Abstract
This study determined the effectiveness of ozone and Fenton’s reagents, and the ozone delivery system, for the degradation of the persistent organic compound p-chlorocresol (4-chloro-3-methylphenol) in tannery wastewater. Laboratory trials demonstrated that removal of p-chlorocresol from wastewater at pH 4.1 using ozone was at least 70% within 2–3 h. Fenton’s reagent did improve the p-chlorocresol removal, but only by 10%. When we normalize the % removal data to account for reaction time and ozone dose, the degradation rates of p-chlorocresol in the ozone only and ozone plus Fenton’s treatments were similar, ~76 mg p-chlorocresol g− O3 h−1. In the pilot trials, the addition of ozone via a venturi delivery system with improved circulation achieved up to 46% removal of the p-chlorocresol in wastewater at pH 4.1 within 24 h; this equated to a removal rate of 3.8 mg p-chlorocresol g− O3 h−1. The large difference in removal rate between the pilot and lab trials was attributed to higher organic load in the wastewater. The addition of Fenton’s reagent increased the removal of the p-chlorocresol to 66% in the venturi injection trial and almost doubled the removal rate (6.7 mg chlorocresol g− O3 h−1). The addition of ozone via a direct blower system was not as effective as the venturi system because of the large loss of ozone from the tank reactor.
1. Introduction
The tannery industry generates wastewater containing ammonium, sulfides, and persistent organic compounds (POCs) including phenolic compounds (i.e., cresols) [,,]. Phenolic compounds such as p-chlorocresol (4-chloro-3-methylphenol) are commonly used in tanneries as an antimicrobial agent. However, p-chlorocresol is considered a priority pollutant by the US Environmental Protection Agency (EPA) due to its toxicity to aquatic life [,].
In Australia, during tannery wastewater treatment, dissolved air flotation (DAF) is used to remove solids, which are disposed of in landfills while the clarified wastewater is applied to land. However, both the solid and liquid streams from tannery DAF systems contain p-chlorocresol. If p-chlorocresol can be removed from wastewater prior to DAF treatment, then the cost of landfill disposal would decrease significantly, and the potential contamination of water bodies from land application of liquid waste disposal could be alleviated.
Many of the organic compounds applied in leather tanning processes resist conventional chemical and biological wastewater treatment processes. For this reason, other methods need to be applied as an alternative to biological and classical physico-chemical processes [,,,]. An effective means of degrading p-chlorocresol into more benign compounds is the use of advanced oxidation procedures (AOPs) such as ozonation, ultraviolet (UV) oxidation, the Fenton process (addition of hydrogen peroxide—H2O2 and iron—Fe2+), and combined processes [,,,,]. The high turbidity of tannery wastewater may exclude UV treatment, and Fenton’s reagents can be a major cost barrier, particularly H2O2 [,,].
Another approach for POC degradation is to use ozone (O3) [,,], a powerful oxidizing agent that can be produced onsite relatively cheaply. Ozone is known to degrade phenolic compounds [], but studies to date have mostly focused on the degradation of pure p-chlorocresol rather than p-chlorocresol in an industrial wastewater environment. Wastewater has a much higher organic load than pure compound; therefore, the efficiency of POC breakdown by O3 is expected to be much lower. In such case, it may be possible to hybridize the oxidation process, for instance by combining O3 oxidation and the Fenton reaction [,]. The Fenton reaction is essentially an oxidation reaction in which H2O2 forms highly reactive hydroxyl radicals (•OH) in the presence of Fe2+ [,]. Again, the combination of O3 oxidation and the Fenton reaction has only been applied to pure phenolic compounds including pure p-chlorocresol, not industrial wastewater [,,,,,].
In addition to the choice of AOP, the delivery method into the wastewater stream is important from both an efficiency and operational perspective. For example, O3 can be delivered to the wastewater stream via dissolution in water, or through direct injection. The dissolution option involves drawing O3 into a water stream with a venturi and delivering that water stream into a wastewater holding tank []. Venturi injection allows O3 to dissolve in the delivery water, giving it excellent contact with the organic material in the wastewater. However, the delivery water must be relatively free of solids to avoid clogging of either the pump or venturi orifice. Direct injection involves pumping O3 into the wastewater. While this technique avoids issues of clogging, the transfer of O3 into the liquid phase is low; hence, the efficiency of degradation can be compromised [].
To the best of our knowledge, no study has evaluated the degradation of p-chlorocresol in tannery wastewater environment. In this study, we evaluated the suitability of O3 oxidation, both alone and in combination with Fenton’s reagent, as a means for degrading p-chlorocresol in tannery wastewater. We also compared dissolved and direct techniques for O3 delivery for removal of p-chlorocresol. All trials were conducted at both laboratory and pilot scales. We designed and constructed a pilot scale system and investigated the optimal operational parameters for the removal of p-chlorocresol from tannery wastewater. The main objective of this work was to develop and propose an effective technology for p-chlorocresol removal suitable for implementation in tannery processing plants.
2. Materials and Methods
2.1. Lab-Scale Trials
2.1.1. Sample Preparation and Tannery Wastewater Collection
Pure p-chlorocresol (4-chloro-3-methylphenol) and surfactant TritonX were purchased from Sigma-Aldrich (USA). According to the manufacturer’s data sheet, solubility of pure p-chlorocresol in water varies from 3.3 g L−1 at pH 5 to 4.2 g L−1 at pH 9. It is highly soluble in organic solvents such as hexane, ethanol, dichloromethane, and acetone. Pure p-chlorocresol was first dissolved in water with the assistance of surfactant TritonX (1 g L−1) to prepare an 850 mg L−1 test solution.
Wastewater samples for the laboratory experiments were collected from the balance tank from a commercial tannery (NSW, Australia) for lab trials. The balance tank is located between the wastewater sump and dissolved air flotation system, where the wastewater is temporally stored for pH adjustment after the removal of large solids. For the pilot trials at the site, wastewater from the main sump was used before any primary treatments. The elemental composition of the wastewater sample was analysed by the Environmental Analysis Laboratory at Southern Cross University, NSW, Australia.
2.1.2. Establish Basic Ozone Treatment Conditions
In the initial trials, 1 L of pure p-chlorocresol test solution (850 mg L−1) and tannery wastewater sample (from balance tank) were treated separately at an O3 dose rate of 0.5 g h−1 using a small O3 generator (1 g h−1) using bottled O2 (Figure 1a) under laboratory conditions at room temperature (≈22 °C), and samples were collected every hour for 3 h. The collected samples were analyzed for p-chlorocresol using the gas chromatographic method described below (Section 2.3). There was a noticeable loss of O3 from the reaction vessel in the first trials, prompting the use of a taller reaction vessel.
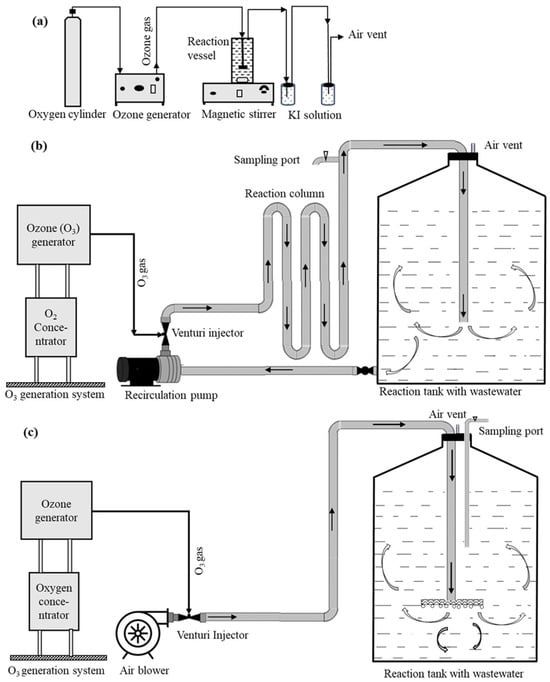
Figure 1.
Schematic diagram of lab setup (a) showing O3 generator (left), 2 L capacity reaction vessel with magnetic stirrer (center), and residual O3 trap (right); pilot system set up at Hide Tanners (b) for venturi reactor system with recirculation pump and 1000 L reaction tank and (c) for blower reactor system and 1000 L reaction tank with a fine bubbler. The black arrows show the direction of flow.
A second series of experiments with wastewater was carried out using a new elongated reaction vessel. The column was 90 mm (Φ) × 640 mm (height) and held 4 L of liquid, allowing for greater contact time between O3 and the wastewater. The liquid sample was agitated using a magnetic stirrer, and the O3 was introduced using a sintered glass bubbler. For this experiment, a series of O3 dose rates (0.4, 0.5, 0.65, and 0.75 g h−1) were tested over a 3 h incubation period.
2.1.3. Combined Ozone Treatment with Fenton’s Oxidation
To improve p-chlorocresol breakdown without increasing the incubation time, we tested the effects of Fenton’s reagent (Fe2+ and H2O2) in combination with O3 on the degradation of p-chlorocresol using an elongated reactor column. The O3 dose rate was fixed at 0.5 g h−1, as this was the most efficient delivery rate from the previous experiment. An Fe2+ concentration of 2 mM was chosen; this was kept constant through all the experiments. The concentrations of H2O2 tested were 1, 10, 50, and 100 mM. In addition, an experiment at 50 mM H2O2 without O3 was also performed. All experiments were run over 3 h.
2.2. Pilot-Scale Field Trials
2.2.1. Dissolved Ozone Delivery by Venturi
In the first field trial, O3 was delivered to the wastewater through a venturi system. Wastewater from the main drainage sump was manually added to a 1 m3 holding tank and then recirculated at 140 L min−1 via an external centrifugal pump (Figure 1b). The venturi drew O3 from a 5 g h−1 O3 generator, which was mixed with the recirculating wastewater and returned to the holding tank via a 4.8 m reaction column (50 mm PVC pipe). A series of trials with O3 and Fenton’s reagents were carried out (Table 1). The ambient temperature during the field trials was approximately 24 °C.

Table 1.
Details of the pilot-scale trials with tannery wastewater using both venturi-based dissolved O3 delivery and direct O3 injection. Concentrations of the Fenton’s reagents are also shown.
Wastewater samples were collected prior to the treated wastewater returning to the holding tank. The final treated wastewater samples were collected after 24 h. The p-chlorocresol levels in the samples were analyzed. The COD (chemical oxygen demand), TOC (total organic carbon), and DOC (dissolved organic carbon) were also determined in the samples (Environmental Analysis Laboratory at Southern Cross University, NSW).
Samples were collected every 2 and 4 h for 24 h. The p-chlorocresol in the samples were analyzed.
2.2.2. Direct Ozone Delivery by Air Blower
The delivery of O3 via a side channel air blower was tested (Figure 1c). The O3 system was also upgraded to a 20 g h−1 generator. To increase contact time, we used 700 L rather than 400 L. The series of trials were carried out in duplicate (Table 1). The samples were collected every 2 or 4 h for 24 h. The p-chlorocresol levels in the samples were analyzed.
2.3. Method for p-Chlorocresol Determination
Due to low molecular weight of p-chlorocresol (4-chloro-3-methylphenol), we focused on a gas chromatographic method. Pure p-chlorocresol (Sigma-Aldrich) was purchased, and a series of standards were prepared in ethanol. Pure compound, which is relatively insoluble, was first dissolved in water with the assistance of the surfactant TritonX (1 g L−1). The developed method relies on gas chromatography with a flame ionization detector (GCFID). The method takes 30 min per sample, with a retention time of 19.75 min. A linear response was found with a limit of detection of approximately 50 µg L−1 (50 ppb). To extract the p-chlorocresol, 50 mL of sample was partitioned against dichloromethane (DCM) and salted with a saturated CaCl2 solution to drive the compound into the organic phase. The DCM solutions were taken to dryness on a rotary evaporator, made up quantitatively with 10 mL of ethanol, and analyzed via GCFID against standards of known p-chlorocresol concentrations. The same procedure was followed for all wastewater samples to identify the p-chlorocresol levels.
2.4. Kinetic Models
The time series data of p-chlorocresol removal in wastewater were fitted to mathematical equations using Pseudo-first-order, Pseudo-second-order (PSO), and Elvoich kinetic models. The linear forms of Pseudo-first-order, Pseudo-second-order, and Elvoich kinetic models can be represented by Equations (1)–(3), respectively:
where qt (mg L−1) and qe (mg L−1) are the amount of p-chlorocresol removed from the wastewater at time t and at equilibrium, respectively; k1 (h−1) and k2 (L mg−1 h−1) are the rate constant of the pseudo-first-order and PSO models, respectively; a (mg L−1 h−1) and b are Elovich constants. The Excel Solver is used to calculate the models’ parameters.
3. Results
The elemental composition of untreated tannery wastewater is presented in Table 2. The average pH of the tannery wastewater was 4.09. It contained an average concentration of 175 mg L−1 of p-chlorocresol, along with high levels of total organic carbon. Significant concentrations of chromium and iron were also found in the tannery wastewater (Table 2).

Table 2.
Elemental composition of untreated wastewater collected from tannery wastewater sump.
3.1. Lab-Scale Optimization
3.1.1. Basic Ozone Treatment
The removal of pure p-chlorocresol was 95% (Figure 2). The concentration of p-chlorocresol in the wastewater (pH 4.09) reduced by 75% within 3 h of treatment at an O3 dose rate of 0.5 g h−1 (Figure 2). This was slower than the pure compound trial at the same O3 dose rate (Figure 2), most likely due to the presence of other organic compounds in the tannery wastewater that were consuming O3.
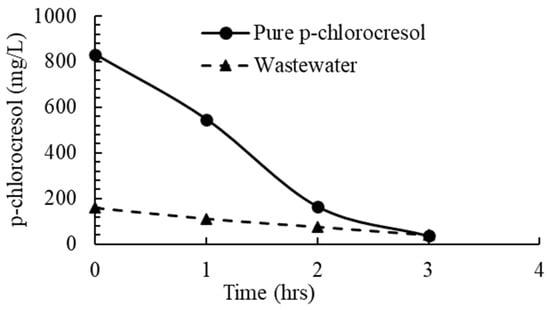
Figure 2.
Concentration of pure p-chlorocresol and p-chlorocresol in tannery wastewater over time at an O3 dose rate of 0.5 g h−1.
Higher O3 dose rates did not induce a linear increase in the p-chlorocresol degradation because there was a significant loss of O3 observed from the small reactor vessel (Figure 1a). Hence, another set of experiments was performed on tannery wastewater in a lab-scale 4 L trial using a modified reactor set up with a taller reaction vessel.
For the modified lab reactor, all O3 dose rates showed a reduction in the p-chlorocresol concentration in wastewater over time (Figure 3a), with the quickest consumption of p-chlorocresol measured at the highest dose rate (0.75 g h−1; Figure 3a). There is a significance difference (p > 0.05; Table S1 in Supplementary Materials) in the p-chlorocresol degradation observed within the O3 dose rates. However, at the two highest dose rates (0.65 and 0.75 g h−1), there was a notable discharge of O3, indicating that the contact time could be improved. At a dose rate of 0.4 g h−1, 42% of the p-chlorocresol in 4 L of wastewater was consumed in 3 h, whereas at 0.75 g h−1 70% of the test POC was consumed within 2 h (Figure 3a). Because different O3 doses, reactor volumes, and treatment times were used, it can be difficult to compare the p-chlorocresol removal between different experiments. To make this comparison easier, we calculated a POC consumption rate which accounts for different experimental conditions, where units are mg p-chlorocresol g− O3 h−1. At the highest O3 dose rate, the consumption of p-chlorocresol was 121 mg chlorocresol g− O3 h−1 (Figure 3a).
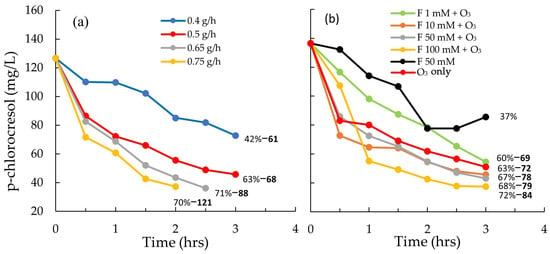
Figure 3.
(a) Degradation of p-chlorocresol in 4 L tannery wastewater at different O3 dosing rates of 0.4, 0.5, 0.65, and 0.75 g h−1 over 3 h. (b) Degradation of p-chlorocresol in 4 L tannery wastewater at different H2O2 dosing rates (1, 10, 50, and 100 mM) over 3 h. The O3 dosing rate was 0.5 g h−1, and the Fe2+ concentration was kept constant at 2 mM. A Fenton’s-only (50 mM) and O3-only treatment (0.5 g h−1) are also shown. Percent reduction is also shown, as well as the p-chlorocresol consumption rate, in units of mg p-chlorocresol g− O3 h−1 (bold black numbers).
3.1.2. Combined Ozone and Fenton’s Reagent Treatment
A significance difference (p > 0.05; Table S1) in the p-chlorocresol degradation is observed in the O3 plus Fenton dose rates. The Fenton’s reagent alone (50 mM H2O2) showed a 37% reduction in p-chlorocresol over three hours, roughly half of the removal achieved with the 0.5 g h−1 O3 dose (Figure 3b). This rules out the use of the Fenton’s reaction as a replacement for O3. When Fenton’s reagent and O3 were combined, no increase in p-chlorocresol removal was found at a H2O2 dose concentration of 1 mM compared to the 0.5 g h−1 O3 dose (Figure 3b). However, at 10, 50, and 100 mM of H2O2, the removal of p-chlorocresol was generally faster, with a lower final concentration. At the highest H2O2 dose rate (100 mM), there was a 10% improvement in the overall removal of p-chlorocresol relative to the O3 only treatment. At this highest H2O2 dose rate, the consumption of p-chlorocresol was 84 mg POC g− O3 h−1, which was higher than the consumption rate with the 0.5 g h−1 O3 alone dose rate (72 mg p-chlorocresol g− O3 h−1; note that if we calculate this at 2 h, the removal rate is around 120 mg p-chlorocresol g− O3 h−1, Figure 3b). Interestingly, the effect of combining Fenton’s reagent and O3 was not additive. In other words, the % removal with the combined O3 and Fenton’s reagent (68%) was less than expected if we were to add the individual consumption rates together (37% and 63% for the 50 mM Fenton’s-only and 0.5 g h−1 O3-only treatments, respectively).
3.2. Pilot Field Trials
The effects of different advanced oxidation processes and delivery systems on the removal of p-chlorocresol in tannery wastewater during 24 h field trials are presented in Table 3. The studies compare O3 treatment alone, O3 combined with Fenton’s reagent, and Fenton’s reagent alone across varying O3 delivery approaches, reactor volumes, and circulation systems.

Table 3.
Removal of p-chlorocresol during the 24 h field trials.
3.2.1. Dissolved Ozone Delivery
Without Fenton’s reagent, dissolved O3 delivered by venturi using a centrifugal pump achieved 33% removal of p-chlorocresol; however, the removal rate (7.5 mg p-chlorocresol g− O3 h−1; Table 3) was much lower than in the lab-based trials (72 mg p-chlorocresol g− O3 h−1; Figure 3b). This is because the O3 dose in the lab trial (Figure 3) per L of wastewater was much higher than for the field trial (0.5 g h−1 for 4 L vs. 5 g h−1 for 400 L).
In addition, for the lab trials, the wastewater was collected from the balance tank where the TOC was less 30% of the TOC in the main sump (Table 2), which is where wastewater was drawn for the pilot trials. The higher organic load would undoubtedly consume O3. With the addition of Fenton’s reagent, an increase in the percent removal of p-chlorocresol was observed (Table 3). Maximum removal was 48% (11.5 mg p-chlorocresol g− O3 h−1; Table 3) at a dose rate of 0.50 mM Fe2+ and 50 mM of H2O2. The combination treatment system appears to be at least additive, in that the % removal with O3 + Fenton’s (48%) was higher than the sum of individual % removals (33.5% for O3 only and 32.3% for Fenton’s only).
Figure 4 shows the comparative removal efficiencies of COD, TOC, DOC, and p-chlorocresol under different treatment conditions in the venturi delivery trials with a centrifugal pump. A significance difference (p > 0.05; Table S2) in the removal efficiencies is observed within the parameters under different treatments. O3 only produced modest removals, with COD, TOC, and DOC each reduced by about 26%, 25%, and 16%, respectively, and p-chlorocresol showed the highest removal at ~33.5. For O3 plus Fenton’s reagent at a lower iron dose (0.25 mM Fe), COD removal remained at ~24%, but mineralization and micropollutant breakdown improved, with TOC, DOC, and p-chlorocresol removals all increasing to ~38–40%. At the higher iron dose (0.50 mM Fe), COD removal rose to ~41%, TOC removal was moderate at ~31%, DOC dropped sharply to ~12%, and p-chlorocresol removal reached the highest level observed (~48%). Overall, these results indicate that the combined O3 and Fenton process is more effective than O3 only for removing p-chlorocresol and bulk organics. However, optimization of Fe and H2O2 dosages is critical, since excessive Fe loading can hinder the mineralization efficiency despite improving target pollutant removal. In addition, the p-chlorocresol accounts for about 8% of the total carbon content of the wastewater (Table 2), and yet the total organic carbon removal was well over 20% for all treatments (Figure 4).
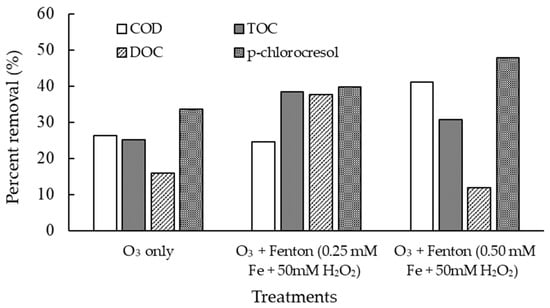
Figure 4.
Removal of COD (chemical oxygen demand), TOC (total organic carbon), DOC (dissolved organic carbon), and p-chlorocresol in the venturi delivery trials with centrifugal pump.
There were no issues observed with clogging of the improved circulation pump (screw pump) due to the high solid contents in the tannery wastewater. By using only O3 through a 20 g/h O3 generator and venturi delivery system with the improved circulation pump unit, the p-chlorocresol was removed by 46% (Table 3), which was higher than that of the venturi delivery system with a centrifugal pump (33.5%; Table 3). The addition of Fenton’s reagents increased the percent removal of p-chlorocresol by 43% compared to O3 only using the improved circulation pump. However, the overall p-chlorocresol removal rate (expressed as mg p-chlorocresol g−1 O3 h−1) decreased by 42–49% in the improved circulation pump system with a 20 g/h O3 generator compared to the centrifugal pump system using a 5 g/h O3 generator (Table 3). This reduction in the p-chlorocresol removal rate was attributed to the larger wastewater volume used (700 L instead of 400 L) in the improved circulation pump system, which also resulted in greater O3 losses with the 20 g/h O3 generator.
3.2.2. Direct Ozone Delivery
The direct delivery system with a 20 g/h O3 unit reduced p-chlorocresol removal by 20% (O3 only) and 17% (O3 plus Fenton’s reagents) compared to the venturi system with an improved circulation pump, which achieved 37% and 55% removal, respectively (Table 3). In contrast, under direct delivery trial, p-chlorocresol removal increased from 33.5% to 37% with O3 alone, and from 48% to 55% with O3 plus Fenton’s reagents, compared to venturi trials using a centrifugal pump. The p-chlorocresol removal rate for O3 alone was nearly the same (~4 mg p-chlorocresol g−1 O3 h−1) using direct delivery and the venturi system with an improved circulation pump. However, with O3 plus Fenton’s reagents, the removal rate decreased from 6.7 to 5.3 mg p-chlorocresol g−1 O3 h−1 (Table 3).
3.3. Kinetics of p-Chlorocresol Removals
The kinetic data of p-chlorocresol removal was fitted with pseudo-first-order, pseu-do-second-order, and Elovich kinetic models. The estimated kinetic models’ parameters are presented in Table 4. The curve fitting of the kinetic models for the removal of the p-chlorocresol are presented in Figure 5.

Table 4.
Estimated kinetic model parameters for removal of p-chlorocresol in wastewater.
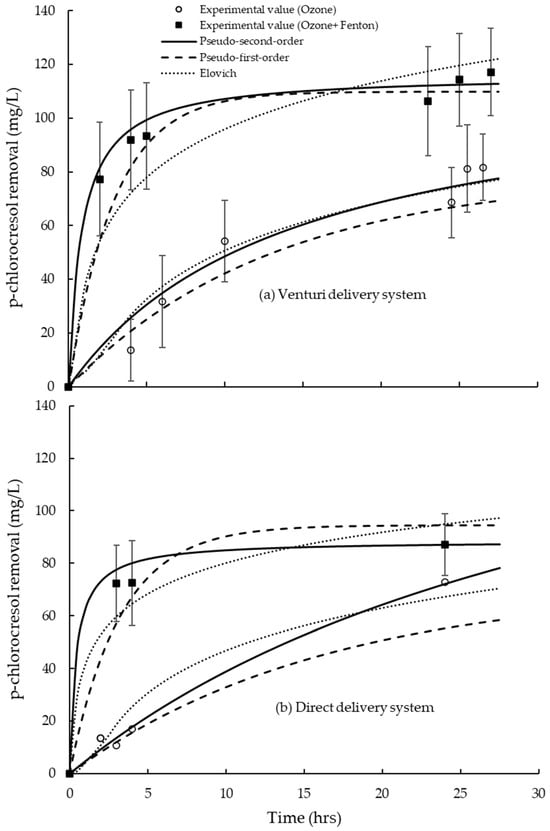
Figure 5.
Removal of the p-chlorocresol in 700 L of tannery wastewater at a 20 g h−1 O3 dosing rate over 24 h using (a) a venturi delivery system with improved circulation pump (screw pump), and (b) direct delivery system with blower in pilot trials. Two replications of each of trial were used. Error bars reflect the variability among different batches of wastewater, but the overall trends remain clear. A Fenton’s dose rate of 0.50 mM Fe2+ and 50 mM of H2O2 was added. Curves are fitted using pseudo-first-order model, pseudo-second-order (PSO) model, and Elovich kinetic models.
A significance difference (p > 0.05; Table S3) in the removal of p-chlorocresol is observed under different treatment conditions (O3, and O3 plus Fenton’s reagent), and delivery systems. The O3 plus Fenton’s reagent showed a significantly higher removal capacity (qe) of p-chlorocresol in wastewater for both the venturi delivery (with an improved circulation pump) and direct delivery (with blower) system compared to O3 only (Table 4; Figure 5). The p-chlorocresol removal using O3 plus Fenton’s reagent was better described by the pseudo-second-order model, with relatively greater qe values and correlation coefficient (R2) (Table 4; Figure 5). The pseudo-first-order and Elovich models exhibited relatively higher correlation coefficients (R2) for O3 only, suggesting a better fit for describing its removal behavior (Table 4; Figure 5).
It was estimated from curve fitting using the PSO equation that a maximum removal of p-chlorocresol in wastewater could be reached up to ≈64% (112 mg/L) within 24 h using O3 plus Fenton’s reagent via venturi delivery (with an improved circulation pump). There could be little to no further removal of p-chlorocresol in wastewater beyond 24 h under the same conditions (Figure 5a). Using O3 only could remove the p-chlorocresol in wastewater up to ≈42% (74 mg/L) in 24 h. However, there could be further removal of the p-chlorocresol in wastewater after 24 h using O3 only (Figure 5a). The p-chlorocresol could be removed up to 17% (31 mg/L) in 5 h by O3 only. However, when the Fenton’s reagents were added with O3, the removal of p-chlorocresol was ≈57% (99 mg/L) within 5 h (Figure 5a).
The PSO model estimated that a maximum ≈ 55% (87 mg/L) removal of p-chlorocresol in wastewater was achieved in 24 h using O3 plus Fenton’s regent with the direct delivery system (Figure 5b). O3 only could remove the p-chlorocresol in wastewater up to ≈38% (72 mg/L) in 24 h. However, there could be further removal of the p-chlorocresol after 24 h using O3 only. O3 only can remove the p-chlorocresol by only 12% (22 mg/L) in 5 h. The removal of p-chlorocresol can be achieved up to 52% (82 mg/L) within 5 h using the Fenton’s reagents plus with O3 (Figure 5b).
4. Discussion
4.1. Influence of Treatment Type on p-Chlorocresol Removal
Ozonation, and the combination of ozonation with Fenton’s reagents, are effective means of destroying p-chlorocresol in pure solutions [,,]. Combining O3 treatment with Fenton’s oxidation reaction is also a promising technique for degrading phenolic compounds in wastewaters [,,,,,]. Our results clearly demonstrate that O3 is a highly effective oxidizing agent for degrading the p-chlorocresol in real tannery wastewater (Figure 5; Table 3). Furthermore, the addition of Fenton’s reagent was clearly beneficial for the p-chlorocresol breakdown, almost doubling the percent removal of our p-chlorocresol compared to O3 only in both the venturi delivery and direct delivery trials (Table 3). For example, the addition of Fenton’s reagent with O3 increased the removal of p-chlorocresol in wastewater up to 66% (Table 3), which indicates a synergistic effect of the O3 plus Fenton process. These results align with the literature showing that hybrid O3/Fenton’s processes are promising for treating phenolic pollutants [,]. This is also consistent with the reports that hybrid AOPs provide synergistic effects by simultaneously generating hydroxyl radicals (•OH) through ozone decomposition and Fenton’s reaction [,]. Our data (Table 3) shows that p-chlorocresol removal increased with higher H2O2 and Fe2+ within the tested window (e.g., at 400 L, increasing Fe2+ from 0.25 to 0.50 mM, with H2O2 50 mM increasing removal from 39.8% to 47.9%). This is consistent with higher transient hydroxyl radical production when the H2O2:Fe2+ ratio is favorable.
4.2. Influence of Treatment Type on COD, TOC, and DOC Removal
When O3 only was applied, moderate removal of COD (≈26%) and TOC (≈27%) was achieved (Figure 4). However, DOC removal remained low (≈17%; Figure 4), indicating that ozonation alone was insufficient for mineralization of dissolved organics. This is consistent with previous findings that O3 only primarily acts through selective oxidation of phenolic compounds rather than complete degradation of organic matter [,]. The addition of Fenton’s reagent substantially improved overall removal. The COD, TOC, and DOC removals increased to ≈38–41% (Figure 4), suggesting a synergistic effect between O3 and hydroxyl radicals generated by the Fenton’s reaction. Hydroxyl radicals are non-selective oxidants that attack a broader range of organic molecules, enhancing both degradation and mineralization [,]. In contrast, increasing the Fe concentration (from 0.25 to 0.50 mM) while keeping H2O2 constant resulted in higher COD (≈42%) removal, but TOC (≈28%) and DOC (≈12%) removals decreased significantly. This may be attributed to the scavenging of hydroxyl radicals by excess Fe2+, which reduces the availability of reactive species for mineralization of bulk organic matter [,].
Multiple studies, including olive-mill and pharmaceutical wastewater tests, reported an improved COD/TOC removal and faster treatment when O3 and Fenton are integrated vs. either alone. Optimizing the treatment sequence, reagent dosage, and pH is critical []. Ozonation often breaks down refractory molecules into smaller, more biodegradable intermediates, thereby increasing the BOD/COD ratio. A subsequent Fenton step can then achieve further mineralization. Sequential or combined O3–Fenton processes are designed to exploit this synergy [].
The very high organic load in the tannery wastewater means that much of the O3 is directed towards the oxidation of other organic material, which was reflected in the removal of COD and TOC (over 25% using O3; over 24–41% using O3 plus Fenton; Figure 4). Similar findings were reported in other studies with ozonation and Fenton oxidation used for the treatment of industrial wastewater [,,]. Nevertheless, we were still able to remove appreciable p-chlorocresol from the tannery wastewater.
4.3. Influence of Wastewater pH on p-Chlorocresol Removal
The pH of our wastewater (4.09; Table 2) may be a limiting factor for the removal of p-chlorocresol, as pH is likely the most critical parameter given that ozone and Fenton’s reagent processes operate optimally in different pH ranges. Ozone can react directly with electron-rich groups (such as double bonds and activated phenols). Under near-neutral to alkaline conditions (pH > 7), it also decomposes to generate hydroxyl radicals (•OH), which are less selective but far more powerful oxidants [,]. The homogeneous Fenton’s reaction (Fe2+ + H2O2 → Fe3+ + •OH + OH−), which involves the use of hydrogen peroxide (H2O2) and iron (Fe2+), produces hydroxyl radical most efficiently at pH ≈ 2.5–3.5. At very low pH values (< 2), H2O2 is stabilized and side reactions occur, while at pH ≈ 3.5–4, iron precipitates as hydroxides, reducing catalytic activity [,]. Therefore, combining O3 and Fenton’s reagent typically increases the overall hydroxyl radical flux, leading to faster oxidation, higher mineralization (TOC removal), and shorter treatment times compared with either process alone []. The oxidation pathway likely favored the degradation of p-chlorocresol, a phenolic compound highly susceptible to hydroxyl radical attack [].
In our study, as the pH (4.09) lies between the optimal windows for ozonation and Fenton processes, p-chlorocresol is oxidized by both molecular O3 and hydroxyl radicals. Under this condition, ozone contributes through direct electrophilic attack and limited hydroxyl radical generation, while Fenton’s regent activity persists (though weaker than at pH ≈ 3) because some Fe2+ remains soluble (less precipitation than at pH > 5.5) [,]. A combined O3 plus Fenton sequence or hybrid (simultaneous) treatment can produce synergies (enhanced hydroxyl radical availability, faster ring-opening/mineralization), but this requires careful control of reagent dosing, pH, and scavengers because partial oxidation can produce more toxic intermediates if mineralization is incomplete [,]. Cresol isomers have been shown to reach high removal (e.g., >80% degradation within 2 h under optimized Fenton conditions in bench studies), but mineralization (TOC) is lower and takes longer [].
4.4. Influence of Delivery System and Reaction Volume on p-Chlorocresol Removal
Improved circulation, such as better mixing, smaller bubbles, and stronger mass transfer, correlates with higher removals and sometimes improved ozone-specific efficiency. At pH 4.5, where radical chemistry is important, transport of O3 into the liquid and rapid mixing to distribute H2O2/Fe2+ are rate-limiting. The type of O3 delivery strongly influenced treatment efficiency. The venturi delivery system with a screw pump (improved circulation) consistently outperformed the direct delivery system with a blower. For instance, O3 plus Fenton’s at 700 L with venturi achieved 66% removal compared to 55% with the blower system (Table 3). Similarly, O3 utilization (7.5 vs. 5.3 mg p-chlorocresol g−1 O3 h−1; Table 3) was higher in the venturi system. This clearly indicates that an increase in the removal rate of p-chlorocresol is due to efficient utilization of O3 by the venturi delivery system with improved circulation. This likely improved O3 dispersion and mixing, enhancing hydroxyl radical production. The improved contact between O3 and water through fine bubble generation in venturi injectors likely explains this performance []. There was more significant loss of O3 from the direct delivery system which contributed to the low rates of the p-chlorocresol breakdown compared to venturi delivery (Table 3). The fine ceramic diffusers for O3 and a tall reaction column may be used to improve the direct delivery system. However, this requires an increase in the pressure of the blower, which could in turn require more air to be pushed through the system, diluting the O3 and reducing its effectiveness. Therefore, reactor design, such as bubble size, contact time, and recirculation, substantially affects apparent synergy. This observation matches reactor-scale studies showing that hydrodynamics strongly influence AOP performance [].
At a higher working volume (700 L) and higher ozone doses (20 g h−1), removal efficiency did not proportionally increase. For example, O3 alone gave 46% removal at 700 L compared to 33.5% at 400 L, but the corresponding values of removal rate were lower (3.8 vs. 7.5 mg p-chlorocresol g−1 O3 h−1). This indicates a decrease in ozone utilization efficiency at larger volumes, likely due to reduced ozone mass transfer and increased scavenging losses []. These results highlight diminishing returns in ozone utilization at higher O3 supply if mass transfer, contact time, or radical coupling are limiting. Efficient radical generation (e.g., via Fenton’s reagent) and effective gas–liquid contact are therefore critical to make each gram of O3 count [].
4.5. Kinetics of p-Chlorocresol Removal
Venturi delivery (with improved circulation) is more effective than direct delivery, providing higher equilibrium p-chlorocresol removal (qe) and better fits to the kinetic models. O3 plus Fenton’s reagent greatly enhances p-chlorocresol removal rate compared to O3 only, regardless of delivery method. The pseudo-second-order model provides the best fit for O3 plus Fenton’s reagent (high R2 ≈ 0.99), suggesting that surface reaction mechanisms dominate in p-chlorocresol degradation. O3 only fits better with the pseudo-first-order or Elovich models, indicating a slower process in p-chlorocresol degradation.
Curve fitting with the PSO equation shows that the removal rate of p-chlorocresol in the wastewater was rapid early on by O3 plus Fenton’s reagents but was then followed by gradual or somewhat slower removal (Figure 5) because ring cleavage and mineralization require successive oxidations. This pattern appears for both Fenton and ozonation []. It is observed that venturi delivery (with improved circulation using a screw pump) can remove the p-chlorocresol up to ≈42% in 24 h with only O3 (Figure 5). When Fenton’s reagent was combined with O3, the removal of p-chlorocresol from wastewater increased significantly, reaching 57% within 5 h and ≈64% within 24 h (Figure 5). This improvement is likely due to enhanced mass transfer achieved through venturi delivery and additional radical generation from the Fenton’s reaction, both of which accelerated and extended the removal process. Removal appears to plateau, suggesting some sort of limit on the reduction of the p-chlorocresol, probably caused by competition with other organics in the wastewater that were also consuming O3. This is why it was seen that the TOC removal using O3 only, and O3 plus Fenton’s reagent, was well over 25% in wastewater, where the p-chlorocresol accounts for about 8% of the TOC in the wastewater (Table 3; Figure 4). It was also observed that the p-chlorocresol removal rate in the wastewater was slower than the pure p-chlorocresol compound trial at the same O3 dose (Figure 2), most likely due to the presence of other organic compounds in the tannery wastewater that were consuming O3. There could be other factors, i.e., the available fraction of p-chlorocresol in the wastewater, that limited its reduction. Most of the p-chlorocresol in the wastewater was removed within 5 h when combining the O3 and Fenton’s treatments (Figure 5), which was probably from the freely available fraction of p-chlorocresol. Some fraction of p-chlorocresol may be strongly bound up in the fats and other solids in the wastewater, which could contribute to the slower removal of p-chlorocresol after 5 h in the O3 plus Fenton’s reagent treatments (Figure 5).
Similar trends in the removal of the p-chlorocresol were observed in the direct delivery system, but the removal was less compared to the venturi delivery system (Figure 5). It is now clear that the addition of Fenton’s reagent with O3 can potentially reduce the time for the removal of p-chlorocresol in tannery wastewater. Other studies also reported that the reaction time for ozonation processes could be shorter to degrade phenolic compounds in wastewater if Fenton or H2O2 is added to the ozonation process [,,,,].
4.6. Intermediates During p-Chlorocresol Degradation
One important aspect regarding the possible toxicity of the reaction or transformation products during the oxidation process needs to be considered. Partial oxidation of chlorophenols can produce chlorocatechols, quinones, and short-chain carboxylic acids. Some intermediates (e.g., certain chlorinated quinones) can be more toxic than the parent compound; therefore, monitoring intermediate formation and TOC or AOX (adsorbable organic halogens) is important. Ozonation alone in some cases increases intermediate toxicity unless radical mineralization proceeds further; combined processes tend to lower overall toxicity when properly optimized [].
In general, all of the aromatic intermediate products (e.g., 4-Methylcatechol, dihydroxyfumaric acid, artaric acid, citric acid, malic acid, etc.) formed during oxidation of phenolic compounds (e.g., cresols) are less toxic compared to cresols [,]. The intermediate products degrade to shorter chains of carboxylic acids with further oxidation. The major degradation pathway of cresol is benzene ring oxidation through electrophilic aromatic substitution reaction by molecular O3 and hydroxyl radicals [].
Other aspects require consideration, such as oxidative treatment of tannery wastewater with O3, which may lead to re-oxidation of chromium (Cr(III) to the Cr(VI). In tannery wastewater, Cr(III) may present as the chromium salt used in the tanning process. Cr(III) in most cases reacts to form insoluble compounds and is commonly considered to be nontoxic []. In our study, Cr(VI) formation was not found during the ozonation of tannery wastewater. Another study reported that effluent ozonation only leads to toxicologically insignificant Cr(VI) concentrations of Cr(III) in wastewater [].
5. Conclusions
In this study, ozonation and ozonation plus Fenton’s reagent were applied to remove the p-chlorocresol from tannery wastewater. Laboratory trials demonstrated that removal of p-chlorocresol from wastewater at pH 4.1 using ozone was over 70% (76 mg p-chlorocresol g− O3 h−1). Fenton’s reagent did improve the p-chlorocresol removal, but only by 10%. A relatively high percentage of p-chlorocresol removal (66%; 6.7 mg p-chlorocresol g− O3 h−1) in the tannery wastewater was found in the ozonation plus Fenton’s reagent process compared to the ozonation-only process (46% removal; 4.8 mg p-chlorocresol g− O3 h−1). At the pH of our tannery wastewater (≈4.5), the O3 plus Fenton process operates in a mixed regime, where direct ozone oxidation occurs in parallel with Fenton-driven hydroxyl radical production. Achieving true synergy and sustaining high ozone-specific removal efficiency require both effective hydrodynamics and optimized reagent stoichiometry.
In our pilot-scale studies, dissolved O3 delivery into wastewater using a venturi with improved circulation was more efficient than direct delivery via an air blower system. The combined use of dissolved O3 and Fenton’s reagent via venturi delivery is recommended as an efficient, effective, and environmentally friendly technique to break down the p-chlorocresol in wastewater. However, ozone utilization efficiency declines at larger volumes, highlighting the importance of optimizing gas–liquid mass transfer for scale-up. For a full-scale tannery effluent treatment, the combination of a high-efficiency gas transfer device with advanced oxidation (O3 plus Fenton) is promising, as it enhances pollutant removal rates and allows for shorter contact times and smaller reactor volumes. This has real operational advantages (such as smaller reactors, lower residence times) for high-load industrial streams. The findings of this study are very useful to guide the implementation of a full-scale commercial system and optimize the operational parameters for p-chlorocresol removal in wastewater.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w17223238/s1, Table S1: Statistical analysis of data for degradation of the p-chlorocresol in 4 L tannery wastewater at O3 only, and O3 plus Fenton dosing rates; Table S2: Statistical analysis of data for removal of COD, TOC, DOC and p-chlorocresol in the venturi delivery trials with centrifugal pump; Table S3: Statistical analysis of data for removal of the p-chlorocresol in 700 L tannery wastewater at a 20 g h-1 O3 dosing rate over 24 h using a venturi delivery system with improved circulation pump (screw pump) and direct delivery system.
Author Contributions
Conceptualization, D.E., A.R., S.M., M.S.R. and D.B.; methodology, D.E., M.S.R. and D.B.; software, M.S.R.; validation, M.S.R., D.B. and D.E.; formal analysis, M.S.R. and D.B.; investigation, M.S.R. and D.B.; resources, D.E., A.R. and S.M.; data curation, M.S.R. and D.E.; writing—original draft preparation, M.S.R.; writing—review and editing, D.E., D.B. and S.M.; visualization, M.S.R.; supervision, D.E. and A.R.; project administration, D.E.; funding acquisition, D.E., A.R. and S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Australian Meat Processor Corporation Ltd. (AMPC), grant number 2022-1058.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare that this study received funding from Australian Meat Processor Corporation Ltd. (AMPC). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
References
- Rajalo, G.; Petrovskaya, T. Selective electrochemical oxidation of sulphides in tannery wastewater. Environ. Technol. 1996, 17, 605–612. [Google Scholar] [CrossRef]
- Szpyrkowicz, L.; Naumczyk, J.; Zilio-Grandi, F. Electrochemical treatment of tannery wastewater using TiPt and Ti/Pt/Ir electrodes. Water Res. 1995, 29, 517–524. [Google Scholar] [CrossRef]
- Xiao, M.; Qi, Y.; Feng, Q.; Li, K.; Fan, K.; Huang, T.; Qu, P.; Gai, H.; Song, H. P-cresol degradation through Fe (III)-EDDS/H2O2 Fenton-like reaction enhanced by manganese ion: Effect of pH and reaction mechanism. Chemosphere 2021, 269, 129436. [Google Scholar] [CrossRef]
- Iliuta, I.; Iliuta, M.C. Intensified phenol and p-cresol biodegradation for wastewater treatment in countercurrent packed-bed column bioreactors. Chemosphere 2022, 286, 131716. [Google Scholar] [CrossRef]
- Singh, R.K.; Kumar, S.; Kumar, S.; Kumar, A. Biodegradation kinetic studies for the removal of p-cresol from wastewater using Gliomastix indicus MTCC 3869. Biochem. Eng. J. 2008, 40, 293–303. [Google Scholar] [CrossRef]
- Dantas, T.L.P.; José, H.J.; Moreira, R.F.P.M. Fenton and Photo-Fenton oxidation of tannery wastewater. Acta Sci. Technol. 2003, 25, 91–95. [Google Scholar]
- Schrank, S.G.; José, H.J.; Moreira, R.F.P.M.; Schröder, H.F. Applicability of Fenton and H2O2/UV reactions in the treatment of tannery wastewaters. Chemosphere 2005, 60, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Sivagami, K.; Sakthivel, K.P.; Nambi, I.M. Advanced oxidation processes for the treatment of tannery wastewater. J. Environ. Chem. Eng. 2018, 6, 3656–3663. [Google Scholar] [CrossRef]
- Lofrano, G.; Meriç, S.; Zengin, G.E.; Orhon, D. Chemical and biological treatment technologies for leather tannery chemicals and wastewaters: A review. Sci. Total Environ. 2013, 461–462, 265–281. [Google Scholar] [CrossRef]
- R Rekhate, C.V.; Srivastava, J.K. Recent advances in ozone-based advanced oxidation processes for treatment of wastewater—A review. Chem. Eng. J. Adv. 2020, 3, 100031. [Google Scholar] [CrossRef]
- Schrank, S.G.; Jose, H.J.; Moreira, R.F.P.M.; Schröder, H.F. Elucidation of the behavior of tannery wastewater under advanced oxidation conditions. Chemosphere 2004, 56, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Rameshraja, D.; Suresh, S. Treatment of tannery wastewater by various oxidation and combined processes. Int. J. Environ. Res. 2011, 5, 349–360. [Google Scholar]
- Asaithambi, P.; Sajjadi, B.; Aziz, A.R.A. Integrated ozone–photo–Fenton process for the removal of pollutant from industrial wastewater. Chin. J. Chem. Eng. 2017, 25, 516–522. [Google Scholar] [CrossRef]
- Pera-Titus, M.; García-Molina, V.; Baños, M.A.; Giménez, J.; Esplugas, S. Degradation of chlorophenols by means of advanced oxidation processes: A general review. Appl. Catal. B Environ. 2004, 47, 219–256. [Google Scholar] [CrossRef]
- Malik, S.N.; Ghosh, P.C.; Vaidya, A.N.; Mudliar, S.N. Hybrid ozonation process for industrial wastewater treatment: Principles and applications: A review. J. Water Process Eng. 2020, 35, 101193. [Google Scholar] [CrossRef]
- Benitez, F.J.; Beltrán-Heredia, J.; Acero, J.L.; Rubio, F.J. Rate constants for the reactions of ozone with chlorophenols in aqueous solutions. J. Hazard. Mater. 2000, 79, 271–285. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Esplugas, S.; Gimenez, J.; Contreras, S.; Pascual, E.; Rodríguez, M. Comparison of different advanced oxidation processes for phenol degradation. Water Res. 2002, 36, 1034–1042. [Google Scholar] [CrossRef]
- Sanchis, S.; Meschede-Anglada, L.; Serra, A.; Simon, F.X.; Sixto, G.; Casas, N.; Garcia-Montaño, J. Solar photo-Fenton with simultaneous addition of ozone for the treatment of real industrial wastewaters. Water Sci. Technol. 2018, 77, 2497–2508. [Google Scholar] [CrossRef]
- Schrank, S.G.; Jose, H.J.; Moreira, R.F.P.M.; Schröder, H.F. Comparison of different advanced oxidation process to reduce toxicity and mineralisation of tannery wastewater. Water Sci. Technol. 2004, 50, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Kow, S.H.; Fahmi, M.R.; Abidin, C.Z.A.; Ong, S.A.; Ibrahim, A.H.; Sabri, S.N.; Razali, N.A. Oxidation of p-cresol by ozonation. Sains Malays. 2018, 47, 1085–1091. [Google Scholar] [CrossRef]
- Kavitha, V.; Palanivelu, K. Destruction of cresols by Fenton oxidation process. Water Res. 2005, 39, 3062–3072. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Costa, R.; Quinta-Ferreira, R.M.; Martins, R.C. Application of ozonation for pharmaceuticals and personal care products removal from water. Sci. Total Environ. 2017, 586, 265–283. [Google Scholar] [CrossRef]
- Zhou, H.; Smith, D.W. Advanced technologies in water and wastewater treatment. Can. J. Civ. Eng. 2001, 28, 49–66. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Nawrocki, J.; Kasprzyk-Hordern, B. The efficiency and mechanisms of catalytic ozonation. Appl. Catal. B Environ. 2011, 99, 27–42. [Google Scholar] [CrossRef]
- Gottschalk, C.; Libra, J.A.; Saupe, A. Ozonation of Water and Waste Water: A Practical Guide to Understanding Ozone and Its Applications; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Walling, C. Fenton’s reagent revisited. Acc. Chem. Res. 1975, 8, 125–131. [Google Scholar] [CrossRef]
- Kirmaci, A.; Duyar, A.; Akgul, V.; Akman, D.; Cirik, K. Optimization of combined ozone/Fenton process on olive mill wastewater treatment. Aksaray Univ. J. Sci. Eng. 2018, 2, 52–62. [Google Scholar] [CrossRef]
- Germán, S.M.; Gabriela, R.M.; Dora, S.C.; Rubí, R.; Reyna, N. Advanced Oxidation Processes: Ozonation and Fenton Processes Applied to the Removal of Pharmaceuticals. In Ecopharmacovigilance: Multidisciplinary Approaches to Environmental Safety of Medicines; Springer: Berlin/Heidelberg, Germany, 2017; pp. 119–142. [Google Scholar]
- Chang, C.Y.; Hsieh, Y.H.; Cheng, K.Y.; Hsieh, L.L.; Cheng, T.C.; Yao, K.S. Effect of pH on Fenton process using estimation of hydroxyl radical with salicylic acid as trapping reagent. Water Sci. 2008, 58, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Fischbacher, A.; von Sonntag, C.; Schmidt, T.C. Hydroxyl radical yields in the Fenton process under various pH, ligand concentrations and hydrogen peroxide/Fe (II) ratios. Chemosphere 2017, 182, 738–744. [Google Scholar] [CrossRef]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef]
- Dai, M.; Niu, Q.; Wu, S.; Lin, Y.; Biswas, J.K.; Yang, C. Hydroxyl radicals in ozone-based advanced oxidation of organic contaminants: A review. Environ. Chem. Lett. 2024, 22, 3059–3106. [Google Scholar] [CrossRef]
- Edwards, J.O.; Curci, R. Fenton type activation and chemistry of hydroxyl radical. In Catalytic Oxidations with Hydrogen Peroxide as Oxidant; Springer: Berlin/Heidelberg, Germany, 1992; pp. 97–151. [Google Scholar]
- Lopez, A.; Mascolo, G.; Detomaso, A.; Lovecchio, G.; Villani, G. Temperature activated degradation (mineralization) of 4-chloro-3-methyl phenol by Fenton’s reagent. Chemosphere 2005, 59, 397–403. [Google Scholar] [CrossRef]
- Rakness, K.L. Ozone in Drinking Water Treatment: Process Design, Operation, and Optimization; American Water Works Association: Denver, CO, USA, 2011. [Google Scholar]
- Fedorov, K.; Dinesh, K.; Sun, X.; Soltani, R.D.C.; Wang, Z.; Sonawane, S.; Boczkaj, G. Synergistic effects of hybrid advanced oxidation processes (AOPs) based on hydrodynamic cavitation phenomenon–A review. Chem. Eng. J. 2022, 432, 134191. [Google Scholar] [CrossRef]
- Beltran, F.J. Ozone Reaction Kinetics for Water and Wastewater Systems; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Kow, S.H.; Fahmi, M.R.; Abidin, C.Z.A.; Ong, S.A. Mechanistic insight into the degradation pathways of p-cresol in ozonation, peroxone, and ozone-persulfate process. Ozone: Sci. Eng. 2020, 43, 507–519. [Google Scholar] [CrossRef]
- Valsania, M.C.; Fasano, F.; Richardson, S.D.; Vincenti, M. Investigation of the degradation of cresols in the treatments with ozone. Water Res. 2012, 46, 2795–2804. [Google Scholar] [CrossRef]
- Celary, P.; Sobik-Szołtysek, J. Vitrification as an alternative to landfilling of tannery sewage sludge. Waste Manag. 2014, 34, 2520–2527. [Google Scholar] [CrossRef] [PubMed]
- Katsoyiannis, I.A.; Gachet, C.; Von Gunten, U. Fate of Cr(III) during ozonation of secondary municipal wastewater effluent. Ozone: Sci. Eng. 2018, 40, 441–447. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).