Modeling Dissolved Organic Carbon in an Estuary Using Optical Properties and Salinity
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Materials
2.3. Sample Collection for Paired Absorbance-DOC Measurements
2.4. Incubation Experiments
2.4.1. Photochemical Experiments
2.4.2. Aging Experiments
2.5. Sample Analysis
2.6. Data Analysis
3. Results
3.1. DOC Concentrations and DOM Composition
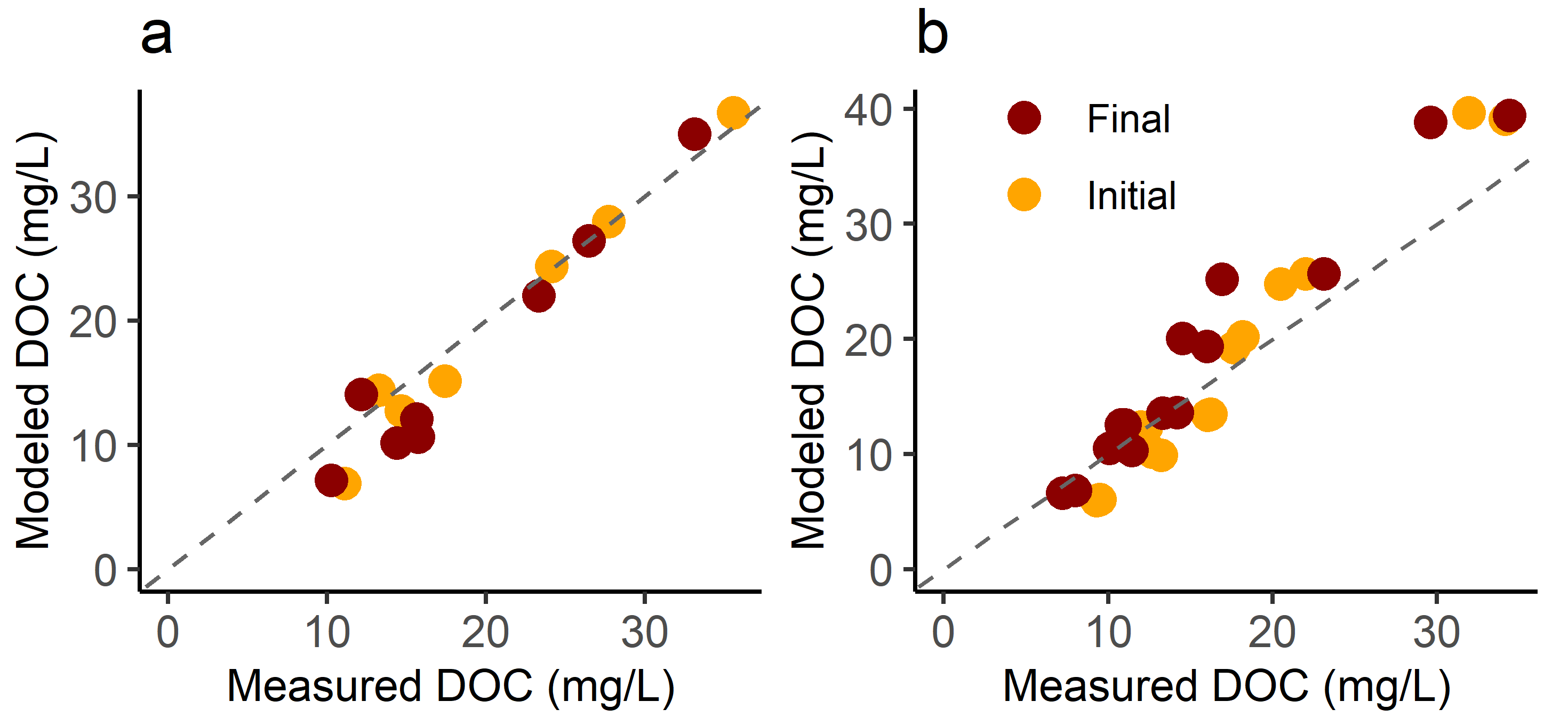
3.2. DOC Models
3.3. Incubation Experiments
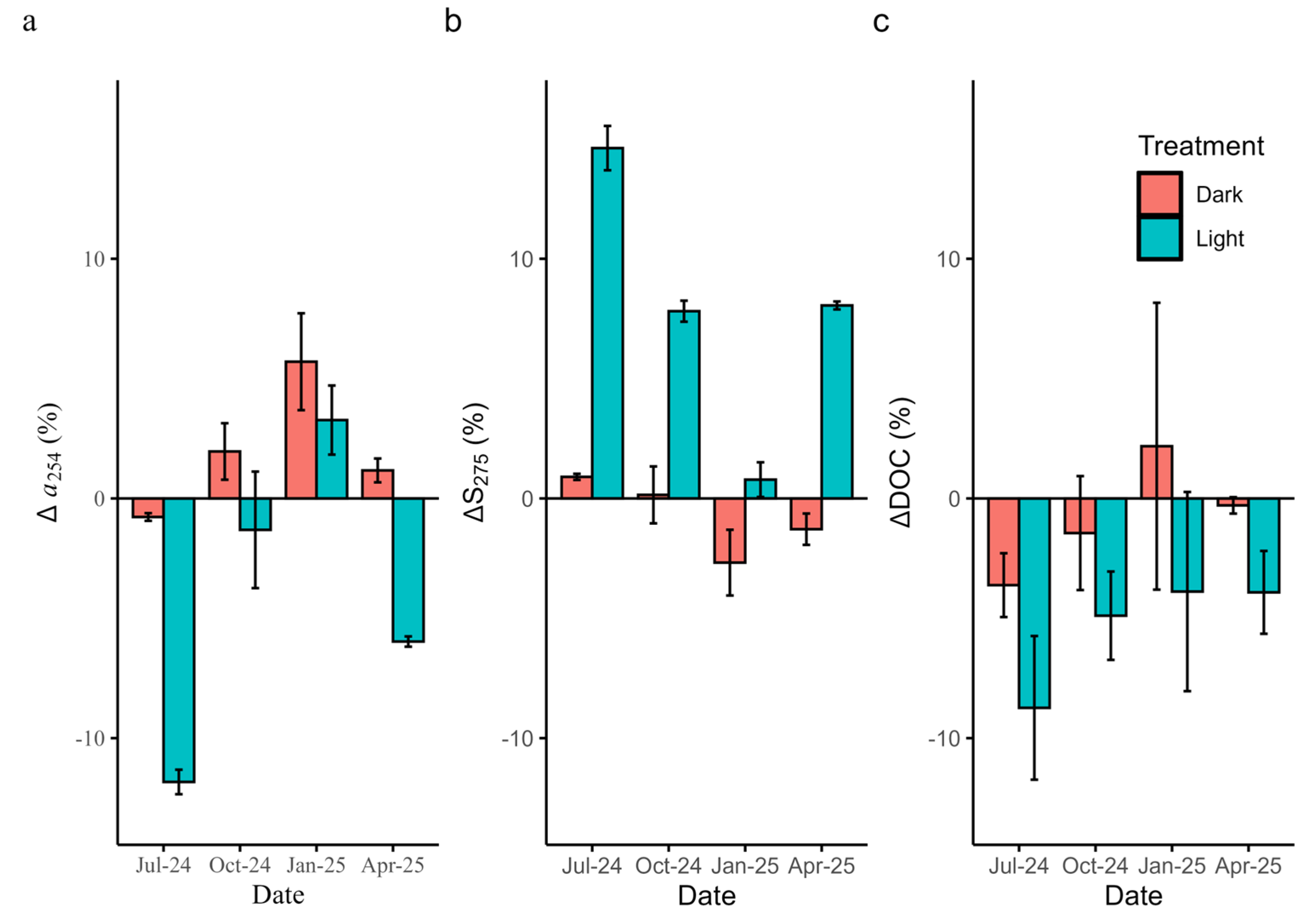
Photochemical Experiments
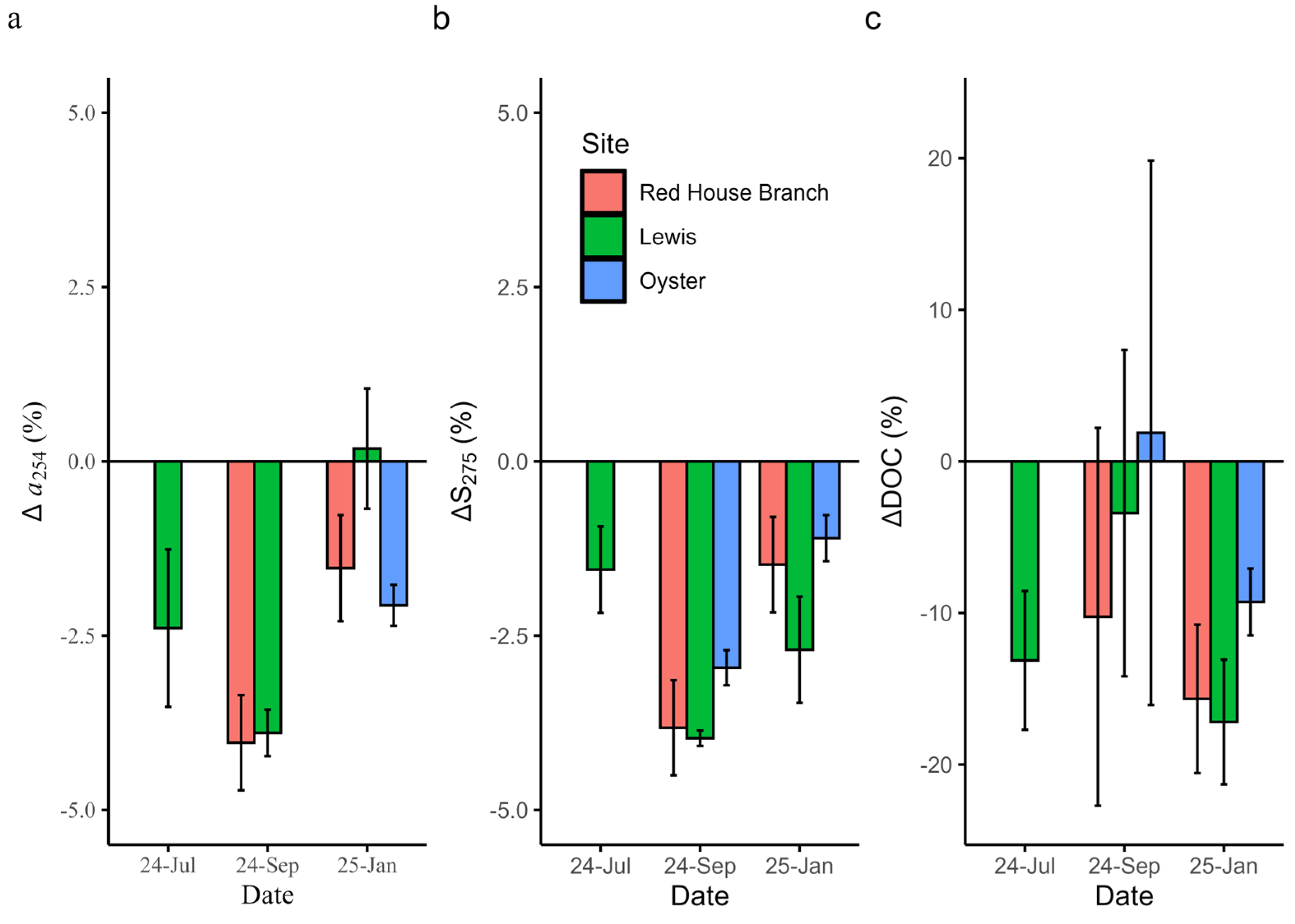
3.4. Aging Experiments
3.5. Effects of Aging and Photolysis on Model Performance
4. Discussion
4.1. DOC Concentrations and DOM Composition
4.2. DOC Models
4.3. Incubation Experiments
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIC | Akaike’s Information Criterion |
| ASW | Artificial Seawater |
| DOM | Dissolved Organic Matter |
| DOC | Dissolved Organic Carbon |
| CDOM | Colored Dissolved Organic Matter |
| HDPE | High-Density Polyethylene |
| MAE | Mean Absolute Error |
| MCE | Mixed Cellulose Ester |
| RMSE | Root Mean Square Error |
| SUVA254 | Specific Ultraviolet Absorbance |
| VIF | Variance Inflation Factor |
References
- Barrón, C.; Duarte, C.M. Dissolved Organic Carbon Pools and Export from the Coastal Ocean. Glob. Biogeochem. Cycles 2015, 29, 1725–1738. [Google Scholar] [CrossRef]
- Hansell, D.A.; Carlson, C.A.; Repeta, D.J.; Schlitzer, R. Dissolved Organic Matter in the Ocean: A Controversy Stimulates New Insights. Oceanography 2009, 22, 202–211. [Google Scholar] [CrossRef]
- Muller, F.L.L.; Tankéré-Muller, S.P.C.; Tang, C.-H. Terrigenous Humic Substances Regulate the Concentrations of Dissolved Fe and Cu (but Not Al, Mn, Ni or Zn) in the Gaoping River Plume. Sci. Total Environ. 2024, 906, 167374. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, S.M.; Tanton, T.W. Mercury in the Aquatic Environment: A Review of Factors Affecting Methylation. Crit. Rev. Environ. Sci. Technol. 2001, 31, 241–293. [Google Scholar] [CrossRef]
- Krachler, R.; Krachler, R.; Valda, A.; Keppler, B.K. Natural Iron Fertilization of the Coastal Ocean by “Blackwater Rivers”. Sci. Total Environ. 2019, 656, 952–958. [Google Scholar] [CrossRef]
- Garrison, C.E.; Roozbehi, S.; Mitra, S.; Corbett, D.R.; Field, E.K. Coastal Microbial Communities Disrupted During the 2018 Hurricane Season in Outer Banks, North Carolina. Front. Microbiol. 2022, 13, 816573. [Google Scholar] [CrossRef]
- Kieber, D.J.; Powers, L.C.; Stubbins, A.; Miller, W.L. Chapter 11—Marine Photochemistry of Organic Matter: Processes and Impacts. In Biogeochemistry of Marine Dissolved Organic Matter, 3rd ed.; Hansell, D.A., Carlson, C.A., Eds.; Academic Press: Amsterdam, The Netherlands, 2024; pp. 507–585. ISBN 978-0-443-13858-4. [Google Scholar]
- Sulzberger, B.; Durisch-Kaiser, E. Chemical Characterization of Dissolved Organic Matter (DOM): A Prerequisite for Understanding UV-Induced Changes of DOM Absorption Properties and Bioavailability. Aquat. Sci. 2009, 71, 104–126. [Google Scholar] [CrossRef]
- Bushaw, K.L.; Zepp, R.G.; Tarr, M.A.; Schulz-Jander, D.; Bourbonniere, R.A.; Hodson, R.E.; Miller, W.L.; Bronk, D.A.; Moran, M.A. Photochemical Release of Biologically Available Nitrogen from Aquatic Dissolved Organic Matter. Nature 1996, 381, 404–407. [Google Scholar] [CrossRef]
- D’Andrilli, J.; Silverman, V.; Buckley, S.; Rosario-Ortiz, F.L. Inferring Ecosystem Function from Dissolved Organic Matter Optical Properties: A Critical Review. Environ. Sci. Technol. 2022, 56, 11146–11161. [Google Scholar] [CrossRef]
- Coble, P.G. Marine Optical Biogeochemistry: The Chemistry of Ocean Color. Chem. Rev. 2007, 107, 402–418. [Google Scholar] [CrossRef]
- Bricaud, A.; Morel, A.; Prieur, L. Absorption by Dissolved Organic Matter of the Sea (Yellow Substance) in the UV and Visible Domains1. Limnol. Oceanogr. 1981, 26, 43–53. [Google Scholar] [CrossRef]
- Conmy, R.N.; Schaeffer, B.A.; Schubauer-Berigan, J.; Aukamp, J.; Duffy, A.; Lehrter, J.C.; Greene, R.M. Characterizing Light Attenuation Within Northwest Florida Estuaries: Implications for RESTORE Act Water Quality Monitoring. Mar. Pollut. Bull. 2017, 114, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Sanwlani, N.; Lee, T.W.Q.; Wong, J.M.C.; Chang, K.Y.W.; Wong, E.W.-S.; Liew, S.C. Dissolved Organic Matter from Tropical Peatlands Reduces Shelf Sea Light Availability in the Singapore Strait, Southeast Asia. Mar. Ecol. Prog. Ser. 2021, 672, 89–109. [Google Scholar] [CrossRef]
- Berezovski, A.; Hessen, D.O.; Andersen, T. Photon Budgets and the Relative Effects of CDOM and Pigment Absorptions on Primary Production Along a Coastal Salinity Gradient. Front. Photobiol. 2025, 2, 1452747. [Google Scholar] [CrossRef]
- Peltzer, E.T.; Fry, B.; Doering, P.H.; McKenna, J.H.; Norrman, B.; Zweifel, U.L. A Comparison of Methods for the Measurement of Dissolved Organic Carbon in Natural Waters. Mar. Chem. 1996, 54, 85–96. [Google Scholar] [CrossRef]
- Forsberg, C. Dissolved Organic Carbon in Some Lakes in Uppland, Sweden. Oikos 1967, 18, 210–216. [Google Scholar] [CrossRef]
- Li, P.; Hur, J. Utilization of UV-Vis Spectroscopy and Related Data Analyses for Dissolved Organic Matter (DOM) Studies: A Review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 131–154. [Google Scholar] [CrossRef]
- Zhang, M.; He, Z. Characteristics of Dissolved Organic Carbon Revealed by Ultraviolet-Visible Absorbance and Fluorescence Spectroscopy: The Current Status and Future Exploration. In Labile Organic Matter—Chemical Compositions, Function, and Significance in Soil and the Environment; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 1–21. ISBN 978-0-89118-963-3. [Google Scholar]
- Hansen, A.M.; Kraus, T.E.C.; Pellerin, B.A.; Fleck, J.A.; Downing, B.D.; Bergamaschi, B.A. Optical Properties of Dissolved Organic Matter (DOM): Effects of Biological and Photolytic Degradation. Limnol. Oceanogr. 2016, 61, 1015–1032. [Google Scholar] [CrossRef]
- Helms, J.R.; Stubbins, A.; Ritchie, J.D.; Minor, E.C.; Kieber, D.J.; Mopper, K. Absorption Spectral Slopes and Slope Ratios as Indicators of Molecular Weight, Source, and Photobleaching of Chromophoric Dissolved Organic Matter. Limnol. Oceanogr. 2008, 53, 955–969. [Google Scholar] [CrossRef]
- Helms, J.R.; Stubbins, A.; Perdue, E.M.; Green, N.W.; Chen, H.; Mopper, K. Photochemical Bleaching of Oceanic Dissolved Organic Matter and Its Effect on Absorption Spectral Slope and Fluorescence. Mar. Chem. 2013, 155, 81–91. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S. Behaviour of the Optical Properties of Coloured Dissolved Organic Matter under Conservative Mixing. Estuar. Coast. Shelf Sci. 2003, 57, 973–979. [Google Scholar] [CrossRef]
- Cao, F.; Tzortziou, M.; Hu, C.; Mannino, A.; Fichot, C.G.; Del Vecchio, R.; Najjar, R.G.; Novak, M. Remote Sensing Retrievals of Colored Dissolved Organic Matter and Dissolved Organic Carbon Dynamics in North American Estuaries and Their Margins. Remote Sens. Environ. 2018, 205, 151–165. [Google Scholar] [CrossRef]
- Fichot, C.G.; Lohrenz, S.E.; Benner, R. Pulsed, Cross-Shelf Export of Terrigenous Dissolved Organic Carbon to the Gulf of Mexico. J. Geophys. Res. Ocean. 2014, 119, 1176–1194. [Google Scholar] [CrossRef]
- Keith, D.J.; Lunetta, R.S.; Schaeffer, B.A. Optical Models for Remote Sensing of Colored Dissolved Organic Matter Absorption and Salinity in New England, Middle Atlantic and Gulf Coast Estuaries USA. Remote Sens. 2016, 8, 283. [Google Scholar] [CrossRef]
- Osburn, C.L.; Boyd, T.J.; Montgomery, M.T.; Bianchi, T.S.; Coffin, R.B.; Paerl, H.W. Optical Proxies for Terrestrial Dissolved Organic Matter in Estuaries and Coastal Waters. Front. Mar. Sci. 2016, 2, 127. [Google Scholar] [CrossRef]
- Avagyan, A.; Runkle, B.R.K.; Kutzbach, L. Application of High-Resolution Spectral Absorbance Measurements to Determine Dissolved Organic Carbon Concentration in Remote Areas. J. Hydrol. 2014, 517, 435–446. [Google Scholar] [CrossRef]
- Carter, H.T.; Tipping, E.; Koprivnjak, J.-F.; Miller, M.P.; Cookson, B.; Hamilton-Taylor, J. Freshwater DOM Quantity and Quality from a Two-Component Model of UV Absorbance. Water Res. 2012, 46, 4532–4542. [Google Scholar] [CrossRef] [PubMed]
- Codden, C.J.; Snauffer, A.M.; Mueller, A.V.; Edwards, C.R.; Thompson, M.; Tait, Z.; Stubbins, A. Predicting Dissolved Organic Carbon Concentration in a Dynamic Salt Marsh Creek via Machine Learning. Limnol. Oceanogr. Methods 2021, 19, 81–95. [Google Scholar] [CrossRef]
- Harvey, E.T.; Kratzer, S.; Andersson, A. Relationships between Colored Dissolved Organic Matter and Dissolved Organic Carbon in Different Coastal Gradients of the Baltic Sea. AMBIO 2015, 44, 392–401. [Google Scholar] [CrossRef]
- Fichot, C.G.; Benner, R. The Spectral Slope Coefficient of Chromophoric Dissolved Organic Matter (S275–295) as a Tracer of Terrigenous Dissolved Organic Carbon in River-Influenced Ocean Margins. Limnol. Oceanogr. 2012, 57, 1453–1466. [Google Scholar] [CrossRef]
- San Sebastian Water Quality Monitoring. Matanzas Riverkeeper. Available online: https://www.matanzasriverkeeper.org/sansebastian_sampling_2024 (accessed on 29 July 2025).
- US Census Bureau. Sunshine State Home to Metro Areas Among Top 10 U.S. Population Gainers From 2022 to 2023. Available online: https://www.census.gov/library/stories/2024/03/florida-and-fast-growing-metros.html (accessed on 11 July 2025).
- Pinto, G.; Bielmyer-Fraser, G.K.; Baynard, C.W.; Casamatta, D.; Closmann, C.; Goldberg, N.; Jones, S.F.; Johnson, A.; Penwell, W.; Pyati, R.; et al. 2024 State of the River Report for the Lower St. Johns River Basin, Florida: Water Quality, Fisheries, Aquatic Life, & Contaminants; Prepared for the City of Jacksonville, Environmental Protection Board; St. Johns Riverkeeper: Jacksonville, FL, USA, 2024. [Google Scholar]
- Gallegos, C.L. Optical Water Quality of a Blackwater River Estuary: The Lower St. Johns River, Florida, USA. Estuar. Coast. Shelf Sci. 2005, 63, 57–72. [Google Scholar] [CrossRef]
- Final Environmental Assessment Guana Tolomato Matanzas National Estuarine Research Reserve Boundary Change; U.S. Department of Commerce, National Oceanic and Atmospheric Administration: Silver Spring, MD, USA, 2019. Available online: https://coast.noaa.gov/data/docs/compliance/gtmnerr-final-ea.pdf (accessed on 22 July 2025).
- Gray, M.W.; Pinton, D.; Canestrelli, A.; Dix, N.; Marcum, P.; Kimbro, D.; Grizzle, R. Beyond Residence Time: Quantifying Factors That Drive the Spatially Explicit Filtration Services of an Abundant Native Oyster Population. Estuaries Coasts 2022, 45, 1343–1360. [Google Scholar] [CrossRef]
- Statistics for San Sebastian River at St. Augustine, FL—USGS Water Data for the Nation. Available online: https://waterdata.usgs.gov/monitoring-location/USGS-02246895/statistics/ (accessed on 22 July 2025).
- Ettensohn, C.A.; Wessel, G.M.; Wray, G. Development of Sea Urchins, Ascidians, and Other Invertebrate Deuterostomes: Experimental Approaches; Elsevier: Amsterdam, The Netherlands, 2004; ISBN 978-0-08-049659-7. [Google Scholar]
- Schafer, T.; Dix, N.; Dunnigan, S.; Reddy, K.R.; Osborne, T.Z. Impacts of Hurricanes on Nutrient Export and Ecosystem Metabolism in a Blackwater River Estuary Complex. J. Mar. Sci. Eng. 2022, 10, 661. [Google Scholar] [CrossRef]
- Iorio, D.D.; Castelao, R.M. The Dynamical Response of Salinity to Freshwater Discharge and Wind Forcing in Adjacent Estuaries on the Georgia Coast. Oceanography 2015, 26, 44–51. [Google Scholar] [CrossRef][Green Version]
- NOAA National Estuarine Research Reserve System Wide Monitoring Program. Available online: http://www.nerrsdata.org (accessed on 17 July 2025).[Green Version]
- Florida Climate Center, Office of the State Climatologist St. Augustine-Precipitation. Available online: https://climatecenter.fsu.edu/products-services/data/precipitation/st-augustine (accessed on 23 July 2025).[Green Version]
- Omanović, D.; Santinelli, C.; Marcinek, S.; Gonnelli, M. ASFit—An All-Inclusive Tool for Analysis of UV–Vis Spectra of Colored Dissolved Organic Matter (CDOM). Comput. Geosci. 2019, 133, 104334. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, L.; Pumpanen, J.; Keinänen, M.; Laudon, H.; Ojala, A.; Palviainen, M.; Kiirikki, M.; Neitola, K.; Berninger, F. Assessment of a Portable UV–Vis Spectrophotometer’s Performance for Stream Water DOC and Fe Content Monitoring in Remote Areas. Talanta 2021, 224, 121919. [Google Scholar] [CrossRef] [PubMed]
- Logozzo, L.; Tzortziou, M.; Neale, P.; Clark, J.B. Photochemical and Microbial Degradation of Chromophoric Dissolved Organic Matter Exported from Tidal Marshes. J. Geophys. Res. Biogeosci. 2021, 126, e2020JG005744. [Google Scholar] [CrossRef]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of Specific Ultraviolet Absorbance as an Indicator of the Chemical Composition and Reactivity of Dissolved Organic Carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef]
- Yan, G.; Labonté, J.M.; Quigg, A.; Kaiser, K. Hurricanes Accelerate Dissolved Organic Carbon Cycling in Coastal Ecosystems. Front. Mar. Sci. 2020, 7, 248. [Google Scholar] [CrossRef]
- Nguyen, H.V.-M.; Hur, J.; Shin, H.-S. Changes in Spectroscopic and Molecular Weight Characteristics of Dissolved Organic Matter in a River During a Storm Event. Water Air Soil Pollut. 2010, 212, 395–406. [Google Scholar] [CrossRef]
- Bittar, T.B.; Berger, S.A.; Birsa, L.M.; Walters, T.L.; Thompson, M.E.; Spencer, R.G.M.; Mann, E.L.; Stubbins, A.; Frischer, M.E.; Brandes, J.A. Seasonal Dynamics of Dissolved, Particulate and Microbial Components of a Tidal Saltmarsh-Dominated Estuary under Contrasting Levels of Freshwater Discharge. Estuar. Coast. Shelf Sci. 2016, 182, 72–85. [Google Scholar] [CrossRef]
- Martineac, R.P.; Vorobev, A.V.; Moran, M.A.; Medeiros, P.M. Assessing the Contribution of Seasonality, Tides, and Microbial Processing to Dissolved Organic Matter Composition Variability in a Southeastern U.S. Estuary. Front. Mar. Sci. 2021, 8, 781580. [Google Scholar] [CrossRef]
- Inamdar, S.P.; Mitchell, M.J. Hydrologic and Topographic Controls on Storm-Event Exports of Dissolved Organic Carbon (DOC) and Nitrate across Catchment Scales. Water Resour. Res. 2006, 42. [Google Scholar] [CrossRef]
- Kalev, S.; Toor, G.S. Concentrations and Loads of Dissolved and Particulate Organic Carbon in Urban Stormwater Runoff. Water 2020, 12, 1031. [Google Scholar] [CrossRef]
- Lambert, T.; Pierson-Wickmann, A.-C.; Gruau, G.; Jaffrezic, A.; Petitjean, P.; Thibault, J.-N.; Jeanneau, L. Hydrologically Driven Seasonal Changes in the Sources and Production Mechanisms of Dissolved Organic Carbon in a Small Lowland Catchment. Water Resour. Res. 2013, 49, 5792–5803. [Google Scholar] [CrossRef]
- Wasko, C.; Nathan, R.; Stein, L.; O’Shea, D. Evidence of Shorter More Extreme Rainfalls and Increased Flood Variability under Climate Change. J. Hydrol. 2021, 603, 126994. [Google Scholar] [CrossRef]
- Shenoy, S.; Gorinevsky, D.; Trenberth, K.E.; Chu, S. Trends of Extreme US Weather Events in the Changing Climate. Proc. Natl. Acad. Sci. USA 2022, 119, e2207536119. [Google Scholar] [CrossRef]
- Fichot, C.G.; Benner, R. A Novel Method to Estimate DOC Concentrations from CDOM Absorption Coefficients in Coastal Waters. Geophys. Res. Lett. 2011, 38. [Google Scholar] [CrossRef]
- Gorman, E.T.; Kubalak, D.A.; Patel, D.; Dress, A.; Mott, D.B.; Meister, G.; Werdell, P.J. The NASA Plankton, Aerosol, Cloud, Ocean Ecosystem (PACE) Mission: An Emerging Era of Global, Hyperspectral Earth System Remote Sensing. In Proceedings of the Sensors, Systems, and Next-Generation Satellites XXIII, Strasbourg, France, 9–12 September 2019; SPIE: Bellingham, WA, USA, 2019; Volume 11151, pp. 78–84. [Google Scholar]
- Alberts, J.J.; Takács, M.; Schalles, J. Ultraviolet-Visible and Fluorescence Spectral Evidence of Natural Organic Matter (NOM) Changes along an Estuarine Salinity Gradient. Estuaries 2004, 27, 296–310. [Google Scholar] [CrossRef]
- Asmala, E.; Bowers, D.G.; Autio, R.; Kaartokallio, H.; Thomas, D.N. Qualitative Changes of Riverine Dissolved Organic Matter at Low Salinities Due to Flocculation. J. Geophys. Res. Biogeosci. 2014, 119, 1919–1933. [Google Scholar] [CrossRef]
- Sholkovitz, E.R. Flocculation of Dissolved Organic and Inorganic Matter during the Mixing of River Water and Seawater. Geochim. Et Cosmochim. Acta 1976, 40, 831–845. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, M.; Korshin, G.V. Effects of Ionic Strength on the Chromophores of Dissolved Organic Matter. Environ. Sci. Technol. 2015, 49, 5905–5912. [Google Scholar] [CrossRef] [PubMed]
- Dalzell, B.J.; Minor, E.C.; Mopper, K.M. Photodegradation of Estuarine Dissolved Organic Matter: A Multi-Method Assessment of DOM Transformation. Org. Geochem. 2009, 40, 243–257. [Google Scholar] [CrossRef]
- Moran, M.A.; Sheldon, W.M.; Zepp, R.G. Carbon Loss and Optical Property Changes during Long-term Photochemical and Biological Degradation of Estuarine Dissolved Organic Matter. Limnol. Oceanogr. 2000, 45, 1254–1264. [Google Scholar] [CrossRef]
- Moran, M.A.; Zepp, R.G. Role of Photoreactions in the Formation of Biologically Labile Compounds from Dissolved Organic Matter. Limnol. Oceanogr. 1997, 42, 1307–1316. [Google Scholar] [CrossRef]
- Cory, R.M.; Kling, G.W. Interactions between Sunlight and Microorganisms Influence Dissolved Organic Matter Degradation along the Aquatic Continuum. Limnol. Oceanogr. Lett. 2018, 3, 102–116. [Google Scholar] [CrossRef]
- Wiegner, T.; Seitzinger, S.; Glibert, P.; Bronk, D. Bioavailability of Dissolved Organic Nitrogen and Carbon from Nine Rivers in the Eastern United States. Aquat. Microb. Ecol. 2006, 43, 277–287. [Google Scholar] [CrossRef]
- Wu, K.; Lu, K.; Dai, M.; Liu, Z. The Bioavailability of Riverine Dissolved Organic Matter in Coastal Marine Waters of Southern Texas. Estuar. Coast. Shelf Sci. 2019, 231, 106477. [Google Scholar] [CrossRef]
- Khoo, C.L.L.; Sipler, R.E.; Fudge, A.R.; Beheshti Foroutani, M.; Boyd, S.G.; Ziegler, S.E. Salt-Induced Flocculation of Dissolved Organic Matter and Iron Is Controlled by Their Concentration and Ratio in Boreal Coastal Systems. J. Geophys. Res. Biogeosci. 2022, 127, e2022JG006844. [Google Scholar] [CrossRef]
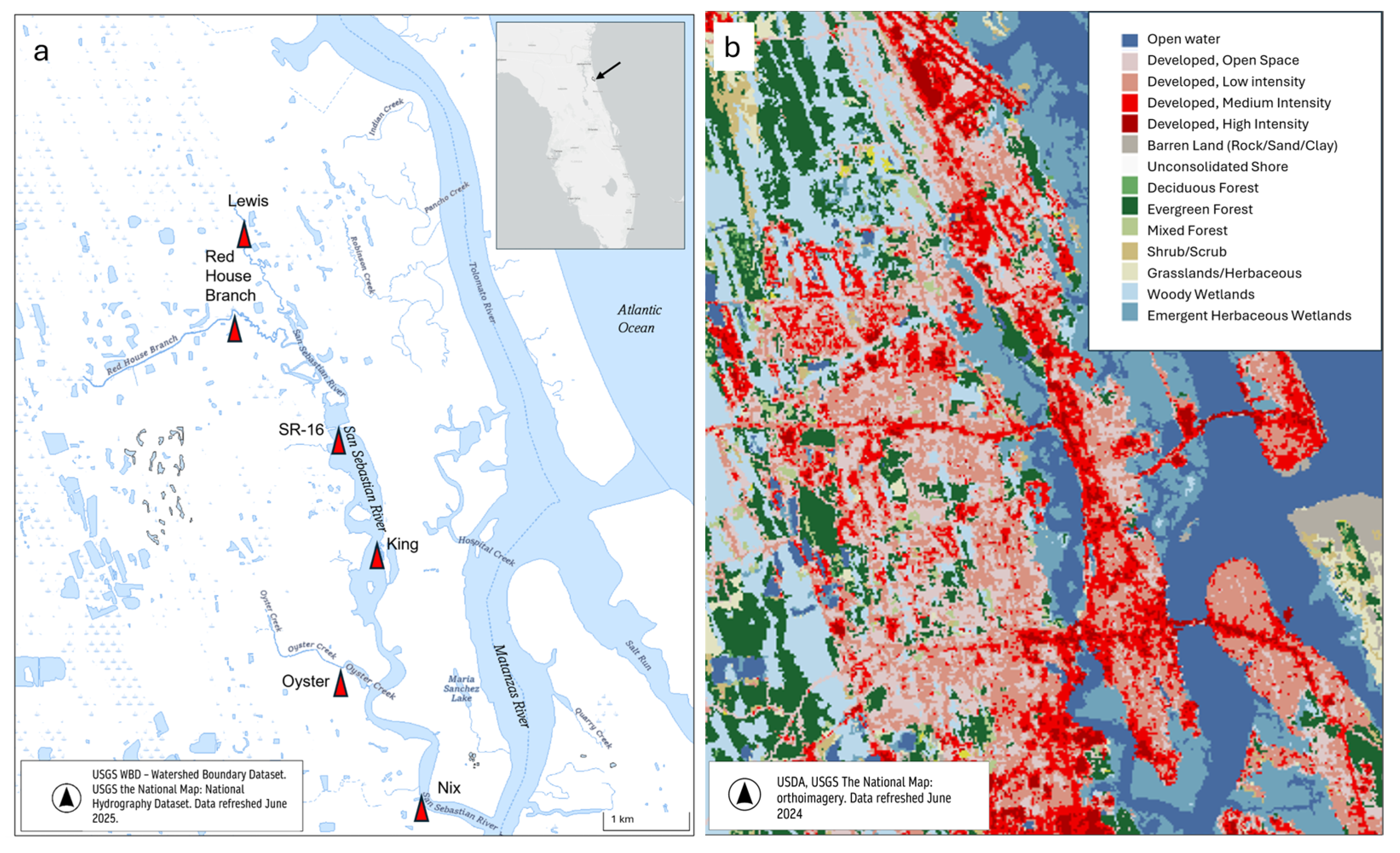
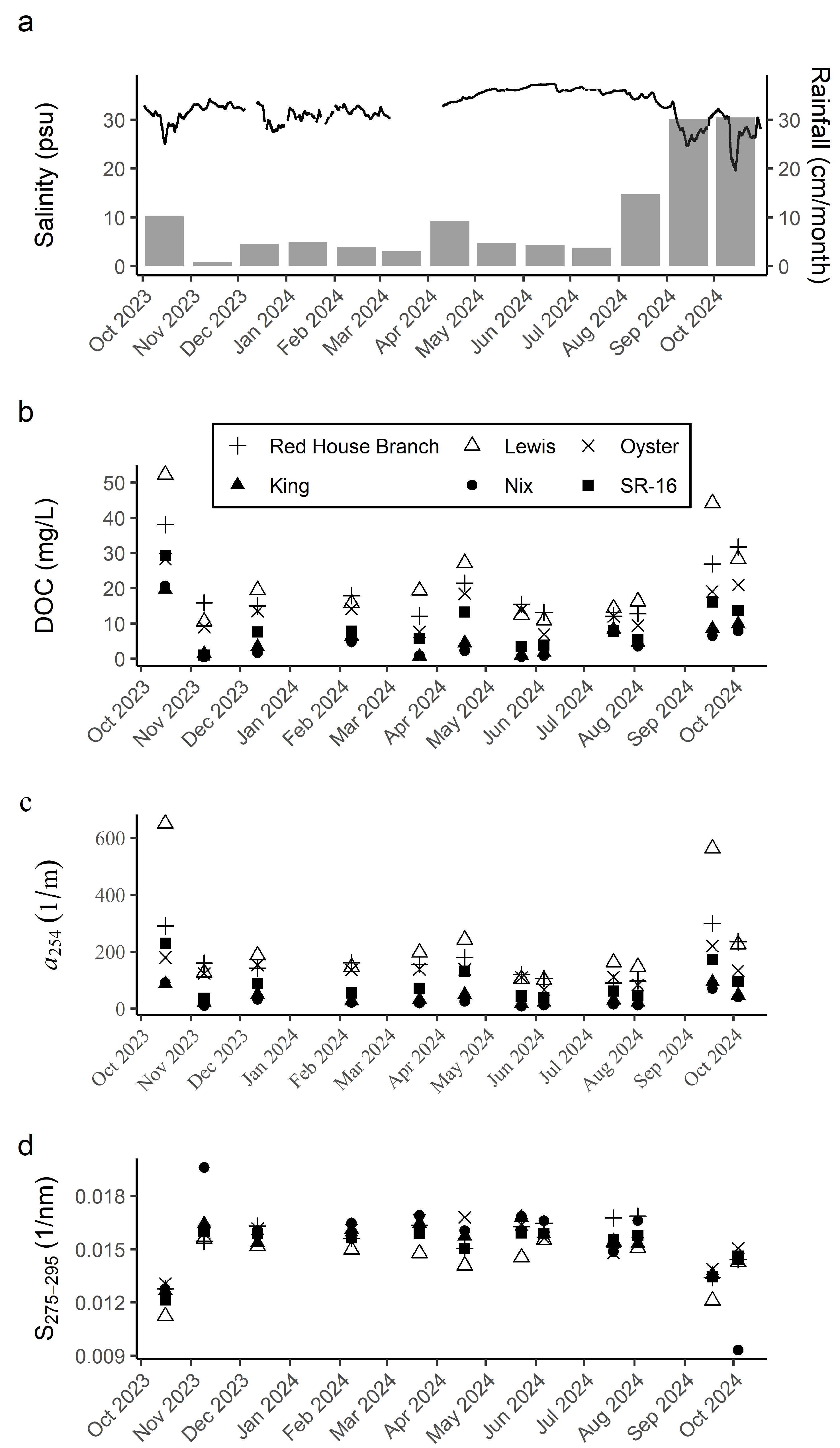

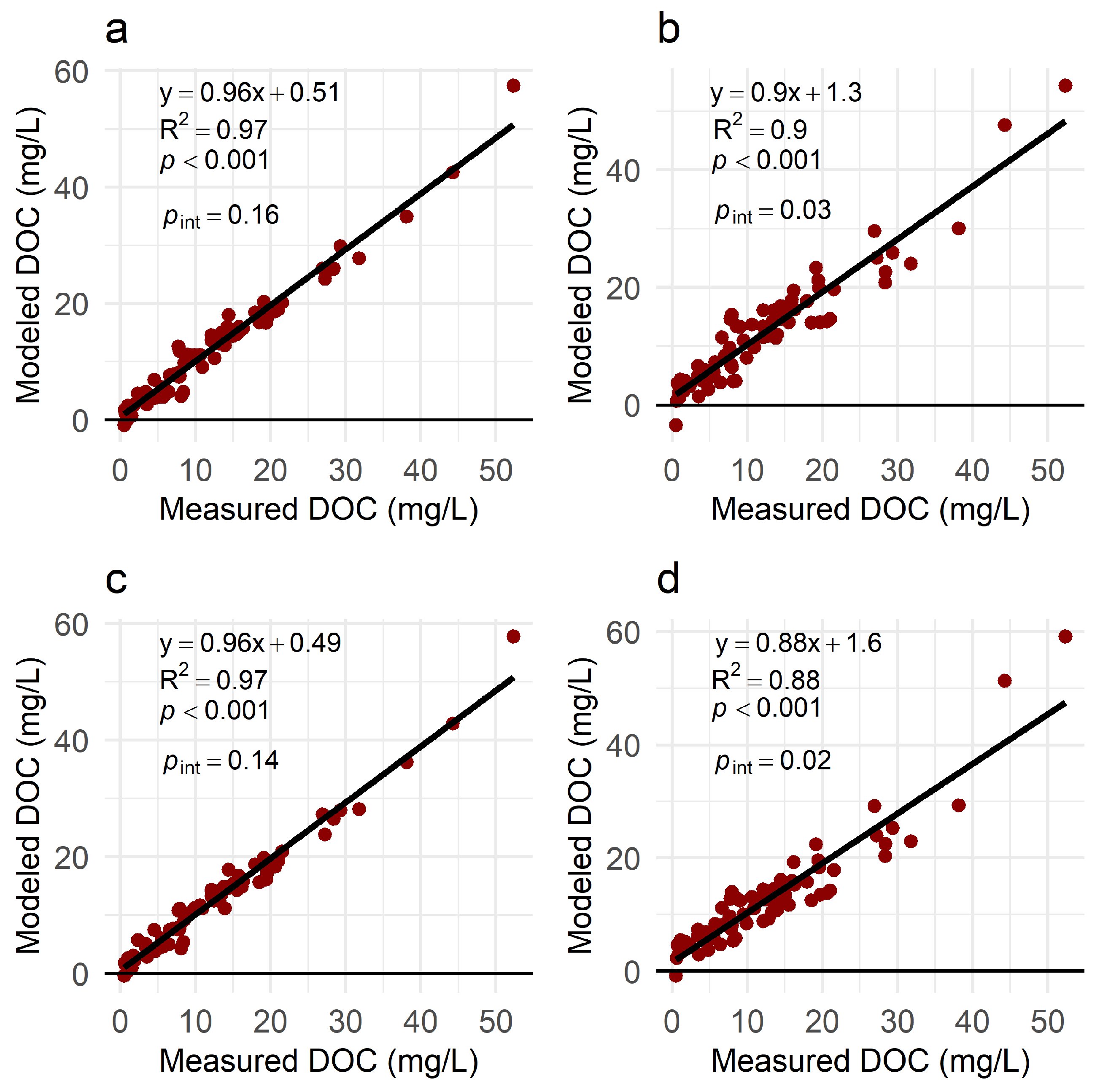
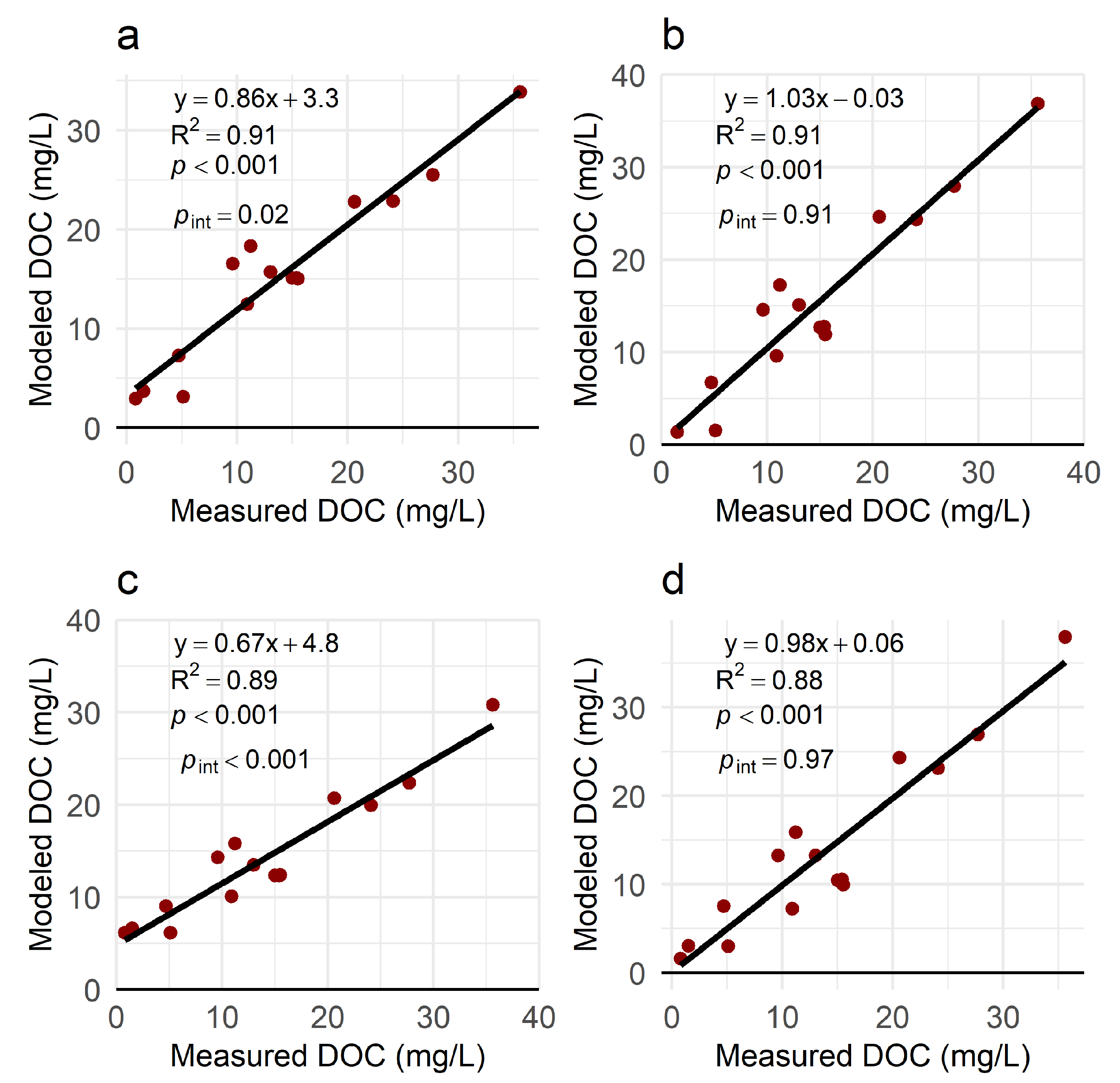
| Sampling Date | Sites | n | Experiment Type |
|---|---|---|---|
| July 2024 | Lewis | n = 5 | Aging |
| July 2024 | Lewis | n = 3 | Photochemical |
| September 2024 | Lewis, Red House Branch, Oyster | n = 3 | Aging |
| October 2024 | Lewis, Red House Branch, Oyster | n = 1 | Photochemical |
| January 2025 | Lewis, Red House Branch, Oyster | n = 3 | Aging |
| January 2025 | Lewis, Red House Branch, Oyster | n = 1 | Photochemical |
| April 2025 | Red House Branch | n = 3 | Photochemical |
| Site | Salinity (psu) | DOC (mg/L) | 254 (1/m) | 280 (1/m) | 325 (1/m) | 355 (1/m) | 440 (1/m) | SR | S275–295 (1/nm) | S350–400 (1/nm) | SUVA254 (L/mg/m) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Red House Branch | 0 (1) | 18.7 (7.8) | 165 (68) | 122 (54) | 64 (32) | 41 (22) | 9.5 (6.1) | 0.90 (0.03) | 0.0156 (0.0014) | 0.0174 (0.0011) | 3.9 (0.9) |
| Lewis | 7 (8) | 21.9 (12.0) | 227 (162) | 172 (129) | 94 (79) | 61 (54) | 15 (15) | 0.88 (0.05) | 0.0146 (0.0015) | 0.0166 (0.0011) | 4.3 (0.7) |
| Oyster | 8 (7) | 14.7 (6.2) | 140 (44) | 102 (33) | 52 (19) | 33 (13) | 7.5 (3.2) | 0.90 (0.07) | 0.0155 (0.0011) | 0.0172 (0.0014) | 4.5 (1.4) |
| SR-16 | 25 (8) | 9.2 (7.5) | 87 (58) | 65 (46) | 34 (27) | 22 (18) | 5.3 (4.9) | 0.92 (0.05) | 0.0152 (0.0012) | 0.0165 (0.0008) | 5.0 (2.8) |
| King | 31 (5) | 5.5 (5.3) | 40 (25) | 30 (19) | 15 (11) | 9.8 (7.3) | 2.5 (1.9) | 0.96 (0.08) | 0.0155 (0.0013) | 0.0162 (0.0014) | 5.4 (4.7) |
| Nix | 33 (4) | 4.6 (5.3) | 28 (24) | 21 (19) | 11 (11) | 6.8 (7.0) | 1.7 (1.8) | 0.97 (0.16) | 0.0157 (0.0025) | 0.0163 (0.0022) | 4.8 (3.2) |
| Model | Fixed Effects | Random Intercepts | AIC (Train) | R2 (Train) | RMSE (Test) mg/L | R2 (Test) | MAE (Test) mg/L | rMAE (Test) % |
|---|---|---|---|---|---|---|---|---|
| Comprehensive Mixed | 0.0613 × − 282 × S275–295 − 0.169 × Salinity +12.9 | Date | 354 | 0.97 | 3.1 | 0.91 | 2.4 | 17% |
| Comprehensive Linear | 0.0606 × − 1609 × S275–295 − 0.159 × Salinity +33.0 | 386 | 0.90 | 2.9 | 0.91 | 2.4 | 17% | |
| Absorbance Mixed | 0.0625 × − 123 × S275–295 +7.38 | Site, Date | 363 | 0.97 | 3.8 | 0.89 | 3.3 | 23% |
| Absorbance Linear | 0.0779 × − 1217 × S275–295 +22.2 | 397 | 0.88 | 3.3 | 0.88 | 2.8 | 20% |
| Response Variable | Factors | df | F Value | p-Value |
|---|---|---|---|---|
| ΔDOC | Treatment | 1 | 23.9 | <0.001 |
| Date | 3 | 5.7 | 0.010 | |
| Site | 2 | 4.0 | 0.043 | |
| Treatment * Date | 3 | 0.4 | 0.764 | |
| Treatment * Site | 2 | 2.7 | 0.107 | |
| Δ | Treatment | 1 | 277.7 | <0.001 |
| Date | 3 | 145.0 | <0.001 | |
| Site | 2 | 7.5 | 0.007 | |
| Treatment * Date | 3 | 26.0 | <0.001 | |
| Treatment * Site | 2 | 0.6 | 0.557 | |
| ΔS275–295 | Treatment | 1 | 761.2 | <0.001 |
| Date | 3 | 131.6 | <0.001 | |
| Site | 2 | 1.2 | 0.330 | |
| Treatment * Date | 3 | 42.8 | <0.001 | |
| Treatment * Site | 2 | 0.8 | 0.456 |
| Response Variable | Factors | df | F Value | p-Value |
|---|---|---|---|---|
| ΔDOC | Treatment | 1 | 0.3 | 0.587 |
| Date | 2 | 6.2 | 0.005 | |
| Site | 2 | 3.3 | 0.048 | |
| Treatment * Date | 2 | 0.9 | 0.404 | |
| Treatment * Site | 2 | 2.7 | 0.084 | |
| Δ | Treatment | 1 | 3.6 | 0.065 |
| Date | 2 | 81.4 | <0.001 | |
| Site | 2 | 20.0 | <0.001 | |
| Treatment * Date | 2 | 0.9 | 0.418 | |
| Treatment * Site | 2 | 0.5 | 0.619 | |
| ΔS275–295 | Treatment | 1 | 0.0 | 0.925 |
| Date | 2 | 67.1 | <0.001 | |
| Site | 2 | 15.5 | <0.001 | |
| Treatment * Date | 2 | 2.8 | 0.073 | |
| Treatment * Site | 2 | 1.2 | 0.319 |
| Experiment Type | Time Point | R2 | RMSE (mg/L) | rMAE (%) |
|---|---|---|---|---|
| Aging | Initial | 0.97 | 3.6 | 17 |
| Aging | Final | 0.94 | 4.1 | 19 |
| Photochemical | Initial | 0.97 | 2.3 | 9.5 |
| Photochemical | Final | 0.93 | 3.1 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Southwell, M.W.; Schindler, C.; Ramirez, F. Modeling Dissolved Organic Carbon in an Estuary Using Optical Properties and Salinity. Water 2025, 17, 3133. https://doi.org/10.3390/w17213133
Southwell MW, Schindler C, Ramirez F. Modeling Dissolved Organic Carbon in an Estuary Using Optical Properties and Salinity. Water. 2025; 17(21):3133. https://doi.org/10.3390/w17213133
Chicago/Turabian StyleSouthwell, Melissa W., Conrad Schindler, and Francisco Ramirez. 2025. "Modeling Dissolved Organic Carbon in an Estuary Using Optical Properties and Salinity" Water 17, no. 21: 3133. https://doi.org/10.3390/w17213133
APA StyleSouthwell, M. W., Schindler, C., & Ramirez, F. (2025). Modeling Dissolved Organic Carbon in an Estuary Using Optical Properties and Salinity. Water, 17(21), 3133. https://doi.org/10.3390/w17213133







