The following abbreviations are used in this manuscript:
| ALARA | As low as reasonably achievable |
| AAHB | Aerobic and anaerobic heterotrophic bacteria |
| AO | Aesthetic objective |
| BTEX | Benzene, toluene, ethylbenzene and xylenes |
| BW30 | Brackish water 30 (a type of reverse osmosis membrane) |
| CFU | Colony-forming unit |
| COD | Chemical oxygen demand |
| DBP | Disinfection by-product |
| DOC | Dissolved organic carbon |
| DWC | Drinking water criteria |
| HAAs | Haloacetic acids |
| HMX | 1,3,5,7-tetranitro-1,3,5,7-tetrazocane |
| HP | Horsepower |
| ICP | Inductively couple plasma |
| INRS | Institut national de la recherche scientifique |
| MTBE | Methyl tert-butyl ether |
| NATO | North Atlantic treaty organization |
| NTU | Nephelometric turbidity unit |
| OG | Operational guidance value |
| PAH | Polycyclic aromatic hydrocarbons |
| PCP | Pentachlorophenol |
| PERC | Tetrachloroethylene |
| PES | Polyethersulfone |
| PFAS | Perfluoroalkyl substances |
| PPCP | Pharmaceuticals and personal care products |
| RDX | 1,3,5-trinitro-1,3,5-triazinane |
| RO | Reverse osmosis |
| ROWPU | Reverse osmosis water purification unit |
| RPM | Revolutions per minute |
| TDS | Total dissolved solids |
| THM | Trihalomethanes |
| TIC | Total inorganic carbon |
| TNT | Trinitrotoluene |
| TOC | Total organic carbon |
| TSS | Total suspended solids |
| UF | Ultrafiltration |
| UV | Ultraviolet |
| VOC | Volatile organic carbon |
| WHO | World health organization |
References
- Katiyar, V.; Tripathi, N.; Patwa, R.; Kotecha, P. Environment friendly packaging plastics. In Polymers for Packaging Applications; Apple Academic Press: Oakville, ON, Canada, 2014; Volume 115. [Google Scholar]
- Boone, C.; Chini, C.M. Comparative life cycle assessment of remote potable water supply for the Department of Defense. Sci. Total Environ. 2024, 949, 174732. [Google Scholar] [CrossRef] [PubMed]
- Gautam, B. Microbiological quality assessment (including antibiogram and threat assessment) of bottled water. Food Sci. Nutr. 2021, 9, 1980–1988. [Google Scholar] [CrossRef] [PubMed]
- Rhodan, M. Bottled Water Company Issues Recall over Possible E. coli Contamination, TIME. 2015. Available online: https://time.com/3931649/niagara-bottled-water-e-coli/ (accessed on 1 July 2024).
- Kasper, M.R.; Lescano, A.G.; Lucas, C.; Gilles, D.; Biese, B.J.; Stolovitz, G.; Reaves, E.J. Diarrhea outbreak during U.S. military training in El Salvador. PLoS ONE 2012, 7, e40404. [Google Scholar] [CrossRef] [PubMed]
- Putnam, S.D.; Sanders, J.W.; Frenck, R.W.; Monteville, M.; Riddle, M.S.; Rockabrand, D.M.; Sharp, T.W.; Frankart, C.; Tribble, D.R. Self-reported description of diarrhea among military populations in operations Iraqi Freedom and Enduring Freedom. J. Travel Med. 2006, 13, 92–99. [Google Scholar] [CrossRef]
- Riddle, M.S.; Rockabrand, D.M.; Schlett, C.; Monteville, M.R.; Frenck, R.W.; Romine, M.; Ahmed, S.F.; Sanders, J.W. A prospective study of acute diarrhea in a cohort of United States military personnel on deployment to the Multinational Force and Observers, Sinai, Egypt. Am. J. Trop. Med. Hyg. 2011, 84, 59–64. [Google Scholar] [CrossRef]
- Cao, F.; Jaunat, J.; Sturchio, N.; Cancès, B.; Morvana, X.; Devos, A.; Barbin, V.; Ollivier, P. Worldwide occurrence and origin of perchlorate ion in waters: A review. Sci. Total Environ. 2019, 661, 737–749. [Google Scholar] [CrossRef]
- Clausen, J.; Robb, J.; Curry, D.; Korte, N. A case study of contaminants on military ranges: Camp Edwards, Massachusetts, USA. Environ. Pollut. 2004, 129, 13–21. [Google Scholar] [CrossRef]
- Bausinger, T.; Bonnaire, E.; Preuß, J. Exposure assessment of a burning ground for chemical ammunition on the Great War battlefields of Verdun. Sci. Total Environ. 2007, 382, 259–271. [Google Scholar] [CrossRef]
- Lafond, S.; Blais, J.F.; Mercier, G.; Martel, R. Counter-current acid leaching process for removal of Cu, Pb, Sb and Zn from shooting range soil. Environ. Technol. 2013, 34, 2377–2387. [Google Scholar] [CrossRef]
- Bhat, S.; Jacobs, J.M.; Hatfield, K.; Prenger, J. Relationships between stream water chemistry and military land use in forested watersheds in Fort Benning, Georgia. Ecol. Indic. 2006, 6, 458–466. [Google Scholar] [CrossRef]
- Iverson, R.M.; Hinckley, B.S.; Webb, R.M.; Hallet, B. Physical effects of vehicular disturbance on arid landscapes. Science 1981, 212, 915–917. [Google Scholar] [CrossRef]
- Whitecotton, R.C.A.; David, M.B.; Darmody, R.G.; Price, D.L. Impact of foot traffic from military training on soil and vegetation properties. Environ. Manag. 2000, 26, 697–706. [Google Scholar] [CrossRef]
- Dong, S.; Page, M.A.; Hur, A.; Hur, K.; Bokenkamp, K.V.; Wagner, E.D.; Plewa, M.J.; Massalha, N. Comparison of estrogenic, spectroscopic, and toxicological analyses of pilot-scale water, wastewaters, and processed wastewaters at select military installations. Environ. Sci. Technol. 2021, 55, 13103–13112. [Google Scholar] [CrossRef] [PubMed]
- Quist, M.C.; Fay, P.A.; Guy, C.S.; Knapp, A.K.; Rubenstein, B.N. Military training effects on terrestrial and aquatic communities on a grassland military installation. Ecol. Appl. 2003, 13, 432–442. [Google Scholar] [CrossRef]
- Stellmann, J.M.; Stellmann, S.D.; Christian, R.; Weber, T.; Tomasallo, C. The extent and patterns of usage of Agent Orange and other herbicides in Vietnam. Nature 2003, 422, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Heberer, T.; Feldmann, D.; Reddersen, K.; Altmann, H.J.; Zimmermann, T. Production of drinking water from highly contaminated surface waters: Removal of organic. inorganic. and microbial contaminants applying mobile membrane filtration units. Acta Hydrochim. Hydrobiol. 2002, 30, 24–33. [Google Scholar] [CrossRef]
- Sharma, S.; Bhattacharya, A. Drinking water contamination and treatment techniques. Appl. Water Sci. 2017, 7, 1043–1067. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Ghosh, P. Nanofiltration and reverse osmosis membranes: Theory and application in separation of electrolytes. Rev. Chem. Eng. 2004, 20, 111–173. [Google Scholar] [CrossRef]
- Pawlak, Z.; Zak, S.; Zablocki, L. Removal of hazardous metals from groundwater by reverse osmosis. Pol. J. Environ. Stud. 2005, 15, 579–583. [Google Scholar]
- Bhattacharya, A. Remediation of pesticides polluted water through membranes. Sep. Purif. Rev. 2006, 35, 1–38. [Google Scholar] [CrossRef]
- Kim, S.; Chu, K.H.; Al-Hamadani, Y.A.J.; Park, C.M.; Jang, M.; Kim, D.H.; Yu, M.; Heo, M.; Yoon, Y. Removal of contaminants of emerging concern by membranes in water and wastewater: A review. Chem. Eng. J. 2018, 335, 896–914. [Google Scholar] [CrossRef]
- Banks, D.; Jun, M.B.; Heo, J.; Her, N.; Park, C.M.; Yoon, Y. Selected advanced water treatment technologies for perfluoroalkyl and polyfluoroalkyl substances: A review. Sep. Purif. Technol. 2020, 231, 115929. [Google Scholar] [CrossRef]
- Nataraj, S.K.; Hosamani, K.M.; Aminabhavi, T.M. Nanofiltration and reverse osmosis thin film composite membrane module for the removal of dye and salts from the simulated mixtures. Desalination 2009, 249, 12–17. [Google Scholar] [CrossRef]
- Ye, L.; You, H.; Su, H. Water treatment technologies for perchlorate: A review. Desalination 2012, 298, 1–12. [Google Scholar] [CrossRef]
- Chen, C.; Guo, L.; Yang, Y.; Oguma, K.; Hou, L. Comparative effectiveness of membrane technologies and disinfection methods for virus elimination in water: A review. Sci. Total Environ. 2021, 801, 149678. [Google Scholar] [CrossRef]
- Park, S.K.; Hu, J.Y. Assessment of the extent of bacterial growth in reverse osmosis system for improving water quality. J. Environ. Sci. Health Part A 2010, 45, 968–977. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, W.; Zhang, W.; Wang, C.; Wang, P. Life cycle assessment of water supply alternatives in water-receiving areas of the south-to-north water diversion project in China. Water Res. 2016, 89, 9–19. [Google Scholar] [CrossRef]
- Tarpani, R.R.Z.; Lapolli, F.R.; Recio, M.A.L.; Gallego-Schmid, A. Comparative life cycle assessment of three alternative techniques for increasing potable water supply in cities in the global south. J. Clean. Prod. 2021, 290, 125871. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Huang, Y.; Nie, Z.; Murray, A.; Li, Y.; Woods-Chabane, G.; Hofmann, R. Experimental validation of a test to estimate the remaining adsorption capacity of granular activated carbon for taste and odour compounds. Environ. Sci. Water Res. Technol. 2019, 5, 609–617. [Google Scholar] [CrossRef]
- Altowayti, W.A.H.; Othman, N.; Shahir, S.; Alshalif, A.F.; Al-Gheethi, A.A.; Al-Towayti, F.A.H.; Saleh, Z.M.; Haris, S.A. Removal of arsenic from wastewater by using different technologies and adsorbents: A review. Int. J. Environ. Sci. Technol. 2022, 19, 9243–9266. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.H.; Babel, S. Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci. Total Environ. 2006, 366, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Amakiri, K.T.; Canon, A.R.; Molinari, M.; Angelis-Dimakis, A. Review of oilfield produced water treatment technologies. Chemosphere 2022, 298, 134064. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.; Sakhiya, A.K.; Anand, A.; Pant, K.K.; Kaushal, P. A state-of-the-art review of various adsorption media employed for the removal of toxic polycyclic aromatic hydrocarbons (PAHs): An approach towards a cleaner environment. J. Water Process Eng. 2022, 47, 102674. [Google Scholar] [CrossRef]
- Bhagyaraj, S.; Al-Ghouti, M.A.; Kasak, P.; Krupa, I. An updated review on boron removal from water through adsorption processes. Emergent Mater. 2021, 4, 1167–1186. [Google Scholar] [CrossRef]
- Jadhav, S.V.; Bringas, E.; Yadav, G.D.; Rathod, V.K.; Ortiz, I.; Marathe, K.V. Arsenic and fluoride contaminated groundwaters: A review of current technologies for contaminants removal. J. Environ. Manag. 2015, 162, 306–325. [Google Scholar] [CrossRef]
- Humbert, H.; Gallard, H.; Suty, H.; Croué, J.P. Natural organic matter and pesticides removal using a combination of ion exchange resin and powdered activated carbon (PAC). Water Res. 2008, 42, 1635–1643. [Google Scholar] [CrossRef]
- Yu, B.; Yuan, Z.; Yu, Z.; Xue-Song, F. BTEX in the environment: An update on sources, fate, distribution, pretreatment, analysis, and removal techniques. Chem. Eng. J. 2022, 435, 134825. [Google Scholar] [CrossRef]
- Gul, A.; Ma’amor, A.; Khaligh, N.G.; Julkapli, N.M. Recent advancements in the applications of activated carbon for the heavy metals and dyes removal. Chem. Eng. Res. Des. 2022, 186, 276–299. [Google Scholar] [CrossRef]
- Wanninayake, D.M. Comparison of currently available PFAS remediation technologies in water: A review. J. Environ. Manag. 2021, 283, 111977. [Google Scholar] [CrossRef]
- El Bouaidi, W.; Enaime, G.; Loudiki, M.; Yaacoubi, A.; Douma, M.; Ounas, A.; Lubken, M. Adsorbents used for microcystin removal from water sources: Current knowledge and future prospects. Processes 2022, 10, 1235. [Google Scholar] [CrossRef]
- Hoslett, J.; Massara, T.M.; Malamis, S.; Ahmad, D.; van den Boogaert, I.; Katsou, E.; Ahmad, B.; Ghazal, H.; Simons, S.; Wrobel, L.; et al. Surface water filtration using granular media and membranes: A review. Sci. Total Environ. 2018, 639, 1268–1282. [Google Scholar] [CrossRef] [PubMed]
- Fuller, M.E.; Farquharson, E.M.; Hedman, P.C.; Chiu, P. Removal of munition constituents in stormwater runoff: Screening of native and cationized cellulosic sorbents for removal of insensitive munition constituents NTO, DNAN, and NQ, and legacy munition constituents HMX, RDX, TNT, and perchlorate. J. Hazard. Mater. 2022, 424, 127335. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Sorial, G.A. Treatment of perchlorate in drinking water: A critical review. Sep. Purif. Technol. 2009, 69, 7–21. [Google Scholar] [CrossRef]
- Paredes, L.; Fernandez-Fontaina, E.; Lema, J.M.; Omil, F.; Carballa, M. Understanding the fate of organic micropollutants in sand and granular activated carbon biofiltration systems. Sci. Total Environ. 2016, 551–552, 640–648. [Google Scholar] [CrossRef]
- Srivastav, A.L.; Patel, N.; Chaudhary, V.K. Disinfection by-products in drinking water: Occurrence. toxicity and abatement. Environ. Pollut. 2020, 267, 115724. [Google Scholar] [CrossRef]
- Sincero, A.P.; Sincero, G.A. Physical-Chemical Treatment of Water and Wastewater; CRC Press: Boca Raton, FL, USA, 2002; 856p. [Google Scholar] [CrossRef]
- Korak, J.A.; Mungan, A.L.; Watts, L.T. Critical review of waste brine management strategies for drinking water treatment using strong base ion exchange. J. Hazard. Mater. 2023, 441, 129473. [Google Scholar] [CrossRef]
- Hijnen, W.A.M.; Beerendonk, E.F.; Medema, G.J. Inactivation credit of UV radiation for viruses. bacteria and protozoan (oo)cysts in water: A review. Water Res. 2006, 40, 3–22. [Google Scholar] [CrossRef]
- Jamaly, S.; Darwish, N.N.; Amed, I.; Hasan, S.W. A short review on reverse osmosis pretreatment technologies. Desalination 2014, 354, 30–38. [Google Scholar] [CrossRef]
- Health Canada, Guidelines for Canadian Drinking Water Quality—Summary Table, Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, ON, Canada. 2022. Available online: https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/ewh-semt/alt_formats/pdf/pubs/water-eau/sum_guide-res_recom/summary-tables-sept-2022-eng.pdf (accessed on 1 July 2024).
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; World Health Organization: Geneva, Switzerland, 2017; 541p, ISBN 978-92-4-154995-0. Available online: https://www.who.int/publications/i/item/9789241549950 (accessed on 1 July 2024).
- Levchuk, I.; Marquez, J.J.R.; Sillanpaa, M. Removal of natural organic matter (NOM) from water by ion exchange—A review. Chemosphere 2018, 192, 90–104. [Google Scholar] [CrossRef]
- Hu, J.; Martin, A.; Shang, R.; Siegers, W.; Cornelissen, E.; Hejman, B.; Rietveld, L. Anionic exchange for NOM removal and the effects on micropollutant adsorption competition on activated carbon. Sep. Purif. Technol. 2014, 129, 25–31. [Google Scholar] [CrossRef]
- Apell, J.N.; Boyer, T.H. Combined ion exchange treatment for removal of dissolved organic matter and hardness. Water Res. 2010, 44, 2419–2430. [Google Scholar] [CrossRef]
- Kumar, K.S.; Kavitha, S.; Parameswari, K.; Sakunthala, A. Environmental occurrence, toxicity and remediation of perchlorate—A review. Chemosphere 2023, 311, 137017. [Google Scholar] [CrossRef]
- Hu, Z.; Jia, Y.; Wu, Y.; Zhang, Y. Occurrence and removal technologies of perchlorate in water: A systematic review and bibliometric analysis. Chemosphere 2024, 364, 143119. [Google Scholar] [CrossRef]
- CEAEQ. Centre D’expertise en Analyse Environnementale du Québec, Méthode D’analyse, Ministère de l’Environnement et Lutte Contre les Changements Climatiques, Gouvernement du Québec, Québec, QC, Canada. 2023. Available online: https://www.ceaeq.gouv.qc.ca/methodes/methode_numer.htm (accessed on 1 July 2024).
- US EPA. United States Environmental Protection, Method EPA 6010B, Inductively Coupled Plasma Atomic Emission Spectrometry. 1996. Available online: https://archive.epa.gov/epawaste/hazard/testmethods/web/html/6_series.html (accessed on 1 July 2024).
- US EPA. United States Environmental Protection, Method EPA 8330B, Nitroaromatics, Nitramines, and Nitrate Esters by High Performance Liquid Chromatography (HPLC). 2006. Available online: https://www.epa.gov/esam/epa-method-8330b-sw-846-nitroaromatics-nitramines-and-nitrate-esters-high-performance-liquid (accessed on 1 July 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).