Complex Effects of Functional Groups on the Cotransport Behavior of Functionalized Fe3O4 Magnetic Nanospheres and Tetracycline in Porous Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Cotransport Experiments
2.3. Interaction Energy
2.4. Molecular Dynamics Simulation
3. Results and Discussion
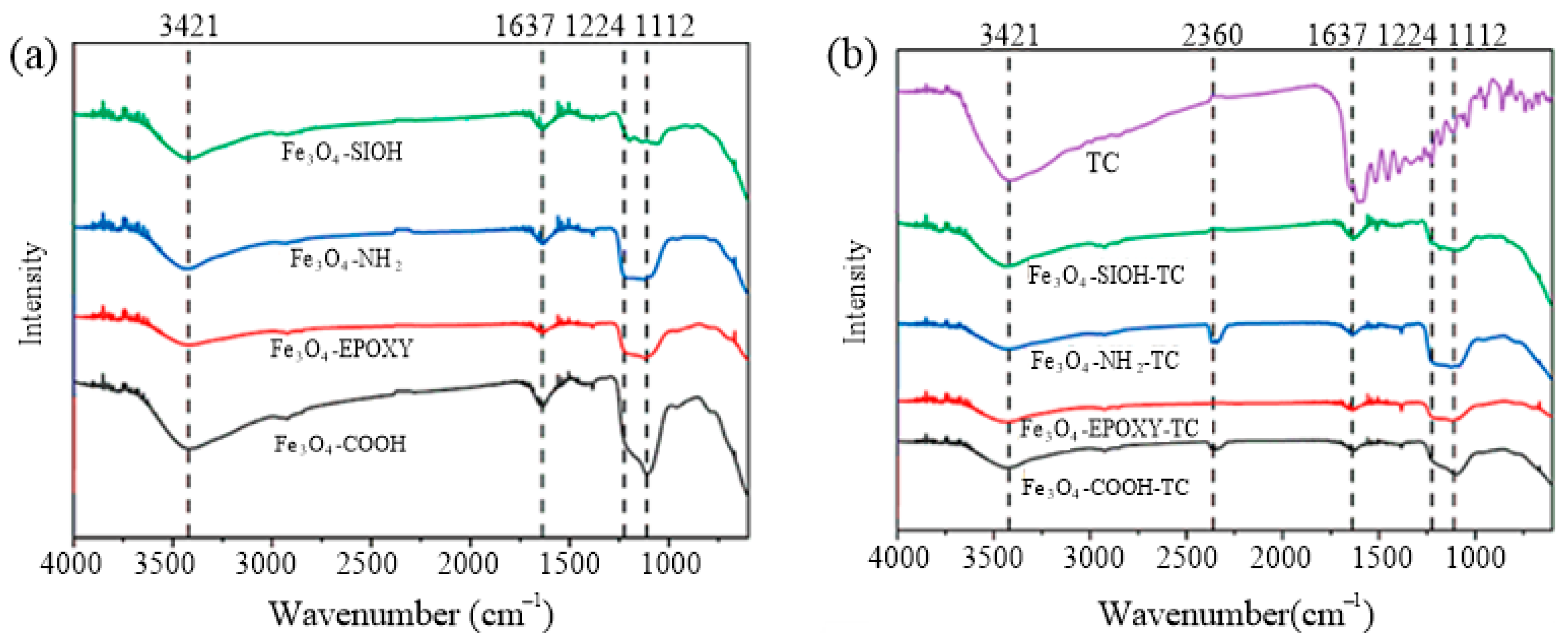
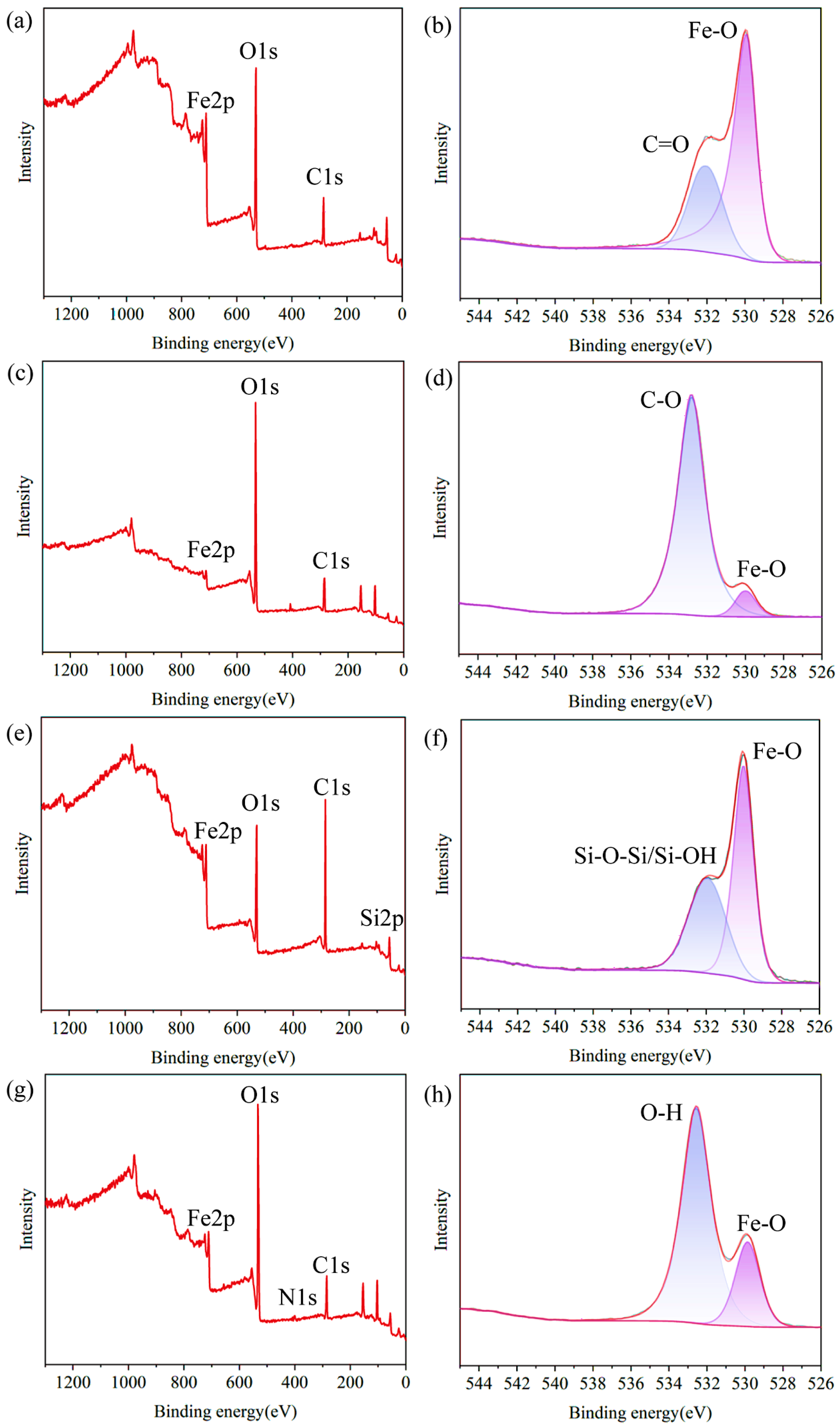
3.1. Characterization of Materials
3.2. XDLVO Results
3.3. The Cotransport of Fe3O4 with Different Functional Group Modifications and TC
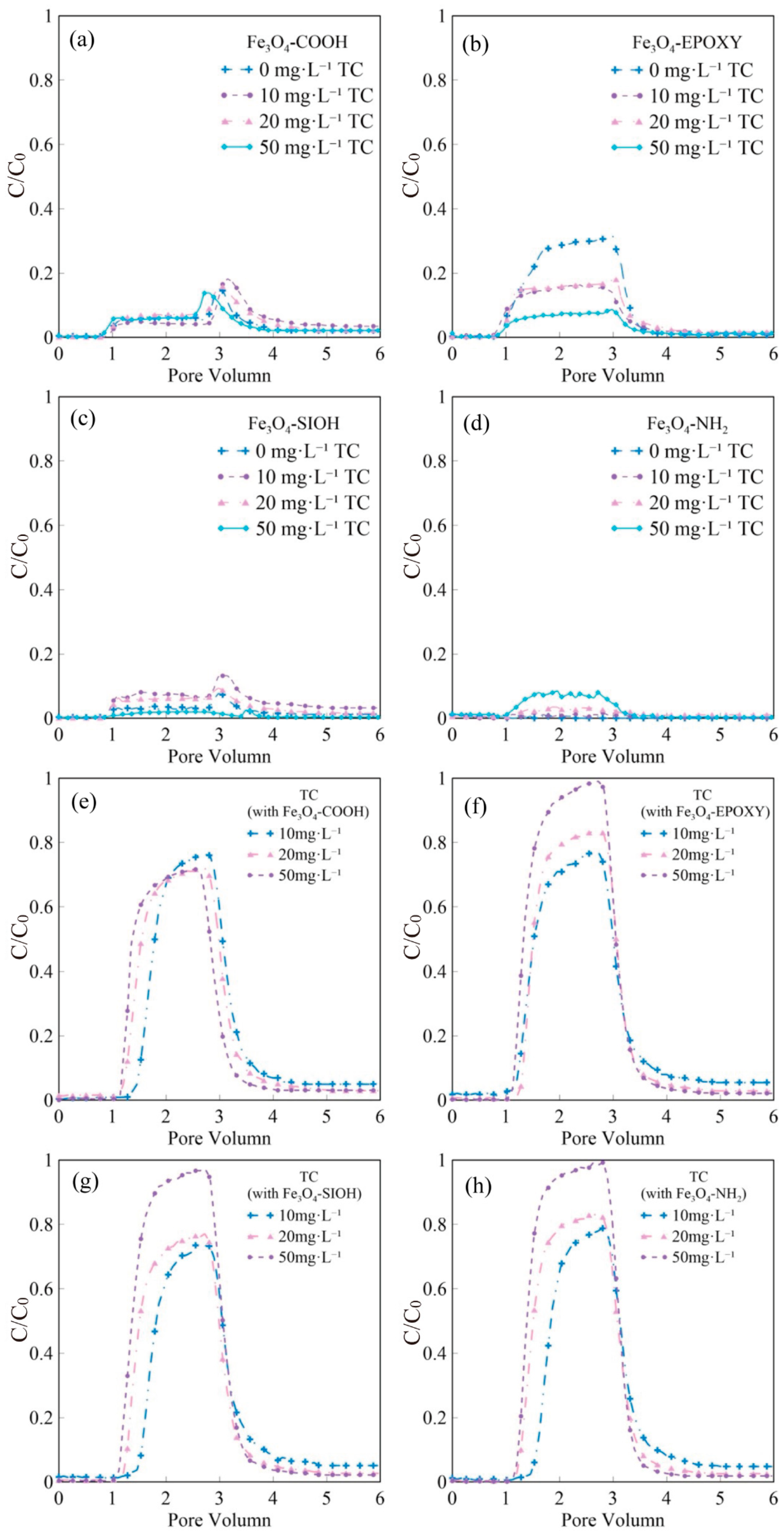
3.3.1. Monotransport of Fe3O4 and TC
3.3.2. Effect of TC on Fe3O4 Transport
3.3.3. Effect of Fe3O4 on TC Transport
3.4. The Results of Molecular Dynamics Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cornelis, G.; Hund-Rinke, K.; Kuhlbusch, T.; Van den Brink, N.; Nickel, C. Fate and bioavailability of engineered nanoparticles in soils: A review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2720–2764. [Google Scholar] [CrossRef]
- Liu, G.; Zhong, H.; Ahmad, Z.; Yang, X.; Huo, L. Transport of engineered nanoparticles in porous media and its enhancement for remediation of contaminated groundwater. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2301–2378. [Google Scholar] [CrossRef]
- Bundschuh, M.; Filser, J.; Lüderwald, S.; McKee, M.S.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Wagner, S. Nanoparticles in the environment: Where do we come from, where do we go to? Environ. Sci. Eur. 2018, 30, 1–17. [Google Scholar] [CrossRef]
- Petosa, A.R.; Jaisi, D.P.; Quevedo, I.R.; Elimelech, M.; Tufenkji, N. Aggregation and deposition of engineered nanomaterials in aquatic environments: Role of physicochemical interactions. Environ. Sci. Technol. 2010, 44, 6532–6549. [Google Scholar] [CrossRef]
- Ali, A.; Zafar, H.; Zia, M.; ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef]
- Imran, M.; Affandi, A.M.; Alam, M.M.; Khan, A.; Khan, A.I. Advanced biomedical applications of iron oxide nanostructures based ferrofluids. Nanotechnology 2021, 32, 422001. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, M.; Singh, A. Synthesis and characterization of iron oxide nanoparticles (Fe2O3, Fe3O4): A brief review. Contemp. Phys. 2021, 62, 144–164. [Google Scholar] [CrossRef]
- Thomas, R.; Ghosh, D.; Pulimi, M.; Nirmala, J.; Anand, S.; Rai, P.K.; Mukherjee, A. Investigating the transport and colloidal behavior of Fe3O4 nanoparticles in aqueous and porous media under varying solution chemistry parameters. Environ. Sci. Pollut. Res. 2023, 30, 118693–118705. [Google Scholar] [CrossRef] [PubMed]
- Lead, J.R.; Wilkinson, K.J. Aquatic colloids and nanoparticles: Current knowledge and future trends. Environ. Chem. 2006, 3, 159–171. [Google Scholar] [CrossRef]
- Baalousha, M. Aggregation and disaggregation of iron oxide nanoparticles: Influence of particle concentration, pH and natural organic matter. Sci. Total Environ. 2009, 407, 2093–2101. [Google Scholar] [CrossRef]
- Bi, Y.; Huang, L.; Song, X.; Sun, T.; Xu, S. Fe-based PRB system with ultrasound synergistically enhances the degradation of tetracycline. Chem. Eng. Process-Process Intensif. 2024, 197, 109687. [Google Scholar] [CrossRef]
- Wang, M.; Song, Y.; Zhang, H.; Lu, T.; Chen, W.; Li, W.; Qi, W.; Qi, Z. Insights into the mutual promotion effect of graphene oxide nanoparticles and tetracycline on their transport in saturated porous media. Environ. Pollut. 2021, 268, 115730. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, T.; Wang, M.; Jin, R.; Song, Y.; Zhou, Y.; Qi, Z.; Chen, W. Inhibitory role of citric acid in the adsorption of tetracycline onto biochars: Effects of solution pH and Cu2+. Colloids Surf. A Physicochem. Eng. Asp. 2020, 595, 124731. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Wang, T.; Huang, R.; Chen, H.L.; Xu, K.M.; Wu, L.G.; Chen, K.P.; Wu, J.C. Comparative study of reactive oxygen species and tetracycline degradation pathways in catalytic peroxodisulfate activation by asymmetric mesoporous TiO2 and the corresponding controlled-release materials. Environ. Pollut. 2024, 348, 123813. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Sun, X.F.; Xing, S.F.; Xia, P.F.; Shi, Y.J.; Wang, S.G. Characterization of the interactions between tetracycline antibiotics and microbial extracellular polymeric substances with spectroscopic approaches. Environ. Sci. Pollut. Res. 2014, 21, 1786–1795. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Gan, C.; Peng, Y.E.; Gan, Y.; He, J.; Du, Y.; Tong, L.; Shi, J.; Wang, Y. Occurrence and source identification of antibiotics and antibiotic resistance genes in groundwater surrounding urban hospitals. J. Hazard. Mater. 2024, 465, 133368. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.L.; Cao, W.; Wu, S.C.; Yang, C.; Cheng, J.H. Removal of tetracycline from aqueous solution by MOF/graphite oxide pellets: Preparation, characteristic, adsorption performance and mechanism. Ecotoxicol. Environ. Saf. 2018, 164, 289–296. [Google Scholar] [CrossRef]
- Zhao, P.; Cui, L.; Zhao, W.; Tian, Y.; Li, M.; Wang, Y.; Chen, Z. Cotransport and deposition of colloidal polystyrene microplastic particles and tetracycline in porous media: The impact of ionic strength and cationic types. Sci. Total Environ. 2021, 753, 142064. [Google Scholar] [CrossRef]
- Gu, C.; Karthikeyan, K.; Sibley, S.D.; Pedersen, J.A. Complexation of the antibiotic tetracycline with humic acid. Chemosphere 2007, 66, 1494–1501. [Google Scholar] [CrossRef]
- Wang, J.; Hu, J.; Zhang, S. Studies on the sorption of tetracycline onto clays and marine sediment from seawater. J. Colloid Interface Sci. 2010, 349, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Giammarco, J.; Mochalin, V.N.; Haeckel, J.; Gogotsi, Y. The adsorption of tetracycline and vancomycin onto nanodiamond with controlled release. J. Colloid Interface Sci. 2016, 468, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, K.; Zhang, H. Adsorption of antibiotics on microplastics. Environ. Pollut. 2018, 237, 460–467. [Google Scholar] [CrossRef]
- Zhang, G.; Cui, J.; Song, J.; Ji, Y.; Zuo, Y.; Jia, H.; Yin, X. Transport of polystyrene nanoplastics with different functional groups in goethite-coated saturated porous media: Effects of low molecular weight organic acids and physicochemical properties. J. Colloid Interface Sci. 2024, 653, 423–433. [Google Scholar] [CrossRef]
- Carstens, J.F.; Bachmann, J.; Neuweiler, I. Effects of organic matter coatings on the mobility of goethite colloids in model sand and undisturbed soil. Eur. J. Soil Sci. 2018, 69, 360–369. [Google Scholar] [CrossRef]
- Ma, J.; Guo, H.; Lei, M.; Li, Y.; Weng, L.; Chen, Y.; Ma, Y.; Deng, Y.; Feng, X.; Xiu, W. Enhanced transport of ferrihydrite colloid by chain-shaped humic acid colloid in saturated porous media. Sci. Total Environ. 2018, 621, 1581–1590. [Google Scholar] [CrossRef]
- He, L.; Xie, L.; Wang, D.; Li, W.; Fortner, J.D.; Li, Q.; Duan, Y.; Shi, Z.; Liao, P.; Liu, C. Elucidating the Role of Sulfide on the Stability of Ferrihydrite Colloids under Anoxic Conditions. Environ. Sci. Technol. 2019, 53, 4173–4184. [Google Scholar] [CrossRef]
- Li, X.; Bi, E. The impacts of Cu(II) complexation on gatifloxacin adsorption onto goethite and hematite. J. Environ. Qual. 2020, 49, 50–60. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, Q.; Lu, T.; Qi, W.; Zhang, H.; Wang, M.; Qi, Z.; Chen, W. Effect of phosphate on the adsorption of antibiotics onto iron oxide minerals: Comparison between tetracycline and ciprofloxacin. Ecotoxicol. Environ. Saf. 2020, 205, 111345. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, K.; Gao, B.; Zhang, G.; Liu, X.; Zhao, Y. Adsorption of tetracycline on soil and sediment: Effects of pH and the presence of Cu (II). J. Hazard. Mater. 2011, 190, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gu, X.; Gao, B.; Zhang, G.; Liu, X.; Wang, X. Adsorption of tetracycline (TC) onto montmorillonite: Cations and humic acid effects. Geoderma 2012, 183, 12–18. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wang, S.; Su, J.F.; Shao, R.Y.; Xing, Q.Y. Biomimetic anti-icing epoxy coating based on controlled-secreting hydrophobic small molecules in microcapsules through shrinkable gel under low temperature. Colloids Surf. A Physicochem. Eng. Asp. 2024, 691, 133892. [Google Scholar] [CrossRef]
- Wu, A.; Zhao, X.; Yang, C.; Wang, J.; Wang, X.; Liang, W.; Zhou, L.; Teng, M.; Niu, L.; Tang, Z.; et al. A comparative study on aggregation and sedimentation of natural goethite and artificial Fe3O4 nanoparticles in synthetic and natural waters based on extended Derjaguin-Landau-Verwey-Overbeek (XDLVO) theory and molecular dynamics simulations. J. Hazard. Mater. 2022, 435, 128876. [Google Scholar] [CrossRef] [PubMed]
- Farias, A.P.S.; Carneiro, C.E.; de Batista Fonseca, I.C.; Zaia, C.T.; Zaia, D.A. The adsorption of amino acids and cations onto goethite: A prebiotic chemistry experiment. Amino Acids 2016, 48, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Zhang, M.; Zhou, Q.; Pan, G. Molecular dynamics simulations of structural transformation of perfluorooctane sulfonate (PFOS) at water/rutile interfaces. Chemosphere 2015, 134, 272–278. [Google Scholar] [CrossRef]
- Sun, H. COMPASS: An ab Initio Force-Field Optimized for Condensed-Phase Applications Overview with Details on Alkane and Benzene Compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Chenoweth, K.; Van Duin, A.C.; Goddard, W.A. ReaxFF reactive force field for molecular dynamics simulations of hydrocarbon oxidation. J. Phys. Chem. A 2008, 112, 1040–1053. [Google Scholar] [CrossRef]
- Yang, K.; Peng, H.; Wen, Y.; Li, N. Re-examination of characteristic FTIR spectrum of secondary layer in bilayer oleic acid-coated Fe3O4 nanoparticles. Appl. Surf. Sci. 2010, 256, 3093–3097. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Z.; Tian, R.; Li, H. Specific ion effects of divalent cations on the aggregation of positively charged goethite nanoparticles in aqueous suspension. Colloids Surf. A Physicochem. Eng. Asp. 2019, 565, 78–85. [Google Scholar] [CrossRef]
- Parolo, M.E.; Savini, M.C.; Valles, J.M.; Baschini, M.T.; Avena, M.J. Tetracycline adsorption on montmorillonite: pH and ionic strength effects. Appl. Clay Sci. 2008, 40, 179–186. [Google Scholar] [CrossRef]
- Qin, Y.; Sun, M.; Liu, H.; Qu, J. AuPd/Fe3O4-based three-dimensional electrochemical system for efficiently catalytic degradation of 1-butyl-3-methylimidazolium hexafluorophosphate. Electrochim. Acta 2015, 186, 328–336. [Google Scholar] [CrossRef]
- Cui, Y.; Kang, W.; Qin, L.; Ma, J.; Liu, X.; Yang, Y. Ultrafast synthesis of magnetic hollow carbon nanospheres for the adsorption of quinoline from coking wastewater. New J. Chem. 2020, 44, 7490–7500. [Google Scholar] [CrossRef]
- Simonsen, M.E.; Sønderby, C.; Li, Z.; Søgaard, E.G. XPS and FT-IR investigation of silicate polymers. J. Mater. Sci. 2009, 44, 2079–20888. [Google Scholar] [CrossRef]
- Idriss, H. On the wrong assignment of the XPS O1s signal at 531–532 eV attributed to oxygen vacancies in photo-and electro-catalysts for water splitting and other materials applications. Surf. Sci. 2021, 712, 121894. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, S.; Yang, D.; Jiang, Y.; Li, Y.; Zhou, C.; Sun, C. Carboxyl Fe3O4 magnetic nanoparticle-based SPE and HPLC method for the determination of six tetracyclines in water. Anal. Bioanal. Chem. 2019, 411, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, R.A.; MacKay, A.A. Sorption of oxytetracycline to iron oxides and iron oxide-rich soils. Environ. Sci. Technol. 2005, 39, 6664–6671. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Karthikeyan, K. Sorption of the antimicrobial ciprofloxacin to aluminum and iron hydrous oxides. Environ. Sci. Technol. 2005, 39, 9166–9173. [Google Scholar] [CrossRef]
- Qin, X.; Liu, F.; Wang, G.; Weng, L.; Li, L. Adsorption of levofloxacin onto goethite: Effects of pH, calcium and phosphate. Colloids Surf. B Biointerfaces 2014, 116, 591–596. [Google Scholar] [CrossRef]
- Gu, C.; Karthikeyan, K. Interaction of tetracycline with aluminum and iron hydrous oxides. Environ. Sci. Technol. 2005, 39, 2660–2667. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, S.; Lin, Q. Influence of metal cation and surface iron oxide on the transport of sulfadiazine in saturated porous media. Sci. Total Environ. 2021, 758, 143621. [Google Scholar] [CrossRef]
- Wu, T.; Xue, Q.; Liu, F.; Zhang, J.; Zhou, C.; Cao, J.; Chen, H. Mechanistic insight into interactions between tetracycline and two iron oxide minerals with different crystal structures. Chem. Eng. J. 2019, 366, 577–586. [Google Scholar] [CrossRef]
- Trivedi, P.; Vasudevan, D. Spectroscopic investigation of ciprofloxacin speciation at the goethite-water interface. Environ. Sci. Technol. 2007, 41, 3153–3158. [Google Scholar] [CrossRef] [PubMed]
- Rakshit, S.; Sarkar, D.; Elzinga, E.J.; Punamiya, P.; Datta, R. Mechanisms of ciprofloxacin removal by nano-sized magnetite. J. Hazard. Mater. 2013, 246–247, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yang, C.; Jiang, Y.; Zhang, X.; Wang, Q.; Dang, Z. Desorption of heavy metals and tetracycline from goethite-coated sands: The role of complexation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 573, 88–94. [Google Scholar] [CrossRef]


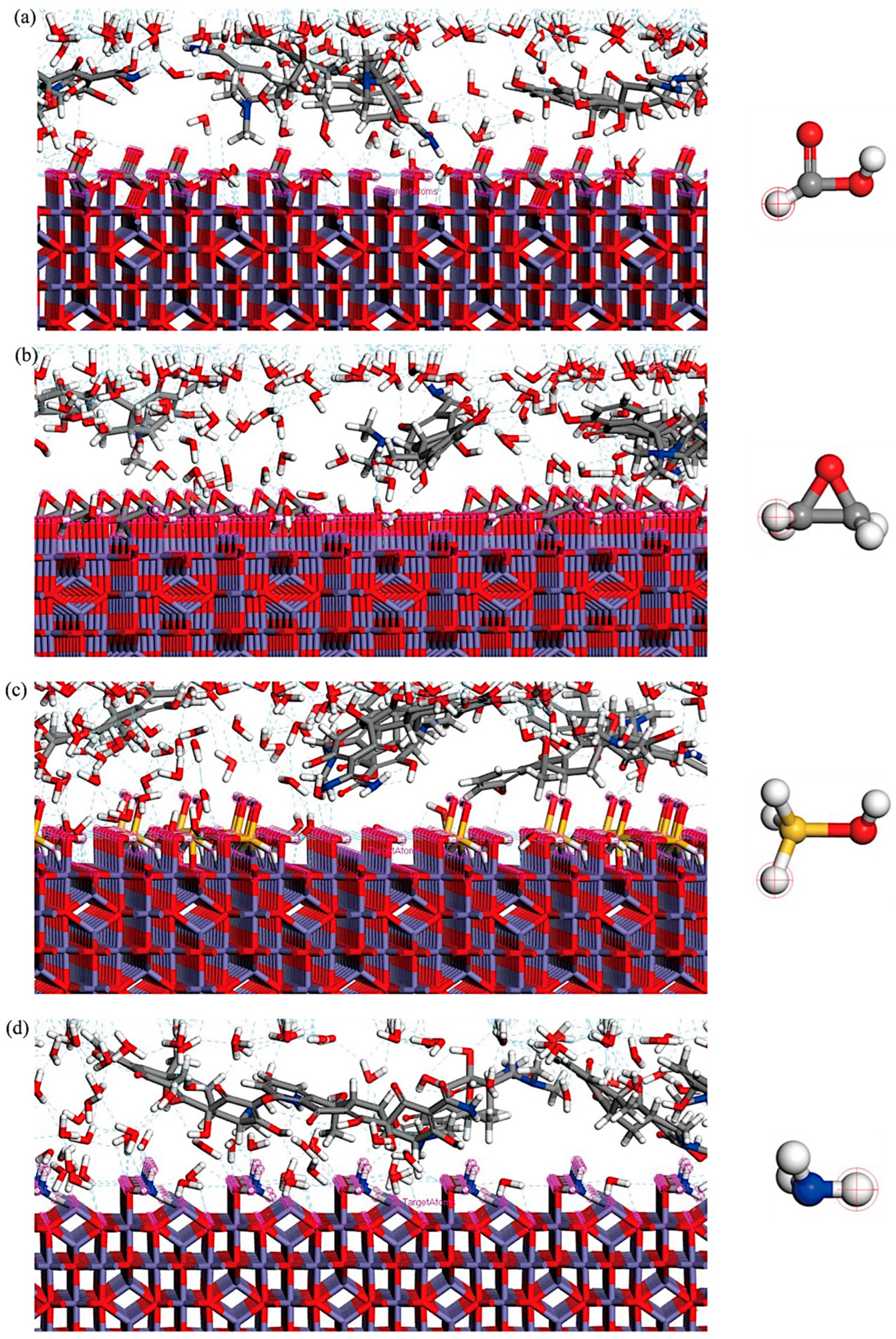
| Energy Type | -COOH/TC | -EPOXY/TC | -SIOH/TC | -NH2/TC |
|---|---|---|---|---|
| Contributions to Total Energy (kcal/mol): | ||||
| Total Energy | −1,209,553.541 | −1,217,209.116 | −1,211,890.528 | −1,218,926.599 |
| Valence Energy (diag. terms) | 9089.448 | 15,453.360 | 10,052.710 | 3309.592 |
| Bond | 2665.652 | 3497.470 | 3870.529 | 2071.052 |
| Angle | 6641.437 | 12,153.575 | 6560.122 | 1677.012 |
| Torsion | −239.040 | −221.967 | −392.768 | −461.485 |
| Inversion | 21.399 | 24.282 | 14.827 | 23.013 |
| Valence Energy (cross terms) | −152.778 | −162.002 | −185.945 | −170.904 |
| Stretch-Stretch | 28.019 | −1.092 | −5.566 | −5.356 |
| Stretch-Bend-Stretch | −127.546 | −107.638 | −129.826 | −114.045 |
| Stretch-Torsion-Stretch | −6.447 | −5.760 | −5.217 | −4.393 |
| Separated-Stretch-Stretch | 1.052 | 0.980 | 0.874 | 0.860 |
| Torsion-Stretch | −24.746 | −33.142 | −24.645 | −23.848 |
| Bend-Bend | 2.101 | 2.584 | 2.033 | 0.954 |
| Torsion-Bend-Bend | −15.906 | −13.288 | −13.174 | −10.278 |
| Bend-Torsion-Bend | −9.307 | −4.646 | −10.424 | −14.798 |
| Non-bond Energy | −1,218,490.210 | −1,232,500.474 | −1,221,757.293 | −1,222,065.287 |
| Van der waals | −949,359.830 | −965,758.372 | −953,107.261 | −958,078.039 |
| Long range correction | −164.921 | −167.228 | −166.904 | −159.944 |
| Electrostatic | −268,965.460 | −266,574.873 | −268,483.128 | −263,827.304 |
| Adsorption energy | −39,943.840 | −40,400.630 | −39,180.480 | −40,666.840 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Wu, M.; Chen, M.; Hao, Y. Complex Effects of Functional Groups on the Cotransport Behavior of Functionalized Fe3O4 Magnetic Nanospheres and Tetracycline in Porous Media. Water 2025, 17, 2889. https://doi.org/10.3390/w17192889
Cui Y, Wu M, Chen M, Hao Y. Complex Effects of Functional Groups on the Cotransport Behavior of Functionalized Fe3O4 Magnetic Nanospheres and Tetracycline in Porous Media. Water. 2025; 17(19):2889. https://doi.org/10.3390/w17192889
Chicago/Turabian StyleCui, Yiqun, Ming Wu, Meng Chen, and Yanru Hao. 2025. "Complex Effects of Functional Groups on the Cotransport Behavior of Functionalized Fe3O4 Magnetic Nanospheres and Tetracycline in Porous Media" Water 17, no. 19: 2889. https://doi.org/10.3390/w17192889
APA StyleCui, Y., Wu, M., Chen, M., & Hao, Y. (2025). Complex Effects of Functional Groups on the Cotransport Behavior of Functionalized Fe3O4 Magnetic Nanospheres and Tetracycline in Porous Media. Water, 17(19), 2889. https://doi.org/10.3390/w17192889







