1. Introduction
Coastal regions are favored for human settlement due to their distinctive geography and aesthetic appeal. Nonetheless, the rise in population, economic expansion, and engineering infrastructure in coastal regions has heightened the demand for fresh groundwater, resulting in diminishing water levels and subsequent seawater intrusion into coastal aquifers. The literature indicates that seawater intrusion has affected around 500 coastal towns globally [
1], encompassing regions such as East Asia [
2,
3,
4,
5], South Asia [
6,
7], the Mediterranean [
8,
9], North America [
10], and Europe [
11,
12].
Seawater intrusion events have resulted in extensive contamination of freshwater aquifers and ecological deterioration along the Chinese coastline. Seawater intrusion in China predominantly occurs in the Bohai Sea region (Shandong, Hebei, Tianjin, and Liaoning) [
4,
13], the Yangtze River Delta, the Pearl River Delta, the Beibu Gulf coastal area, Hainan, and the central and western plains of Taiwan [
3,
14].
Current research on seawater intrusion encompasses inquiry and assessment, modeling of seawater intrusion [
15], and strategies for its prevention and control [
16]. Methods for investigating and evaluating seawater intrusion encompass water chemistry techniques [
17,
18], isotope analysis [
19], and geophysical approaches [
20], among others. The water chemistry approach serves as the most direct and complete indicator; however, its drawbacks include an excessive number of indicators, susceptibility to human impact, interference from hydrogeological circumstances, and inconsistencies among indicators. The extensive application of various approaches is the prevailing trend in the investigation of seawater intrusion [
13,
21,
22,
23].
Laizhou Bay, recognized as a critical region experiencing seawater intrusion in China, has been adversely impacted in terms of residential water consumption and industrial and agricultural output [
24,
25,
26,
27,
28,
29]. The south coast of Laizhou Bay is a hot research area for seawater intrusion and saline (brine) water intrusion, and the methods used to study it are mostly hydrogeochemical and isotope geochemical methods, etc. In the case of saline (brine) water, there are debates on the evaporation and concentration of ancient seawater and the dissolution of evaporite rocks in sediments [
4,
30,
31,
32,
33,
34,
35]. Since the discovery of seawater intrusion in the study area located on the east coast of Laizhou Bay in the 1970s, scholars have also carried out a great deal of research. Currently, it is generally agreed that groundwater salinization along the east coast of Laizhou Bay is mainly caused by seawater intrusion, and the presence of brine has not been found [
25,
36]. In the 1990s, Xue Yuqun and Yin Zesheng examined the current state of seawater intrusion, the transport dynamics at the saline-freshwater interface, as well as the causes, mechanisms, and classifications of seawater intrusion [
25,
29]. They employed various methodologies, including water chemistry analysis, groundwater isotope research, statistical groundwater assessments, monitoring section construction, numerical simulations, remote sensing interpretation, ecological investigations, and modeling, resulting in significant insights and accomplishments. In recent years, certain scholars conducted predictive modeling in the study area [
37], performed a disaster risk assessment of seawater intrusion in the coastal zone of Laizhou City [
38], and proposed control measures for seawater intrusion [
39]; however, they neglected to analyze the water chemistry affected by seawater intrusion.
Groundwater serves as the primary water source in the study area, a significant grain-producing region and mining development zone in Laizhou City. The 2017–2020 Yantai City Water Resources Bulletin indicates that groundwater constitutes over 50% of the total water supply in the study region. Seawater intrusion has significantly affected the water supply for agricultural, industrial, and residential use in the studied region. This research project aims to assess the existing condition of the chemical properties of groundwater in the study area due to persistent seawater intrusion. To accomplish these objectives, a comparative schematic map illustrating the progression of seawater intrusion over the years was created, and the origins of groundwater salinity and recharge were examined utilizing hydrochemical and isotopic techniques. The study area is crucial for water resource management, prevention and control of saltwater intrusion disasters, and conservation of the coastal ecological environment.
2. Study Area
The study area is situated in the northern region of Laizhou City, Yantai City, Shandong Province, along the eastern coast of Laizhou Bay (
Figure 1). The study area exhibits a continental climate within the northern temperate East Asian monsoon zone, characterized by an average annual precipitation of 616.05 mm and an average temperature of 12.6 °C from 1970 to 2021.
The research area’s topography is predominantly elevated in the southeast and depressed in the northwest, characterized primarily by low hills and plains, with an elevation range of 0–478 m. The primary stratigraphy of the study area comprises the Neoproterozoic Jiao Dong Rock Group, the Paleoproterozoic Jingshan Group, and the Powder Mountain Group, alongside the Cenozoic Paleocene and Quaternary. Additionally, a significant expanse of magmatic intrusion affects the exposed strata, with the Quaternary predominantly constituting the principal aquifer, which includes Quaternary alluvial deposits, fine sand, coarse sand, and pebble gravels. The primary aquifers consist of Quaternary alluvial and floodplain medium and fine sands, coarse sands, and pebble gravel. Typically, there exists a singular aquifer layer, though there may be two to four levels, with a close hydraulic relationship between them, and thickness varying from 2 to 30 m. In the southern region of the city, the ancient Yuan dynasty rock formation is visible, and the aquifer consists of carbonate rock, with the water level depth varying from 14.50 to 19.00 m. In the eastern and southeastern regions of the city, granite and metamorphic rock are exposed, with weathering fissure water as the primary source. The area is characterized by limited tectonic water management, and the thickness of the weathering zone typically ranges from 30 to 40 m. With the exception of a portion of the karst fissure water, the groundwater is predominantly submerged. Groundwater discharge routes mostly consist of runoff into the ocean and artificial extraction [
40,
41].
Groundwater recharge primarily originates from the infiltration of atmospheric precipitation, supplemented by surface water recharge during flood and irrigation events. The flow direction of groundwater runoff aligns with that of surface water systems, predominantly towards the north and northwest. The watershed for surface water coincides with that of groundwater, with the exception of the Xiaogu River, which discharges into Jiaozhou Bay to the south; all other rivers flow northward and northwestward into the Bohai Sea. The coastal region of the study area features extensive mariculture bases, a significant gold mining zone in the north, and a marble mining area in the south. The predominant land use type is arable land, primarily cultivated with winter wheat and maize.
4. Results and Discussion
Groundwater chemistry in fresh groundwater and seawater intrusion areas has different characteristics, and analyzing the characteristics of groundwater chemistry is of great significance for seawater intrusion studies. Groundwater was categorized into freshwater TDS (g/L) < 1, brackish water TDS (g/L) 1–3, and saline water TDS (g/L) > 3 based on TDS values [
49]. The groundwater samples were categorized. Among the 115 samples in the 2020 water-abundance period, 55 samples were fresh water, accounting for 47.83%, 52 samples were brackish water, accounting for 45.22%, and eight samples were saline water, accounting for 6.95%.
4.1. Hydrochemical Composition
The pH value (
Table 3) of the groundwater samples varied from 6.18 to 8.88, with an average value of 7.07. The pH value in the study area tended to be stable in general, and showed a pattern of change from weakly acidic to weakly alkaline; the TDS value varied from 276 mg/L to 6674.9 mg/L, with an average value of 1346.16 mg/L, and the maximum value was about 24.2 times the minimum value.
The changes of cation concentration in the groundwater samples in the study area were as follows: TDS < 1 g/L (
Table 4), Ca
2+ > Na
+ > Mg
2+ > K
+ in descending order, with Ca
2+ occupying the main advantage; 1 g/L < TDS < 3 g/L (
Table 5), Ca
2+ > Na
+ > Mg
2+ > K
+ in descending order, with Ca
2+ occupying the main advantage; some samples with Na
+ > Ca
2+ > K
+, and some samples with Na
+ > Ca
2+ > K
+. Ca
2+ > Na
+ > Mg
2+ > K
+, with Ca
2+ dominating, and Na
+ > Ca
2+ in some of the samples; the concentrations of cations in groundwater with TDS > 3 g/L (
Table 6) were, in descending order, Na
+ > Ca
2+ > Mg
2+ > K
+, and Mg
2+ > Ca
2+ in some of the samples.
The anion concentration of groundwater samples varied as follows: TDS < 1 g/L (
Table 4), HCO
3− > Cl
− > SO
42−, SO
42−, in which the concentration of some samples was higher than that of Cl
− or HCO
3−; TDS > 1 g/L, Cl
− and SO
42− > HCO
3−, SO
42− and Cl
− concentration of different samples had their own advantages compared to each other; SO
42− and Cl
− concentrations of different samples had their own advantages.
4.2. Water Chemistry Type
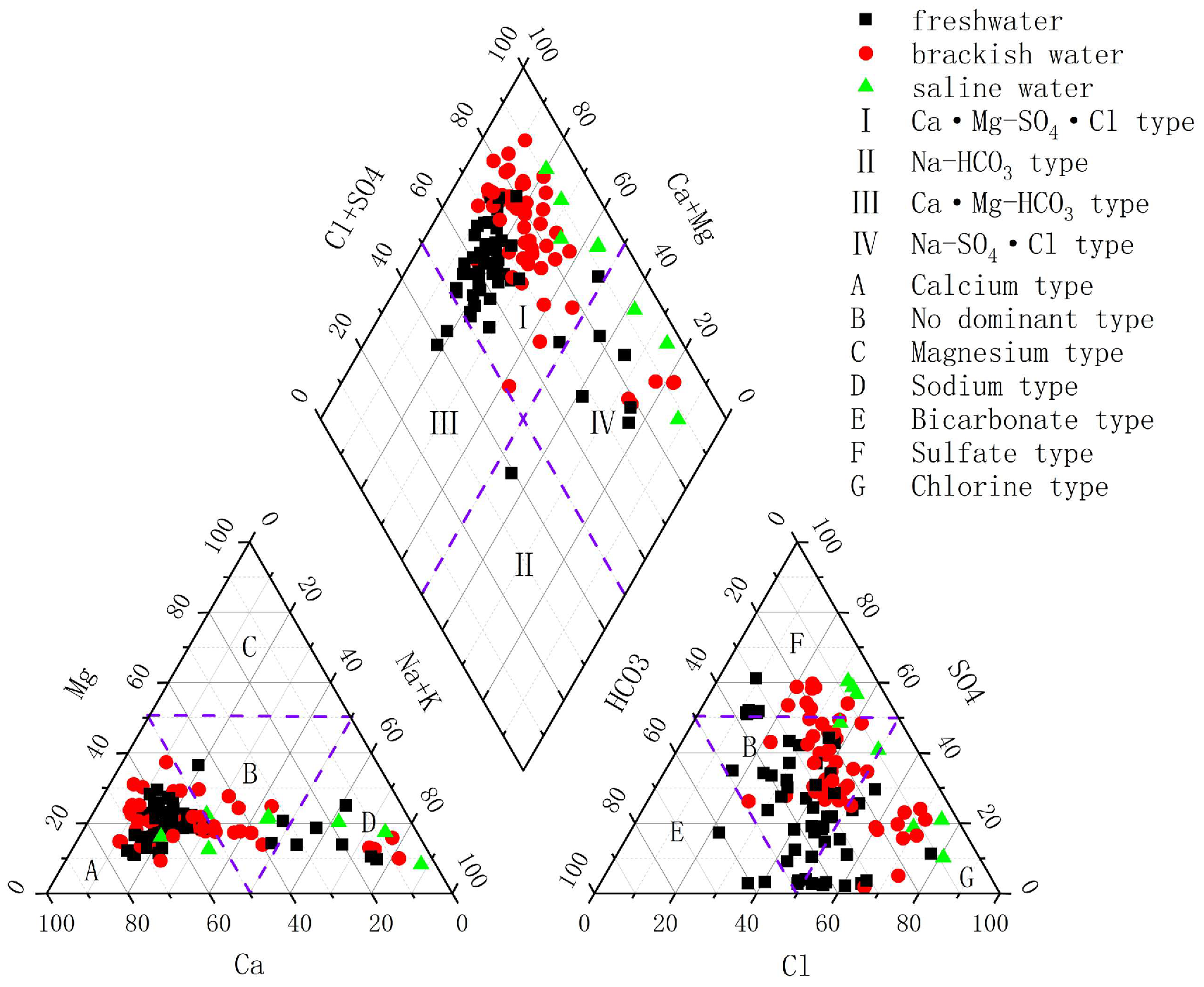
From
Figure 4, it can be seen that the cations of freshwater samples of groundwater samples are dominated by Ca
2+, and there is no obvious dominant anion; the cations of brackish water samples are dominated by Ca
2+, and the anions are dominated by Cl
− and SO4
2−; and the cations of saline water samples are dominated by Na
+ and Ca
2+, and the anions are dominated by Cl
− and SO
42−.
Combined with the results of previous research and the results of the present analysis, it is believed that under the influence of aerosol diffusion by the sea breeze [
40,
50], the chemical type of the unpolluted natural groundwater in the northern part of Laizhou City is the Ca-HCO
3·Cl type. The Piper diagram reveals that cations in freshwater and brackish water samples are dominated by Ca
2+, with groundwater samples concentrated in Zone I. Based on the characteristics of major ions, it is inferred that the higher Ca
2+ concentration in brackish water may result from cation exchange [
40]. Combined with Shukarev classification, from freshwater to saline water, the groundwater chemistry transitions from Ca-HCO
3·Cl-type water to Ca·Na-SO
4·Cl-type water, and finally to Na-Cl-type water.
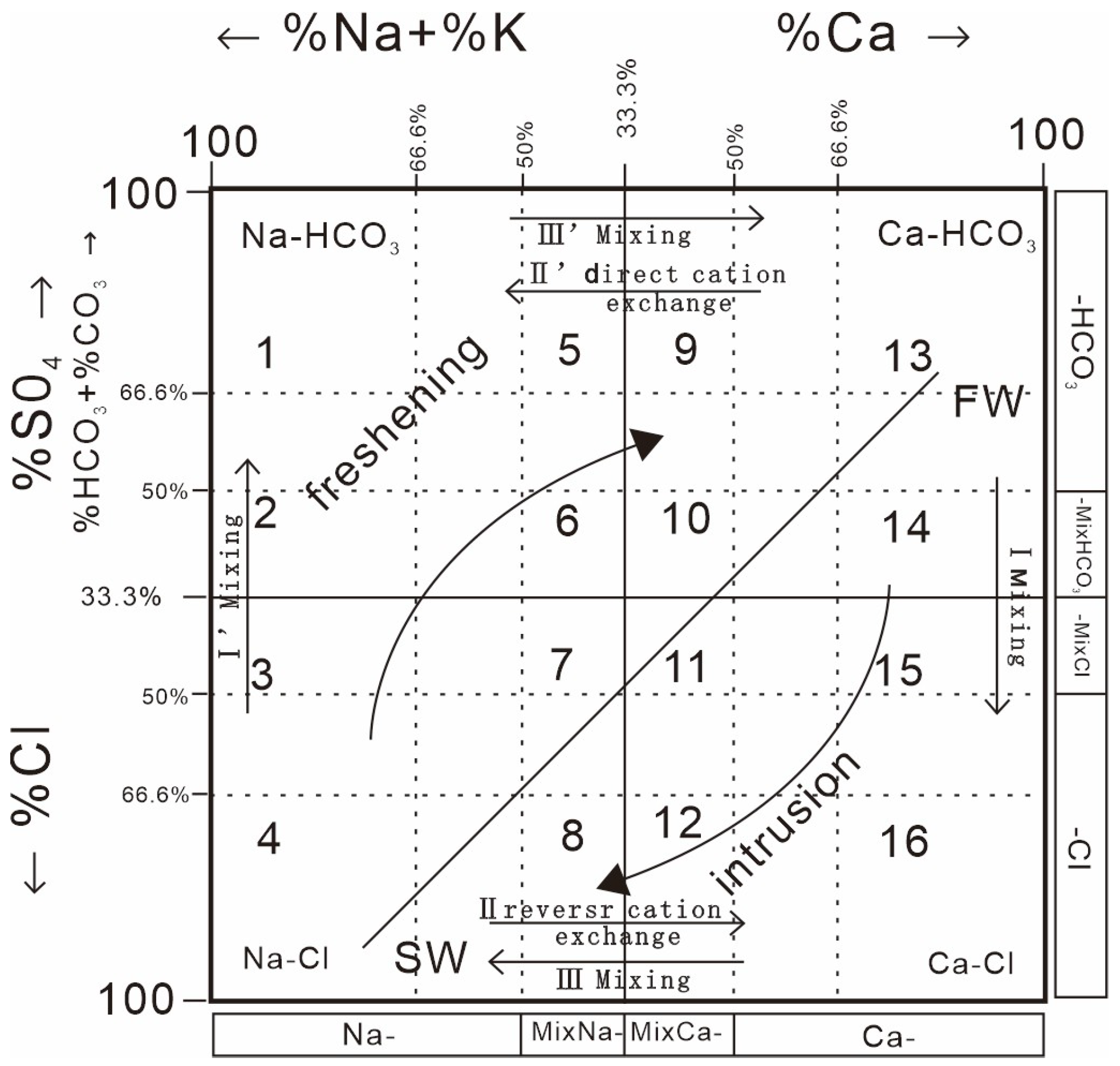
Groundwater samples occupy 40 percent of the freshening phase and 60 percent of the intrusion phase, with intrusion dominating. Groundwater samples occupy 13 of the 16 phases in the hydrochemical evolution diagram (
Figure 5). The main hydrochemical facies of 115 groundwater samples are Ca-MixCl, Ca-MixHCO
3/MixSO
4, Ca-HCO
3/SO
4, Ca-Cl, and Na-Cl, which accounted for 83.48% of the total samples. There were 27 samples of Ca-MixCl phase, 26 samples of Ca-MixHCO
3/MixSO
4 phase, 11 samples of Ca-MixHCO
3 phase, and 15 samples of Ca-MixSO
4 phase. Also, 46.09% of the total samples were Ca-MixHCO
3/MixSO
4 and Ca-MixCl facies samples, 46.09% of the total samples were Ca-HCO
3/MixSO
4 facies samples of 17 samples, 15 samples were Ca-SO
4 facies, and only two samples were Ca-HCO
3 facies. There were 15 Ca-Cl facies samples and 11 Na-Cl facies samples. Groundwater hydrochemical facies are characterized by complexity and variability.
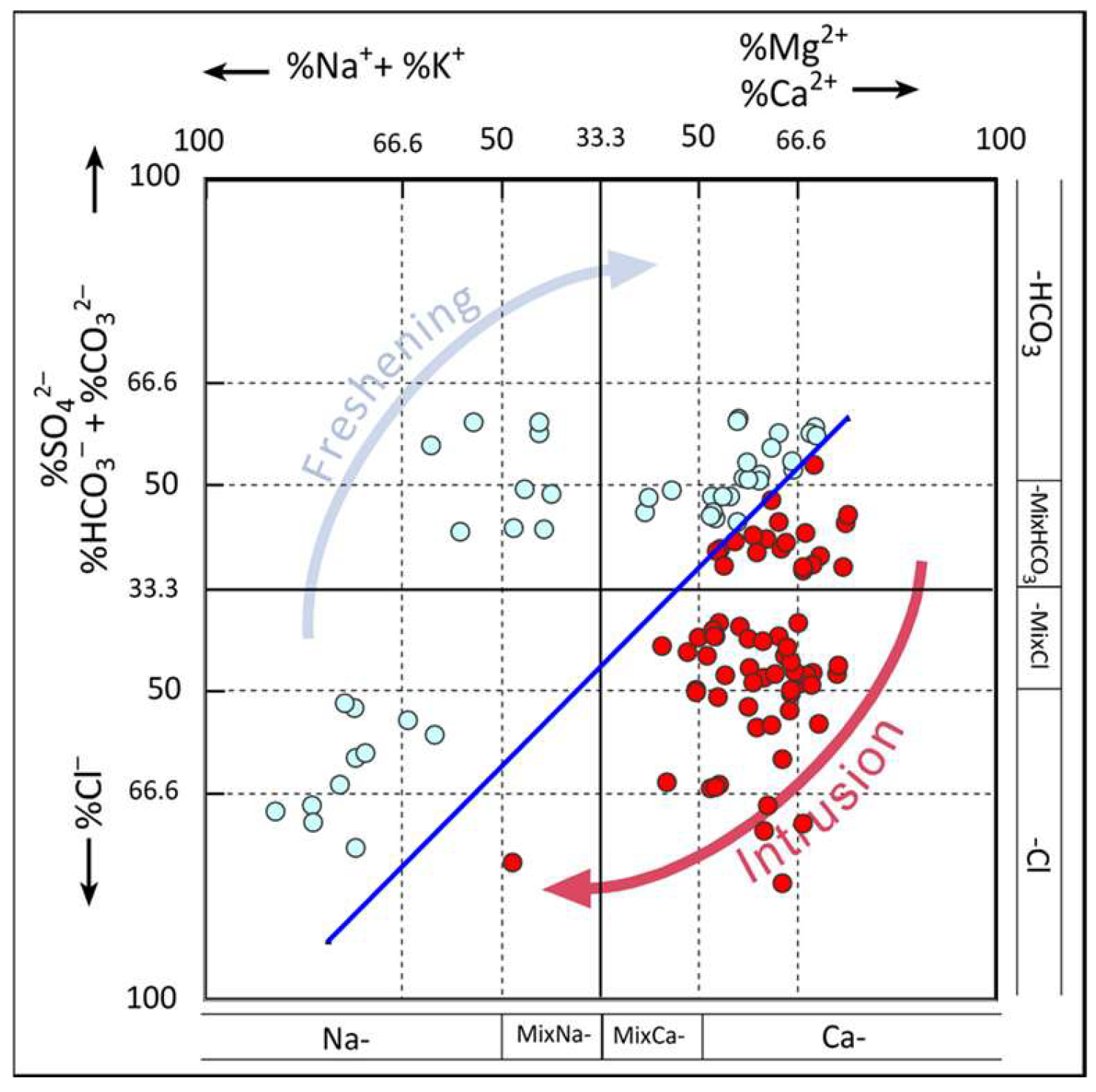
As can be seen from
Figure 6, most of the groundwater samples in the study area are evolving from Ca-HCO
3-type water to Ca-Cl-type water, with mixing dominating the invasion phase. During the desalination stage, positive cation exchange has a greater influence on the groundwater chemistry type. Only very few areas of the study area exhibit Na-HCO
3-type water, indicating that there is no obvious trend of desalination of brackish water in the study area, which may be attributed to insufficient groundwater recharge.
4.3. Hydrogeochemical Variations
The Na/Cl ratio has no specific pattern in the scatter plot (
Figure 6a). The range of Na/Cl ratios in the study area is 0.11–1.88, and 75.65% of the groundwater samples have Na/Cl ratios less than 0.86, indicating that the study area is mainly affected by seawater intrusion [
51]. Furthermore, 21.74% of the groundwater samples have Na/Cl ratios equal to or greater than 1, and areas with high Na/Cl ratios (>1) may be contaminated by anthropogenic activities [
51].
The Mg/Cl ratios in the scatter plots showed a decrease with the increase of Cl
− concentration and converged around the seawater ratio line (
Figure 6b). Typical Mg/Ca ratios for seawater range from 4.5 to 5, while freshwater Mg/Ca ratios are less than 1 [
52]. The Mg/Ca ratios in the study area ranged from 0.14 to 2.35 and did not tend to be close to the seawater Mg/Ca ratio line with increasing Cl
− concentration (
Figure 6c), indicating that a considerable number of samples had excess Ca
2+ and deficient Mg
2+, which was presumably affected by the mixing of seawater and freshwater, as well as by the influence of Mg
2+ and the influence of seawater and freshwater mixing. It is assumed that Mg
2+ is affected by the mixing of seawater and freshwater, as well as by the cation exchange.
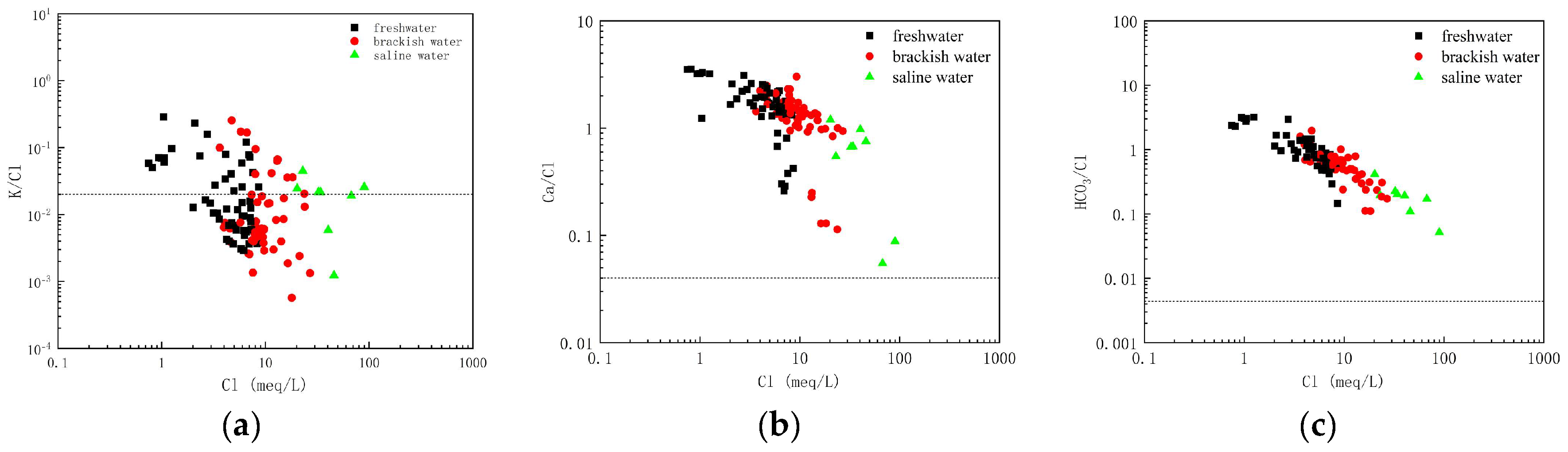
The K/Cl ratio in the scatter plot shows an increase with Cl
− concentration and a certain degree of proximity to the seawater dilution line (
Figure 7a), suggesting that the K
+ may be primarily sourced from invaded seawater and influenced by cation exchange.
The Ca/Cl ratios all show a pattern of decreasing with increasing Cl
− concentration in the scatter plots, and the Ca/Cl ratios of some samples were higher than the seawater ratio line (
Figure 7b). Ca
2+ is inferred to be affected by seawater intrusion and cation exchange in conjunction with water chemistry components and the location of groundwater Piper diagrams.
The HCO
3/Cl ratios in the scatter plots all show a pattern of decreasing with increasing Cl
− concentration, and HCO
3− is higher than the seawater ratio line in the scatter plots of the groundwater samples and shows a certain linear pattern (
Figure 7c), which indicates that HCO
3− is mainly derived from freshwater and mineral dissolution, and that the content is affected by seawater intrusion mainly because of the mixing of seawater and freshwater from the groundwater in the aquifer.
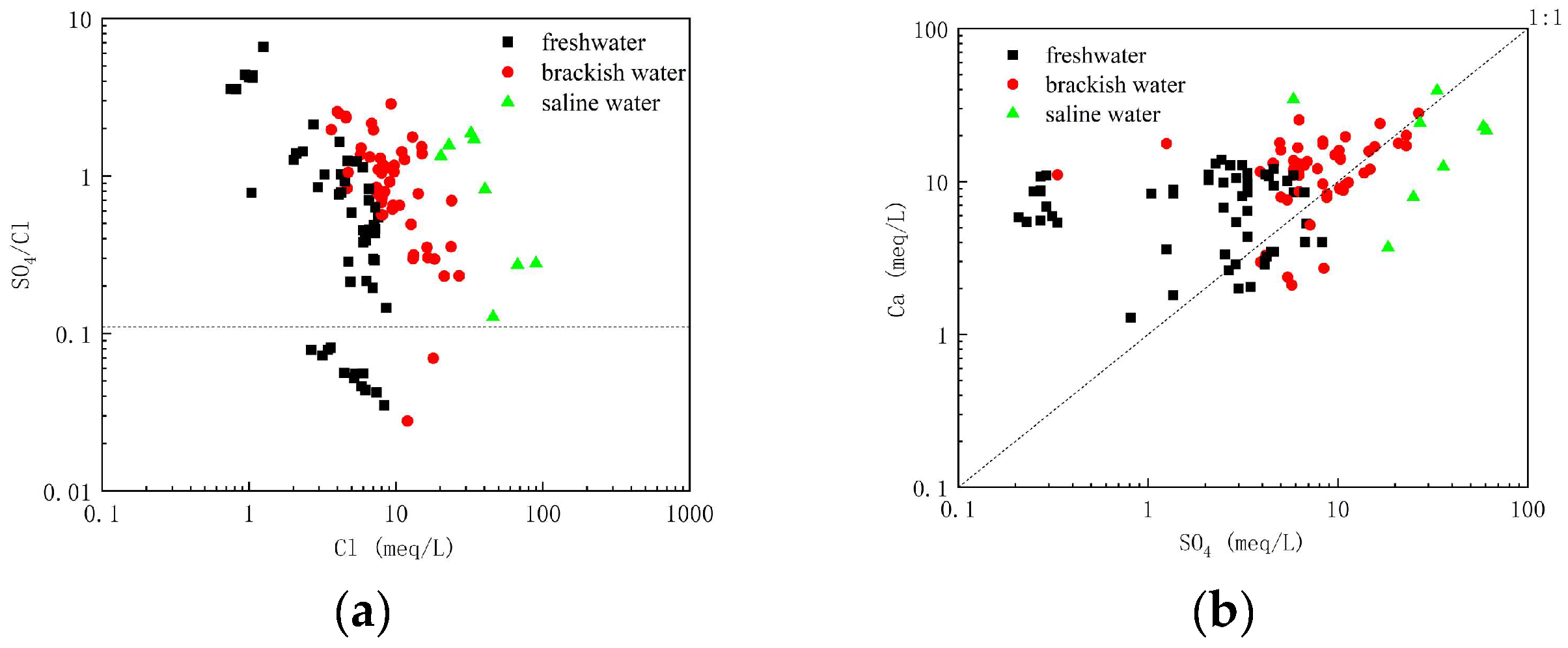
The SO
4/Cl ratios in the scatter plot show an increase in Cl
− concentration and a certain degree of closeness to the seawater ratio line of the pattern (
Figure 8a), indicating that SO
42− originated from invaded seawater. Some samples fell above the 1:1 line, suggesting that dissolution of gypsum is one of the sources of SO
42; in addition, some samples were far above the 1:1 line (
Figure 8b), suggesting that they may be affected by human activities (mariculture).
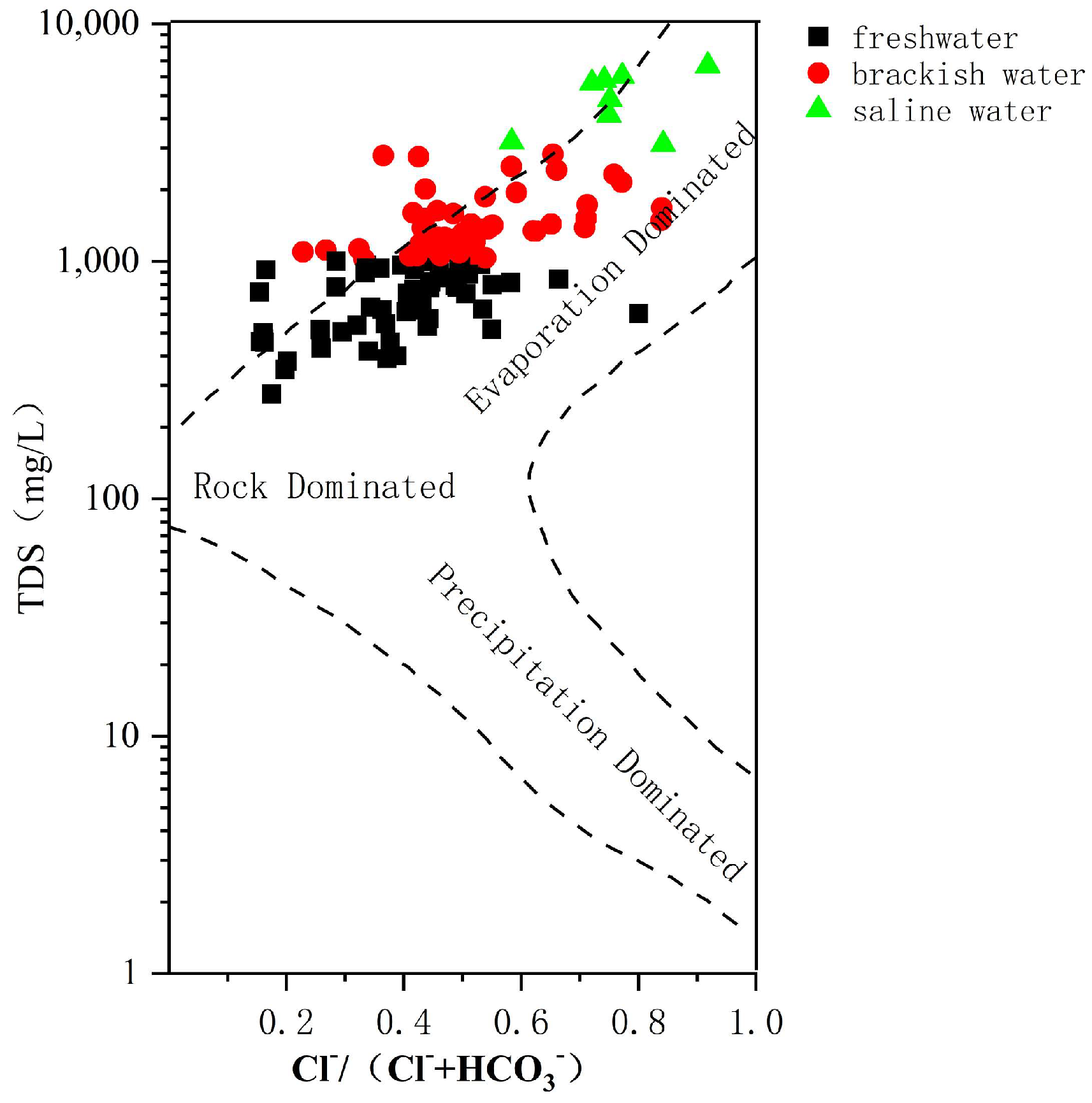
From
Figure 9, it can be seen that most of the groundwater samples fall within the rock-dominated and evaporation-dominated zones. Freshwater and brackish water are dominated by rock, and saline water is mainly affected by evaporation dominance. As TDS increases, groundwater changes from rock-dominated to evaporation-dominated. It shows the same pattern as the southern coast of Leizhou Bay [
33].
4.4. Scope of Seawater Intrusion
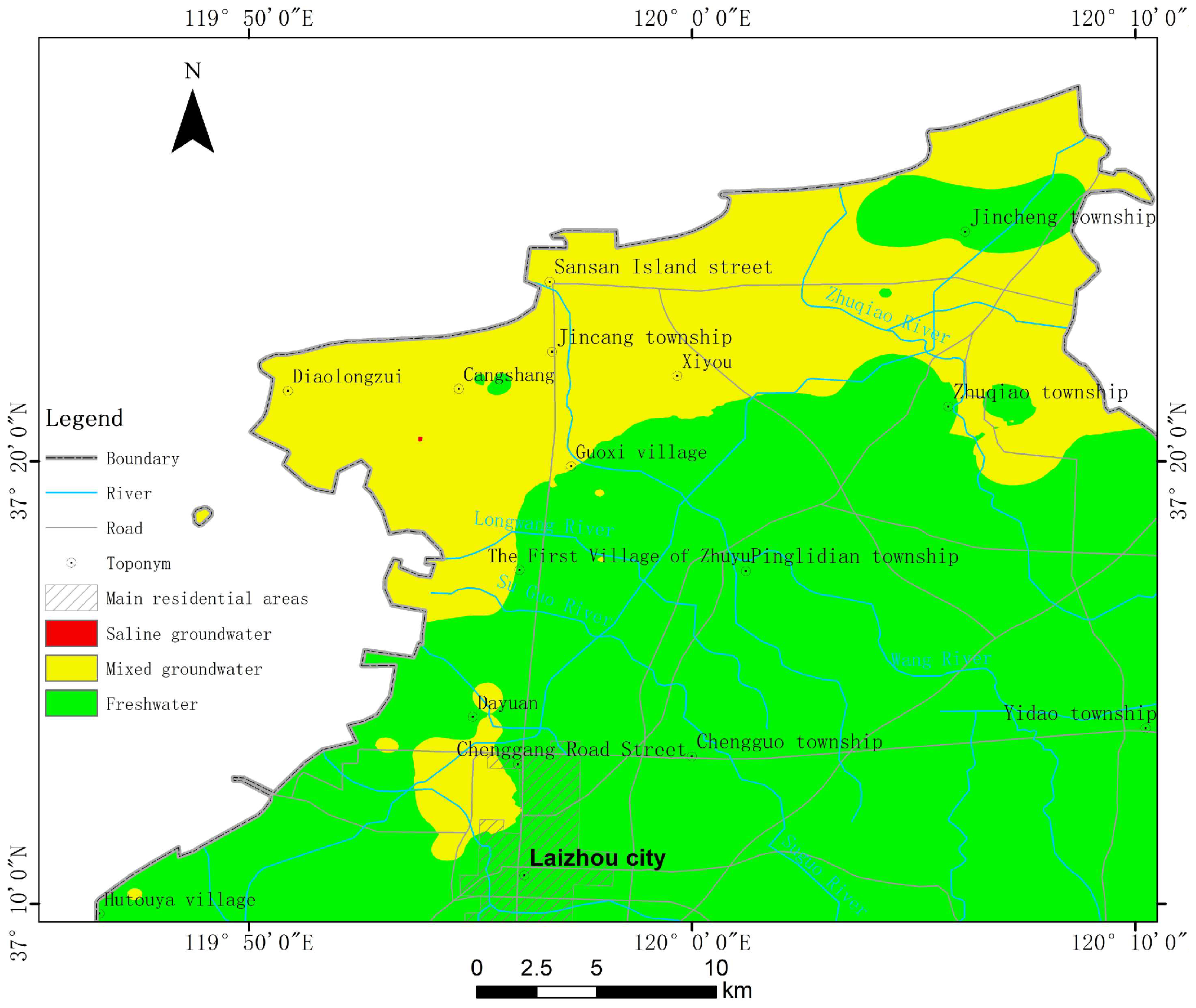
The distribution of
GQISWI values for the 115 samples ranged from 49.34 to 85.34, with a mean value of 74.18 and a median value of 76.33. The main types of groundwater in the study area are freshwater and mixed groundwater (
Figure 10), and only one area with
GQISWI values lower than 50 exists in the southwestern part of Cangshang. Mixed groundwater is mainly distributed in the western part of the city of Laizhou, along the area from Zhuyueyi village, over the west to the northern part of Zhuqiao town.
4.5. Sources of Groundwater Recharge
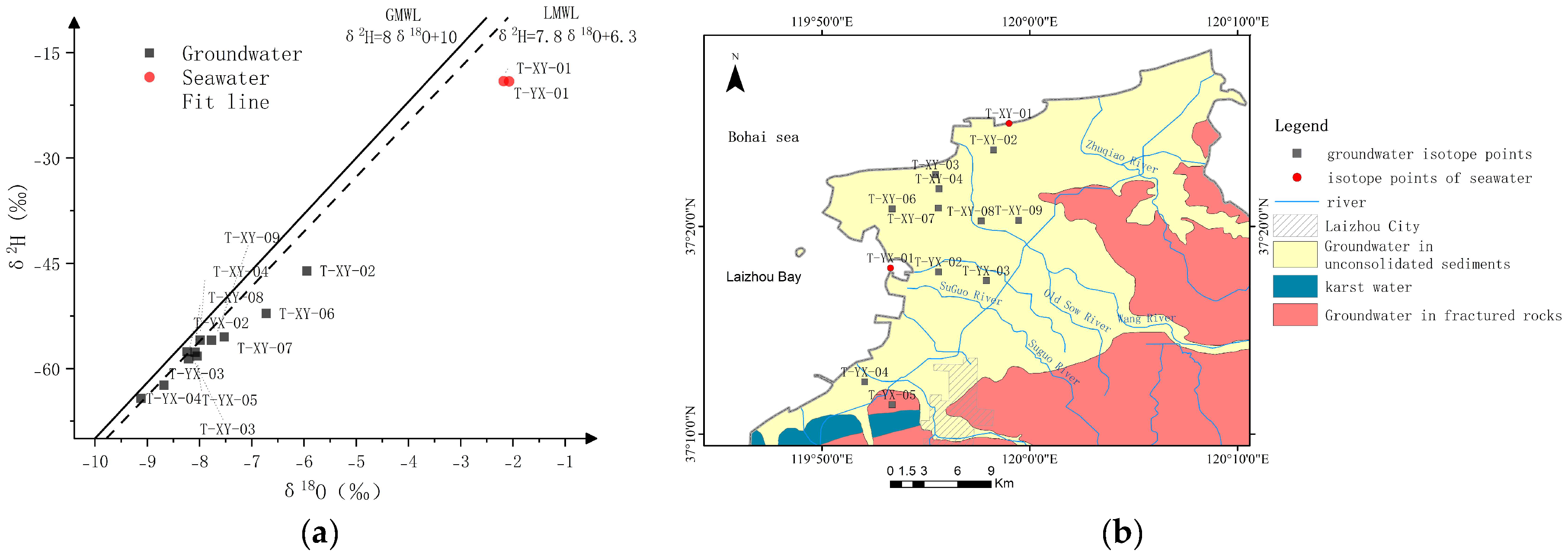
The theoretical values of δ2H and δ18O of seawater are both 0, but the near-shore surface seawater is affected by land surface water and groundwater, which will deviate from the theoretical values. Two surface seawater hydrogen and oxygen isotope samples were collected: T-XY-01 had a δ2H value of −19.07 ‰ and a δ18O value of −2.18 ‰; T-YX-01 had a δ2H value of −19.10 ‰ and a δ18O value of −2.07 ‰.
The δ
2H value of groundwater samples: −9.12 ‰~−5.94 ‰, mean value −7.85 ‰; δ
18O value: −62.40 ‰~−46.08 ‰, mean value −56.76 ‰. It can be seen that the hydrogen and oxygen isotope values of the groundwater samples all fall near or below the global atmospheric precipitation line and the regional atmospheric precipitation line (
Figure 11), which indicates that atmospheric precipitation is the main source of groundwater.
In addition, points 7, 6, and 2 deviate from the regional atmospheric precipitation line, and, according to the location of the sampling points, this is probably due to the fact that the groundwater in the region is subjected to the effect of evaporation and fractionation.
5. Conclusions
The distribution of groundwater samples in Piper’s trilinear map indicates the complexity of groundwater chemistry types in the study area. From freshwater to saline water, the groundwater chemistry transitions from Ca-HCO3·Cl-type water to Ca·Na-SO4·Cl-type water, and finally to Na-Cl-type water. The hydrochemical evolution diagram shows that most of the groundwater samples in the study area are in the invasive stage, and the hydrochemical phases of the groundwater samples are complex. Ca-MixCl and Ca-Cl phases are the major hydrochemical phases of the groundwater samples.
The main ions in the groundwater in the study area are controlled by seawater intrusion: Na+, Cl−, K+, Mg2+, SO42− are mainly derived from seawater intrusion, and are affected by human activities, cation exchange, and water–rock interactions; HCO3− is mainly derived from freshwater and mineral dissolution, and its content is affected by seawater intrusion; changes in Ca2+ content are affected by seawater intrusion, cation exchange, and mineral dissolution. As TDS increases, groundwater changes from rock-dominated to evaporation-dominated.
The current status of seawater intrusion in the study area was evaluated using the seawater intrusion groundwater quality index (GQISWI), and seawater intrusion was mainly concentrated in the northern part of the study area. In view of the extent of seawater intrusion in the past, it is recommended that the monitoring of seawater intrusion in the northern part of the study area be strengthened and that groundwater recharge to the study area be increased.
Hydrogen and oxygen isotope data of groundwater and seawater showed that the main source of recharge in the study area is atmospheric precipitation.