Effect of Hydraulic Projects on the Phytoplankton Community Structure in the Mainstream of the Ganjiang River
Abstract
1. Introduction
2. Material and Method
2.1. Study Area and Sample Site Selection
2.2. Sample Collection and Analysis
2.3. Statistical Analysis
3. Results
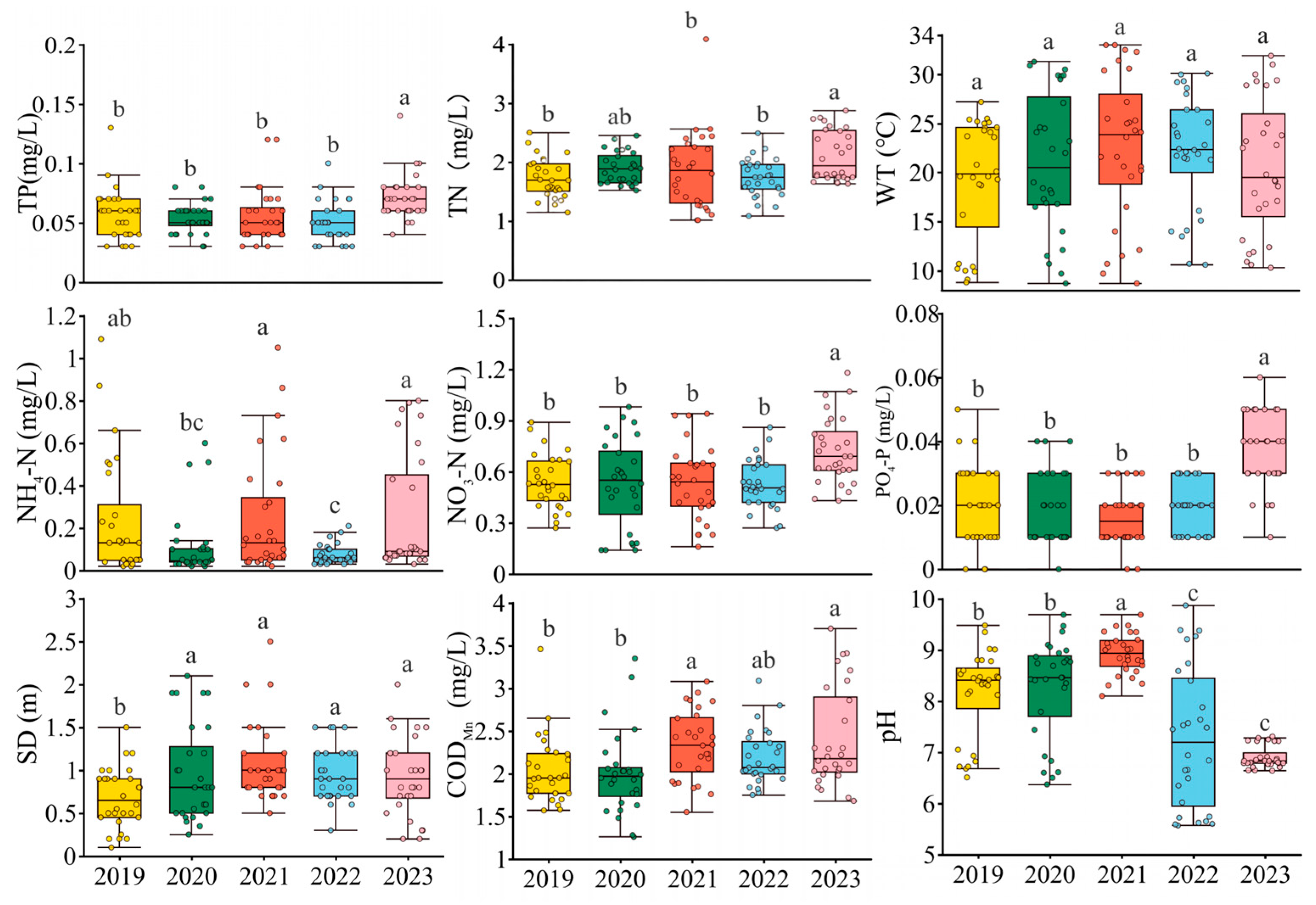
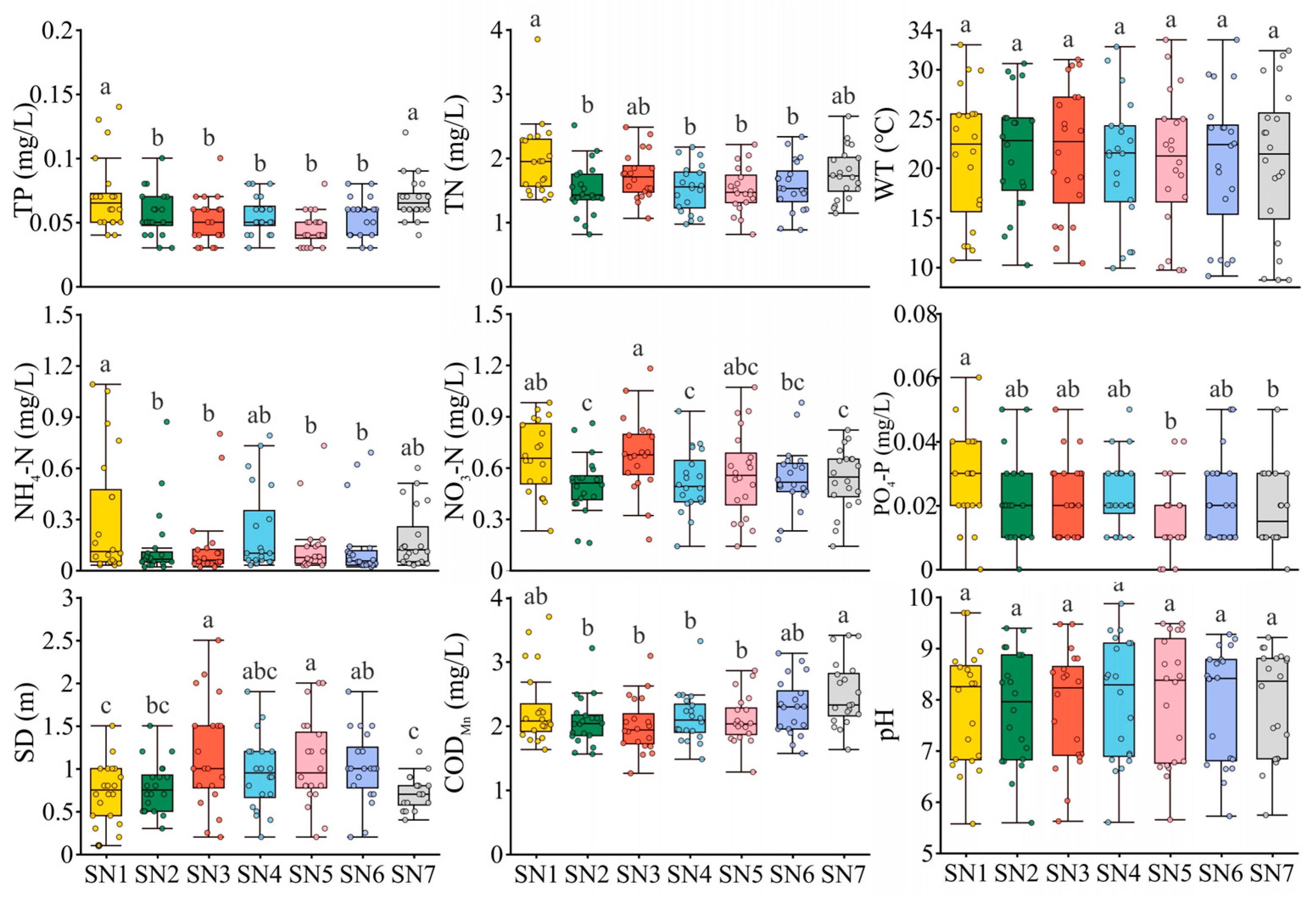
3.1. Physicochemical Factors
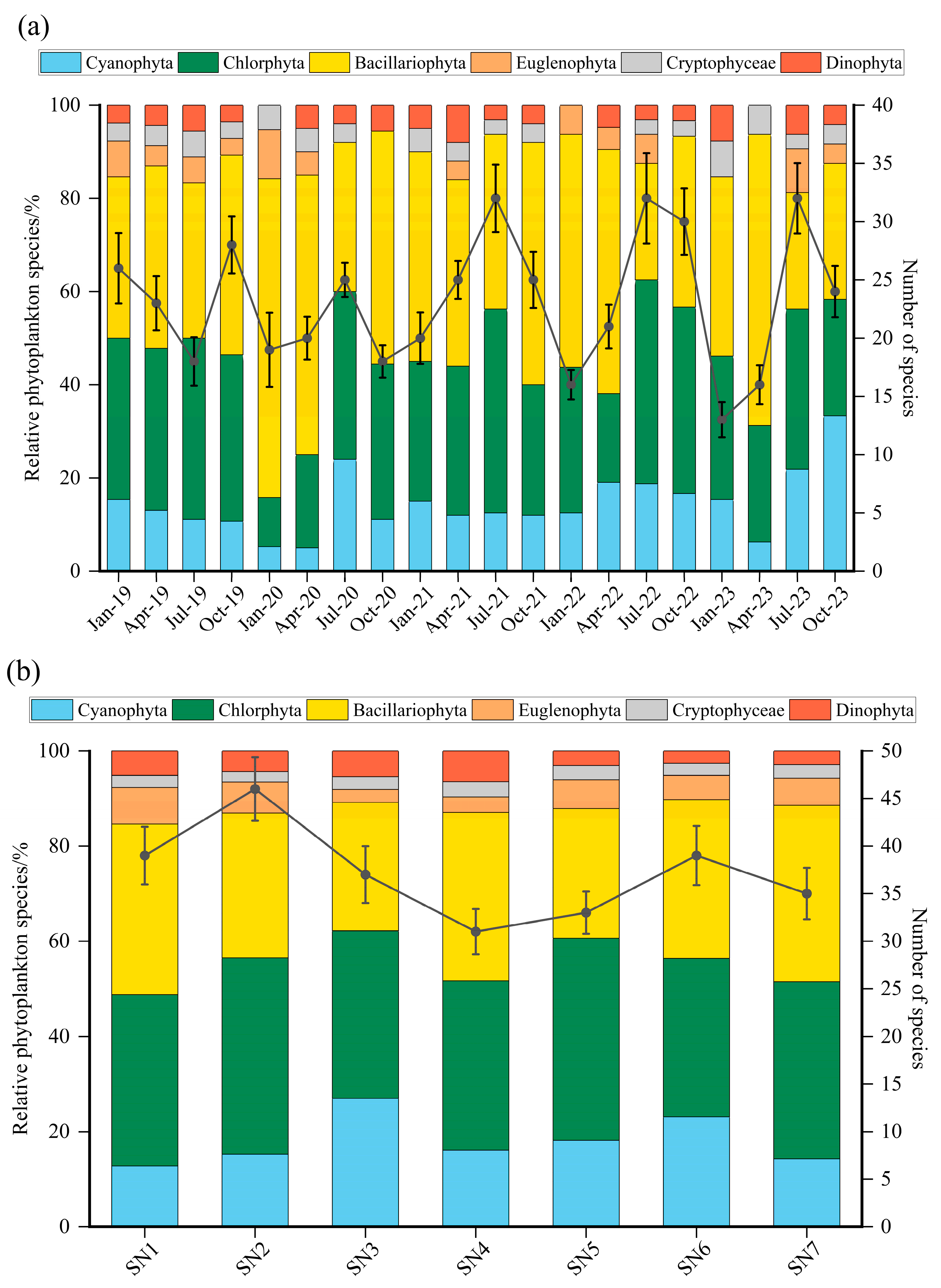
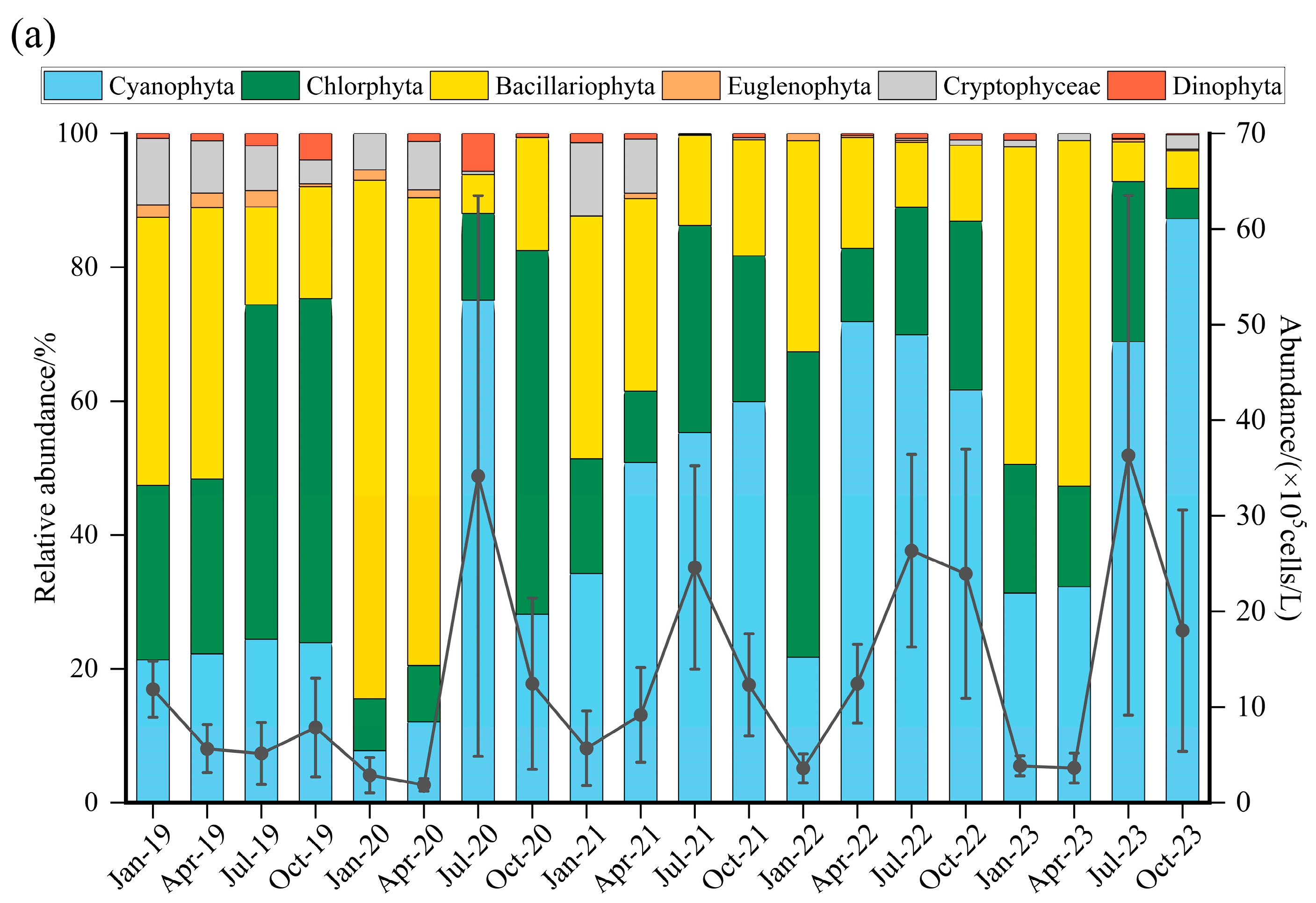
3.2. Phytoplankton Composition
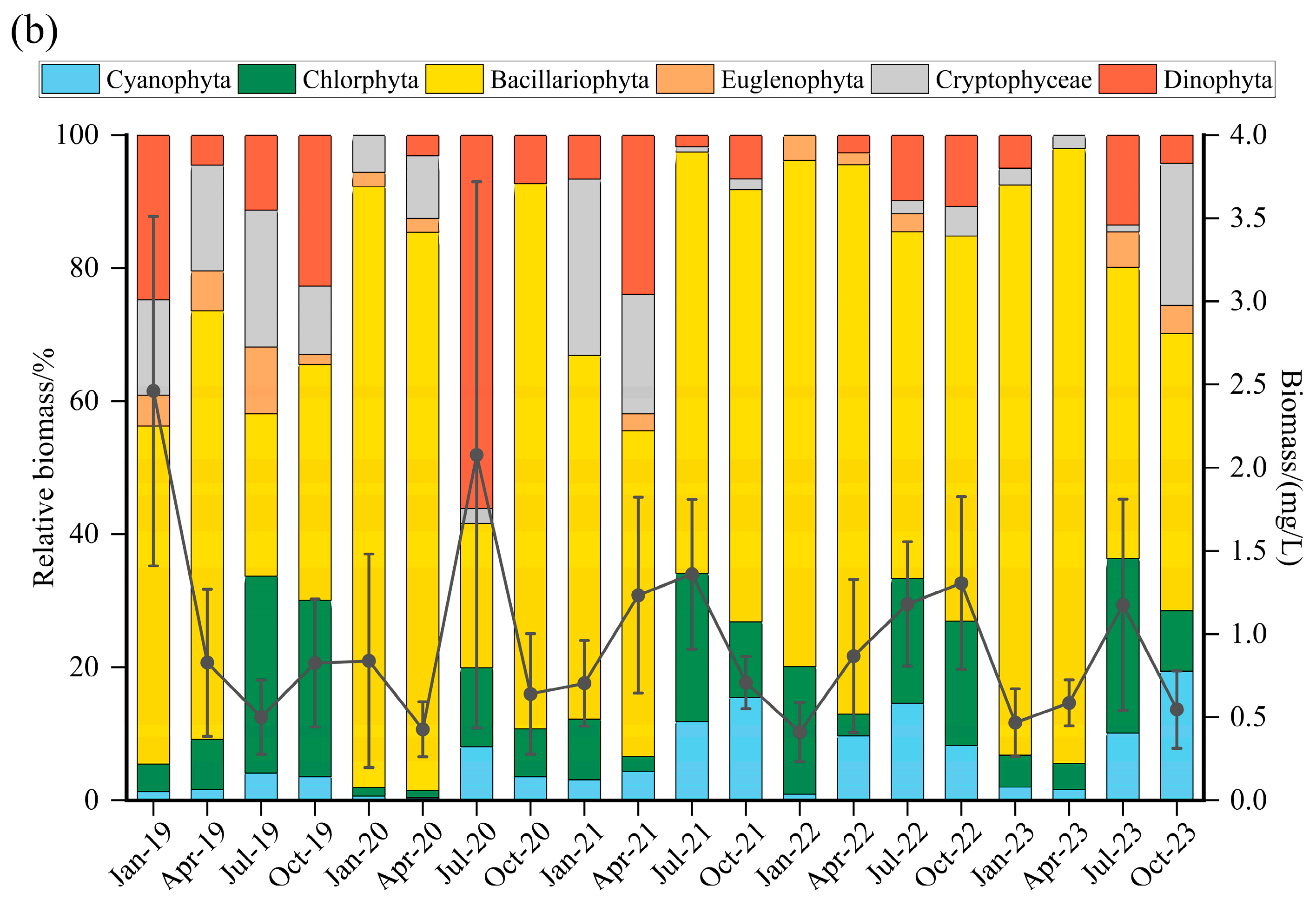
3.3. Seasonal Variation in Phytoplankton
3.4. Spatial Variation in Phytoplankton
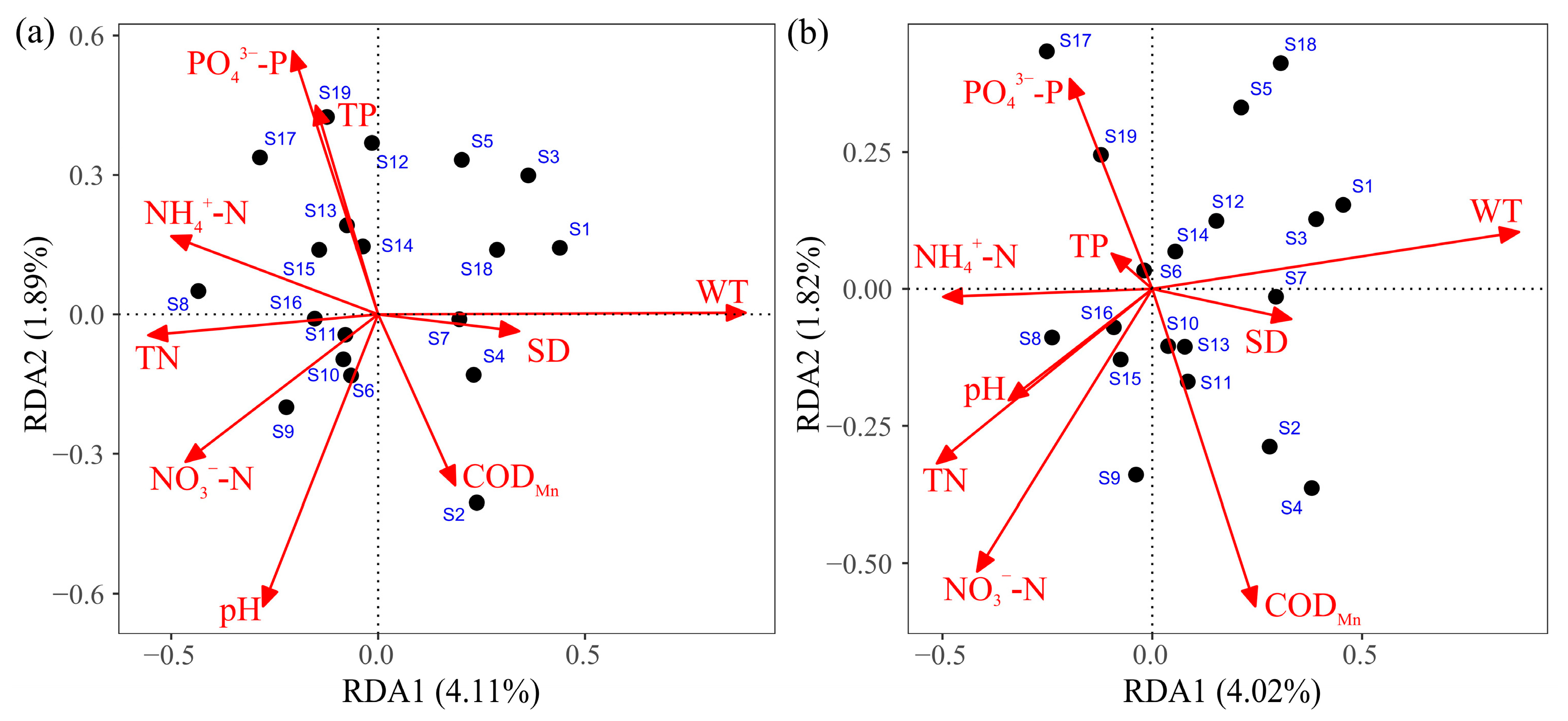
3.5. The Influence of Physicochemical Factors on Phytoplankton
4. Discussion
4.1. Structural Characteristics of Phytoplankton Communities
4.2. Relationship Between Phytoplankton Community Structure and Physicochemical Factors
4.3. Impact of Hydraulic Project on Phytoplankton Community Structure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gogoi, P.; Das, S.K.; Das Sarkar, S.; Chanu, T.N.; Manna, R.K.; Sengupta, A.; Raman, R.K.; Samanta, S.; Das, B.K. physicochemical factors Driving Phytoplankton Assemblage Pattern and Diversity: Insights from Sundarban Eco-Region, India. Ecohydrol. Hydrobiol. 2021, 21, 354–367. [Google Scholar] [CrossRef]
- Hochfeld, I.; Hinners, J. Phytoplankton Adaptation to Steady or Changing Environments Affects Marine Ecosystem Functioning. Biogeosciences 2024, 21, 5591–5611. [Google Scholar] [CrossRef]
- Jin, X.; Xie, H.; Zhao, X.; He, D.; Zhan, A.; Cai, Y.; Wu, N.; Zhang, X.; Yang, J.; Wang, Y.; et al. Aquatic Ecosystem Health Assessment in China Based on Metacommunity Theory: From Theory to Practice. Carbon Res. 2025, 4, 16. [Google Scholar] [CrossRef]
- Zarfl, C.; Lumsdon, A.E.; Berlekamp, J.; Tydecks, L.; Tockner, K. A Global Boom in Hydropower Dam Construction. Aquat. Sci. 2015, 77, 161–170. [Google Scholar] [CrossRef]
- Soomro, A.G.; Shah, S.A.; Memon, A.H.; Alharabi, R.S.; Memon, D.; Panhwar, S.; Keerio, H.A. Cascade Reservoirs: An Exploration of Spatial Runoff Storage Sites for Water Harvesting and Mitigation of Climate Change Impacts, Using an Integrated Approach of GIS and Hydrological Modeling. Sustainability 2022, 14, 13538. [Google Scholar] [CrossRef]
- Yang, M.; Zhu, L.; Liu, J.; Zhang, Y.; Zhou, B. Influence of Water Conservancy Project on Runoff in the Source Region of the Yellow River and Wetland Changes in the Lakeside Zone, China. J. Groundw. Sci. Eng. 2023, 11, 333–346. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, W.; Xu, B.; Xu, Z. Riverine Sulfate Sources and Behaviors in Arid Environment, Northwest China: Constraints from Sulfur and Oxygen Isotopes. J. Environ. Sci. 2024, 137, 716–731. [Google Scholar] [CrossRef]
- Wang, X.; Wang, P.; Wang, C.; Chen, J.; Hu, B.; Yuan, Q.; Du, C.; Xing, X. Cascade Damming Impacts on Microbial Mediated Nitrogen Cycling in Rivers. Sci. Total Environ. 2023, 903, 166533. [Google Scholar] [CrossRef]
- Bao, Y.; Sun, M.; Wang, Y.; Lu, J.; Wu, Y.; Chen, H.; Li, S.; Qin, Y.; Wang, Z.; Wen, J.; et al. Impact of Cascade Reservoir on the Sources of Organic Matter in Sediments of Lancang River. iScience 2025, 28, 111681. [Google Scholar] [CrossRef]
- Peñuelas, J.; Sardans, J.; Rivas-ubach, A.; Janssens, I.A. The Human-induced Imbalance between C, N and P in Earth’s Life System. Glob. Change Biol. 2012, 18, 3–6. [Google Scholar] [CrossRef]
- Grumbine, R.E.; Xu, J. Mekong Hydropower Development. Science 2011, 332, 178–179. [Google Scholar] [CrossRef]
- Orr, S.; Pittock, J.; Chapagain, A.; Dumaresq, D. Dams on the Mekong River: Lost Fish Protein and the Implications for Land and Water Resources. Glob. Environ. Change 2012, 22, 925–932. [Google Scholar] [CrossRef]
- Bai, H.; Wang, Y.; Song, J.; Kong, F.; Zhang, X.; Li, Q. Characteristics of Plankton Community Structure and Its Relation to physicochemical factors in Weihe River, China. Ecol. Environ. Sci. 2022, 31, 117–130. [Google Scholar] [CrossRef]
- Jiangxi Provincial Water Resources Department (Ed.) Encyclopedia of Rivers and Lakes in Jiangxi, 1st ed.; Chang Jiang Chu Ban She: Wuhan, China, 2010; ISBN 978-7-80708-942-1. [Google Scholar]
- Chen, C.; Zhang, Y.; Xiang, Y.; Wang, L. Study on Runoff Responses to Land Use Change in Ganjiang Basin. J. Nat. Resour. 2014, 29, 1758–1769. [Google Scholar] [CrossRef]
- Han, H.; Sun, J. An Analysis of the Meteorological and Hydrological Drought Propagation Characteristics in Ganjiang River Basin. China Rural Water Hydropower 2022, 64, 101–106. [Google Scholar] [CrossRef]
- Hu, F.; Hou, J.; Luo, J.; Hu, W. Analysis of Pollution Loading and Water Environmental Capacity of Gan River in Nanchang. Environ. Sci. Technol. 2010, 33, 192–195+205. Available online: https://lib.cqvip.com/Qikan/Article/Detail?id=1001415721 (accessed on 25 September 2025).
- Cao, B.; Lei, B.; Yu, J. Research on the Basin Water Temperature Impact of Important Hydro Junction Constructions of Ganjing Mainstream in Jiangxi Province. Jiangxi Chem. Ind. 2014, 30, 35–38. [Google Scholar] [CrossRef]
- Baird, R.B.; Eaton, A.D.; Rice, E.W. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association, American Water Works Association, Water Environment Federation, Eds.; American Public Health Association: Washington, DC, USA, 2017; ISBN 978-0-87553-287-5. [Google Scholar]
- Guo, T.; Fu, Z.; Zhou, C.; Chen, J.; OuYang, S.; Wu, X. Diversity of Eukaryotic Phytoplankton in Poyang Lake Based on Environmental DNA Metabarcoding. J. Hydroecol. 2023, 44, 67–75. [Google Scholar] [CrossRef]
- Hu, H.; Wei, Y. The Freshwater Algae of China: Systematics, Taxonomy and Ecology; Science Press: Beijing, China, 2006; ISBN 7-03-016633-7. [Google Scholar]
- Hillebrand, H.; Dürselen, C.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume Calculation for Pelagic and Benthic Microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Liu, J.; Zou, H.; Deng, F.; Liu, Y.; Li, W.; Xu, J.; Liu, S.; Wu, Q.; Zhang, X.; Weng, F.; et al. Phytoplankton Functional Groups in Poyang Lake: Succession and Driving Factors. J. Ocean. Limnol. 2024, 42, 1764–1776. [Google Scholar] [CrossRef]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R; Use R! 2nd ed.; Springer: Cham, Switzerland, 2018; ISBN 978-3-319-71404-2. [Google Scholar]
- Wang, Y.; Chen, L.; Niu, Y.; Yu, H.; Luo, M. Spatio-Temporal Variation in Phytoplankton Community and Its Influencing Factors in Danjiangkou Reservoir. J. Lake Sci. 2016, 28, 1057–1065. [Google Scholar] [CrossRef][Green Version]
- Zhang, C.; Lei, G.; Zhao, F.; Chen, K.; Zhang, C.; Lu, C.; Luo, Q.; Song, J.; Chen, K.; Ye, J.; et al. Functional Trait-Based Phytoplankton Biomass and Assemblage Analyses in the Pre-Growing Season for Comprehensive Algal Bloom Risk Assessment. Water Res. 2024, 257, 121755. [Google Scholar] [CrossRef]
- Wang, J.; Xia, Y.; Yu, X.; Liu, J.; Li, H.; Chen, Y. Temporal and Spatial Distribution Characteristics and Water Quality Evaluation of Planktonic Algae in the Middle and Lower Reaches of Ganjiang River. J. Ecol. Rural Environ. 2023, 39, 1031–1041. [Google Scholar] [CrossRef]
- Zhao, X.; Fang, T.; Yang, K.; Li, J.; Liang, Y.; Lu, W. Community Structure Characteristics of Phytoplankton and Related physicochemical factors in Summer in Tuohu Lake, Anhui, China. Plant Sci. J. 2018, 36, 687–695. [Google Scholar] [CrossRef]
- Peimin, P.; Yuhong, L.; Jinfang, Z.; Yongbing, M.; Zhengkui, L.; Xiaoying, C. Eutrophication Control in Local Area through Phytoplankton Population Regulation by Eco-Remediation: A Case Study on Aqua-Eco-Remediation Engineering in Lake Hongfeng, Guizhou Province. J. Lake Sci. 2012, 24, 503–512. [Google Scholar] [CrossRef]
- Yu, H.; Ji, H.; Li, Y.; Qi, J.; Ma, B.; Hu, C.; Qu, J. Long-Term Succession in Cyanobacteria and Aquatic Plant Communities: Insights from Sediment Analysis. Engineering 2025, in press. [Google Scholar] [CrossRef]
- Xia, J.; Hu, H.; Gao, X.; Kan, J.; Gao, Y.; Li, J. Phytoplankton Diversity, Spatial Patterns, and Photosynthetic Characteristics under Environmental Gradients and Anthropogenic Influence in the Pearl River Estuary. Biology 2024, 13, 550. [Google Scholar] [CrossRef]
- Zhang, H.; Peng, Y.; Zou, X.; Zhang, T.; Wu, C.; Lin, X.; Qiao, Y.; Yang, H. Characteristics of Phytoplankton Functional Groups and Their Relationships with physicochemical factors in Xinfengjiang. China Environ. Sci. 2022, 42, 380–392. [Google Scholar] [CrossRef]
- Jin, X.; Wu, H.; Chen, Z.; Song, H.; He, Y. Phosphorus Fractions Sorption Characteristics and Its Release in the Sediments of Yangtze Estuary Reservoir China. Environ. Sci. 2015, 36, 448–456. [Google Scholar]
- Yuan, H.; Zhang, R.; Li, Q.; Lu, Q.; Chen, J. Bacterially Mediated Phosphorus Cycling Favors Resource Use Efficiency of Phytoplankton Communities in a Eutrophic Plateau Lake. Water Res. 2025, 277, 123300. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Kang, S. Analysis and Reflection of the Exceeding De-Sign Standard Flood in Poyang Lake Basin in 2020. China Flood Drought Manag. 2021, 31, 45–48. [Google Scholar] [CrossRef]
- Li, W.; Jiang, M.; Xu, L.; Hu, S.; You, H.; Zhou, Q.; Chen, Z.; Zhang, L. Spatial and Temporal Characteristics of Phytoplankton in Lake Poyang and Its Response to Extreme Flood and Drying Events. J. Lake Sci. 2024, 36, 1001–1013. [Google Scholar] [CrossRef]
- Huang, Z.; Pan, B. Research on the Impact of Flood Process on Aquatic Ecosystem. J. Xi’an Univ. Technol. 2020, 36, 300–306. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, Y.; Lin, Y.; Chen, Q.; Zhang, J.; Ding, J.; Ma, H. Characteristic of Phytoplankton Community Structure and Its Driving Factors along the Cascade Reservoirs in the Lancang River. J. Lake Sci. 2023, 35, 530–539. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Z.; Cao, B.; Li, J.; Peng, L. Distribution Characteristics of Nitrogen and Phosphorus Nutrients in Main Rivers of Ganjiang River during Wet and Dry Seasons. Environ. Monit. China 2023, 39, 21–32. [Google Scholar] [CrossRef]
- Liu, J.; Xu, M.; Huang, X.; Chen, Q.; Luo, Q. Investigation and Research on Water Pollution Sources along Riverside of Ganjiang River (Nanchang Reach). Water Resour. Hydropower Eng. 2010, 41, 13–18. [Google Scholar] [CrossRef]
- Breton, E.; Goberville, E.; Sautour, B.; Ouadi, A.; Skouroliakou, D.-I.; Seuront, L.; Beaugrand, G.; Kléparski, L.; Crouvoisier, M.; Pecqueur, D.; et al. Multiple Phytoplankton Community Responses to Environmental Change in a Temperate Coastal System: A Trait-Based Approach. Front. Mar. Sci. 2022, 9, 914475. [Google Scholar] [CrossRef]
- Li, C.; Feng, W.; Chen, H.; Li, X.; Song, F.; Guo, W.; Giesy, J.P.; Sun, F. Temporal Variation in Zooplankton and Phytoplankton Community Species Composition and the Affecting Factors in Lake Taihu—A Large Freshwater Lake in China. Environ. Pollut. 2019, 245, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Grill, G.; Lehner, B.; Thieme, M.; Geenen, B.; Tickner, D.; Antonelli, F.; Babu, S.; Borrelli, P.; Cheng, L.; Crochetiere, H.; et al. Mapping the World’s Free-Flowing Rivers. Nature 2019, 569, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Tang, S.; Cheng, K.; Cai, Z.; Chen, G.; Zhou, J. Microbial Community Composition and Metabolic Potential during a Succession of Algal Blooms from Skeletonema Sp. to Phaeocystis Sp. Front. Microbiol. 2023, 14, 1147187. [Google Scholar] [CrossRef]
- Jung, E.; Joo, G.-J.; Kim, H.G.; Kim, D.-K.; Kim, H.-W. Effects of Seasonal and Diel Variations in Thermal Stratification on Phytoplankton in a Regulated River. Sustainability 2023, 15, 16330. [Google Scholar] [CrossRef]
- Chang, F.; Hou, P.; Wen, X.; Duan, L.; Zhang, Y.; Zhang, H. Seasonal Stratification Characteristics of Vertical Profiles and Water Quality of Lake Lugu in Southwest China. Water 2022, 14, 2554. [Google Scholar] [CrossRef]
- Bestion, E.; Haegeman, B.; Alvarez Codesal, S.; Garreau, A.; Huet, M.; Barton, S.; Montoya, J.M. Phytoplankton Biodiversity Is More Important for Ecosystem Functioning in Highly Variable Thermal Environments. Proc. Natl. Acad. Sci. USA 2021, 118, e2019591118. [Google Scholar] [CrossRef] [PubMed]
- Konopka, A.; Brock, T.D. Effect of Temperature on Blue-Green Algae (Cyanobacteria) in Lake Mendota. Appl. Environ. Microbiol. 1978, 36, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Shurin, J.B.; Efroymson, R.A.; Mathews, T.J. Heterogeneity in Nitrogen Sources Enhances Productivity and Nutrient Use Efficiency in Algal Polycultures. Environ. Sci. Technol. 2018, 52, 3769–3776. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wang, J.; Wang, H.; Liu, J.; Yao, Q.; Yu, Z.; Ran, X. Trends in Nutrients in the Changjiang River. Sci. Total Environ. 2023, 872, 162268. [Google Scholar] [CrossRef]
- Schindler, D.W.; Hecky, R.E.; Findlay, D.L.; Stainton, M.P.; Parker, B.R.; Paterson, M.J.; Beaty, K.G.; Lyng, M.; Kasian, S.E.M. Eutrophication of Lakes Cannot Be Controlled by Reducing Nitrogen Input: Results of a 37-Year Whole-Ecosystem Experiment. Proc. Natl. Acad. Sci. USA 2008, 105, 11254–11258. [Google Scholar] [CrossRef]
- Tang, B.; He, Y.; Yang, G.; Zhang, L.; Sun, X.; Ai, H.; He, Q.; Li, H. Identification of Phytoplankton Growth Limiting Factors during Flood Seasons in Tributary of the Three Gorges Reservoir. J. Southwest Univ. (Nat. Sci. Ed.) 2023, 45, 138–151. [Google Scholar] [CrossRef]
- Wu, X.; Liu, H.; Ru, Z.; Tu, G.; Xing, L.; Ding, Y. Meta-Analysis of the Response of Marine Phytoplankton to Nutrient Addition and Seawater Warming. Mar. Environ. Res. 2021, 168, 105294. [Google Scholar] [CrossRef]
- Liang, Z.; Soranno, P.A.; Wagner, T. The Role of Phosphorus and Nitrogen on Chlorophyll a: Evidence from Hundreds of Lakes. Water Res. 2020, 185, 116236. [Google Scholar] [CrossRef]
- Wang, B.; Yang, X.; Li, S.-L.; Liang, X.; Li, X.-D.; Wang, F.; Yang, M.; Liu, C.-Q. Anthropogenic Regulation Governs Nutrient Cycling and Biological Succession in Hydropower Reservoirs. Sci. Total Environ. 2022, 834, 155392. [Google Scholar] [CrossRef]
- Wang, L.; Tan, L.; Cai, Q. Distinct Differences of Vertical Phytoplankton Community Structure in Mainstream and a Tributary Bay of the Three Gorges Reservoir, China. Front. Plant Sci. 2024, 15, 1381798. [Google Scholar] [CrossRef]
- Luo, Q.; Zhu, L.; Li, D.; Zu, Z.; Chen, K.; Wang, J.; Yi, Y. Role of Hydraulic Residence Time in Shaping Phytoplankton Community Assembly in the Upper Yellow River Cascade Reservoirs. Front. Environ. Sci. 2025, 13, 1551988. [Google Scholar] [CrossRef]
- Atazadeh, E.; Gell, P.; Mills, K.; Barton, A.; Newall, P. Community Structure and Ecological Responses to Hydrological Changes in Benthic Algal Assemblages in a Regulated River: Application of Algal Metrics and Multivariate Techniques in River Management. Environ. Sci. Pollut. Res. 2021, 28, 39805–39825. [Google Scholar] [CrossRef]
- Dai, L.; Zhang, Q.; Ren, Y.; Chen, L.; Jiang, W.; Dai, H.; Tang, Z. Water Temperature Stratification Characteristics of Xiluodu Reservoir in Ecological Regulation Period under the Condition of Inflow Change. J. Water Resour. Hydraul. Eng. 2023, 34, 132–139. [Google Scholar] [CrossRef]
- Huang, Q.; Li, N.; Li, Y. Long-Term Trend of Heat Waves and Potential Effects on Phytoplankton Blooms in Lake Qiandaohu, a Key Drinking Water Reservoir. Environ. Sci. Pollut. Res. 2021, 28, 68448–68459. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, H.; Wang, Z.; Tian, Y.; Tian, W.; Liu, Z. Temperature Orchestrates Phytoplankton Community and Environment in Mountain Stream for Enhancing Resource Use Efficiency. Front. Mar. Sci. 2025, 12, 1565858. [Google Scholar] [CrossRef]
- Chen, Q.; Shi, W.; Huisman, J.; Maberly, S.C.; Zhang, J.; Yu, J.; Chen, Y.; Tonina, D.; Yi, Q. Hydropower Reservoirs on the Upper Mekong River Modify Nutrient Bioavailability Downstream. Natl. Sci. Rev. 2020, 7, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Fortin, N.; Munoz-Ramos, V.; Bird, D.; Lévesque, B.; Whyte, L.; Greer, C. Toxic Cyanobacterial Bloom Triggers in Missisquoi Bay, Lake Champlain, as Determined by next-Generation Sequencing and Quantitative PCR. Life 2015, 5, 1346–1380. [Google Scholar] [CrossRef]
- Kim, J.Y.; Atique, U.; Mamun, M.; An, K.-G. Long-Term Interannual and Seasonal Links between the Nutrient Regime, Sestonic Chlorophyll and Dominant Bluegreen Algae under the Varying Intensity of Monsoon Precipitation in a Drinking Water Reservoir. Int. J. Environ. Res. Public Health 2021, 18, 2871. [Google Scholar] [CrossRef]
- Wijewardene, L.; Wu, N.; Qu, Y.; Guo, K.; Messyasz, B.; Lorenz, S.; Riis, T.; Ulrich, U.; Fohrer, N. Influences of Pesticides, Nutrients, and Local Environmental Variables on Phytoplankton Communities in Lentic Small Water Bodies in a German Lowland Agricultural Area. Sci. Total Environ. 2021, 780, 146481. [Google Scholar] [CrossRef] [PubMed]
- Smucker, N.J.; Beaulieu, J.J.; Nietch, C.T.; Young, J.L. Increasingly Severe Cyanobacterial Blooms and Deep Water Hypoxia Coincide with Warming Water Temperatures in Reservoirs. Glob. Change Biol. 2021, 27, 2507–2519. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Liu, J.; Zhou, S.; Huang, Y.; Liu, G.; Chen, Y.; Xia, Y.; He, T.; Li, W. Effect of Hydraulic Projects on the Phytoplankton Community Structure in the Mainstream of the Ganjiang River. Water 2025, 17, 3126. https://doi.org/10.3390/w17213126
Zhu J, Liu J, Zhou S, Huang Y, Liu G, Chen Y, Xia Y, He T, Li W. Effect of Hydraulic Projects on the Phytoplankton Community Structure in the Mainstream of the Ganjiang River. Water. 2025; 17(21):3126. https://doi.org/10.3390/w17213126
Chicago/Turabian StyleZhu, Jie, Jinfu Liu, Shiyu Zhou, Yezhi Huang, Guangshun Liu, Yuwei Chen, Yu Xia, Ting He, and Wei Li. 2025. "Effect of Hydraulic Projects on the Phytoplankton Community Structure in the Mainstream of the Ganjiang River" Water 17, no. 21: 3126. https://doi.org/10.3390/w17213126
APA StyleZhu, J., Liu, J., Zhou, S., Huang, Y., Liu, G., Chen, Y., Xia, Y., He, T., & Li, W. (2025). Effect of Hydraulic Projects on the Phytoplankton Community Structure in the Mainstream of the Ganjiang River. Water, 17(21), 3126. https://doi.org/10.3390/w17213126





