Temporal and Spatial Distribution Characteristics and Source Analysis of Antibiotic Resistance Gene Pollution in Dongliao River Basin, China
Abstract
1. Introduction
2. Sampling Point Setting and Sampling Method
2.1. Sampling Point Setting
2.2. Sample Collection Method
2.3. Detection of Routine Physical and Chemical Indicators of the Samples
2.4. ARGs Detection in Samples
2.5. ARGs Pollution Source Analysis Method
3. Results and Discussion
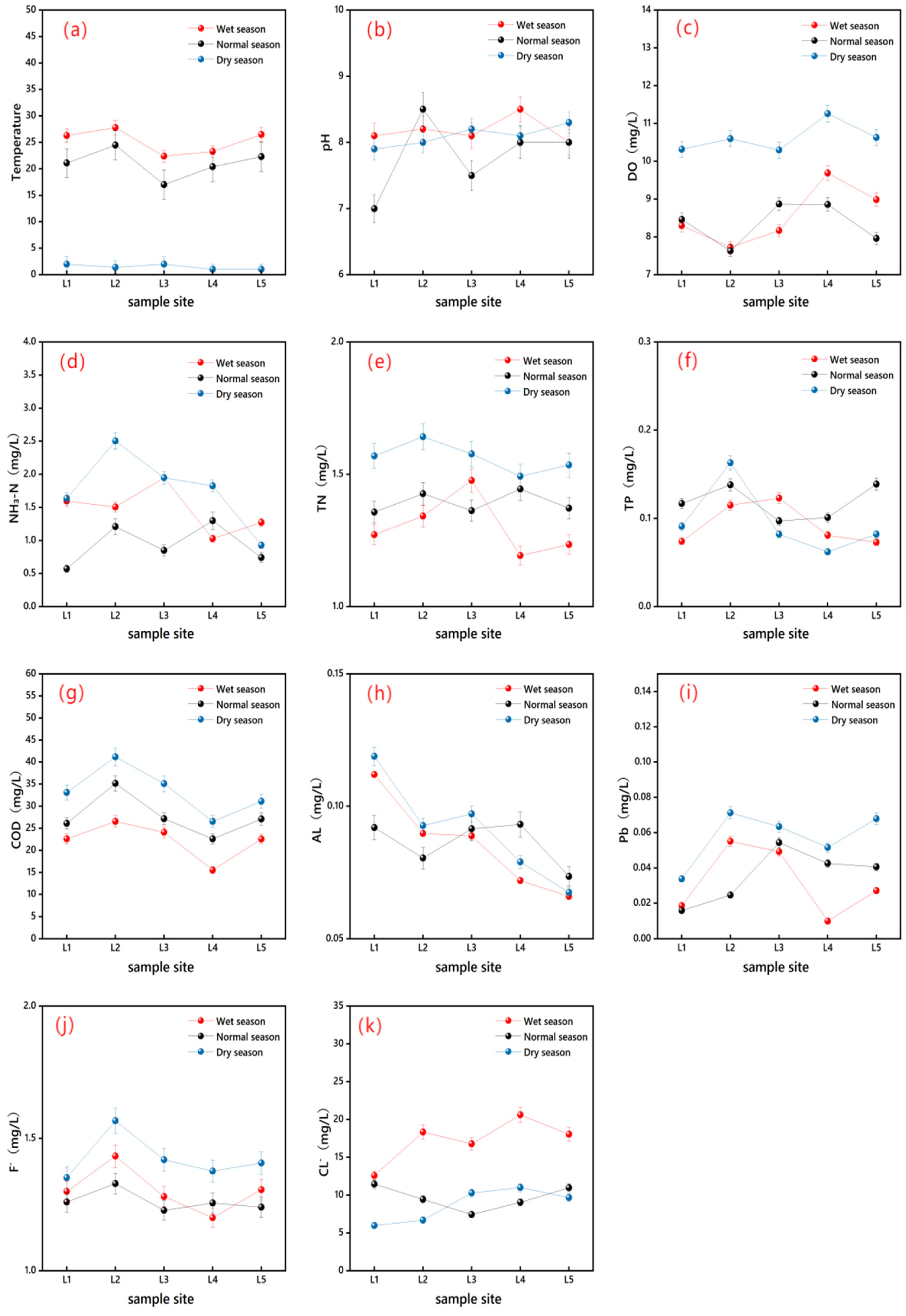
3.1. Physical and Chemical Indexes of Surface Water
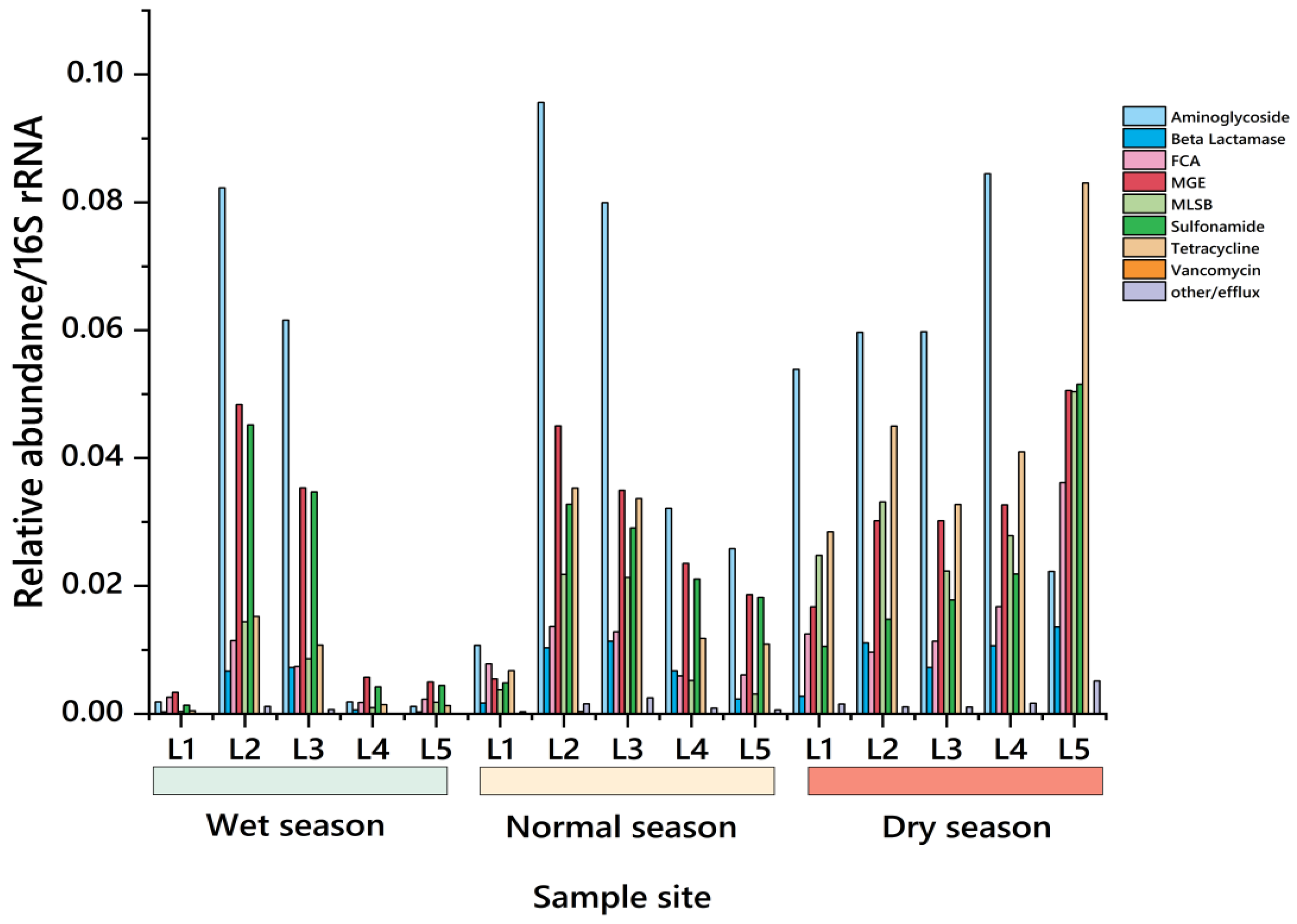
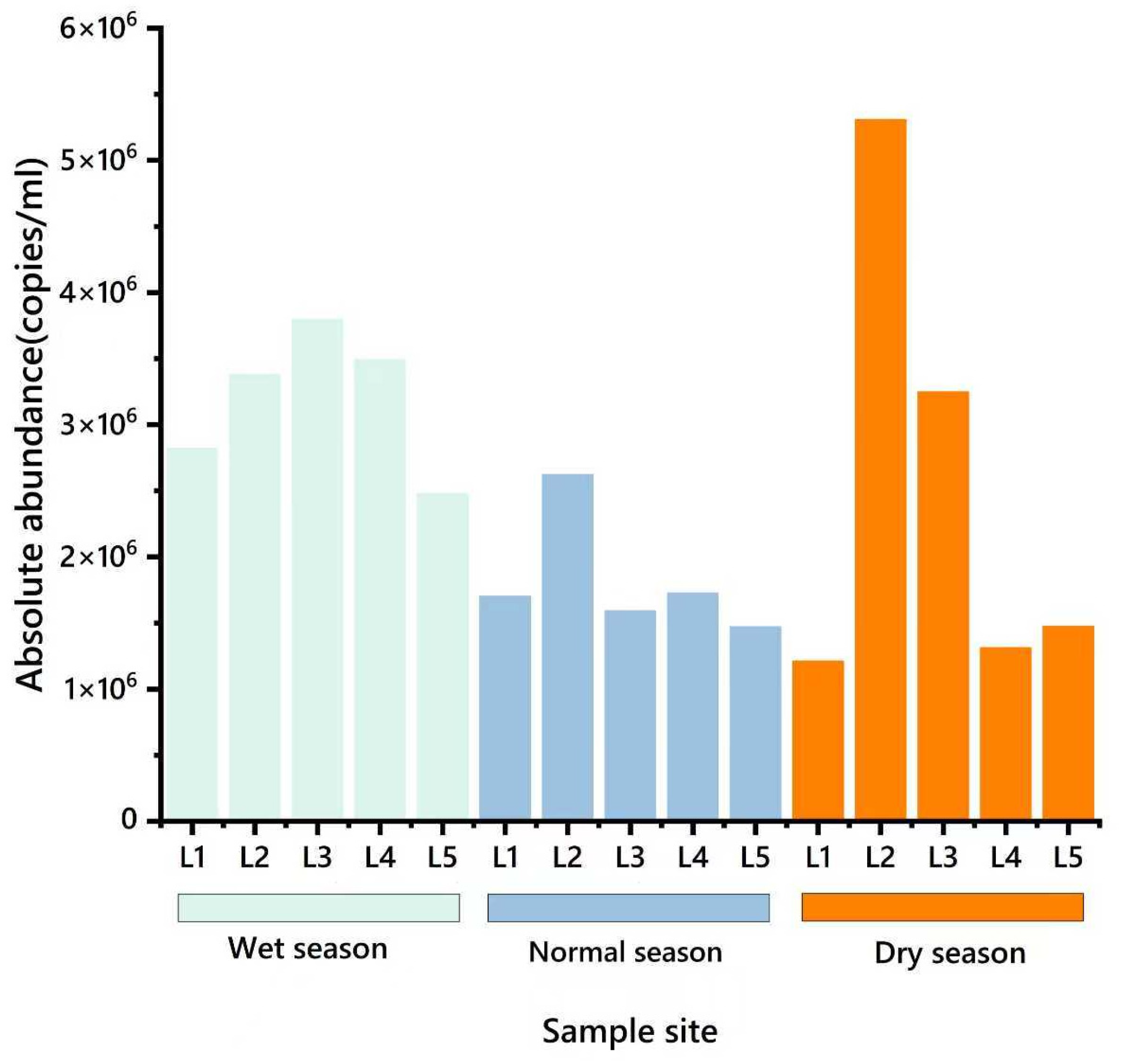
3.2. Spatial and Temporal Distribution Characteristics of ARGs in the Water Environment
3.2.1. Temporal Distribution Characteristics of ARGs in the River Basin
3.2.2. Spatial Distribution Characteristics of ARGs in Watershed
3.3. Correlation Analysis Between ARGs and Environmental Factors
3.4. ARGs Pollution Source Analysis
3.4.1. Establishment of the PCA–MLR Model
- log10(ARGi): Log-transformed abundance of the i-th ARG (dependent variable);
- β0: the intercept;
- βj: the regression coefficient;
- k: Number of principal components (k = 3 in this study);
- ε: the error;
- PCj: jth principal component score (independent variable).
3.4.2. Model Validation and Reliability Assessment
4. Conclusions
- (1)
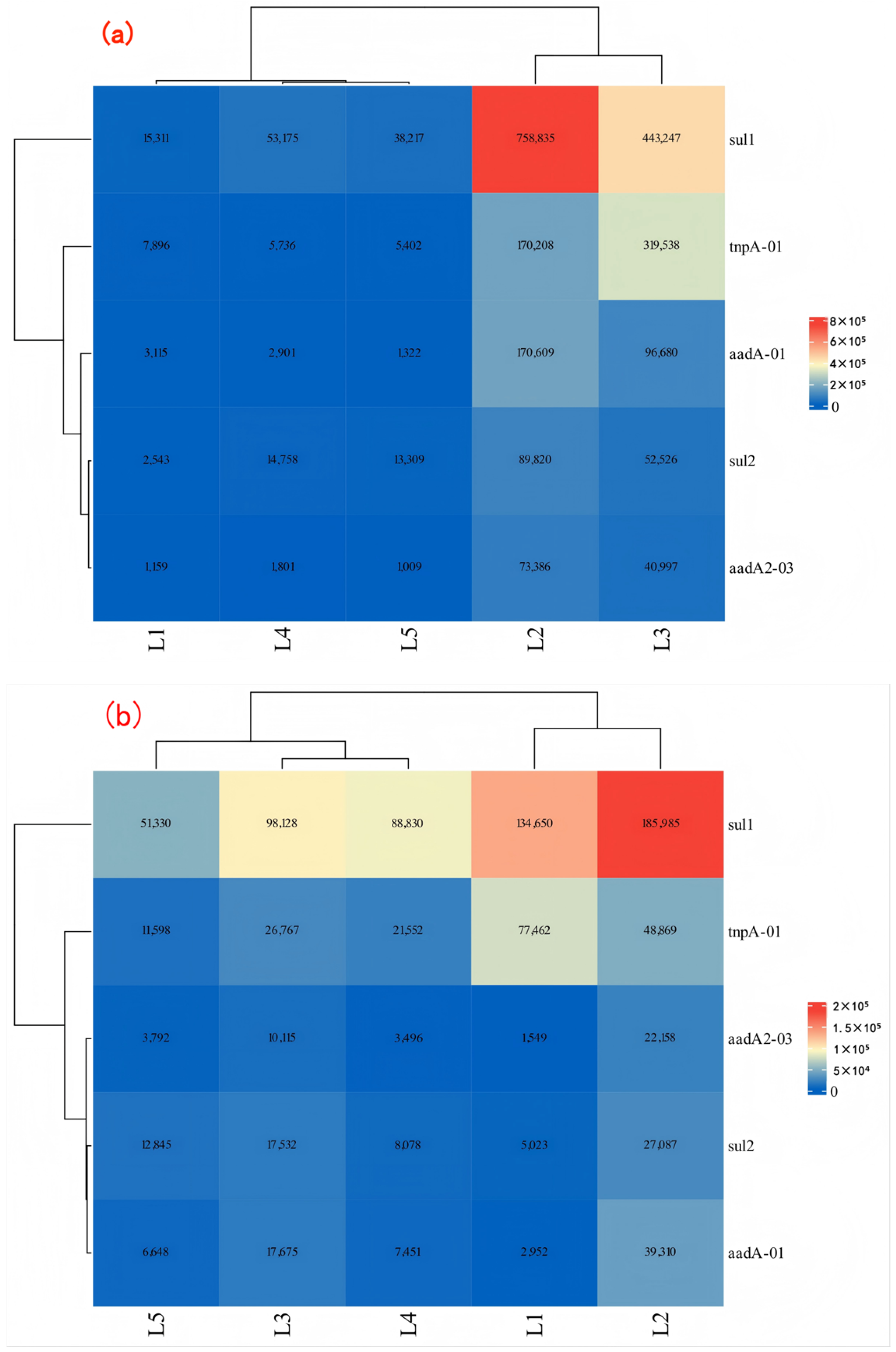
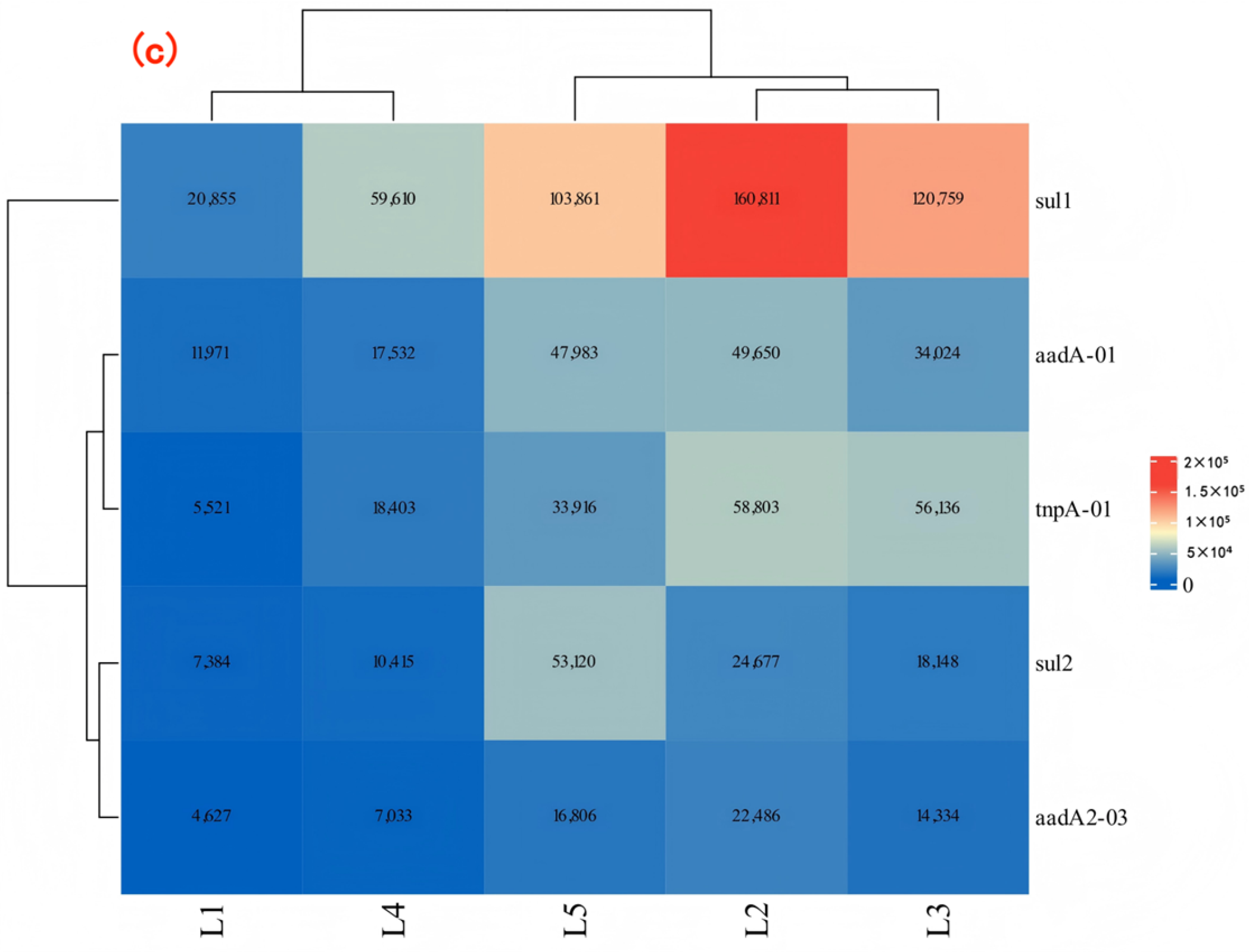
- In the water environment of the Dongliao River Basin, the pollution of ARGs has shown a wide distribution trend. The monitoring data showed that the relative abundance of the seven kinds of ARGs in surface water was detected, with the relative abundances of ARGs of SAs, AMs, and TCs being relatively large. The data showed that the absolute abundance of sul1 was large, which indicates that it contributed a lot to ARGs pollution in the Dongliao River basin.
- (2)
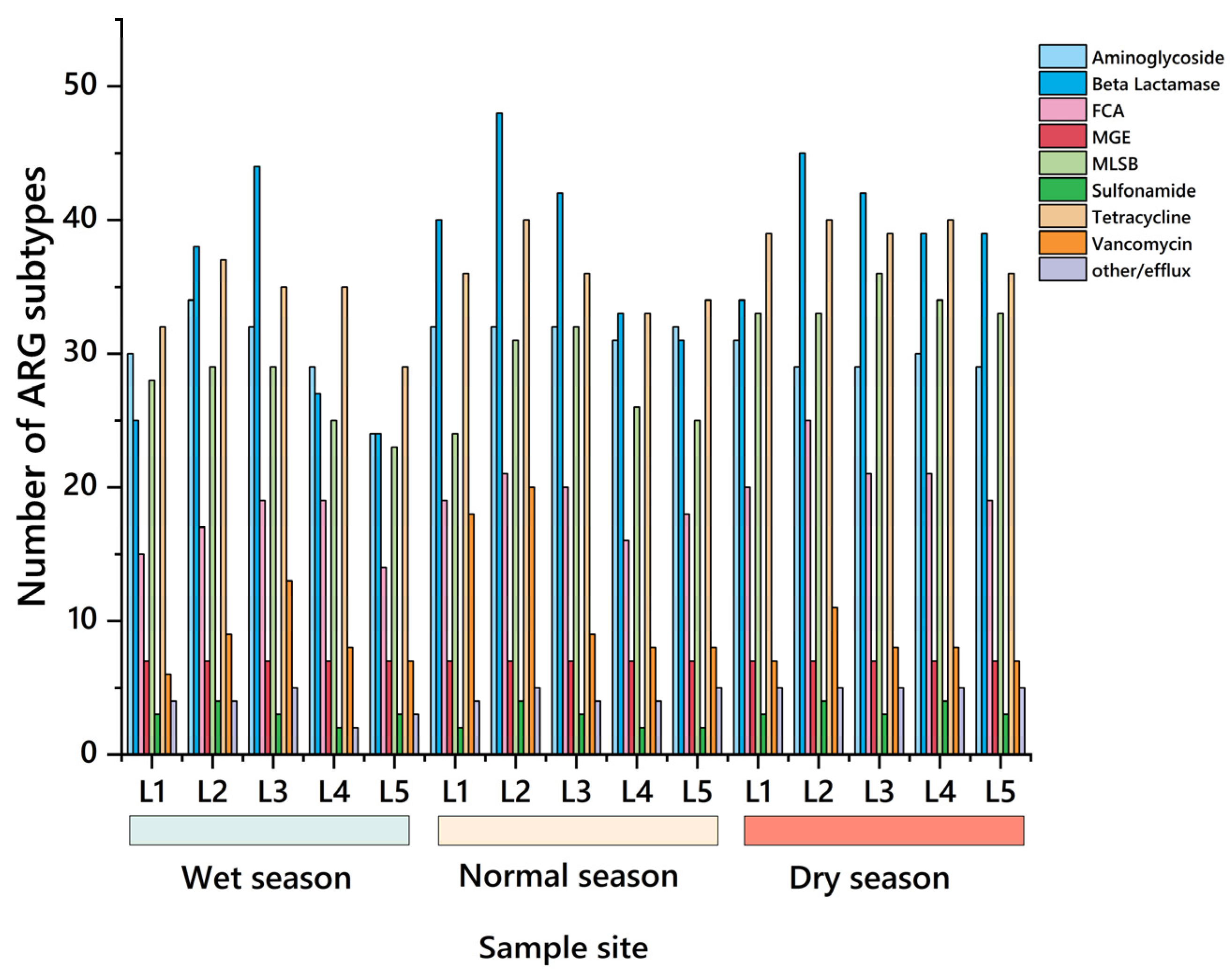
- The detected subtypes results showed that the number of gene subtypes was the highest in the dry season, with 180–200 at each sampling point, and 7 MGEs were detected at the same time, among which β-lactams and MLSB were more, with 39–50 and 30–36 gene subtypes. Therefore, in the water environment of the Dongliao River basin, β-β-lactam ARGs had high diversity, which might pose a potential danger to the ecological environment of the basin.
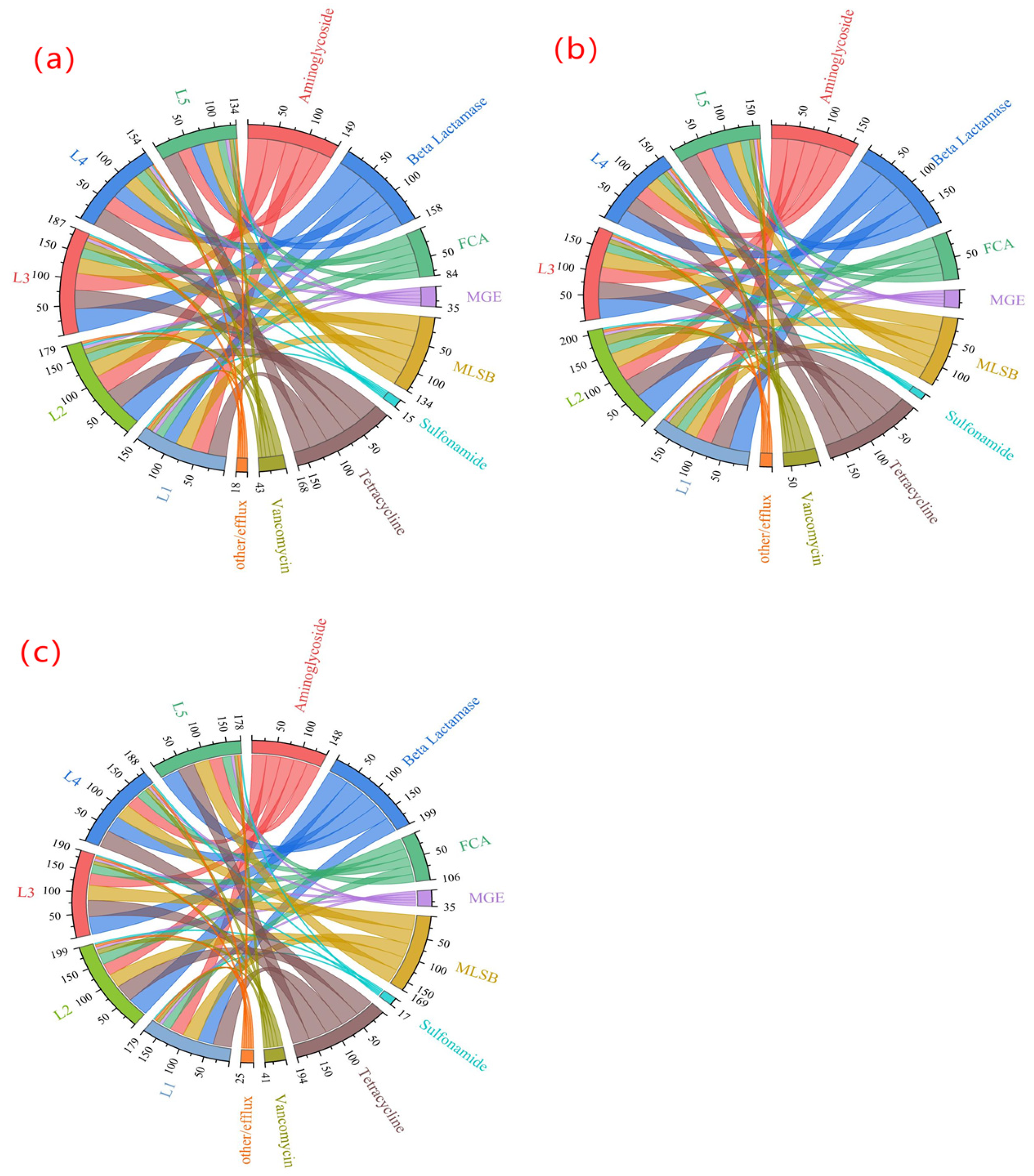
- (3)
- The total number of genes detected in the wet season was 134–187, and MGEs were 7, among which the ARGs subtypes of L2 and L3 were the most, reaching 180 and 184. The total number of genes detected in the normal season was 162–208, and MGEs were still 7. The ARG subtypes of L2 and L3 were the most, with 208 and 185. The total number of genes detected in the dry season was 180–200, with 7 MGEs, and the ARGs subtypes of L2 and L3 were the most, with 200 and 199. Therefore, it can be seen that in the three water periods, the number of ARG subtypes at sampling points L2 and L3 was always at a high level, which clearly pointed out that the ARG pollution problem was particularly prominent in densely populated areas of agriculture and animal husbandry.
- (4)
- Analysis of river physicochemical indicators and resistance gene abundance using the PCA–MLR model revealed that sulfonamide resistance genes (sul1, sul2) were primarily driven by nutrient pollution. Aminoglycoside resistance genes (aadA2–03, aadA–01) exhibited sensitivity to temperature gradients, with significant proliferation during high–temperature seasons. The PCA–MLR model provides a scientific basis for pollution source control, risk assessment, and precision management. Future studies are recommended to expand sample size, incorporate nonlinear models, and integrate biological factors to further enhance the model’s predictive capability and applicability.
- (5)
- This study marked the beginning of long–term monitoring research on ARGs in the Dongliao River. It had limitations such as insufficient sampling points and the absence of ARG detection in sediments. Future research will incorporate sediment and vertical profile samples. Should further investigation into ARGs in the Dongliao River water environment be required, continuous monitoring will need to be maintained.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jarab, A.S.; Mukattash, T.L.; Nusairat, B.; Shawaqfeh, M.; Abu Farha, R. Patterns of Antibiotic Use and Administration in Hospitalized Patients in Jordan. Saudi Pharm. J. 2018, 26, 764–770. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, M. Occurrence and removal of antibiotic resistance genes in municipal wastewater and rural domestic sewage treatment systems in eastern China. Environ. Int. 2013, 55, 9–14. [Google Scholar] [CrossRef]
- Gallagher, M.T.; Reisinger, A.J. Effects of ciprofloxacin on metabolic activity and algal biomass of urban stream biofilms. Sci. Total Environ. 2019, 706, 135728. [Google Scholar] [CrossRef]
- Gracia–Marín, E.; Rico, A.; Fabregat–Safont, D.; López, F.J.; Hernández, F.; Pitarch, E.; Bijlsma, L. Comprehensive study on the potential environmental risk of temporal antibiotic usage through wastewater discharges. Chemosphere 2023, 346, 140587. [Google Scholar] [CrossRef] [PubMed]
- Gros, M.; Mas–Pla, J.; Sànchez–Melsió, A.; Čelić, M.; Castaño, M.; Rodríguez–Mozaz, S.; Borrego, C.M.; Balcázar, J.L.; Petrović, M. Antibiotics, antibiotic resistance and associated risk in natural springs from an agroecosystem environment. Sci. Total Environ. 2022, 857, 159202. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.P.; Zhao, S.; Chen, Y.R.; Yang, J.; Hou, L.J.; Liu, M.; Yang, Y. Antibiotic resistance genes in sediments of the Yangtze Estuary: From 2007 to 2019. Sci. Total Environ. 2020, 744, 140713. [Google Scholar] [CrossRef]
- Na, G.; Zhang, K.; Gao, H.; Li, R.; Jin, S.; Zhao, F.; Zhang, H.; Li, S. Occurrence and distribution characteristics of antibiotic resistance genes in sediments between urban and rural of the Liaohe River Basin, China. Environ. Sci. Pollut. Res. 2021, 28, 54002–54014. [Google Scholar] [CrossRef]
- Li, J.; Feng, S.; Liu, X.; Jia, X.; Qiao, F.; Guo, J.; Deng, S. Effects of Traditional Chinese Medicine and its Active Ingredients on Drug–Resistant Bacteria. Front. Pharmacol. 2022, 13, 837907. [Google Scholar] [CrossRef]
- Guo, Z.F.; Boeing, W.J.; Xu, Y.Y.; Borgomeo, E.; Liu, D.; Zhu, Y.G. Data–driven discoveries on widespread contamination of freshwater reservoirs by dominant antibiotic resistance genes. Water Res. 2022, 229, 119466. [Google Scholar] [CrossRef]
- Jiang, L.; Zhai, W.; Wang, J.; Li, G.; Zhou, Z.; Li, B.; Zhuo, H. Antibiotics and antibiotic resistance genes in the water sources of the Wuhan stretch of the Yangtze River: Occurrence, distribution, and ecological risks. Environ. Res. 2023, 239, 117295. [Google Scholar] [CrossRef]
- Su, S.; Li, C.; Yang, J.; Xu, Q.; Qiu, Z.; Xue, B.; Wang, S.; Zhao, C.; Xiao, Z.; Wang, J.; et al. Distribution of Antibiotic Resistance Genes in Three Different Natural Water Bodies–A Lake, River and Sea. Int. J. Environ. Res. Public Health. 2020, 17, 552. [Google Scholar] [CrossRef]
- Givens, C.E.; Kolpin, D.W.; Hubbard, L.E.; Meppelink, S.M.; Cwiertny, D.M.; Thompson, D.A.; Lane, R.F.; Wilson, M.C. Simultaneous Stream Assessment of Antibiotics, Bacteria, Antibiotic Resistant Bacteria, and Antibiotic Resistance Genes in an Agricultural Region of the United States. Sci. Total Environ. 2023, 904, 166753. [Google Scholar] [CrossRef]
- Jiang, L.; Hu, X.; Xu, T.; Zhang, H.; Sheng, D.; Yin, D. Prevalence of Antibiotic Resistance Genes and Their Relationship with Antibiotics in the Huangpu River and the Drinking Water Sources, Shanghai, China. Sci. Total Environ. 2013, 458–460, 267–272. [Google Scholar] [CrossRef]
- Muoghalu, C.; Kaboggoza, H.C.; Semiyaga, S.; Lebu, S.; Lui, C.; Niwagaba, C.; Nansubuga, F.; Manga, M. Antibiotic Resistant Bacteria (ARB) and Genes (ARGs) in Urban Wastewater Treatment Plants: Influencing Factors, Mechanisms, and Removal Efficiency. Environ. Pollut. 2025, 383, 126851. [Google Scholar] [CrossRef] [PubMed]
- Silvester, R.; Perry, W.B.; Webster, G.; Rushton, L.; Baldwin, A.; Pass, D.A.; Byrnes, N.A.; Farkas, K.; Heginbothom, M.; Craine, N.; et al. Metagenomic Profiling of Hospital Wastewater: A Comprehensive National Scale Analysis of Antimicrobial Resistance Genes and Opportunistic Pathogens. J. Infect. 2025, 90, 106503. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhang, S.; Zhu, R.; Zhao, M.; Zhang, Y.; Wang, Y.; Liao, X.; Wu, Y.; Mi, J. Distribution and Driving Factors of Antibiotic Resistance Genes in Treated Wastewater from Different Types of Livestock Farms. Sci. Total Environ. 2022, 849, 157837. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, A.K.T.; Schachner–Groehs, I.; Linke, R.B.; Farnleitner, A.H. Correspondence on “Tracking Sources and Dissemination of Indicator Antibiotic Resistance Genes at a Watershed Scale”. Be Aware of DNA Loss during Extraction from Water Samples! ACS EST Water 2024, 4, 4658–4660. [Google Scholar] [CrossRef]
- Wiesner–Friedman, C.; Beattie, R.E.; Stewart, J.R.; Hristova, K.R.; Serre, M.L. Identifying Sources of Antibiotic Resistance Genes in the Environment Using the Microbial Find, Inform, and Test Framework. Front. Microbiol. 2023, 14, 1223876. [Google Scholar] [CrossRef]
- Zhang, A.N.; Hou, C.J.; Negi, M.; Li, L.G.; Zhang, T. Online Searching Platform for the Antibiotic Resistome in Bacterial Tree of Life and Global Habitats. FEMS Microbiol. Ecol. 2020, 96, fiaa107. [Google Scholar] [CrossRef]
- Wang, H.; Hou, L.; Liu, Y.; Liu, K.; Zhang, L.; Huang, F.; Wang, L.; Rashid, A.; Hu, A.; Yu, C. Horizontal and Vertical Gene Transfer Drive Sediment Antibiotic Resistome in an Urban Lagoon System. J. Environ. Sci. 2020, 102, 11–23. [Google Scholar] [CrossRef]
- Sanganyado, E.; Gwenzi, W. Antibiotic Resistance in Drinking Water Systems: Occurrence, Removal, and Human Health Risks. Sci. Total. Environ. 2019, 669, 785–797. [Google Scholar] [CrossRef]
- Zhu, D.; Xiang, Q.; Yang, X.R.; Ke, X.; O’Connor, P.; Zhu, Y.G. Trophic Transfer of Antibiotic Resistance Genes in a Soil Detritus Food Chain. Environ. Sci. Technol. 2019, 53, 7770–7781. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.C.; Shuai, X.Y.; Lin, Z.J.; Zheng, J.; Chen, H. Comprehensive Profiling and Risk Assessment of Antibiotic Resistance Genes in a Drinking Water Watershed by Integrated Analysis of Air–Water–Soil. J. Environ. Manag. 2023, 347, 119092. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Pan, X.; Grossart, H.P.; Lin, L.; Shi, J.; Yang, Y. Vertical and Horizontal Distributions of Clinical Antibiotic Resistance Genes and Bacterial Communities in Danjiangkou Reservoir, China. Environ. Sci. Pollut. Res. 2021, 28, 61163–61175. [Google Scholar] [CrossRef]
- Wang, S.; Fang, L.; Sun, X.; Lu, W. Occurrence and Distribution of Antibiotic Resistance Genes in Urban Rivers with Black–Odor Water of Harbin, China. Environ. Res. 2024, 259, 119497. [Google Scholar] [CrossRef]
- Li, L.G.; Huang, Q.; Yin, X.; Zhang, T. Source Tracking of Antibiotic Resistance Genes in the Environment—Challenges, Progress, and Prospects. Water Res. 2020, 185, 116127. [Google Scholar] [CrossRef]
- HJ 91.2–2022; Technical Specifications for Surface Water Environmental Quality Monitoring. Standards Press of China: Beijing, China, 2022.
- HJ 442.4–2020; Technical Specification for Offshore Environmental Monitoring–Part 4: Offshore Sediment Monitoring. Standards Press of China: Beijing, China, 2020.
- HJ/T 91–2002; Technical Specifications Requirements for Monitoring of Surface Water and Waste Water. Standards Press of China: Beijing, China, 2002.
- GB 3838–2002; Environmental Quality Standards for Surface Water. Standards Press of China: Beijing, China, 2002.
- Ogola, H.J.O. Wastewater–Driven Nutrient Enrichment Restructures Viral Community Assembly, Host Interactions, and Ecological Function along the Nakivubo–Lake Victoria Interface in Uganda. Sci. Total Environ. 2025, 993, 180002. [Google Scholar] [CrossRef]
- Fernanda, P.A.; Liu, S.; Yuan, T.; Ramalingam, B.; Lu, J.; Sekar, R. Diversity and Abundance of Antibiotic Resistance Genes and Their Relationship with Nutrients and Land Use of the Inflow Rivers of Taihu Lake. Front. Microbiol. 2022, 13, 1009297. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Zhou, X.; Huang, L.; Wang, G.; Liao, J.; Dai, R. From “Resistance Genes Expression” to “Horizontal Migration” as Well as over Secretion of Extracellular Polymeric Substances: Sludge Microorganism’s Response to the Increasing of Long–Term Disinfectant Stress. J. Hazard. Mater. 2024, 469, 133940. [Google Scholar] [CrossRef]
- Yu, K.; Chai, B.; Zhuo, T.; Tang, Q.; Gao, X.; Wang, J.; He, L.; Lei, X.; Chen, B. Hydrostatic Pressure Drives Microbe–Mediated Biodegradation of Microplastics in Surface Sediments of Deep Reservoirs: Novel Findings from Hydrostatic Pressure Simulation Experiments. Water Res. 2023, 242, 120185. [Google Scholar] [CrossRef]
- Chen, A.; Zhang, T.; Cheng, F.; Yang, H.; Guo, Z.; Zhao, S.; Zhang, Y.-N.; Qu, J. Comprehensive Analysis and Risk Assessment of Antibiotic Contaminants, Antibiotic–Resistant Bacteria, and Resistance Genes: Patterns, Drivers, and Implications in the Songliao Basin. Environ. Pollut. 2024, 361, 124852. [Google Scholar] [CrossRef] [PubMed]
- Omeyer, L.C.M.; Duncan, E.M.; Aiemsomboon, K.; Beaumont, N.; Bureekul, S.; Cao, B.; Carrasco, L.R.; Chavanich, S.; Clark, J.R.; Cordova, M.R.; et al. Corrigendum to “Priorities to Inform Research on Marine Plastic Pollution in Southeast Asia”. Sci. Total Environ. 2022, 841, 159595. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Guan, F.; Chen, B.; Luo, P.; Guo, C.; Wu, G.; Ye, Y.; Zhou, Q.; Fang, H. Spatial and Seasonal Variations of Antibiotic Resistance Genes and Antibiotics in the Surface Waters of Poyang Lake in China. Ecotoxicol. Environ. Saf. 2020, 196, 110543. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, F.; Li, Y.; Yue, C.; Zhang, Y.; Zhou, P.; Mu, J. Influence of Anthropogenic Disturbances on Antibiotic Resistance Gene Distributions along the Minjiang River in Southeast China. J. Environ. Manag. 2022, 323, 116154. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Na, G.; Gao, H.; Wang, L.; Bao, C.; Yao, Z. Fate of Sulfonamide Resistance Genes in Estuary Environment and Effect of Anthropogenic Activities. Sci. Total Environ. 2015, 527–528, 429–438. [Google Scholar] [CrossRef]
- Tian, M.; He, X.; Feng, Y.; Wang, W.; Chen, H.; Gong, M.; Liu, D.; Clarke, J.L.; van Eerde, A. Pollution by Antibiotics and Antimicrobial Resistance in LiveStock and Poultry Manure in China, and Countermeasures. Antibiotics 2021, 10, 539. [Google Scholar] [CrossRef]
- Zhang, L.; Du, S.; Zhang, X.; Lyu, G.; Dong, D.; Hua, X.; Zhang, W.; Guo, Z. Occurrence, Distribution, and Ecological Risk of Pharmaceuticals in a Seasonally Ice–Sealed River: From Ice Formation to Melting. J. Hazard. Mater. 2020, 389, 122083. [Google Scholar] [CrossRef]
- White, D.; Lapworth, D.J.; Civil, W.; Williams, P. Tracking Changes in the Occurrence and Source of Pharmaceuticals within the River Thames, UK; from Source to Sea. Environ. Pollut. 2019, 249, 257–266. [Google Scholar] [CrossRef]
- Czatzkowska, M.; Wolak, I.; Harnisz, M.; Korzeniewska, E. Impact of Anthropogenic Activities on the Dissemination of ARGs in the Environment—A Review. Int. J. Environ. Res. Public Health 2022, 19, 12853. [Google Scholar] [CrossRef]
- Lu, X.M.; Peng, X.; Xue, F.; Qin, S.; Ye, S.; Dai, L.B. Distance Dilution of Antibiotic Resistance Genes of Sediments in an Estuary System in Relation to Coastal Cities. Environ. Pollut. 2021, 281, 116980. [Google Scholar] [CrossRef]
- Adelowo, O.O.; Fagade, O.E.; Agersø, Y. Antibiotic Resistance and Resistance Genes in Escherichia Coli from Poultry Farms, Southwest Nigeria. J. Infect. Dev. Ctries. 2014, 8, 1103–1112. [Google Scholar] [CrossRef]
- Su, H.; Liu, S.; Hu, X.; Xu, X.; Xu, W.; Xu, Y.; Li, Z.; Wen, G.; Liu, Y.; Cao, Y. Occurrence and Temporal Variation of Antibiotic Resistance Genes (ARGs) in Shrimp Aquaculture: ARGs Dissemination from Farming Source to Reared Organisms. Sci. Total. Environ. 2017, 607–608, 357–366. [Google Scholar] [CrossRef]
- Ramessar, K.; Egbewale, O.S.; Kumar, A.; Olaniran, A.O. Prevalence and Removal of Antibiotic–Resistant Bacteria and Genes in Treated Effluents of Four Wastewater Treatment Plants and Receiving Surface Waters. J. Water Process. Eng. 2025, 77, 108432. [Google Scholar] [CrossRef]
- Li, Z.; Cai, H.; Xu, B.; Dong, Q.; Jia, K.; Lin, Z.; Wang, X.; Liu, Y.; Qin, X. Prevalence, Antibiotic Resistance, Resistance and Virulence Determinants of Campylobacter Jejuni in China: A Systematic Review and Meta–Analysis. One Health 2025, 20, 100990. [Google Scholar] [CrossRef]
- Rahman, A.U.; Valentino, V.; Sequino, G.; Ercolini, D.; De Filippis, F. Comparative Analysis of Antibiotic–Administered vs. Antibiotic–Free Farming in Meat Production: Implications for Health, Environment, and Antibiotic Resistance. Food Microbiol. 2025, 133, 104877. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Cui, Y.; Tian, W.; Wei, H.; Chen, Q.; Liu, B.; Zhang, D.; Xie, B. Vessel Transport of Antibiotic Resistance Genes across Oceans and Its Implications for Ballast Water Management. Chemosphere 2020, 253, 126697. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, W.; Xu, T.; Zheng, B.; Yin, D. Occurrence and Distribution of Antibiotic Resistance Genes in the Water and Sediments of Qingcaosha Reservoir, Shanghai, China. Environ. Sci. Eur. 2019, 31, 81–89. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, X.; Niu, Z.; Lu, D.; Sun, X.; Zhao, S.; Hou, L.; Liu, M.; Yang, Y. Antibiotic Resistance Genes (ARGs) and Their Associated Environmental Factors in the Yangtze Estuary, China: From Inlet to Outlet. Mar. Pollut. Bull. 2020, 158, 111360. [Google Scholar] [CrossRef]
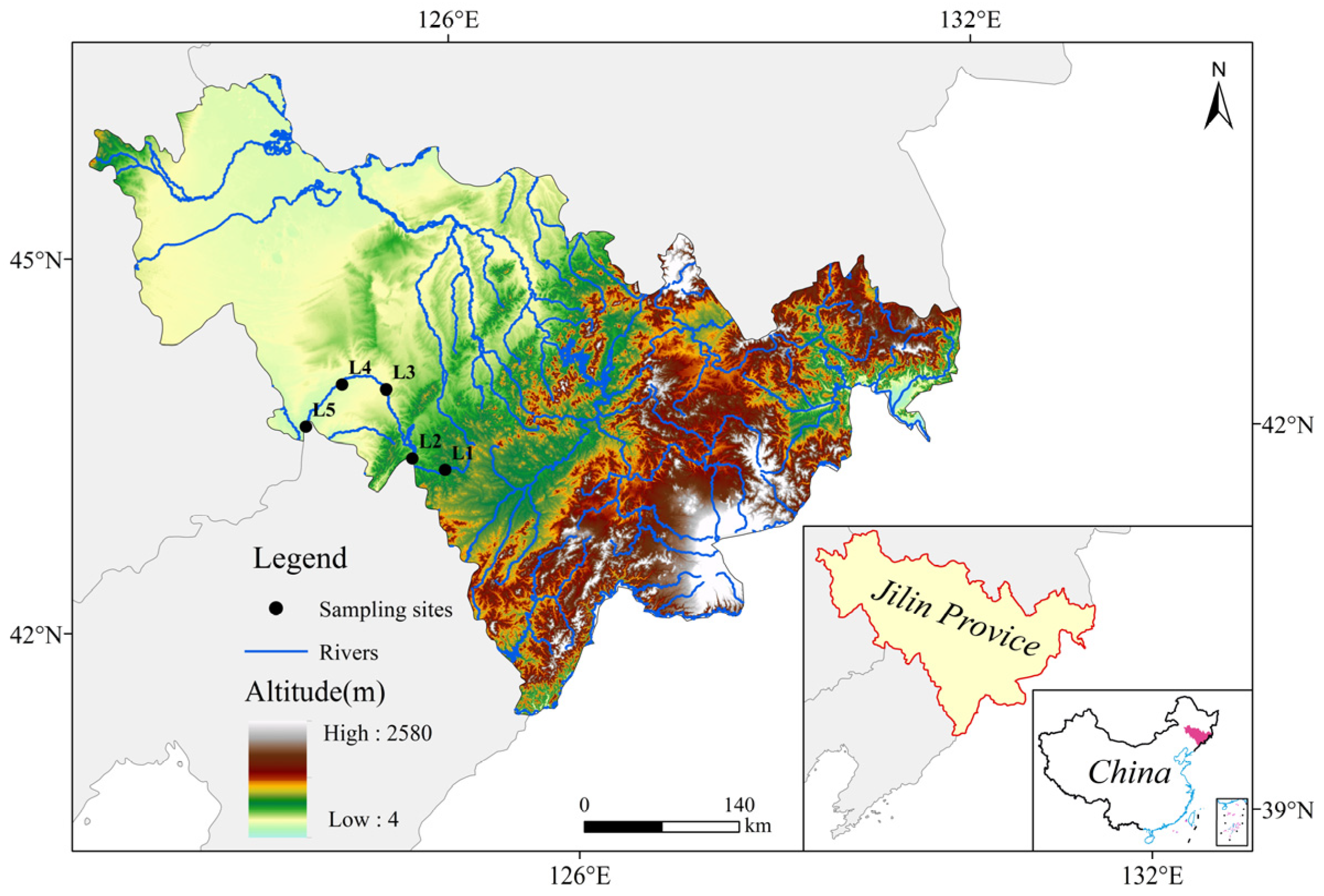

| Name of Sampling Point | Number of Sampling Points | Longitude and Latitude |
|---|---|---|
| Dashou village | L1 | (42°54′51″ N, 125°11′47″ E) |
| Heqing | L2 | (43°2′46″ N, 124°51′11″ E) |
| Chengzishang | L3 | (43°37′34″ N, 124°40′33″ E) |
| Zhoujiahekou | L4 | (43°42′57″ N, 124°11′20″ E) |
| Sishuang Bridge | L5 | (43°25′12″ N, 123°43′0″ E) |
| Principal Component | Eigenvalue | Variance Contribution Rate (%) | Cumulative Contribution Rate (%) | Primary Environmental Gradients |
|---|---|---|---|---|
| PC1 | 4.653 | 42.3 | 42.3 | Temperature–Season |
| PC2 | 3.157 | 28.7 | 71.0 | Nutrient pollution |
| PC3 | 1.673 | 15.2 | 86.2 | Heavy metal pollution |
| Environmental Factors | PC1 | PC2 | PC3 | Togetherness |
|---|---|---|---|---|
| DO | 0.350 | −0.250 | 0.200 | 0.228 |
| pH | −0.400 | 0.350 | 0.250 | 0.343 |
| Temperature | 0.450 | −0.150 | 0.100 | 0.234 |
| TN | 0.150 | 0.400 | 0.250 | 0.244 |
| TP | 0.200 | 0.350 | −0.350 | 0.294 |
| NH4+–N | 0.250 | 0.300 | −0.250 | 0.212 |
| COD | 0.300 | 0.450 | 0.150 | 0.317 |
| Al | 0.150 | 0.250 | 0.400 | 0.254 |
| Pb | 0.280 | 0.320 | 0.450 | 0.386 |
| F− | 0.200 | 0.280 | 0.350 | 0.251 |
| Cl− | −0.300 | −0.200 | 0.300 | 0.230 |
| Gene | Intercept (β0) | PC1 (β1) | PC2 (β2) | PC3 (β3) | R2 | Adjusted R2 | F | p |
|---|---|---|---|---|---|---|---|---|
| sul1 | 73,250.4 | −8623.5 | 24,851.2 | 12,305.6 | 0.742 | 0.672 | 10.5 | <0.001 |
| sul2 | 28,184.0 | −5241.8 | 18,934.7 | 15,627.3 | 0.689 | 0.6004 | 8.13 | <0.01 |
| aadA2–03 | 16,641.9 | 15,482.3 | 12,076.4 | 6835.2 | 0.715 | 0.638 | 9.21 | <0.001 |
| aadA–01 | 33,484.1 | 18,756.9 | 19,245.8 | 9124.7 | 0.758 | 0.692 | 11.5 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Zheng, Y.; Wang, L.; Cong, Q. Temporal and Spatial Distribution Characteristics and Source Analysis of Antibiotic Resistance Gene Pollution in Dongliao River Basin, China. Water 2025, 17, 3168. https://doi.org/10.3390/w17213168
Lu H, Zheng Y, Wang L, Cong Q. Temporal and Spatial Distribution Characteristics and Source Analysis of Antibiotic Resistance Gene Pollution in Dongliao River Basin, China. Water. 2025; 17(21):3168. https://doi.org/10.3390/w17213168
Chicago/Turabian StyleLu, Hai, Yang Zheng, Lijun Wang, and Qiao Cong. 2025. "Temporal and Spatial Distribution Characteristics and Source Analysis of Antibiotic Resistance Gene Pollution in Dongliao River Basin, China" Water 17, no. 21: 3168. https://doi.org/10.3390/w17213168
APA StyleLu, H., Zheng, Y., Wang, L., & Cong, Q. (2025). Temporal and Spatial Distribution Characteristics and Source Analysis of Antibiotic Resistance Gene Pollution in Dongliao River Basin, China. Water, 17(21), 3168. https://doi.org/10.3390/w17213168






