Abstract
The efficient removal of natural organic matter (NOM) through a coagulation process is crucial for improving the quality of drinking water. Recent studies have focused on the interaction between NOM and coagulants during the floc formation and aging process. Therefore, based on the relevant literature from the past few decades, this review focuses on changes in floc activity during floc formation and aging at a molecular level. It systematically clarifies the mechanisms and factors influencing floc formation and aging and summarizes the characterization techniques for NOM and flocs. Notably, the interaction between NOM and coagulant flocs is determined by the presence of carboxyl groups and hydroxyl groups on NOM and hydroxyl groups (η-OH) and water molecules (η-OH2) on coagulant flocs. Aging involves the transformation of coagulant species and an increase in floc crystallinity, which leads to the absorption or release of organic matter. Although numerous analytical techniques currently offer new insights into the interaction between coagulants and NOM, in situ characterization techniques remain limited. This review provides a theoretical foundation for the full life cycle assessment of NOM in coagulation processes, which is of great significance for advancing drinking water technologies and achieving carbon neutrality goals.
1. Introduction
Natural organic matter (NOM) is a complex organic substance that is abundant in surface water and groundwater. It mainly originates from biological activities and the flushing of soil by rainfall [1]. NOM can basically be classified into three major divisions, allochthonous NOM (e.g., humic acid (HA), fulvic acid (FA) and humin), autochthonous NOM (e.g., EPS secreted by algae, plants and bacteria) and anthropogenic NOM [1]. Although NOM is non-toxic in itself, its presence in drinking water sources poses a hazard. On the one hand, NOM can not only change the color, odor, taste and other sensory properties of the water sources, but also combine with pollutants to pose a greater threat to humans [2,3]. On the other hand, NOM can react with disinfectants during the disinfection process to produce disinfection by-products (DBPs), such as trihalomethanes (THMs), haloacetic acids (HAAs) and haloacetonitriles (HANs), which are carcinogenic, teratogenic and mutagenic [4,5]. Furthermore, NOM plays a crucial role in the global carbon cycle, including the reservation and sequestration of organic carbon [6]. HA is reported to occupy about 25% of the global carbon content and account for 67.6% of the carbon in the Earth’s biosphere [7]. Therefore, efficiently removing NOM is key to guaranteeing the safety of drinking water and is also a necessary pathway to achieve carbon neutrality.
Coagulation is one of the most widely used water treatment processes in the world, and it plays an important role in the removal of NOM. Commonly used coagulants include metal salts such as aluminum, iron and titanium salts. In addition, organic polymer flocculants are usually added as an aid in the coagulation process to enhance the removal efficiency of NOM [8,9]. Following extensive deliberation throughout the 1960s, a consensus was gradually reached regarding the coagulation mechanism of traditional coagulants in water treatment [10]. Coagulants can produce multiple species through hydrolysis. These species interact with organic or particulate matter via mechanisms such as electrostatic neutralization, adsorption bridging and sweep precipitation [11,12,13,14]. Subsequently, numerous studies have extensively investigated the effects of pH [15], coagulant type [16], turbidity [17] and organic species on coagulation removal efficiency [18]. Beyond discussions on hydrolysis species, the floc formation process has also gradually attracted attention [19,20,21]. Actually, the characteristics of flocs directly affect the settling performance of flocs, which in turn affects coagulation removal efficiency. Nevertheless, the formation of flocs and the mechanism of their interaction with NOM at a molecular scale have not yet been fully elucidated. Therefore, this review focuses on changes in floc activity during the interaction between NOM and coagulant, revealing the effect of NOM properties on the floc formation process from a molecular perspective.
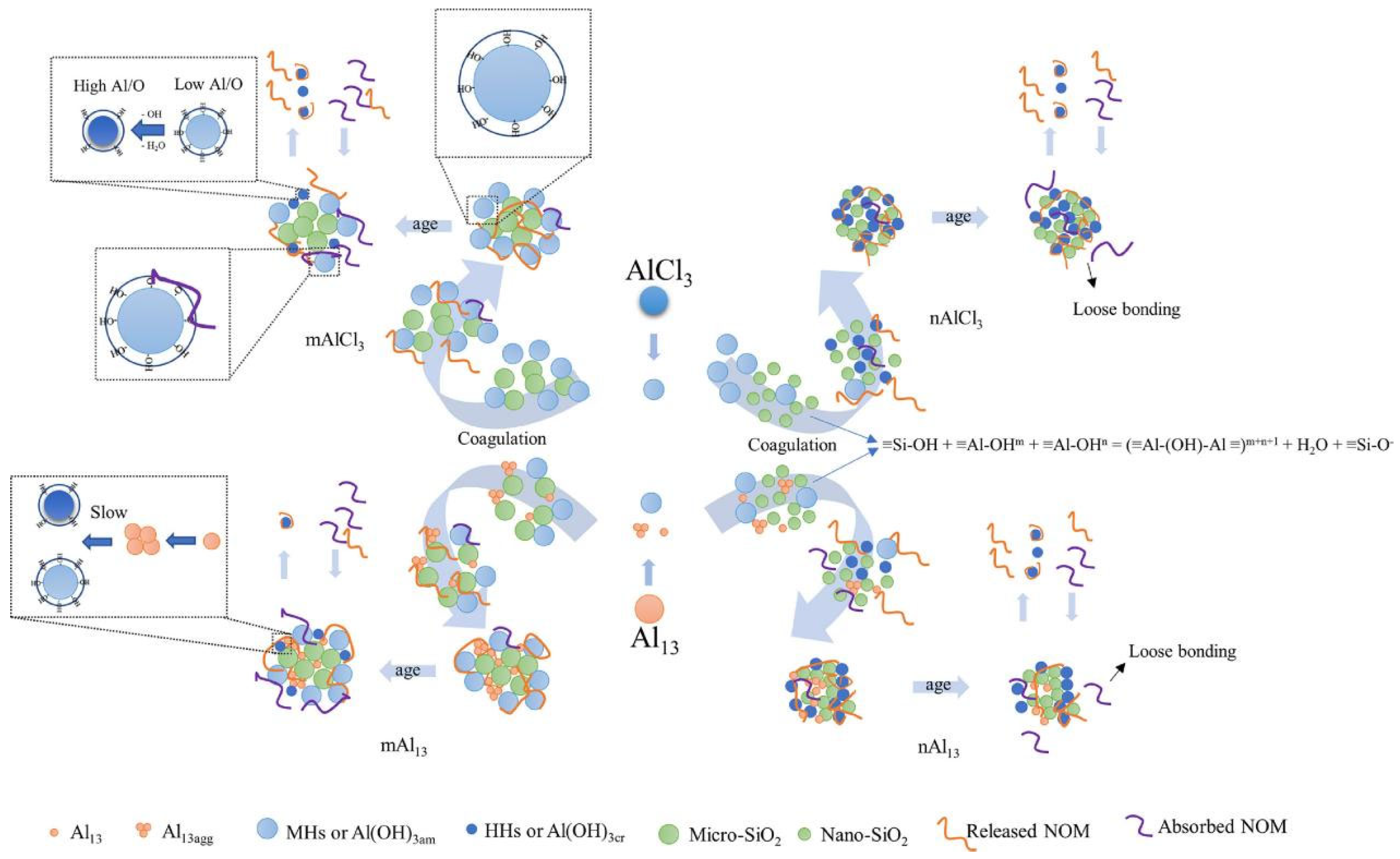
In the practical water treatment process, flocs need to be left in the sedimentation tank for a few days before they are discharged. Therefore, the stability of the flocs during this retention period can also directly affect the final water treatment effect. It has been suggested that flocs undergo aging during the sedimentation process, leading to changes in floc activity and morphology. The aging process causes some of the adsorbed pollutants to be released back into the aquatic environment, thereby resulting in secondary pollution [22,23]. Particularly, the aging process of aluminum salt flocs has been systematically investigated. Previous studies have revealed that Al(OH)3 indeterminate precipitates convert from amorphous to regular crystalline structures during floc aging, resulting in the release of organic matter and Al(OH)3 nanoparticles [24]. Meanwhile, the degree of organic matter release is closely related to the type of organic matter. Humic substances, which are highly aromatic and unsaturated, are more likely to be released during aging than protein-like substances [25]. Conversely, organic matter can also influence the aging process of flocs. For instance, organic acids are observed to retard the crystallization of aluminum hydroxide [26]. Additionally, anions such as chloride ions, nitrate ions, sulfate ions, and phosphate ions inhibit the crystal transformation of aluminum or iron hydroxides to varying degrees [27,28]. Consequently, research on floc aging is necessary to provide new insights into the optimization of coagulation process.
Therefore, this review comprehensively summarizes existing literature on floc formation and floc aging, with a particular focus on changes in floc activity during the interaction between NOM and coagulants. The mechanisms of floc aging as well as the influencing factors are systematically elaborated. Moreover, novel techniques for characterizing changes in NOM and floc during floc aging process are also summarized to provide technical support for the development of coagulation morphology. Finally, the review presents an advanced perspective on the development of coagulation treatment technology. This review establishes a theoretical foundation for improving floc utilization technology and the full life cycle assessment of NOM in drinking water treatment processes. This is highly significant for enhancing drinking water treatment technologies, ensuring drinking water safety and reducing carbon emissions in drinking water treatment plants.
2. Floc Formation
In the water treatment process, adding coagulants can destabilize colloidal particles, resulting in the formation of flocs through a series of physicochemical processes. Commonly used coagulants are mainly inorganic coagulants such as aluminum, iron and titanium salts. While in recent years, inorganic–organic composite coagulants have also received extensive attention. Therefore, this section summarizes the hydrolysis processes, coagulation mechanisms and influencing factors of commonly used coagulants to enhance understanding of the coagulation process from a microscopic perspective. The focus is on changes in floc formation and floc activity during the interaction between coagulant hydrolysis species and NOM. The hydrolysis–polymerization processes of typical metal coagulants are shown in Figure 1 and Figure 2.
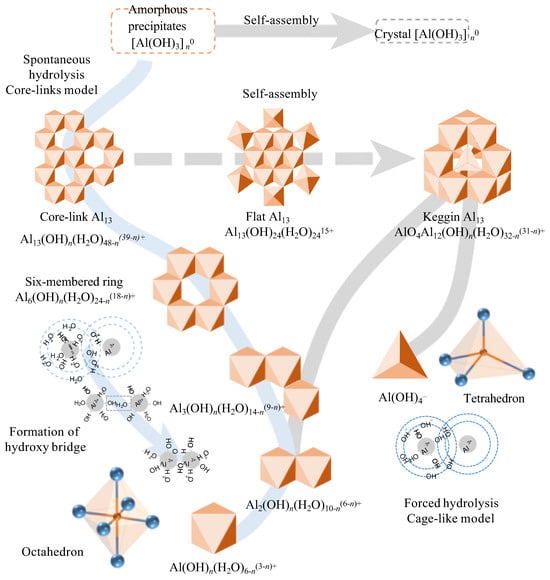
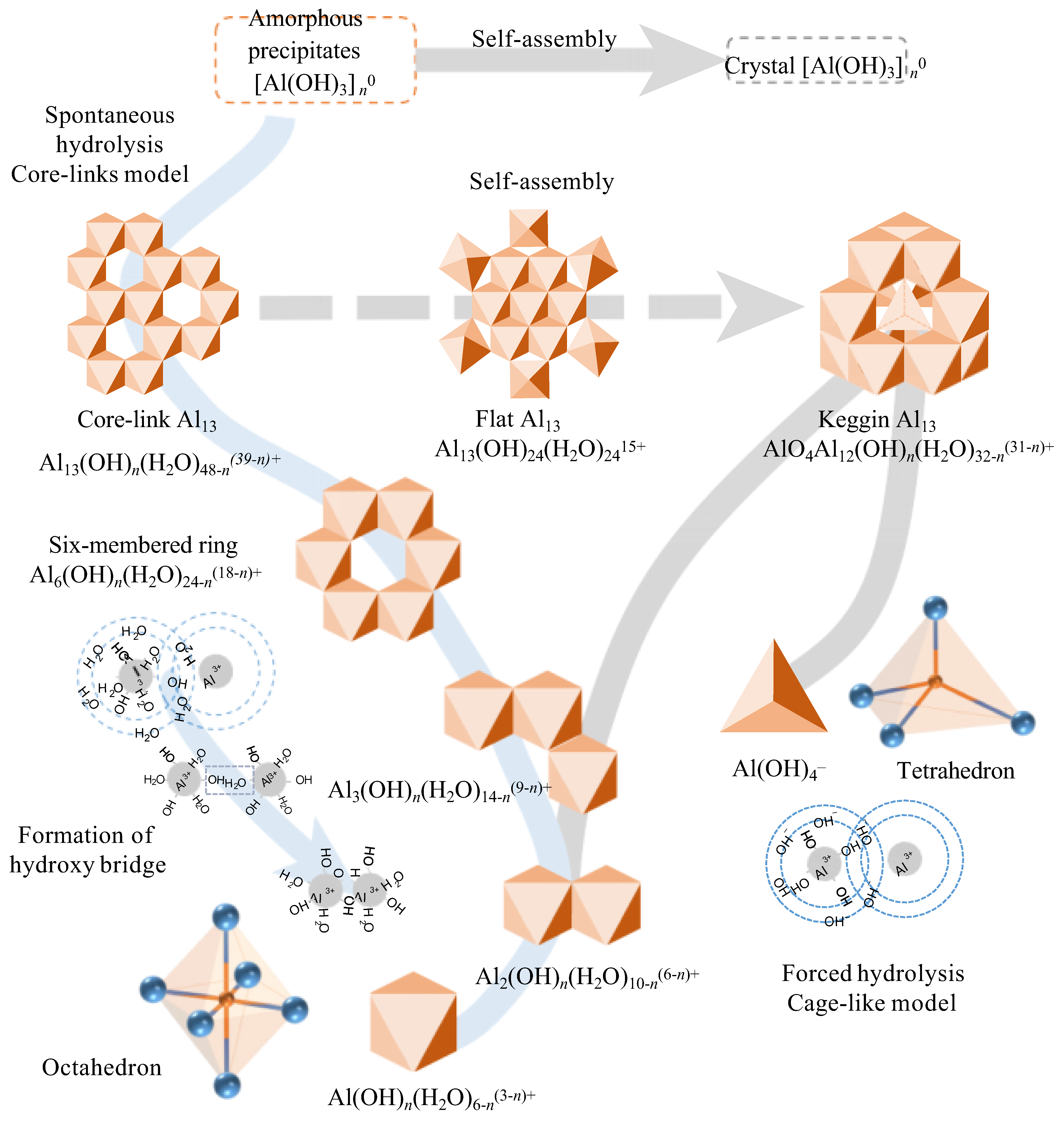
Figure 1.
Schematic diagram of hydrolysis process of aluminum. An octahedron represents an aluminum hydration structure, and a tetrahedron represents an AlO4 structure in the diagram (Reproduced from Ref. [29], with permission from Springer Nature, 2024).
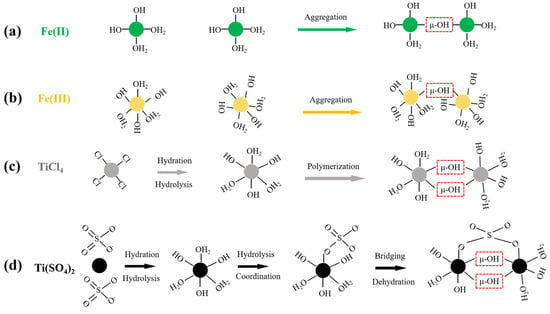
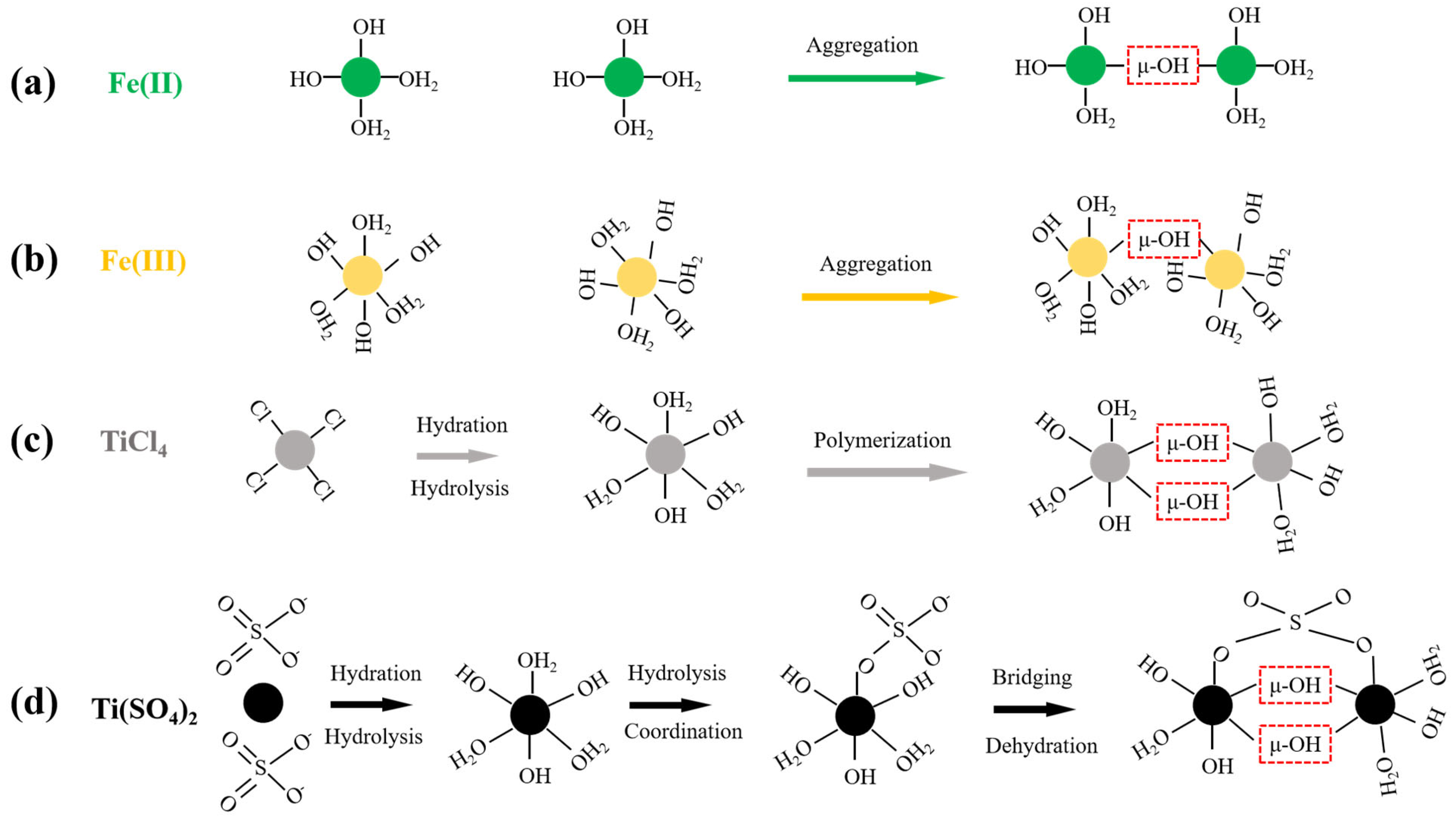
Figure 2.
The schematics of Fe(II) (a) and Fe (III) coagulation (b) as well as the hydrolysis reactions of TiCl4 (c) and Ti(SO4)2 (d). The structure enclosed by the red dashed box in the figure is the μ-OH bond.
2.1. Aluminum-Based Coagulants
Aluminum-based coagulants are one of the most commonly used coagulants, which can be divided into traditional aluminum-based coagulants (e.g., AlCl3, Al2(SO4)3) and inorganic polymer coagulants (e.g., polyaluminum chloride, PACl; polyaluminum sulfate, PAS). There are currently two models that illustrate the hydrolysis of aluminum (Figure 1): the “cage-like” model and the “core-links” model. Initially, these two models were considered contradictory. However, with the development of detection technologies and the discovery of new aluminum hydrolysis structures in recent years, they are now regarded as complementary [30]. Specifically, the two models are applicable under different conditions. The “core-links” model is applicable under neutral pH conditions, where the transformation of polynuclear aluminum species undergoes a continuous speciation change process: from small polymers with a linear shape to medium polymers with a plane shape, to large polymers with a stereoscopic conformation. As this model consists of multiple continuous transient processes, many aluminum species proposed under this theory remain undetected. Nevertheless, recent studies have pointed out that water molecules (η-OH2) and hydroxy groups (η-OH) are the active sites of these aluminum species. Consequently, the hydrolysis products of aluminum salts can be simplified as precipitates connected by hydroxy bridges (μ-OH), formed by η-OH2 and η-OH [31]. The specific reaction is as follows:
> Al-OH2 + HO-Al < → > Al-(μ-H3O2)-Al < → > Al-(μ-OH)-Al < + H2O
Meanwhile, the “cage-like” model depicts the forced hydrolysis of aluminum salts in an alkaline environment. Through self-assembly, sub-steady-state speciation of polynuclear aluminum forms, characterized by a central tetrahedral AlO4 unit [30].
The coagulation efficiency is closely related to the species of coagulant hydrolysis products. Based on the kinetic differences in their reactions with the ferron reagent, the hydrolyzed aluminum species can be classified as monomeric aluminum species (Ala), medium-polymerized aluminum species (Alb) and colloidal or solid aluminum species (Alc) [32,33]. Correspondingly, their respective mechanisms for NOM removal are complexation, charge neutralization and sweep flocculation, respectively [34]. Kong et al. compared the removal efficiency of humic acid (HA) using AlCl3 (Ala), PACl1 (Alb) and PACl2 (Alc) [20]. The results indicated that AlCl3 could maintain an HA removal rate of over 40% across the entire pH range of 4 to 9. PACl1; however, it could only achieve an excellent HA removal rate under acidic conditions, while PACl2 was effective only within the pH range of 6–9. This phenomenon occurs because AlCl3 can generate a variety of aluminum species in situ, including Al1 to Al20, which facilitate HA removal through the synergistic effects of charge neutralization, surface complexation and sweep flocculation. Furthermore, the hydrolysis product of PACl1 is mainly Al13, and its primary mechanisms for HA removal are charge neutralization and electrostatic clustering. Studies have indicated that its ability to neutralize charges decreases under alkaline conditions, leading to poor HA removal performance [35]. While the reason PACl2 exhibits poor HA removal efficiency under acidic conditions is that excessive Alc causes charge reversal on the floc surface, leading to restabilization.
Al13 is a stable intermediate in aluminum hydrolysis products, with the molecular formula Al13O4(OH)24(H2O)7. It has received extensive attention due to its excellent stability and highly efficient coagulation performance [36,37]. Previous studies have demonstrated that Al13 exhibits superior coagulation performance in the removal of both NOM and clay particles compared to traditional aluminum salt coagulants [38,39]. Moreover, the amount of NOM released upon the breakdown of Al13 flocs is smaller than that of conventional coagulants such as AlCl3 [34]. The fundamental reason for these results is that Al13 possesses a higher charge neutralization ability and a more stable chemical structure [40]. Nevertheless, Al13 exhibits poor performance in removing small-molecular-weight organic substances. Zong et al. have indicated that compared with AlCl3, Al13 struggles to remove salicylic acid across the entire pH range of 6 to 8 [15]. This is fundamentally due to the decomposition of Al13 induced by salicylic acid under acidic conditions and the limited adsorption capacity of Al13 for small hydrophilic molecules under alkaline conditions.
At specific pH levels, the deprotonation of water molecules within the Al13 structure reduces its surface charge, causing Al13 to aggregate and form new aluminum species known as Al13 aggregates (Al13agg) [41]. It has been suggested that Al13agg can be generated in situ during the coagulation process. To verify this hypothesis, Wang et al. investigated the effects of in situ Al13agg and pre-formed Al13agg on the removal of clay particles [42]. The results indicated that under neutral and alkaline conditions, the in situ Al13agg exhibited superior performance. This is because Al13 can exert a charge neutralization effect in the early stage of coagulation, and then undergo in situ aggregation to form in situ Al13agg, which can play a role in electrostatic clustering and bridging, promoting the formation of larger flocs and improving particle removal performance [37]. However, different results were observed in the process of organic matter removal. The pre-formed Al13agg effectively removed all molecular weight components of humic acid across the entire pH range, whereas in situ Al13agg only exhibited good performance under acidic and neutral conditions [35]. This difference in performance is due to their distinct coagulation mechanisms: pre-formed Al13agg primarily plays the role of adsorption and complexation, whereas in situ Al13agg exerts a charge neutralization effect. In comparison, the charge neutralization effect is sensitive to changes in pH, thus in situ Al13agg performs poorly under alkaline conditions.
2.2. Iron-Based Coagulants
Iron-based coagulants are widely utilized and can be employed across a broader pH and temperature range than aluminum salts. It has been reported that the ferric salt hydrolysis products possess more active hydroxyl groups on their surfaces, which promotes the floc growth and facilitates the formation of larger flocs. In addition, ferric salts not only perform excellently in the floc formation process but also exhibit high stability during floc aging, with a lower released ion concentration than aluminum salts [43]. Moreover, iron-based coagulants have been found to be more effective than aluminum salts at removing unsaturated and oxidized NOM substances. Since HA contains components with such structures, iron-based coagulants are thus more efficient at removing HA [44].
As shown in Figure 2a,b, the hydrolysis and polymerization processes of ferrous and ferric coagulants are similar. Both cations can form μ-OH bonds through the combination of η-OH sites and η-OH2 sites on their surfaces, followed by gradual polymerization. The reaction process can be simplified as follows:
> Fe-OH2 + HO-Fe < → > Fe-(μ-H3O2)-Fe < → > Fe-(μ-OH)-Fe < + H2O
However, their final hydrolysis products are distinct. During the initial stage of ferrous ion hydrolysis, Fe(OH)2 nanoparticles form rapidly. Subsequently, the ferrous ions on the surface oxidize to form ferric ions. After aging, γ-FeOOH is formed. In contrast, the hydrolysis process of ferric salts results in the formation of amorphous, spherical Fe(OH)3 precipitates. These differences contribute to the distinct coagulation performance of ferrous and ferric coagulants [45].
Similarly, the ferric hydrolysis species can be categorized as Fea, Feb, and Fec based on the ferron assay. Dong et al. employed FeCl3, PFC10, and PFC22 to investigate the performance of Fea, Feb and Fec in removing HA [46]. The results indicated that FeCl3 had a higher removal efficiency for dissolved organic matter than PFC10 and PFC22, due to its superior charge neutralization capability. Nevertheless, previous studies have demonstrated that Feb is the most effective iron salt coagulant [47]. Therefore, PFC10 with a higher Feb content should exhibit better coagulation performance. However, this is inconsistent with the results observed by Dong et al. [46]. The fundamental reason for this discrepancy is that, in addition to HA, kaolin particles are also present in the aqueous solution. During the coagulation process, HA preferentially reacts with the coagulant through adsorption to form Fe-HA complexes. These complexes then react with kaolin via charge neutralization and sweep flocculation. Consequently, FeCl3, containing Fea, Feb and Fec simultaneously, can exert synergistic effects, resulting in excellent coagulation removal performance.
Among iron salt coagulants, ferrous salts are more cost-effective than ferric salts, but they require conversion to the ferric ion in order to exert a coagulation effect. Compared with the direct addition of ferric salts, oxidizing ferrous ions to ferric ions using oxidants (e.g., hydrogen peroxide, potassium permanganate, ozone, or oxygen) has been reported to achieve better performance. This is ascribed to the ability of oxidants to effectively oxidize specific functional groups (e.g., double bonds) in NOM [48]. In addition, the flocs formed by ferrous and ferric iron are distinct in size and shape. Although ferric iron flocs form rapidly initially, they ultimately end up small due to their spherical shape and fewer surface active sites. In contrast, flocs formed by ferrous iron are planar with more active sites, resulting in a larger final size [45].
Although inorganic coagulants have advantages of low cost and simplicity in use, their coagulation efficiency is limited. Therefore, numerous studies in recent years have focused on preparing inorganic–organic composite coagulants, which can combine the charge neutralization effect of inorganic components with the hydrophobic interaction and bridging effect of organic components to achieve high coagulation efficiency. Polyacrylic acid (PAA) is a commonly used organic polymer material in water treatment. It is environmentally friendly and biodegradable, and its flexible linear chain structure makes it an ideal material for preparing inorganic–organic composite coagulants. Therefore, Yue et al. compounded Fe3+ with PAA to develop a novel composite coagulant, Fe-PAA [9]. Results implied that the PAA-Fe composite agent prepared at a Fe3+/COOH ratio of 1:1 exhibited excellent performance in removing HA over the pH range of 5–9. Furthermore, the primary mechanisms for the removal of organic matter by Fe-PAA are charge neutralization and bridging, which dominate the coagulation process under acidic conditions. However, complexation and bridging play a dominant role under neutral and alkaline conditions.
2.3. Titanium-Based Coagulants
Although aluminum and iron salts are widely used in water treatment, they have unavoidable drawbacks. For instance, aluminum salts can be harmful to humans, and using aluminum and iron salts generates significant amounts of sludge, leading to secondary pollution. Conversely, titanium-based coagulants offer several advantages, including effective NOM removal, rapid floc growth and reduced sensitivity to temperature. Additionally, recycling titanium sludge originating from coagulation is feasible [16,49]. The hydrolysis processes of two typical titanium salts are illustrated in Figure 2c,d. As for TiCl4, it forms titanium chloride dihydrate or titanium chloride pentahydrate through hydrolysis at different temperature and pH levels, respectively. Subsequently, hydroxyl groups gradually replace the chlorine groups that are coordinated with the titanium, forming Ti-OH complexes. These complexes then combine via surface η-OH and η-OH2 active sites to form μ-OH bonds, which gradually polymerize into polynuclear Ti-OH complexes. These complexes ultimately convert into hydrated titanium dioxide colloidal particles. Conversely, the hydrolysis process of Ti(SO4)2 is obviously distinct. When Ti(SO4)2 dissolves in water, Ti4+ ions quickly coordinate with H2O molecules, followed by rapid hydrolysis to form mononuclear hydroxyl complexes. Since one of the coordinated water molecules is replaced by a sulfate ion, a bidentate complex is formed through subsequent dehydration and bridging. Subsequently, continuous dehydration occurs to generate Ti-O-Ti clusters containing SO42−. Ultimately, polymerized Ti-O-Ti clusters are formed via dehydration or desulfurization reactions [50]. Although the hydrolysis pathways of the two titanium salts differ, they ultimately form μ-OH bonds and aggregate through the following reaction:
> Ti-OH2 + HO-Ti < → > Ti-(μ-H3O2)-Ti < → > Ti-(μ-OH)-Ti < + H2O
Similar to other metal coagulants, the coagulation mechanism of titanium salts under acidic conditions is charge neutralization. However, they are more sensitive to pH changes because their isoelectric point is much lower than that of traditional aluminum or iron coagulants [51]. Analogously, bridging and sweeping play a dominant role under neutral or alkaline conditions. Zhao et al. compared the coagulation effects of titanium chloride, ferric chloride and aluminum sulfate. The results indicated that TiCl4 exhibited the best removal performance for absorbance at 254 nm (UV254) and dissolved organic matter (DOC), due to its superior charge neutralization capacity. However, the hydrolysis of titanium salts releases significant amounts of H+, resulting in a low pH value in the effluent [51]. Therefore, subsequent studies have developed polytitanium tetrachloride (PTC) and polytitanium sulfate (PTS) coagulants to address this issue. Additionally, PTC/PTS can still maintain high removal efficiency for NOM, but their coagulation process is dominated by sweep flocculation [49,52]. To overcome the limitation of single metal salt coagulants, researchers have developed titanium-iron/aluminum composite coagulants, which also exhibit high efficiency in NOM removal [53,54].
In summary, different coagulants have their own respective advantages and disadvantages. Therefore, Table 1 summarizes the coagulation mechanisms and applicable conditions of commonly used metal coagulants. To achieve optimal coagulation performance, researchers need to select appropriate coagulants according to water quality conditions.

Table 1.
The coagulation mechanisms, optimal conditions and NOM removal efficiency of commonly used metal coagulants.
3. Influencing Factors of Floc Activity
Besides the type of coagulant, floc activity is also directly related to coagulation removal efficiency. Numerous factors can influence floc activity, including dosing methods [61], hydraulic conditions [62] and the characteristics of NOM [22]. These factors can influence coagulation efficiency by altering the coagulation mechanism, changing the morphology of hydrolysis and affecting the activity of functional groups on the floc surfaces.
3.1. Dosing Methods
In the context of in situ treatment of reservoirs and lakes, it is sometimes not feasible to add all the required coagulants at once due to limitations in volume of the water body. Therefore, different dosing methods and intervals must be considered. Sun et al. explored the impact of various coagulant dosing frequencies on UV254 removal and discovered that the value of UV254 decreased further with each addition of coagulant, due to humic substances reacting only with fresh coagulant hydrolysis products [61]. Liu and Yu also observed a similar phenomenon and they attributed it to the presence of more η-OH2 active sites on the surface of the newly formed hydrolyzed precipitates of the coagulant [31]. Furthermore, it has been further found that humic substances can be rapidly removed in the early stages of coagulation, which can be ascribed to the nanoparticles generated by the rapid hydrolysis of the coagulant [56,58]. These nanoparticles are highly active and can quickly adsorb HA until saturation is reached. As the activity of flocs decreases over time, shorter dosing intervals are more favorable for removing humic substances from aquatic environments [58]. Actually, coagulants are often mixed with organic coagulant aids to enhance the removal efficiency of NOM and particles in practical water treatments. Therefore, researchers have investigated the impact of the dosing sequence of metal coagulants and coagulant aids on coagulation efficiency. The results showed that the sequence of dosing metal coagulants first, followed by coagulant aids, yielded better performance [63,64]. The reason for this phenomenon is that the coagulant aid dosed first competes with NOM for the coagulant, whereas it can only exert its bridging effect when dosed later. Additionally, adding NOM at different stages of coagulation also impacts the removal efficiency. Studies have discovered that the removal efficiency of HA added during the floc breakage stage is lower than that of HA added at the beginning of coagulation. This is because HA molecules can cover the active sites on the surface of broken flocs, decreasing their regrowth ability [56]. Consequently, this phenomenon re-emphasizes the importance of active sites on floc surfaces.
3.2. Hydraulic Conditions
Different hydraulic conditions can also have a significant impact on the growth rate and size of flocs. Although high-intensity hydraulic shear can promote floc growth in the initial stage of coagulation, flocs are relatively small at equilibrium [59,62]. Nevertheless, observations reveal that flocs formed under high-hydraulic intensity exhibit fewer branches and greater strength. Subsequent studies have concluded that the presence or absence of hydraulic shear only affects the time taken for floc formation and has no significant impact on the final efficiency of NOM removal by coagulation [65]. However, prolonged hydraulic shear causes organic matter to be released from the flocs, with the amount released increasing as the intensity of the hydraulic shear increases. The reason is that aluminum salt flocs are interconnected by μ-OH as bridges. As the intensity of hydraulic shear increases, these μ-OH bonds break and the η-OH2 active sites on the floc surfaces gradually convert to η-OH after multiple instances of breakage and re-formation, thereby reducing the floc activity and leading to the release of NOM [39,64]. Liu and Yu further investigated the effect of breakage times on the floc activity and found that floc size gradually decreased with increasing breakage times [31]. This is primarily because the hydraulic shear diminishes the active sites on the floc surfaces, reducing the adsorption and complexation capacity of flocs.
3.3. Type of NOM, Number and Position of Functional Groups
NOM is a complex substance comprising a variety of aliphatic and aromatic compounds. It has been shown that NOM with an aromatic structure exhibits a higher coagulation removal efficiency than that containing aliphatic hydrocarbons [66]. Furthermore, increasing the chain length of aliphatic hydrocarbons or the number of benzene rings is beneficial for improving coagulation efficiency due to changes in hydrophilicity [67]. Actually, NOM can be categorized as hydrophilic, amphiphilic and hydrophobic components through membrane filtration. Following their interaction with coagulants, hydrophobic NOM has been found to possess a higher coagulation removal rate [65,68]. However, He et al. found a different phenomenon that no significant difference in the removal of hydrophilic versus hydrophobic NOM molecules, which may be attributed to the fact that the NOM in their study was derived from the same water source and the samples were identical [40]. In contrast, prior studies investigating the impact of hydrophobicity on NOM removal efficiency may have used synthetic model compounds or compounds extracted from various water sources. Consequently, the components of NOM vary depending on the source.
Previous studies have primarily revealed coagulation mechanisms involved in removing different organic compounds from a macroscopic perspective. However, with the continuous advancement of science and technology, the emergence of high-end instruments provides an opportunity to explore the transformation of organics during coagulation at a molecular level. For instance, using Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR-MS), NOM can be further subdivided into substances such as lipids, proteins/amino sugars, carbohydrates, unsaturated hydrocarbons, lignin, tannins, and polycyclic aromatic hydrocarbons substances [69]. Further research has revealed that the primary component of HA in natural water bodies is lignin [40]. It has been reported that aluminum flocs are more inclined to absorb NOM with reduced and saturated structures [66]. Moreover, lignin-like compounds with a high number of carboxyl groups can be efficiently removed through coagulation, whereas unsaturated and oxidized lipids, proteins and carbohydrates are more difficult to remove due to their high polarity and low carboxyl group content [66]. Additionally, it has been discovered that sulfur-containing NOM molecules, which may originate from surfactants or their metabolites and by-products, are challenging to remove through coagulation [70,71].
Since NOM is prevalent in natural waters, the impact of light conditions on NOM should not be overlooked. Previous studies have demonstrated that light conditions can lead to the transformation and degradation of NOM, resulting in a decrease in fluorescence intensity [72]. Simultaneously, the photochemical transformation of NOM is associated with a reduction in molecular weight and aromatic substances content, leading to an increase in aliphatic compounds. For instance, it has been shown that humic substances can decompose into low-molecular-weight acids under simulated light irradiation [73]. Furthermore, Hu et al. found that the coagulation efficiency initially decreased with longer irradiation duration (e.g., DOC removal decreased from 74% to 55% after 48 h of simulated light exposure). This is because light irradiation can transform carboxyl groups on NOM into hydroxyl groups and convert polyphenolics in NOM into compounds with lower aromaticity [32]. Moreover, light irradiation can alter the coagulation mechanism. When the irradiation time exceeded 36 h, the coagulation mechanism shifted from charge neutralization to sweep flocculation.
The above results suggest that the presence of carboxyl groups in organic matter significantly influences removal efficiency. Song et al. further demonstrated a positive correlation between the number of carboxyl groups in benzene rings and the coagulation removal efficiency of NOM [67]. Subsequently, using Kendrick mass defect (KMD) analysis to examine the molecular structure of organic matter before and after coagulation removal. It was also observed that the removed NOM molecules contained more carboxyl groups and had larger molecular masses than the molecules that resisted coagulation [40]. Moreover, the position of the carboxyl groups within the benzene ring also affects coagulation efficiency. Compounds with ortho-carboxyl groups on the benzene ring exhibit better removal efficiency than those with meta- or para-carboxyl groups [29]. Similarly, hydroxyl groups can play the same role, but Al3+ is more likely to bind with carboxyl groups than with phenolic hydroxyl groups [74]. Additionally, Jin et al. also found that in the process of coagulation, carboxyl groups respond more rapidly than aliphatic hydroxyl groups at pH 7, as revealed by two-dimensional correlation spectroscopy (2D-COS) analysis [75].
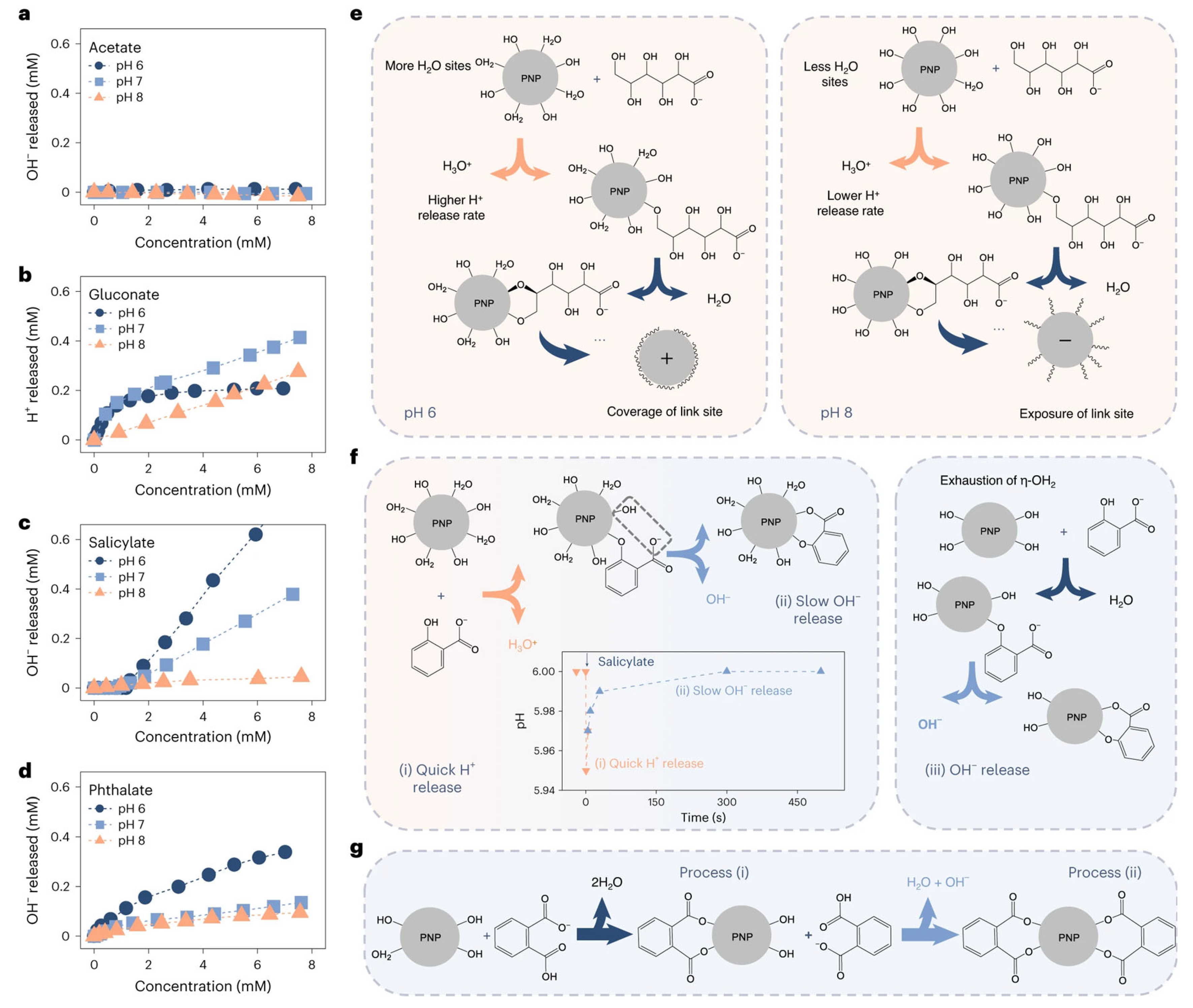
The fundamental reason coagulants are selective towards organic matter is the varying strength of the bonds formed between their surface-active sites and the different functional groups of organic compounds. Liu et al. elucidated the mechanism by which the carboxyl and hydroxyl groups of organic matter interact with the η-OH and η-OH2 active sites of amorphous aluminum hydroxide precipitates [29], as illustrated in Figure 3. Gluconate binds more strongly than acetate to the η-OH2 sites on aluminum precipitates due to the presence of more hydroxyl groups (Figure 3e), resulting in higher coagulation efficiency. In contrast, deprotonated carboxyl groups (-COO−) in acetate are unable to react with aluminum precipitates, leading to lower coagulation efficiency. Salicylate, which contains aromatic structures, also exhibits a similar phenomenon. First, phenolic hydroxyl groups bind preferentially to η-OH2 and rapidly release H+. Subsequently, the deprotonated carboxyl groups replace η-OH sites on the aluminum precipitates surfaces, thus slowly releasing OH−. These different release rates of H+ and OH− exactly demonstrate that phenolic hydroxyl groups bind more easily to aluminum precipitates than deprotonated carboxyl groups. Since more than 20% of the carboxyl groups in phthalates remain undissociated at pH 6, they react with aluminum precipitates in a manner similar to salicylates (Figure 3g).
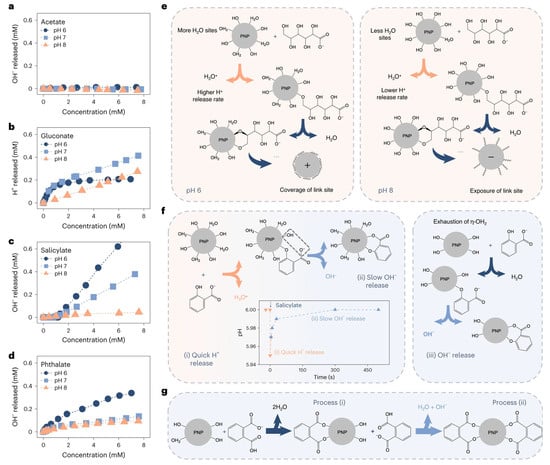
Figure 3.
Reaction between aluminum hydroxide precipitates and functional groups. Titration curves for the aluminum precipitates on addition of acetate (a), gluconate (b), salicylate (c) and phthalate (d); The corresponding schematic of the adsorption of the four organic matter by precipitated nanoparticles (PNP) (e–g) (Reproduced from Ref. [29], with permission from Springer Nature, 2024).
4. Floc Aging
In the coagulation process, flocs are typically removed through sedimentation and filtration. However, in recent years, with the proposal of the dual-carbon goals and the advocacy of resource recycling and reuse of solid waste, the reuse of flocs has attracted people’s attention. For instance, flocs can adsorb and remove pollutants, as well as participating in sludge reflux in wastewater treatment plants, which can not only improve the concentration of particles in raw water and enhance the pollutant removal efficiency, but also reduce the coagulant dosage. This addresses the issue of poor coagulation efficiency in low turbidity water [76]. However, research has found that flocs are not stable during aging. With the increase in time, the size distribution of flocs becomes narrower [77], which is not attributed to the erosion of particles from the floc surface, but rather caused by the contraction of the constituent nanoparticles [78]. Furthermore, the activity of flocs declines during the aging process, resulting in increased residual aluminum or iron concentrations and the release of adsorbed pollutants. For example, Yu et al. found that the concentrations of aluminum and HA in the supernatant increased sharply after 15 d of aging [23]. Moreover, the adsorption capacity for phosphate and fluoride ions diminishes with aging [79,80]. Gao et al. observed that the efficiency of flocs in removing colored substances decreased with prolonged aging time during the coagulation treatment of dyeing and printing wastewater [81]. Furthermore, floc aging can also lead to more severe membrane fouling in the coagulation-membrane process for HA removal [82,83]. Consequently, research on floc aging is of great importance and necessity. This review summarizes the fundamental mechanisms and the affecting factors of floc aging, as well as the relevant analytical methods involved in aging process. The aim is to fill the research gap in floc aging and provide a theoretical basis for subsequent studies.
4.1. The Mechanism of Floc Aging
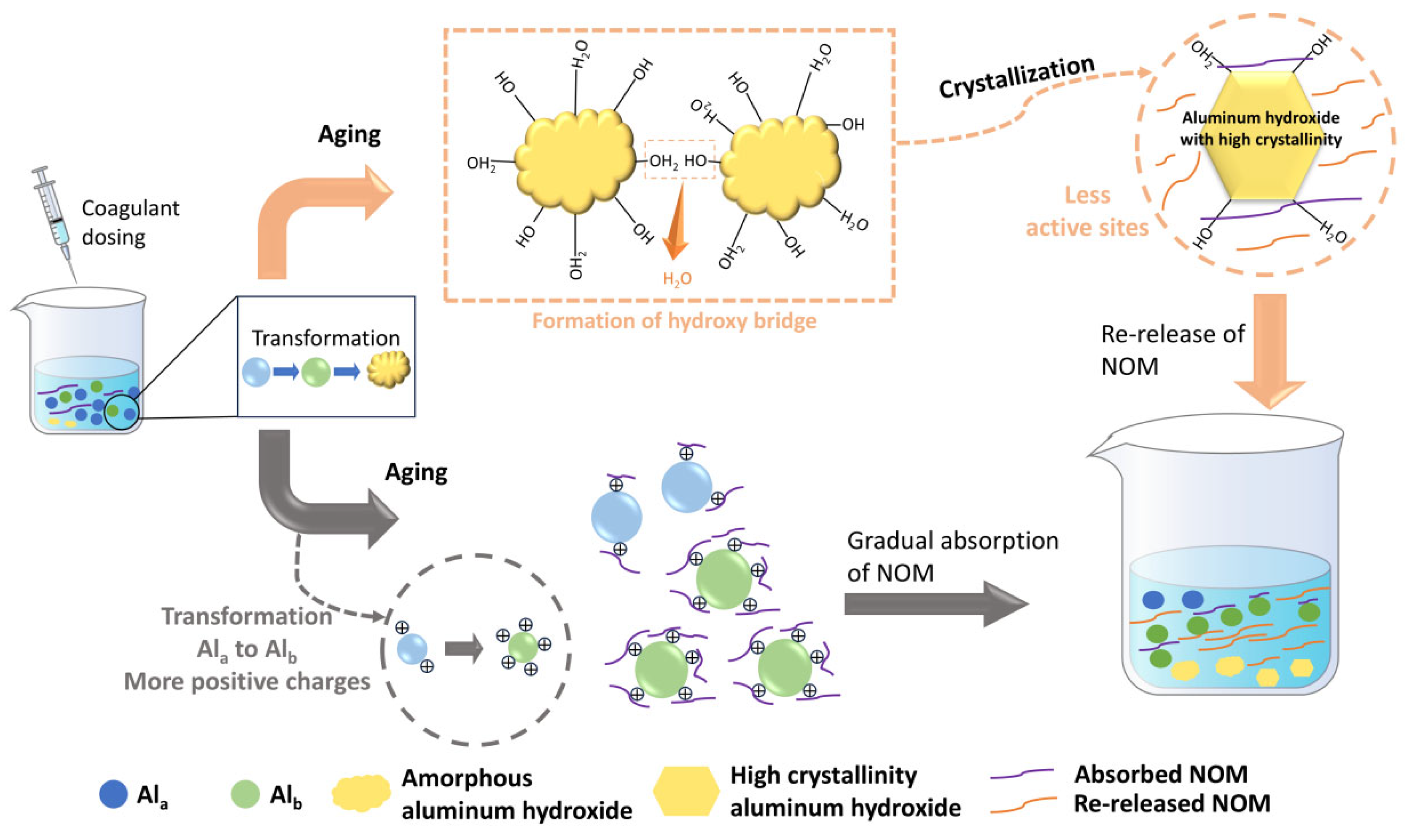
Although relatively few studies on the mechanism of floc aging have been conducted to date, this review has systematically summarized the existing research and put forward our own hypotheses. As shown in Figure 4, the key to changes in floc activity during floc aging lies in changes to the species and crystal structure of the coagulant. As mentioned above, aluminum-based coagulants undergo a series of reactions such as hydrolysis, polymerization and precipitation, thereby forming various aluminum species such as Ala, Alb and Alc. However, these aluminum species are not stable and undergo transformation during aging. For example, Ala can transform into Alb, which can subsequently become Alc. As for amorphous precipitated Alc, it can also crystallize into aluminum hydroxide with high crystallinity [79]. Since Ala, Alb and Alc correspond to coagulation mechanisms of electric double layer compression, charge neutralization and sweep flocculation, respectively, changes in aluminum species alter the pollutant removal mechanism, leading to the adsorption or release of organic matter. Moreover, the mechanism underlying the change in the crystallinity of amorphous aluminum hydroxide precipitates has also been further revealed. Actually, hydroxyl bridging reactions can occur between amorphous aluminum hydroxide precipitates with the elimination of water molecules, leading to the detachment of some aluminum hydroxide nanoparticles from the flocs and the release of organic matter [24]. Similarly, the transformation of Fea/Feb to Fec is also observed in iron flocs. Song et al. found that combining aged iron flocs with potassium persulfate (PS) could effectively enhance the removal of low-molecular-weight NOM (0.5–10 kDa) under acidic conditions. This is because the high crystallinity of the aged flocs can sufficiently activate PS to generate more hydroxyl radicals [84]. All of the above studies have confirmed that crystallization of flocs is one of the main characteristics of floc aging. Therefore, a hypothesis can be put forward that aged flocs possess some mineral-like properties, meaning their interaction with NOM resembles that between minerals and NOM [85].
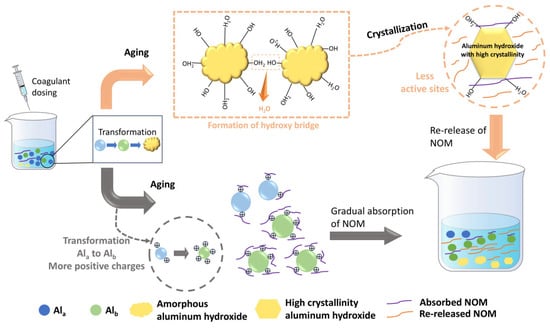
Figure 4.
The illustration of floc aging mechanism.
4.2. Affecting Factors
The aging process of flocs is affected by many factors, including pH, aging time, co-existing pollutants and types of coagulants. These factors can alter the crystallinity of aging flocs, leading to various degrees of pollutant release.
4.2.1. pH
pH is a core factor that influences coagulation, playing a crucial role in both pollutant removal and floc aging. The influence of pH on floc activity is mainly reflected in changes to the coagulation mechanism and variations in the potential of organic matter. For instance, charge neutralization is the primary mechanism for HA removal under acidic conditions, whereas bridging and sweeping coagulation predominate under neutral and alkaline conditions [51,60]. Due to the higher floc strength formed under the electrostatic neutralization mechanism compared to that formed under the sweeping effect, flocs formed under acidic conditions exhibit greater stability during aging and release fewer organic substances. Additionally, the pKa values of the different functional groups on NOM surfaces result in distinct surface potentials under various pH conditions, thereby affecting their binding with coagulants. For instance, the deprotonation processes of carboxyl and hydroxyl groups are strongly pH-dependent. Compared with phenolic hydroxyl groups, the pKa value of carboxyl groups in NOM is lower [74], enabling them to form surface complexes with Al(OH)3 under neutral conditions [67]. However, when the pH value exceeds 6, approximately 94% of acetate becomes deprotonated and is therefore difficult to bind with aluminum salt flocs [29]. Furthermore, pH also affects the change in crystallinity during floc aging. Yu et al. found that as pH increased, the amount of dissolved aluminum rose correspondingly [24]. Further studies have indicated that this phenomenon is caused by the conversion of the η-OH2 active sites on amorphous aluminum hydroxide precipitates to η-OH sites under alkaline conditions. This promotes hydroxyl-bridge reactions, which subsequently increase floc crystallinity [23]. Afterward, an increase in crystallinity leads to a decrease in floc activity, resulting in the release of organic matter. Therefore, floc activity can be restored by adjusting the pH level to acidic levels. For example, Yu et al. found that adjusting the pH to 5 and then refluxing the floc improved pollutant removal [23]. Song et al. also discovered that under acidic conditions, hydrogen ions can disrupt floc structures, creating more sites for NOM adsorption and enhancing NOM degradation by potassium persulfate [84].
4.2.2. Aging Time
The aging of flocs is a relatively slow process, making aging time a crucial factor. Wu et al. found that aging for 24 h did not result in a loss of floc active sites, while Sun et al. observed the re-release of organics after 3 d of aging [56,61]. Further studies have indicated that as aging time increases, the pH value of the supernatant rises correspondingly, while the strength of η-OH and η-OH2 functional groups on the floc surface weakens. This occurs because aging process involves floc dehydration and crystallization, during which hydroxyl bridge reactions consume H+ or release OH− [80]. Ding et al. conducted a detailed analysis of iron species at different stages of ferrihydrite aging. The results showed that the iron concentration in the flocs underwent three stages: a significant and rapid decrease within 24 h (Stage I), a gradual increase from 24 to 48 h (Stage II) and an entry into a plateau after 48–196 h (Stage III) [86]. The reason for the above phenomenon is the release and recrystallization of iron ions in the flocs. Since the main component of the amorphous precipitates formed by iron salt coagulants is exactly ferrihydrite, this research provides a theoretical reference for explaining the aging mechanism of iron salt flocs.
4.2.3. Co-Existing Pollutants
Actually, natural water bodies are complex and multi-component systems, so emerging pollutants such as particulate matter, microplastics and nanoplastics (MNPs), perfluorinated compounds and antibiotics present in them can all affect the floc aging process. It has been shown that flocs containing particulate matter of various sizes exhibit differences in the types and amounts of organic matter absorbed or released during the aging process. Figure 5 illustrates how particle size affects the floc activity. Although flocs containing nano-silica (nSiO2) and micro-silica (mSiO2) both release humic substances during aging process, the humic substances released by nSiO2 flocs are more aromatic. Furthermore, mSiO2 flocs tend to absorb low-molecular-weight neutrals (LWM-N), whereas nSiO2 flocs struggle to absorb macromolecules and LWM-N. This is attributed to the varying number of ≡Si-OH groups distributed on the surfaces of particulate matter with distinct sizes. It has been reported that ≡Si-OH can promote hydroxyl-bridge reactions with ≡Al-OH, thereby reducing floc activity. This is further supported by the observation that more OH− is released from mSiO2 flocs [25]. Therefore, the mSiO2 system with fewer ≡Si-OH groups provides more adsorbable ≡Al-OH active sites for NOM, enabling it to absorb more LWM-N during aging. Moreover, ≡Si-OH groups tend to adsorb water molecules, which may hinder the binding between NOM and flocs. The lower floc strength formed by nSiO2 compared to that formed by mSiO2 further substantiates this point [87]. Consequently, nSiO2 adsorbs bound water more easily than mSiO2, leading to a higher release of organic matter from the nano-system [88].
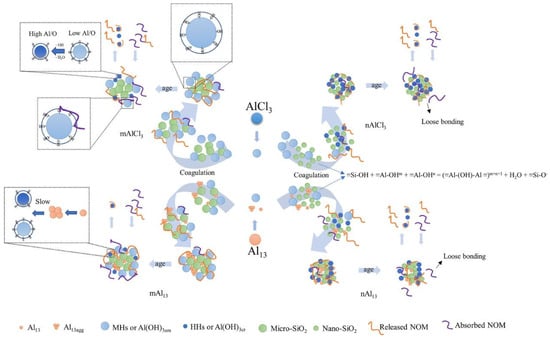
Figure 5.
Schematic of particle size effects on organic matter release during aging process (Reproduced from ref. [25], with permission from Elsevier, 2022).
Yan et al. also found that the extent of organic matter released from flocs formed by different aluminum-based coagulants containing MNPs varied [89]. MNPs-AlCl3 flocs tend to release humic substances and microbial metabolites, whereas MNPs-Al13 flocs are more prone to releasing microbial metabolites such as amino acids, sugars and lipids. Furthermore, the sequence of functional group changes induced by the interaction between MNPs and AlCl3/Al13 also differed. In the MNPs-Al13 system, the reactivity order of functional groups is aromatic C-H > C-OH > C-O > NH2 > aromatic C-C > aliphatic C-H. Conversely, in the MNPs-AlCl3 system, the reactivity order is C-O > HC=O > NH2 > C-OH > aliphatic C-H. The reason for these differences is that the floc aging mechanisms vary between the two systems. Hydrophobic and π-π interactions occur between polystyrene particles and NOM during aging in the AlCl3-NOM-MNPs system. However, new Al-O bonds form during the aging process in the Al13-NOM-NMPs system, accompanied by carbonyl bridging reactions and crystallization.
Additionally, F− and PO43− ions also influence the floc aging process. Zhang et al. investigated the influence of the coexistence of HA and F− on the aging process [80]. Results showed that over time, F− ions weakened the Al-O or Ti-O-Ti bonds in the floc structure, allowing H+ or H2O to attack the flocs and destroy their structure. Moreover, F− ion can compete with hydroxide ions for binding sites or replace hydroxyl groups in hydrolysis products, thereby causing the release of hydroxide ions. Berkowitz et al. also observed a similar phenomenon when treating phosphorus-containing wastewater, where aged flocs released phosphate [79]. Xu et al. further investigated the impact of phosphorus-containing wastewater on the crystallization of floc aging [90]. The results showed that phosphate could hinder or destroy the crystal structure of aged flocs. This may be due to the fact that phosphate occupies the active sites (η-OH/-η-OH2) on iron hydroxide precipitates during the coagulation process, thereby affecting the hydroxyl bridging reaction, which is the core of the floc aging process.
4.2.4. Types of Coagulant
Different coagulant species exhibit varying binding abilities with organic matter, thus the type of coagulant significantly influences floc stability during aging. Studies have demonstrated that both aluminum- and iron-based coagulants tend to release highly aromatic humic substances during aging [25,84]. However, aluminum-based coagulants release more, as aluminum flocs have lower strength, making their structure unstable during aging. Furthermore, conventional aluminum coagulants (e.g., Al2(SO4)3, AlCl3) release more NOM than inorganic polymer coagulants such as PACl during aging, possibly due to the stable structure of Al13 in PACl and its stronger binding to aromatic compounds than AlCl3 dose [91]. Additionally, it has been found that the fractal dimension (Df) of PACl flocs increases during aging, whereas that of AlCl3 decreases, which indicates that the type of coagulant affects the variation in floc structure during aging [89]. When treating fluorine-containing wastewater with PACl and PATC, Zhang et al. found that the flocs formed by the two coagulants did not release F− ions in the same pattern during aging [80]. The release amount of F− ions from PATC flocs was lower than from PACl flocs, which is ascribed to the co-existence of Al-OH, Ti-OH, and Al-O-Ti bonds in PATC flocs. This results in a more complex and stable floc structure, providing more sites for F− ions to combine with [53].
4.3. Characterization Methods in Mechanism Analysis
4.3.1. Characterization of Organic Matter
To analyze the interaction mechanism between organic matter and coagulants during floc formation and aging process, instruments such as spectroscopy, chromatography and mass spectrometry are commonly employed to characterize changes in the structure and morphology of organic matter, coagulants and flocs. The methods and their related applications are listed in Table 2. Ultraviolet-visible spectroscopy (UV-Vis) is frequently utilized to characterize NOM due to its advantages, such as convenience, a rapid response time and high sensitivity. UV-Vis spectral data can reveal changes in the dynamic/static environments of NOM [18,92]. For example, the ratio of absorbance at 254 nm (UV254) to dissolved organic carbon (DOC) is known as SUVA254 and is commonly used as an indicator of the aromatic hydrocarbon content of humic substances. Moreover, absorption ratios such as E2/E3 (250/365 nm), E2/E4 (265/465 nm) and E4/E6 (465/665 nm) can be employed to evaluate variations in the molecular weight, aromaticity, polarity, humicity and hydrophobicity of NOM [58,93]. Additionally, the UV spectral slope (Dslope), which is derived from a logarithmic transformation, can also partially reflect aromaticity, the source of NOM and the binding status between NOM and metal ions [35].

Table 2.
Commonly used characterization methods.
Fluorescence spectroscopy can characterize the chemical composition of NOM, and one of the most commonly employed fluorescence analysis techniques is the fluorescence excitation-emission matrix (EEM). Previous studies have indicated that EEM spectra can be divided into five regions: aromatic protein I (region I), aromatic protein II (region II), fulvic acid-like (region III), soluble microbial byproduct (region IV) and humic acid-like (region V) [97]. With advances in analytical techniques, EEM spectra combined with parallel factor analysis (PARAFAC) and two-dimensional correlation spectroscopy (2D-COS) are now widely employed to further assess changes in the composition of NOM. PARAFAC analysis divides the fluorescence spectral data of NOM into four fractions: C1 (fulvic acids), C2 (humic acids), C3 (aromatic proteins II, i.e., tryptophan-like proteinaceous substances) and C4 (aromatic proteins I) [98]. Therefore, a large number of studies have used the EEM-PARAFAC analysis to evaluate the removal efficiency of NOM in sludge-containing wastewater and algae-containing water [94,96], as well as to explore its role in multi-pollutant systems treated by coagulation-oxidation processes [48,95,99]. In contrast, 2D-COS analysis unfolds spectra along two dimensions to enhance spectral resolution and capture subtle changes in infrared (IR), UV-Vis and fluorescence spectra. For instance, combining Fourier transform infrared spectroscopy (FTIR) with the 2D-COS enables the analysis of the sequential changes in the reactivity order of the functional groups in the MNPs-NOM system [57,89], while combining UV-Vis spectroscopy with the 2D-COS can obtain the release characteristics of UV-active substances during the floc aging process [25].
Mass spectrometry can reveal the species, molecular weights and molecular formulas of NOM at a molecular level. Commonly used types of mass spectrometry in NOM research include pyrolysis gas chromatography-mass spectrometry (pyrolysis-GC-MS), liquid chromatography-mass spectrometry (LC-MS) and FT-ICR-MS. Among them, FT-ICR-MS has received extensive attention due to its characteristics of high resolution, small sample requirement and high accuracy. The atomic counts of C, H, O, N, P and S elements in NOM can be obtained through FT-ICR-MS; therefore, the specific molecular formula of NOM can be deduced subsequently. By plotting the H/C and O/C atomic ratios of NOM molecules in a two-dimensional ordination diagram (Van Krevelen plots), NOM can be further subdivided into seven categories such as lipids, proteins, carbohydrates, unsaturated hydrocarbons, lignin, tannins and aromatics. The specific classification method is as follows: lipids (1.5 < H/C ≤ 2.0, 0 ≤ O/C ≤ 0.3), proteins (1.5 < H/C ≤ 2.2, 0.3 < O/C ≤ 0.67), carbohydrates (1.5 < H/C ≤ 2.4, 0.67 ≤ O/C < 1.2), unsaturated hydrocarbons (0.7 < H/C ≤ 1.5, O/C < 0.1), lignin (0.7 < H/C ≤ 1.5, 0.1 < O/C < 0.67), tannins (0.6 ≤ H/C ≤ 1.5, 0.67 < O/C ≤ 1.0), and aromatics (0.2 < H/C ≤ 0.7, O/C ≤ 0.67) [100]. In addition, the double bond equivalent (DBE) and nominal oxidation state of carbon (NOSC) analysis methods can be used to characterize the degree of unsaturation of NOM molecules and the oxidation state of carbon elements in organic matter, respectively [101,102].
4.3.2. Characterization of Flocs
It is also essential to characterize the flocs. A scanning electron microscope (SEM) and a dynamic laser scattering instrument (DLS) can be employed to observe the morphology and size variations of flocs at different stages. It has been demonstrated that the size of flocs produced by different coagulants varies significantly. For example, titanium coagulants form larger flocs than iron and aluminum coagulants, which is primarily attributed to the stronger charge neutralization ability of titanium coagulants [16]. Regarding different species of aluminum coagulants, the order of their average floc particle sizes is Alc > Ala > Alb [103,104]. Compared with ferric flocs, ferrous flocs are larger, which is ascribed to the difference in the number of active sites on their surfaces [45].
The fractal dimension (Df), strength factor (Sf) and recovery factor (Rf) are used to measure the density, strength and regrowth ability of flocs, respectively. The values of these parameters can not only reveal the strength of the association between organic matter and coagulant, but also uncover the coagulation mechanisms involved in organic matter removal. It has been reported that the flocs formed by the sweep flocculation have the largest fractal dimension, followed by those formed by charge neutralization and bridging [105].
The values of Sf and Rf are both closely related to multiple factors. It is generally believed that the larger the floc size, the smaller the strength factor [46,106]. However, the opposite phenomenon was observed when studying the properties of flocs formed by three coagulants (titanium salt, iron salt, and aluminum salt) with NOM [107]. There are two possible reasons for this phenomenon. Firstly, the coagulation mechanism. The adsorption of iron salt hydrolysate plays a significant role in the coagulation process. And studies have shown that flocs formed predominantly via adsorption possess lower strength, making iron flocs weaker than titanium flocs. Secondly, there is the surface charge of the flocs. It has been pointed out that floc strength is inversely proportional to the number of surface charges on flocs [107]. The number of charges on flocs formed by titanium salt is smaller than that formed by iron salt, which in turn is smaller than that formed by aluminum salt. Regarding the value of Rf, it is influenced by the coagulation mechanism. Numerous studies have proved that flocs formed by charge neutralization are fully recoverable, while those formed by sweep flocculation exhibit limited regrowth ability following the breakage stage [34,46]. The fundamental reason for the irrecoverability of flocs after breakage is the loss of active sites on the floc surface. Higher hydraulic intensity results in fewer active sites on the floc surface, thereby reducing its adsorption/complexation capacity and leading to an inability to recover the original floc size [16].
X-ray diffraction (XRD) analysis can be utilized to characterize the crystallization of aged flocs. It has been reported that fresh aluminum flocs exhibit an XRD peak at 40°, indicating that these flocs exist as amorphous, micro and nanocrystals, or as semi-crystalline mixtures [80]. In contrast, aluminum flocs exhibit an obvious crystalline structure of gibbsite after 15 d of aging [24]. Furthermore, this crystallization phenomenon becomes more pronounced with increasing pH. Similarly, the aging process of iron salt flocs is also accompanied by a transformation from amorphous to ordered structures [108]. Nevertheless, compared with iron salts, the flocs formed by ferrous ions are more likely to transform into crystals during the aging process, which is ascribed to the lower nucleation barrier of ferrous ions [109,110].
Additionally, infrared spectroscopy and thermogravimetric differential thermal analysis (TG-DTA) can also reflect changes in the functional groups and composition of the flocs. It is evident that the weight loss of freshly precipitated flocs is greater than that of aged flocs. Studies have pointed out that the weight loss at 130–150 °C is mainly caused by the loss of bound water. Meanwhile the dehydration at around 330 °C is attributed to the recombination of nanoparticles [24]. Furthermore, the FTIR spectra of flocs also change at different aging stages. Peaks at 1640 cm−1 and 530 cm−1 represent the adsorbed water and coordinated water on the floc surface, respectively [111,112]. With the increase in aging time, a new peak appears at 460 cm−1, which indicates the formation of Al-O bonds (AlO6) [113]. This phenomenon further confirms that the flocs will crystallize during the aging process.
5. Conclusions and Prospect
The efficient removal of NOM remains critical to coagulation treatment. This review summarizes the changes in floc activity during NOM-coagulant interactions in detail and reveals that the factors affecting floc activity include the type of coagulant, the dosing method and hydraulic conditions, as well as the type of NOM, and the number and position of functional groups at the molecular level. Beyond floc formation, the aging process significantly affects the final organic removal efficiency. pH, aging time, coexisting pollutants and coagulant type all impact the release of organics during floc aging. Furthermore, the fundamental mechanism of floc aging involves changes in coagulant morphology and floc crystal forms.
5.1. Bottleneck
Although the coagulation process plays a vital role in removing NOM, it inevitably faces multiple developmental bottlenecks.
- (1)
- There exists a contradiction between the coagulant performance and its environmental adaptability. Although inorganic coagulants are low-cost, the residual metal ions may pose health risks. Moreover, they exhibit low removal efficiency for small-molecule hydrophilic organic compounds. Even with enhanced coagulation techniques, removal efficiency can only be marginally improved, while simultaneously increasing acid consumption and sludge production. The synthesis process of organic polymer flocculants consumes high energy, involves highly toxic monomers and exhibits poor biodegradability. Although natural polymer flocculants are environmentally friendly, their low charge density and susceptibility to pH fluctuations limit their practical application.
- (2)
- The composition, molecular weight and hydrophilicity/hydrophobicity of NOM fluctuate significantly depending on water source, season and environmental conditions, making it difficult to optimize coagulant selection and dosage.
- (3)
- The large-scale production of new flocculants faces bottlenecks, making industrial-scale promotion difficult. Furthermore, the ecological toxicity of the new flocculant has not been fully studied, and its life-cycle carbon emissions have not been incorporated into process optimization considerations.
- (4)
- Although numerous analytical techniques currently offer new perspectives for exploring the interaction between coagulants and NOM from the standpoint of functional groups and molecular structures, there remains a lack of in situ characterization techniques to reveal the interaction processes between the active sites (η-OH2 and η-OH) on floc surfaces and various functional groups on NOM surfaces during floc formation and aging. Additionally, current detection methods for coagulant hydrolysis species mainly include the ferron timed complex colorimetric method, nuclear magnetic resonance (NMR) and ESI-TOF-MS, etc., which still have limitations in the instantaneous capture of hydrolysis species.
5.2. Prospect
Therefore, future prospects can be advanced through the following aspects:
- (1)
- Design targeted coagulants and develop environmentally responsive materials to enhance the selectivity and stability of coagulants. Furthermore, incorporate the environmental impacts across the entire chain of flocculant production, use and disposal into consideration, establishing an economic-environmental benefit coupling model to advance green process design.
- (2)
- Develop an intelligent coagulation system integrating online spectroscopy, machine learning and dynamic control algorithms to achieve adaptive coagulant dosing under fluctuating water quality conditions.
- (3)
- Develop high-resolution mass spectrometry and surface-enhanced Raman spectroscopy to achieve molecular fingerprint identification and in situ monitoring of organic compounds. Additionally, establish a functional group-active site interaction model based on quantum chemistry and develop multiscale simulation tools to achieve precise prediction of process parameters.
- (4)
- Current research on floc aging is still in its preliminary stage, with existing studies mainly focusing on the aging of aluminum salt flocs. Therefore, there is an urgent need to conduct in-depth investigations into the aging processes of other commonly used coagulants, such as iron salts and titanium salts. Actual water treatment systems are more diverse and complex. Hence, future studies should focus more on the characteristics of floc formation and aging in multiple pollutant systems and examine the effects of emerging pollutants, such as antibiotics, personal care products and biochar-derived organic matter. Regarded that one of the characteristics of floc aging is the transformation of crystal forms, reference can be made to the crystal form transformation processes of minerals and the interaction processes between minerals and various substances to reveal the floc aging mechanism.
- (5)
- In existing water treatment plants, the setting parameters of coagulation process is only based on the formation of flocs, which the aging process of flocs is neglected. However, from the summary in this review, it can be found that there is secondary release of organic matter and aluminum/iron during floc aging, which poses a potential threat to water quality safety. Therefore, future water treatment plants need to optimize coagulation process parameters by integrating both floc formation and aging processes, ensuring not only the rapid formation of flocs but also high stability of flocs during aging.
- (6)
- Except revealing the pollutant release mechanisms during the floc aging process, the special utilization of aging flocs deserves further investigation. For example, aged flocs can be used to remove fluoride ions, phosphate ions and arsenate ions. In particular, the coagulation process generates large amounts of aluminum sludge and iron sludge, the effective utilization of which is crucial for drinking water treatment plants to achieve energy conservation, emission reduction and carbon neutrality.
Author Contributions
Conceptualization, H.S. and H.L.; writing—original draft preparation, H.L. and J.S.; writing—review and editing, H.S.; data curation, H.L. and Z.K.; supervision, H.S.; funding acquisition, H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 52300003).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ali, I.; Tan, X.; Li, J.Y.; Peng, C.S.; Naz, I.; Duan, Z.P.; Ruan, Y.L. Interaction of Microplastics and Nanoplastics with Natural Organic Matter (NOM) and The Impact of NOM on The Sorption Behavior of Anthropogenic Contaminants—A Critical Review. J. Clean. Prod. 2022, 376, 134314. [Google Scholar] [CrossRef]
- Liu, M.; Lu, Q.; Siddique, M.S.; Yu, W. Molecular-Weight Dependent Promotion and Competition Effects of Natural Organic Matter on Dissolved Black Carbon Removal by Coagulation. Chemosphere 2024, 356, 141940. [Google Scholar] [CrossRef]
- Sun, H.; Jiao, R.; Gao, Y.N.; Wang, D.S. Insight into The Aggregation Kinetics of Nanoparticles in Aqutic Environments: Combined Effects of Protein Corona and Particle Size. Sep. Purif. Technol. 2025, 362, 131899. [Google Scholar] [CrossRef]
- Wan, Y.; Huang, X.; Shi, B.; Shi, J.; Hao, H. Reduction of Organic Matter and Disinfection Byproducts Formation Potential By Titanium, Aluminum and Ferric Salts Coagulation for Micro-Polluted Source Water Treatment. Chemosphere 2019, 219, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Wang, L.; Tian, Y.; Li, J.; Zhou, Q.; Li, A.; Hua, M.; Pan, Y. Effects of Ozonation-Enhanced Coagulation on Effluent Organic Matter and Disinfection Byproducts. J. Clean. Prod. 2025, 490, 144801. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, C.; Xi, M.; Ma, H.; Jiang, H. Multi-Scale Modeling of Natural Organic Matter-Heavy Metal Cations Interactions: Aggregation and Stabilization Mechanisms. Water Res. 2023, 238, 120007. [Google Scholar] [CrossRef] [PubMed]
- Rebhum, M.; Lurie, M. Control of Organic Matter by Coagulation and Floc Separation. Water Sci. Technol. 1993, 27, 1–20. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Wei, X.; Huang, L.; Zhang, J.; Wu, Y.; Zhang, Y.; Xiang, Y. A Comprehensive Study on The Performance and Mechanism of Microplastics Removal by Enhanced Coagulation Methods. J. Water Process Eng. 2023, 56, 104238. [Google Scholar] [CrossRef]
- Yue, Y.; An, G.; Lin, L.; Demissie, H.; Yang, X.; Jiao, R.; Wang, D. Design and Coagulation Mechanism of a New Functional Composite Coagulant in Removing Humic Acid. Sep. Purif. Technol. 2022, 292, 121016. [Google Scholar] [CrossRef]
- Wang, D.; Tang, H. Quantitative Model of Coagulation with Inorganic Polymer Flocculant PACl: Application of the PCNM. J. Environ. Eng. 2006, 132, 434–441. [Google Scholar] [CrossRef]
- Amirtharajah, A.; Mills, K.J. Rapid-mix Design for Mechanisms of Alum Coagulation. J. Am. Water Work. Assoc. 1982, 744, 210–216. [Google Scholar] [CrossRef]
- Letterman, R.D.; Iyer, D.R. Modeling the Effects of Hydrolyzed Aluminum and Solution Chemistry on Flocculation kinetics. Environ. Sci. Technol. 1985, 198, 673–681. [Google Scholar] [CrossRef]
- Tang, H.X.; Stumm, W. The Coagulation Behavior of Fe(III) Polymeric Species. I, II. Water Res. 1987, 21, 123–128. [Google Scholar]
- Dentel, S.K. Application of The Precipitation-Charge Neutralization Model of Coagulation. Environ. Sci. Technol. 1988, 227, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Jin, X.; Li, Y.; Shang, Y.; Wang, Y.; Jin, P.; Wang, X.; Guo, F.; Li, D. The Coagulation Behavior and Mechanism of Low-Coagulability Organic Matter (LCOM). Sep. Purif. Technol. 2024, 328, 125055. [Google Scholar] [CrossRef]
- Zhao, Y.; Phuntsho, S.; Gao, B.; Yang, Y.; Kim, J.; Shon, H. Comparison of a Novel Polytitanium Chloride Coagulant with Polyaluminium Chloride: Coagulation Performance and Floc Characteristics. J. Environ. Manag. 2015, 147, 194–202. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, D.; Xu, Z.; Liu, Y.; Jiao, R.; Wang, D. Study on The Effects of Organic Matter Characteristics on The Residual Aluminum and Flocs in Coagulation Processes. J. Environ. Sci. 2018, 63, 307–317. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, H.; Zheng, L.; Liu, Z.; Korshin, G.; Yan, M. A Universal Model to Predict DOC Removal by Coagulation Based on UV-Visible Absorption Spectrum. Water Res. 2025, 286, 124160. [Google Scholar] [CrossRef]
- Duan, J.; Gregory, J. Coagulation by Hydrolysing Metal Salts. Adv. Colloid Interface Sci. 2003, 100–102, 475–502. [Google Scholar] [CrossRef]
- Kong, Y.; Ma, Y.; Ding, L.; Ma, J.; Zhang, H.; Chen, Z.; Shen, J. Coagulation Behaviors of Aluminum Salts Towards Humic Acid: Detailed Analysis of Aluminum Speciation and Transformation. Sep. Purif. Technol. 2021, 259, 118137. [Google Scholar] [CrossRef]
- Wang, W.; Li, R.; Bu, F.; Gao, Y.; Gao, B.; Yue, Q.; Yang, M.; Li, Y. Coagulation and Membrane Fouling Mechanism of Al Species in Removing Humic Acid: Effect of pH and a Dynamics Process Analysis. Sep. Purif. Technol. 2023, 309, 123130. [Google Scholar] [CrossRef]
- Chi, J.; Sun, H.; Lin, H.; Wang, Y.; Wu, L.; Sun, H. Effect of pH and Organic Matter Species on The Aging Process of Flocs. Liaoning Chem. Ind. 2025, 54, 913–919. [Google Scholar]
- Yu, J.; Xu, H.; Sun, H.Y.; Jin, Z.Y.; Wang, D.S. Mechanism on The Effects of Floc Aging and pH Adjustment on Reflux Feed Water and Coagulation. China Environ. Sci. 2022, 42, 4612–4620. [Google Scholar]
- Yu, W.; Xu, L.; Lei, K.; Gregory, J. Effect of Crystallization of Settled Aluminum Hydroxide Precipitate on “Dissolved Al”. Water Res. 2018, 143, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xu, H.; Wang, D.; Sun, H.; Jiao, R.; Liu, Y.; Jin, Z.; Zhang, S. Variations in NOM During Floc Aging: Effect of Typical Al-Based Coagulants and Different Particle Sizes. Water Res. 2022, 218, 118486. [Google Scholar] [CrossRef] [PubMed]
- Violante, A.; Huang, P.M. Influence of Inorganic and Organic-Ligands on The Formation of Aluminum Hydroxides and Oxyhydroxides. Clay Clay Miner. 1985, 33, 181–192. [Google Scholar] [CrossRef]
- Duffy, S.J.; Vanloon, G.W. Characterization of Amorphous Aluminum Hydroxide by The Ferron Method. Environ. Sci. Technol. 1994, 28, 1950–1956. [Google Scholar] [CrossRef]
- Violante, A.; Violante, P. Influence of pH, Concentration, and Chelating Power of Organic-Anions on the Synthesis of Aluminum Hydroxides and Oxyhydroxides. Clays Clay Miner. 1980, 28, 425–434. [Google Scholar] [CrossRef]
- Liu, M.; Graham, N.; Gregory, J.; Elimelech, M.; Yu, W. Towards a Molecular-Scale Theory for the Removal of Natural Organic Matter by Coagulation with Trivalent Metals. Nat. Water 2024, 2, 285–294. [Google Scholar] [CrossRef]
- Bi, S.; Wang, C.; Cao, Q.; Zhang, C. Studies on The Mechanism of Hydrolysis and Polymerization of Aluminum Salts in Aqueous Solution: Correlations Between The “Core-Links” Model and “Cage-Like” Keggin-Al13 Model. Coordin. Chem. Rev. 2004, 248, 441–455. [Google Scholar] [CrossRef]
- Liu, M.; Yu, W. Surface Chemical Groups of Flocs Are Key Factors for The Growth of Flocs in Sweep Coagulation: A Case Study of Surface Occupation by Humic Acid. ACS EST Eng. 2022, 2, 2301–2310. [Google Scholar] [CrossRef]
- Hu, C.; Liu, H.; Qu, J.; Wang, D.; Ru, J. Coagulation Behavior of Aluminum Salts in Eutrophic Water: Significance of Al13 Species and pH Control. Environ. Sci. Technol. 2006, 40, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Chen, G.; Hu, C.; Liu, Z.; Liu, H.; Qu, J. Speciation Matching Mechanisms Between Orthophosphate and Aluminum Species During Advanced P Removal Process. Sci. Total Environ. 2018, 642, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Peng, S.; Nan, J.; He, C.; Qi, F.; Ji, X.; Li, W.; Sun, D. Effect of Al Species of Polyaluminum Chlorides on Floc Breakage and Re-Growth Process: Dynamic Evolution of Floc Properties, Dissolved Organic Matter and Dissolved Al. Chemosphere 2020, 249, 126449. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; An, G.; Liu, L.; Lin, L.; Jiao, R.; Wang, D. Pre-Aggregation of Al13 in Optimizing Coagulation for Removal of Humic Acid. Chemosphere 2021, 277, 130268. [Google Scholar] [CrossRef]
- Wang, D.; Wang, S.; Huang, C.; Chow, C.W.K. Hydrolyzed Al(III) Clusters: Speciation Stability of Nano-Al13. J. Environ. Sci. 2011, 23, 705–710. [Google Scholar] [CrossRef]
- Liu, L.; Lu, S.; Jin, Z.; Lou, J.; Zhang, W.; Wang, D. Hydrolysis of Al13 and Its Coagulation Mechanism: Role of Speciation Stability and Transformation. Water Res. 2025, 281, 123672. [Google Scholar] [CrossRef]
- Song, J.; Jin, P.; Jin, X.; Wang, X.C. Synergistic Effects of Various in Situ Hydrolyzed Aluminum Species for the Removal of Humic Acid. Water Res. 2019, 148, 106–114. [Google Scholar] [CrossRef]
- Xu, W.; Gao, B.; Yue, Q.; Wang, Q. Effect of Preformed and Non-Preformed Al13 Species on Evolution of Floc Size, Strength and Fractal Nature of Humic Acid Flocs in Coagulation Process. Sep. Purif. Technol. 2011, 78, 83–90. [Google Scholar] [CrossRef]
- He, Y.; Jarvis, P.; Huang, X.; Shi, B. Unraveling the Characteristics of Dissolved Organic Matter Removed by Aluminum Species Based on FT-ICR MS Analysis. Water Res. 2024, 255, 121429. [Google Scholar] [CrossRef]
- An, G.; Yue, Y.; Wang, P.; Liu, L.; Demissie, H.; Jiao, R.; Wang, D. Deprotonation and Aggregation of Al13 Under Alkaline Titration: A Simulating Study Related to Coagulation Process. Water Res. 2021, 203, 117562. [Google Scholar] [CrossRef]
- Wang, P.; Jiao, R.; Liu, L.; Xiao, F.; An, G.; Wang, D. Optimized Coagulation Pathway of Al13: Effect of In-Situ Aggregation of Al13. Chemosphere 2019, 230, 76–83. [Google Scholar] [CrossRef]
- Xing, B.; Graham, N.; Yu, W. Transformation of Siderite to Goethite by Humic Acid in the Natural Environment. Commun. Chem. 2020, 3, 38. [Google Scholar] [CrossRef]
- Li, S.; Liu, M.; Meng, F.; Hu, X.; Yu, W. Removal of F− and Organic Matter from Coking Wastewater by Coupling Dosing FeCl3 and AlCl3. J. Environ. Sci. 2021, 110, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Graham, N.J.D.; Deng, W.; Liu, M.; Liu, T.; Yu, W. The Formation of Planar Crystalline Flocs of γ-FeOOH in Fe(II) Coagulation and The Influence of Humic Acid. Water Res. 2020, 185, 116250. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Gao, B.; Yue, Q.; Sun, S.; Wang, Y.; Li, Q. Floc Properties and Membrane Fouling of Different Monomer and Polymer Fe Coagulants in Coagulation-Ultrafiltration Process: The Role of Fe (III) Species. Chem. Eng. J. 2014, 258, 442–449. [Google Scholar] [CrossRef]
- Jiang, J.; Graham, N. Preparation and Characterisation of An Optimal Polyferric Sulphate (PFS) As a Coagulant for Water Treatment. J. Chem. Technol. Biotechnol. 1998, 73, 351–358. [Google Scholar] [CrossRef]
- Yang, B.; Graham, N.; Liu, P.; Liu, M.; Gregory, J.; Yu, W. Atomic-Level Structural Differences Between Fe(III) Coprecipitates Generated by The Addition of Fe(III) Coagulants and By the Oxidation of Fe(II) Coagulants Determine Their Coagulation Behavior in Phosphate and DOM Removal. Environ. Sci. Technol. 2023, 57, 12489–12500. [Google Scholar] [CrossRef]
- Zhao, Y.; Phuntsho, S.; Gao, B.; Shon, H. Polytitanium Sulfate (PTS): Coagulation Application and Ti Species Detection. J. Environ. Sci. 2017, 52, 250–258. [Google Scholar] [CrossRef]
- Gan, Y.; Li, J.; Zhang, L.; Wu, B.; Huang, W.; Li, H.; Zhang, S. Potential of Titanium Coagulants for Water and Wastewater Treatment: Current Status and Future Perspectives. Chem. Eng. J. 2021, 406, 126837. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, B.; Shon, H.; Cao, B.; Kim, J. Coagulation Characteristics of Titanium (Ti) Salt Coagulant Compared with Aluminum (Al) and Iron (Fe) Salts. J. Hazard. Mater. 2011, 185, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Chekli, L.; Galloux, J.; Zhao, Y.X.; Gao, B.Y.; Shon, H.K. Coagulation Performance and Floc Characteristics of Polytitanium Tetrachloride (PTC) Compared with Titanium Tetrachloride (TiCl4) and Iron Salts in Humic Acid-Kaolin Synthetic Water Treatment. Sep. Purif. Technol. 2015, 142, 155–161. [Google Scholar] [CrossRef]
- Liu, B.; Gao, Y.; Pan, J.; Feng, Q.; Yue, Q.; Guo, K.; Gao, B. Coagulation Behavior of Polyaluminum-Titanium Chloride Composite Coagulant with Humic Acid: A Mechanism Analysis. Water Res. 2022, 220, 118633. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, B.; Gao, Y.; Wang, Y.; Yue, Q.; Guo, K.; Gao, B. Insight into Control Mechanism of Polymeric Ferric Titanium Composite Coagulant on Membrane Fouling: Role of Natural Organic Matters. Sep. Purif. Technol. 2023, 322, 124255. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, H.; Li, Q.; Cheng, C.; Shen, H.; Zhang, Z.; Zhang, Z.; Wang, H. Removal of Refractory Organics in Wastewater by Coagulation/Flocculation with Green Chlorine-Free Coagulants. Sci. Total Environ. 2021, 787, 147654. [Google Scholar] [CrossRef]
- Wu, M.; Yu, W.; Qu, J.; Gregory, J. The Variation of Flocs Activity During Floc Breakage and Aging, Adsorbing Phosphate, Humic Acid and Clay Particles. Water Res. 2019, 155, 131–141. [Google Scholar] [CrossRef]
- Shao, Y.; Liu, B.; Guo, K.; Gao, Y.; Yue, Q.; Gao, B. Coagulation Performance and Mechanism of Different Hydrolyzed Aluminum Species for the Removal of Composite Pollutants of Polyethylene and Humic Acid. J. Hazard. Mater. 2024, 465, 133076. [Google Scholar] [CrossRef]
- Yu, J.; Xu, H.; Yang, X.; Sun, H.; Jin, Z.; Wang, D. Floc Formation and Growth During Coagulation Removing Humic Acid: Effect of Stirring Condition. Sep. Purif. Technol. 2022, 302, 122084. [Google Scholar] [CrossRef]
- Huang, X.; Gao, B.; Sun, Y.; Yue, Q.; Wang, Y.; Li, Q. Effects of Solution pH and Synthetic Method on Destabilization Process of Polytitanium-Silicate-Chloride. J. Hazard. Mater. 2016, 311, 230–236. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Gao, B.Y.; Yue, Q.; Zhao, Y. The Disinfection By-Products Precursors Removal Efficiency and The Subsequent Effects on Chlorine Decay for Humic Acid Synthetic Water Treated by Coagulation Process and Coagulation-Ultrafiltration Process. Chem. Eng. J. 2012, 193–194, 59–67. [Google Scholar] [CrossRef]
- Sun, H.; Jiao, R.; Yang, Q.; Yu, J.; Wang, D. Aggregation and Settling Characteristics of Particulate Matter and DOM In a Southern China Reservoir: Influence of Hydraulic Conditions and Dosing Methods. Process Saf. Environ. 2022, 166, 500–511. [Google Scholar] [CrossRef]
- Jiao, R.; Sun, H.; Xu, S.; He, Y.; Xu, H.; Wang, D. Aggregation, Settling Characteristics and Destabilization Mechanisms of Nano-Particles Under Different Conditions. Sci. Total Environ. 2022, 827, 154228. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Gao, B.; Yue, Q.; Wang, Y.; Li, Q.; Zhao, S.; Sun, S. Effect of Dosing Sequence and Raw Water pH on Coagulation Performance and Flocs Properties Using Dual-Coagulation of Polyaluminum Chloride and Compound Bioflocculant in Low Temperature Surface Water Treatment. Chem. Eng. J. 2013, 229, 477–483. [Google Scholar] [CrossRef]
- Huang, X.; Gao, B.; Rong, H.; Yue, Q.; Zhang, Y.; Teng, P. Effect of Using Polydimethyldiallylammonium Chloride as Coagulation Aid on Polytitanium Salt Coagulation Performance, Floc Properties and Sludge Reuse. Sep. Purif. Technol. 2015, 143, 64–71. [Google Scholar] [CrossRef]
- Mallya, D.S.; Abdikheibari, S.; Dumée, L.F.; Muthukumaran, S.; Lei, W.; Baskaran, K. Removal of Natural Organic Matter from Surface Water Sources by Nanofiltration and Surface Engineering Membranes for Fouling Mitigation—A Review. Chemosphere 2023, 321, 138070. [Google Scholar] [CrossRef]
- Ma, G.; Xu, H.; Yang, X.; An, G.; Yang, Q.; Wang, X.; Wang, D. Molecular Investigation on Changing Behaviors of Natural Organic Matter by Coagulation with Non-Targeting Screen Using High-Resolution Mass Spectrometry. J. Hazard. Mater. 2022, 424, 127408. [Google Scholar] [CrossRef]
- Song, J.; Jin, X.; Wang, X.C.; Jin, P. Preferential Binding Properties of Carboxyl and Hydroxyl Groups with Aluminium Salts for Humic Acid Removal. Chemosphere 2019, 234, 478–487. [Google Scholar] [CrossRef]
- Finkbeiner, P.; Moore, G.; Pereira, R.; Jefferson, B.; Jarvis, P. The Combined Influence of Hydrophobicity, Charge and Molecular Weight on Natural Organic Matter Removal by Ion Exchange and Coagulation. Chemosphere 2020, 238, 124633. [Google Scholar] [CrossRef]
- Wang, K.; Li, P.; He, C.; Shi, Q.; He, D. Hydrologic Heterogeneity Induced Variability of Dissolved Organic Matter Chemistry Among Tributaries of The Three Gorges Reservoir. Water Res. 2021, 201, 117358. [Google Scholar] [CrossRef]
- He, Y.; Huang, X.; Van Leeuwen, J.; Feng, C.; Shi, B. Compositional and Structural Identification of Organic Matter Contributing to High Residual Soluble Aluminum After Coagulation. Sci. Total Environ. 2024, 907, 168005. [Google Scholar] [CrossRef]
- Yuan, Z.; He, C.; Shi, Q.; Xu, C.; Li, Z.; Wang, C.; Zhao, H.; Ni, J. Molecular Insights into The Transformation of Dissolved Organic Matter in Landfill Leachate Concentrate During Biodegradation and Coagulation Processes Using ESI FT-ICR MS. Environ. Sci. Technol. 2017, 51, 8110–8118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, F.; Li, T.; Xie, K.; Yao, H.; Xing, B.; Li, Z.; Bai, Y. Simulated Photo-Degradation of Dissolved Organic Matter in Lakes Revealed by Three-Dimensional Excitation-Emission Matrix with Regional Integration and Parallel Factor Analysis. J. Environ. Sci. 2020, 90, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Slavik, I.; Kostrowski, D.; Uhl, W. Effect of Solar Radiation on Natural Organic Matter Composition in Surface Waters and Resulting Impacts on Drinking Water Treatment. Environ. Technol. 2023, 44, 1549–1565. [Google Scholar] [CrossRef] [PubMed]
- Adusei-Gyamfi, J.; Ouddane, B.; Rietveld, L.; Cornard, J.; Criquet, J. Natural Organic Matter-Cations Complexation and Its Impact on Water Treatment: A Critical Review. Water Res. 2019, 160, 130–147. [Google Scholar] [CrossRef]
- Jin, P.; Song, J.; Wang, X.C.; Jin, X. Two-Dimensional Correlation Spectroscopic Analysis on The Interaction Between Humic Acids and Aluminum Coagulant. J. Environ. Sci. 2018, 64, 181–189. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, B.; Wen, Q.; Chen, C. Evaluation of Enhanced Coagulation Combined with Densadeg-Ultrafiltration Process in Treating Secondary Effluent: Organic Micro-Pollutants Removal, Genotoxicity Reduction, and Membrane Fouling Alleviation. J. Hazard. Mater. 2020, 396, 122697. [Google Scholar] [CrossRef]
- Bardossy, G.; White, J.L. Carbonate Inhibits the Crystallization of Aluminum Hydroxide in Bauxite. Science 1979, 203, 355–356. [Google Scholar] [CrossRef]
- Francois, R.J. Ageing of Aluminum Hydroxide Flocs. Water Res. 1987, 21, 523–531. [Google Scholar] [CrossRef]
- Berkowitz, J.; Anderson, M.A.; Amrhein, C. Influence of Aging on Phosphorus Sorption to Alum Floc in Lake Water. Water Res. 2006, 40, 911–916. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, Y.; Fan, L.; Zheng, X.; Wang, J.; Jiang, C.; Xu, S.; Xu, H.; Wang, D. Coagulation Effect of Polyaluminum-Titanium Chloride Coagulant and The Effect of Floc Aging in Fluoride Removal: A Mechanism Analysis. Sep. Purif. Technol. 2023, 325, 124674. [Google Scholar] [CrossRef]
- Gao, B.; Liu, B.; Chen, T.; Yue, Q. Effect of Aging Period on The Characteristics and Coagulation Behavior of Polyferric Chloride and Polyferric Chloride-Polyamine Composite Coagulant for Synthetic Dying Wastewater Treatment. J. Hazard. Mater. 2011, 187, 413–420. [Google Scholar] [CrossRef]
- Yu, W.; Graham, N.; Yang, Y.; Zhou, Z.; Campos, L.C. Effect of Sludge Retention on UF Membrane Fouling: The Significance of Sludge Crystallization and EPS Increase. Water Res. 2015, 83, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Liu, M.; Zhang, X.; Graham, N.; Qu, J. Effect of Pre-Coagulation Using Different Aluminium Species on Crystallization of Cake Layer and Membrane Fouling. npj Clean Water 2019, 2, 17. [Google Scholar] [CrossRef]
- Song, Q.; Yang, B.; Liu, M.; Song, S.; Graham, N.; Yu, W. Floc Aging: Crystallization and Improving Low Molecular Weight Organic Removal in Re-Coagulation. Water Res. 2023, 243, 120328. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Xiang, H.; Jiang, J.; Chen, W. Interfacial Interactions Between Minerals and Organic Matter: Mechanisms and Characterizations. Chemosphere 2024, 359, 142383. [Google Scholar] [CrossRef]
- Ding, L.; Han, B.; Jia, R.R.; Yang, X.; Liang, X.J.; Guo, X.T. Molecular Insights into the Synergistic Inhibition of Microplastics Derived Dissolved Organic Matter and Anions on The Transformation of Ferrihydrite. Environ. Sci. Technol. 2025, 59, 4104–4112. [Google Scholar] [CrossRef]
- Sun, H.; Jiao, R.; Xu, H.; An, G.; Wang, D. The Influence of Particle Size and Concentration Combined With pH on Coagulation Mechanisms. J. Environ. Sci. 2019, 82, 39–46. [Google Scholar] [CrossRef]
- Sun, H.; Jiao, R.; An, G.; Xu, H.; Wang, D. Influence of Particle Size on The Aggregation Behavior of Nanoparticles: Role of Structural Hydration Layer. J. Environ. Sci. 2021, 103, 33–42. [Google Scholar] [CrossRef]
- Yan, Y.; Xu, H.; Wang, Z.; Chen, H.; Yang, L.; Sun, Y.; Zhao, C.; Wang, D. Effect of Surface Functional Groups of Polystyrene Micro/Nano Plastics on The Release of NOM From Flocs During the Aging Process. J. Hazard. Mater. 2024, 472, 134421. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Z.; Graham, N.J.D.; Yu, W. Exploring the Influence of Aquatic Phosphate on Fe Floc Dynamics in Water Treatment. Water Res. 2024, 262, 122146. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, H.; Hu, C.; Qu, J. Effect of Aluminum Speciation and Structure Characterization on Preferential Removal of Disinfection Byproduct Precursors by Aluminum Hydroxide Coagulation. Environ. Sci. Technol. 2009, 43, 5067–5072. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yu, H.Q. Advances in the Characterization and Monitoring of Natural Organic Matter Using Spectroscopic Approaches. Water Res. 2021, 190, 116759. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.; Zhou, K.; Zhang, Z.; Peng, C.; Chen, W. Elucidating the Structural Variation of Membrane Concentrated Landfill Leachate During Fenton Oxidation Process Using Spectroscopic Analyses. Environ. Pollut. 2020, 256, 113467. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Huang, Y.; Ao, J.; Wu, Y.; Bu, L.; Zhou, S.; Deng, L.; Shi, Z. A Molecular-Level Mechanism Analysis of PFS Coagulation Behaviors: Differences in Natural Organic Matter and Algal Organic Matter. Sep. Purif. Technol. 2023, 314, 123485. [Google Scholar] [CrossRef]
- Yang, B.; Rashid, S.; Graham, N.; Li, G.; Yu, W. In-Depth Study of The Removal of Mn(II) By Fe(VI) Treatment and The Profound Influence of NOM on Floc Formation and Properties. Water Res. 2023, 247, 120840. [Google Scholar] [CrossRef]
- Duan, S.; Dong, H.; Jiang, C.; Liang, H.; Jiang, L.; Xu, Q.; Cheng, X.; Qiang, Z. Removal of Particulate Matter and Dissolved Organic Matter from Sedimentation Sludge Water During Pre-Sedimentation Process: Performances and Mechanisms. J. Environ. Sci. 2025, 148, 409–419. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence Excitation-Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Murphy, K.; Stedmon, C.; Wenig, P.; Bro, R. OpenFlor—An Online Spectral Library of Auto-Fluorescence by Organic Compound in the Environment. Anal. Methods 2014, 6, 658. [Google Scholar] [CrossRef]
- Luo, M.; Wang, Z.; Zhang, C.; Song, B.; Li, D.; Cao, P.; Peng, X.; Liu, S. Advanced Oxidation Processes and Selection of Industrial Water Source: A New Sight from Natural Organic Matter. Chemosphere 2022, 303, 135183. [Google Scholar] [CrossRef]
- Kim, S.; Kramer, R.W.; Hatcher, P.G. Graphical Method for Analysis of Ultrahigh-Resolution Broadband Mass Spectra of Natural Organic Matter, The Van Krevelen Diagram. Anal. Chem. 2003, 75, 5336–5344. [Google Scholar] [CrossRef]
- Larowe, D.E.; Van Cappellen, P. Degradation of Natural Organic Matter: A Thermodynamic Analysis. Geochim. Cosmochim. Acta 2011, 75, 2030–2042. [Google Scholar] [CrossRef]
- Zhang, X.; Han, J.; Zhang, X.; Shen, J.; Chen, Z.; Chu, W.; Kang, J.; Zhao, S.; Zhou, Y. Application of Fourier Transform Ion Cyclotron Resonance Mass Spectrometry to Characterize Natural Organic Matter. Chemosphere 2020, 260, 127458. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Wei, Q.; Wang, D.; Zhu, Z.; Tang, H. Coagulation of Humic Acid: The Performance of Preformed and Non-Preformed Al Species. Colloids Surface A 2007, 296, 141–148. [Google Scholar] [CrossRef]
- Yan, M.; Wang, D.; Qu, J.; He, W.; Chow, C.W.K. Relative Importance of Hydrolyzed Al(III) Species (Ala, Alb, and Alc) During Coagulation with Polyaluminum Chloride: A Case Study with The Typical Micro-Polluted Source Waters. J. Colloid Interface Sci. 2007, 316, 482–489. [Google Scholar] [CrossRef]
- Li, T.; Zhu, Z.; Wang, D.; Yao, C.; Tang, H. Characterization of Floc Size, Strength and Structure Under Various Coagulation Mechanisms. Powder Technol. 2006, 168, 104–110. [Google Scholar] [CrossRef]
- Jarvis, P.; Jefferson, B.; Gregory, J.; Parsons, S.A. A Review of Floc Strength and Breakage. Water Res. 2005, 39, 3121–3137. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, B.; Shon, H.; Kim, J.; Yue, Q.; Wang, Y. Floc Characteristics of Titanium Tetrachloride (TiCl4) Compared with Aluminum and Iron Salts in Humic Acid-Kaolin Synthetic Water Treatment. Sep. Purif. Technol. 2011, 81, 332–338. [Google Scholar] [CrossRef]
- Furcas, F.; Mundra, S.; Lothenbach, B.; Angst, U. Speciation Controls the Kinetics of Iron Hydroxide Precipitation and Transformation at Alkaline pH. Environ. Sci. Technol. 2024, 58, 19851–19860. [Google Scholar] [CrossRef]
- Sheng, A.; Li, X.; Arai, Y.; Ding, Y.; Rosso, K.M.; Liu, J. Citrate Controls Fe(II)-Catalyzed Transformation of Ferrihydrite by Complexation of The Labile Fe(III) Intermediate. Environ. Sci. Technol. 2020, 54, 7309–7319. [Google Scholar] [CrossRef]
- Sheng, A.; Liu, J.; Li, X.; Luo, L.; Ding, Y.; Chen, C.; Zhang, X.; Wang, C.; Rosso, K.M. Labile Fe(III) Supersaturation Controls Nucleation and Properties of Product Phases from Fe(II)-Catalyzed Ferrihydrite Transformation. Geochim. Cosmochim. Acta 2021, 309, 272–285. [Google Scholar] [CrossRef]
- Li, G.; Liu, Y.; Liu, D.; Liu, L.; Liu, C. Synthesis of Flower-Like Boehmite (AlOOH) Via a Simple Solvothermal Process Without Surfactant. Mater. Res. Bull. 2010, 45, 1487–1491. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Z.; Wang, B.; Zhang, S.; Yang, R. Synthesis Neutral Rare Earth Complexes of Diethylenetriamine-N,N″-Bis(Acetyl-Isoniazid)-N,N′,N″-Triacetic Acid as Potential Contrast Enhancement Agents for Magnetic Resonance Imaging. Chem. Pharm. Bull. 2006, 54, 1203–1206. [Google Scholar] [CrossRef]
- Hou, H.; Xie, Y.; Yang, Q.; Guo, Q.; Tan, C. Preparation and Characterization of γ-AlOOH Nanotubes and Nanorods. Nanotechnology 2005, 16, 741. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).