A Study on the Use of Copper Ions for Bacterial Inactivation in Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Cultivation
2.2. Copper Ion Generation
2.3. Experimental Design
2.4. Effect of Growth Stage of Bacteria
2.5. Effect of Temperature
2.6. Effects of Organic Matter
2.7. Effects of pH, Bicarbonate, and Water Hardness
2.8. Disinfection of Bacterial Cultures
2.9. Culture-Based Enumeration and Disinfection Kinetics
2.10. ATP Analysis
2.11. Flow Cytometry
2.12. Statistical Analysis
3. Results and Discussion
3.1. Effect of Growth Phase
3.2. Effect of Organic Matter
3.3. Effect of Temperature
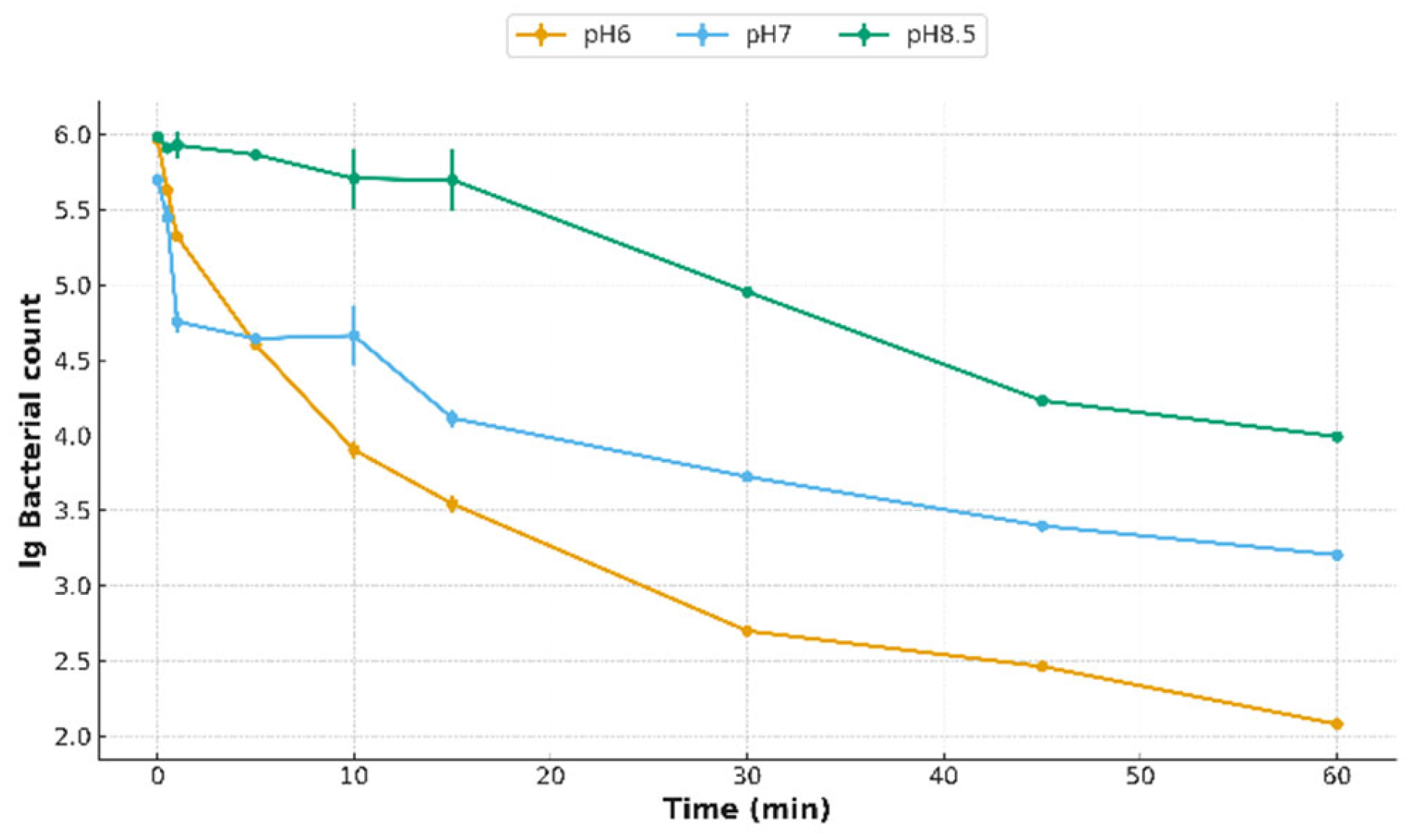
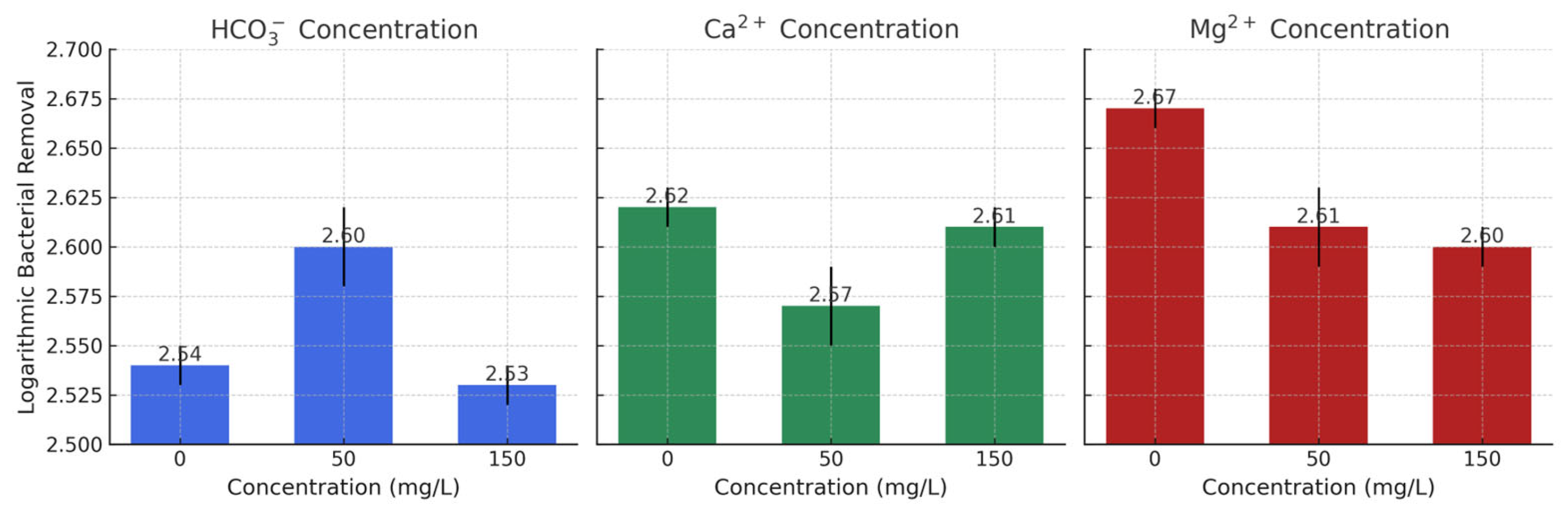
3.4. Effects of pH, Bicarbonate, and Water Hardness
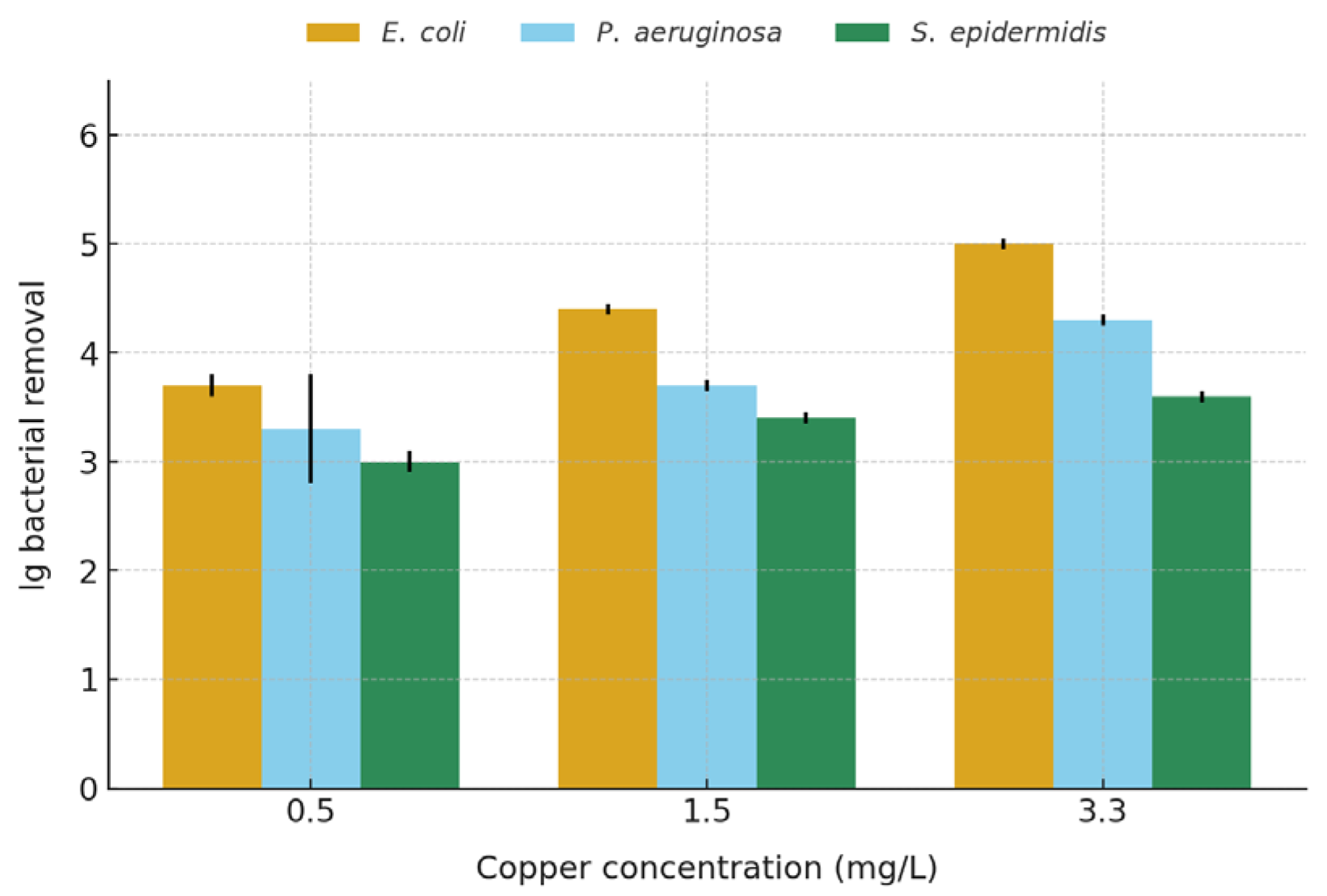
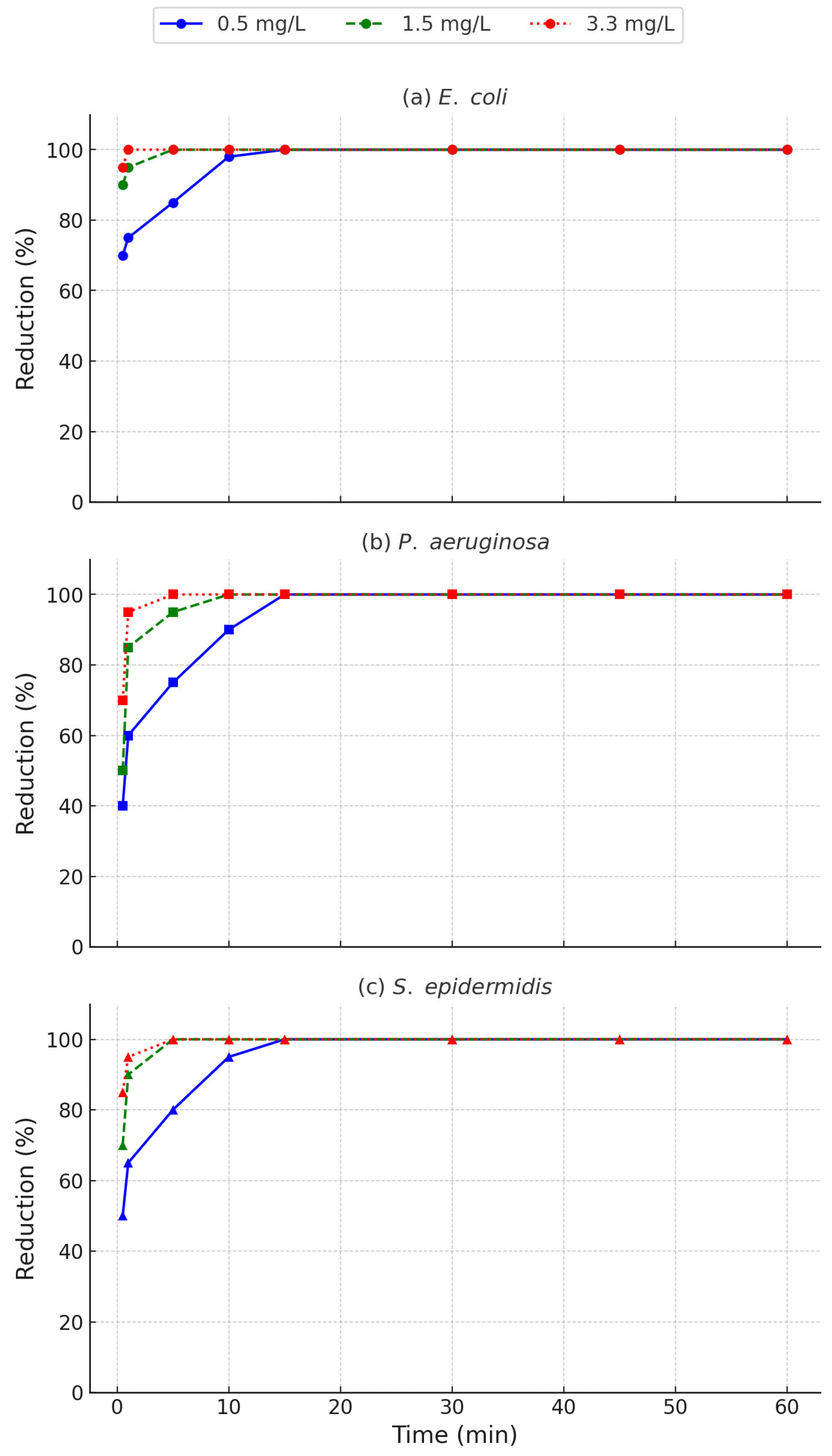
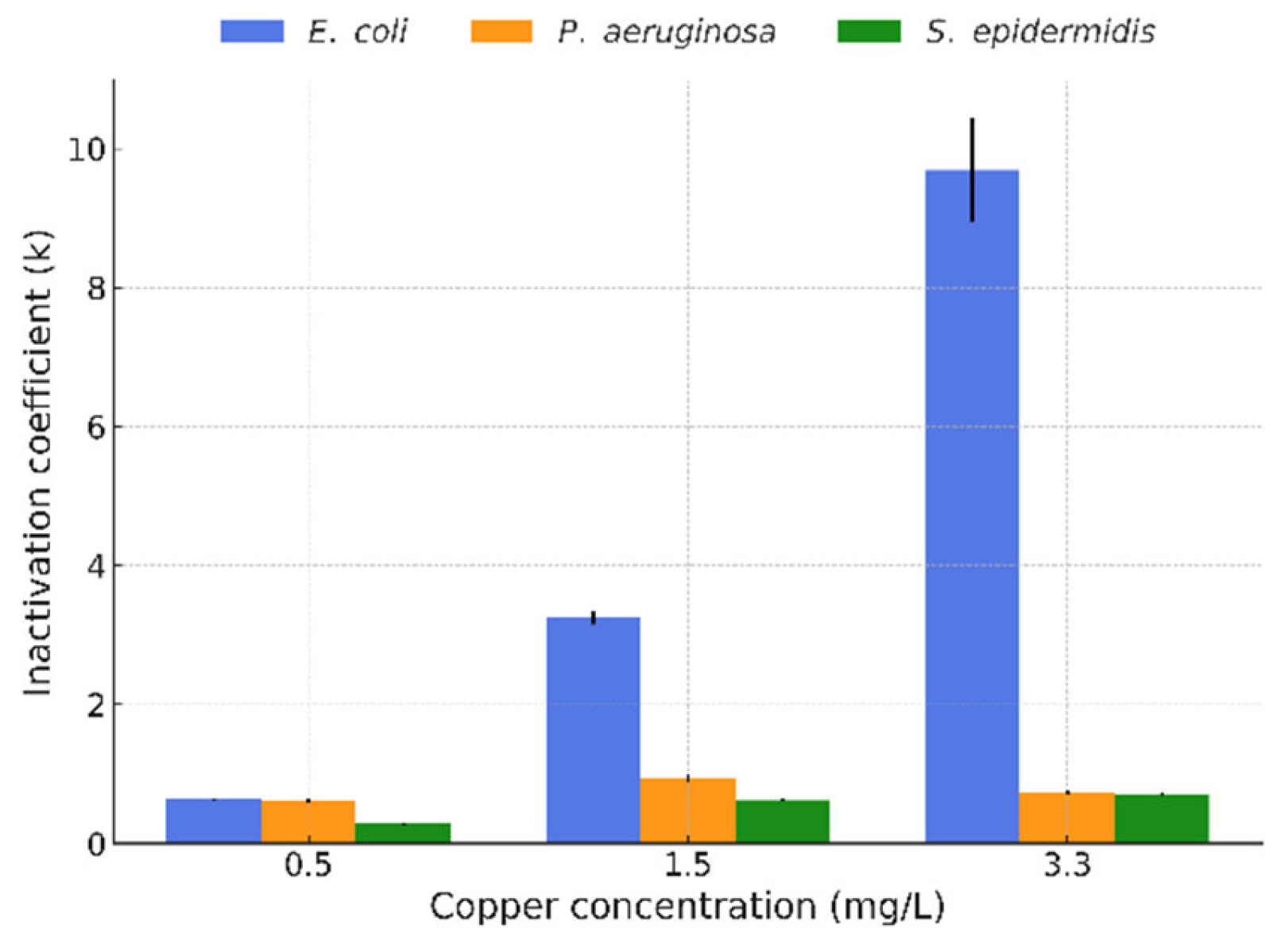
3.5. Effects of Copper Ion Concentration on Bacterial Inactivation and Kinetic Modelling
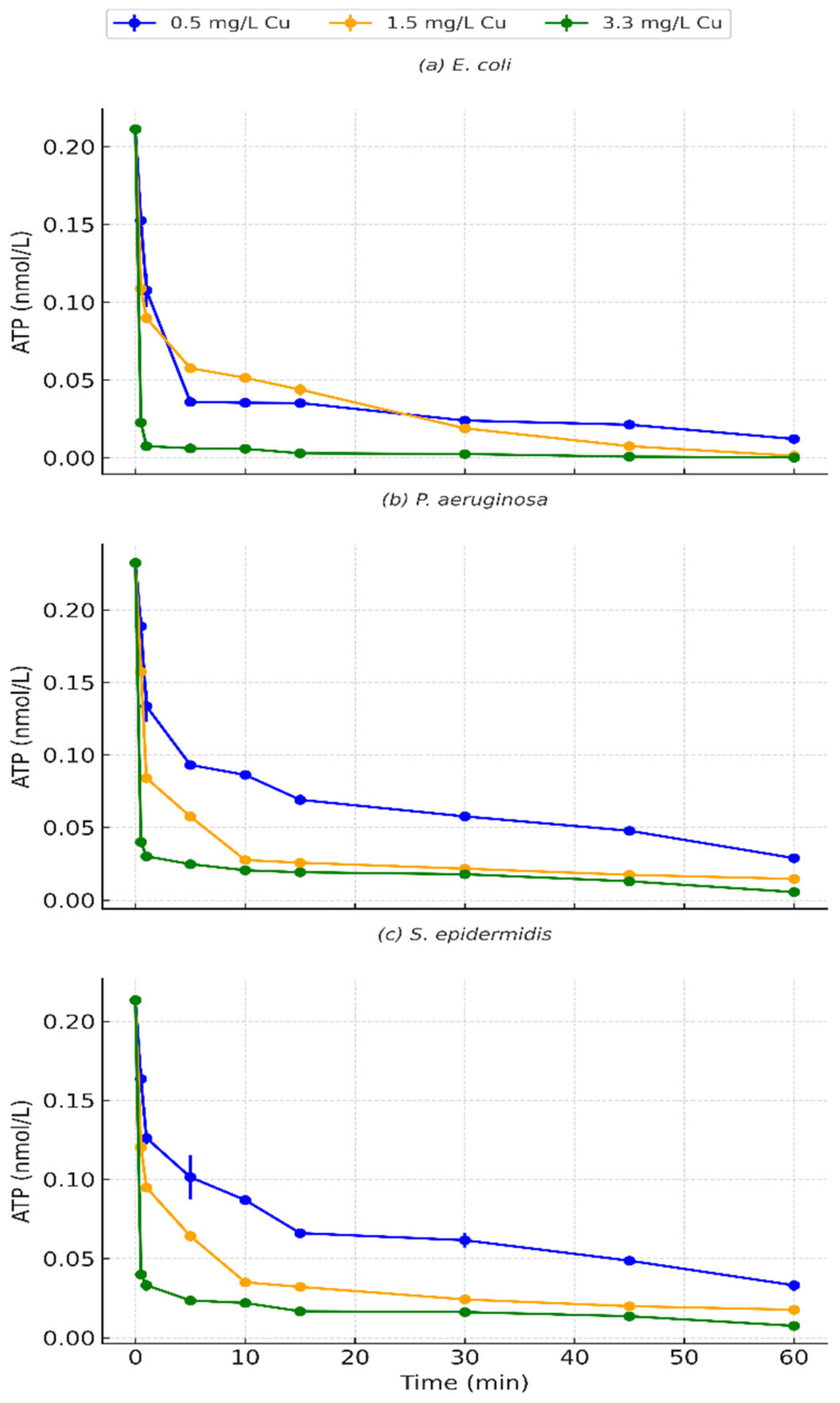
3.6. ATP Reduction Patterns
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Drinking-Water; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Ashbolt, N.J. Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology 2004, 198, 229–238. [Google Scholar] [CrossRef]
- United Nations. Sustainable Development Goal 6: Ensure Availability and Sustainable Management of Water and Sanitation for All; United Nations: New York, NY, USA, 2022. [Google Scholar]
- Li, L.; Mendis, N.; Trigui, H.; Oliver, J.D.; Faucher, S.P. The importance of the viable but non-culturable state in human bacterial pathogens. Front. Microbiol. 2014, 5, 258. [Google Scholar] [CrossRef]
- Richardson, S.D.; Plewa, M.J.; Wagner, E.D.; Schoeny, R.; DeMarini, D.M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research. Mutat. Res. 2007, 636, 178–242. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.D. The viable but non-culturable state in bacteria. J. Microbiol. 2005, 43, 93–100. [Google Scholar] [PubMed]
- Vincent, M.; Duval, R.E.; Hartemann, P.; Engels-Deutsch, M. Contact killing and antimicrobial properties of copper. Appl. Microbiol. Biotechnol. 2014, 98, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Santo, C.E.; Morais, P.V.; Grass, G. Isolation and characterization of bacteria resistant to metallic copper surfaces. Appl. Environ. Microbiol. 2010, 76, 1341–1348. [Google Scholar] [CrossRef]
- Liu, R.; Gunawan, C.; Barraud, N.; Rice, S.A.; Harry, E.J.; Amal, R. Understanding, monitoring, and controlling biofilm growth in drinking water distribution systems. Environ. Sci. Technol. 2020, 54, 11953–11972. [Google Scholar] [CrossRef]
- Dziewulski, D.M.; Kulakov, L.A.; Wardell, J.; Colbourne, J.S. Use of copper–silver ionization for Legionella control in hospital water systems. J. Hosp. Infect. 2015, 91, 971–976. [Google Scholar] [CrossRef]
- Lin, Y.E.; Stout, J.E.; Yu, V.L. Controlling Legionella in hospital drinking water: An evidence-based review of disinfection methods. Infect. Control Hosp. Epidemiol. 2011, 32, 166–173. [Google Scholar] [CrossRef]
- Aldsworth, T.G.; Carrington, C.; Flegg, J.; Stewart, G.S.A.B.; Dodd, C.E.R. Bacterial adaptation to environmental stress: The implications for food processing. Leatherhead Food RA Food Ind. J. 1998, 1, 136–144. [Google Scholar]
- Ferro, S. Challenges in Designing Electrochemical Disinfection Systems for Reducing Microbial Contamination in Drinking Water Distribution Networks. Water 2025, 17, 754. [Google Scholar] [CrossRef]
- Li, R.; Dai, H.; Wang, W.; Peng, R.; Yu, S.; Zhang, X.; Huo, Z.-Y.; Yuan, Q.; Luo, Y. Local Electric Field-Incorporated In-Situ Copper Ions Eliminating Pathogens and Antibiotic Resistance Genes in Drinking Water. Antibiotics 2024, 13, 1161. [Google Scholar] [CrossRef]
- Xu, L.; Sigler, A.; Chernatynskaya, A.; Rasmussen, L.; Lu, J.; Sahle-Demessie, E.; Westenberg, D.; Yang, H.; Shi, H. Study of Legionella pneumophila Treatment with Copper in Drinking Water by Single Cell-ICP-MS. Anal. Bioanal. Chem. 2024, 416, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Hammes, F.; Egli, T. New method for assimilable organic carbon determination using flow-cytometric enumeration and a natural microbial consortium as inoculum. Environ. Sci. Technol. 2005, 39, 3289–3294. [Google Scholar] [CrossRef] [PubMed]
- Prest, E.I.; Hammes, F.; van Loosdrecht, M.C.M.; Vrouwenvelder, J.S. Biological stability of drinking water: Controlling factors, methods, and challenges. Front. Microbiol. 2016, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Van der Wielen, P.W.J.J.; van der Kooij, D. Effect of water composition, distance and season on the adenosine triphosphate concentration in unchlorinated drinking water in the Netherlands. Water Res. 2010, 44, 4860–4867. [Google Scholar] [CrossRef]
- Magic-Knezev, A.; van der Kooij, D. Optimisation and significance of ATP analysis for measuring active biomass in granular activated carbon filters used in water treatment. Water Res. 2004, 38, 3971–3979. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; APHA: Washington, DC, USA, 2017. [Google Scholar]
- Valkonen, M.; Penttinen, R.; Vaara, M.; Kukkonen, J. Effect of temperature on the efficacy of disinfectants against Legionella pneumophila biofilms. J. Appl. Microbiol. 2020, 128, 1219–1229. [Google Scholar]
- LeChevallier, M.W.; Cawthon, C.D.; Lee, R.G. Factors promoting survival of bacteria in chlorinated water supplies. Appl. Environ. Microbiol. 1988, 54, 649–654. [Google Scholar] [CrossRef]
- Landeen, L.K.; Yahya, M.T.; Gerba, C.P. Efficacy of copper and silver ions and reduced levels of free chlorine in inactivation of Legionella pneumophila. Appl. Environ. Microbiol. 1989, 55, 3045–3050. [Google Scholar] [CrossRef]
- Liu, H.; Chen, T.; Wang, J.; Wang, C. Effect of pH on copper corrosion and release in drinking water systems. Corros. Sci. 2012, 65, 536–543. [Google Scholar]
- Tipping, E. Humic substances in soil, sediment, and water: Their behavior and impact on the environment. Environ. Sci. Technol. 2002, 36, 248A–249A. [Google Scholar]
- Watson, H.E. A note on the variation of the rate of disinfection with change in the concentration of the disinfectant. J. Hyg. 1908, 8, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Haas, C.N.; Joffe, J. Disinfection under dynamic conditions: Modification of the Chick–Watson model. Environ. Sci. Technol. 1994, 28, 1367–1370. [Google Scholar] [CrossRef] [PubMed]
- Najm, I.N. Modeling Water Treatment Plant Performance; AWWA Research Foundation: Denver, CO, USA, 2006. [Google Scholar]
- ISO 9308-1:2014; Water Quality—Enumeration of Escherichia coli and Coliform Bacteria—Part 1: Membrane Filtration Method. International Organization for Standardization: Geneva, Switzerland, 2014.
- APHA. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- ISO 16266:2006; Water Quality—Detection and Enumeration of Pseudomonas aeruginosa—Method by Membrane Filtration. International Organization for Standardization: Geneva, Switzerland, 2006.
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021. [Google Scholar]
- Hammes, F.; Berney, M.; Wang, Y.; Vital, M.; Köster, O.; Egli, T. Flow-cytometric total bacterial cell counts as a descriptive microbiological parameter for drinking water treatment processes. Water Res. 2008, 42, 269–277. [Google Scholar] [CrossRef]
- Berney, M.; Hammes, F.; Bosshard, F.; Weilenmann, H.U.; Egli, T. Assessment and interpretation of bacterial viability by using the LIVE/DEAD BacLight kit in combination with flow cytometry. Appl. Environ. Microbiol. 2007, 73, 3283–3290. [Google Scholar] [CrossRef]
- Hammes, F.; Egli, T. Cytometric methods for measuring bacteria in water: Advantages, pitfalls and applications. Anal. Bioanal. Chem. 2010, 397, 1083–1095. [Google Scholar] [CrossRef]
- Grass, G.; Rensing, C.; Solioz, M. Metallic Copper as an Antimicrobial Surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef]
- Choi, S.H.; Lee, J.H.; Kim, S.J.; Park, H.D. The CusCFBA Efflux System Plays a Role in Escherichia coli’s Survival in Toxic Copper Environments. FEMS Microbiol. Lett. 2018, 365, fnx282. [Google Scholar] [CrossRef]
- Macomber, L.; Hausinger, R.P. Mechanisms of nickel and copper toxicity in microorganisms. Metallomics 2011, 3, 1153–1162. [Google Scholar] [CrossRef]
- Santo, C.E.; Lam, E.W.; Elowsky, C.G.; Quaranta, D.; Domaille, D.W.; Chang, C.J.; Grass, G. Bacterial Killing by Dry Metallic Copper Surfaces. Appl. Environ. Microbiol. 2011, 77, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Outten, F.W.; Huffman, D.L.; Hale, J.A.; O’Halloran, T.V. The independent cue and cus systems confer copper tolerance during aerobic and anaerobic growth in Escherichia coli. J. Biol. Chem. 2001, 276, 30670–30677. [Google Scholar] [CrossRef] [PubMed]
- Warnes, S.L.; Caves, V.; Keevil, C.W. Mechanism of Copper Surface Toxicity in Escherichia coli O157:H7 and Salmonella Involves Immediate Membrane Depolarization Followed by Slower Rate of DNA Destruction, Which Differs from That Observed for Gram-Positive Bacteria. Environ. Microbiol. 2012, 14, 1730–1743. [Google Scholar] [CrossRef] [PubMed]
- Molteni, C.; Abicht, H.K.; Solioz, M. Killing of Bacteria on Copper Surfaces via Respiratory Inhibition Is in the Millisecond Range. Appl. Environ. Microbiol. 2010, 76, 4099–4101. [Google Scholar] [CrossRef]
- De Schamphelaere, K.A.C.; Vasconcelos, F.M.; Tack, F.M.G.; Allen, H.E.; Janssen, C.R. Effect of dissolved organic matter source on acute copper toxicity to Daphnia magna. Environ. Toxicol. Chem. 2004, 23, 1248–1255. [Google Scholar] [CrossRef]
- Linbo, T.L.; Sadler, R.M.; Hoffman, J.L.; Venables, W.Y.; McIntyre, J.K.; Baldwin, D.H. Effects of water hardness, alkalinity, and dissolved organic carbon on the toxicity of copper to the lateral line of developing fish (Danio rerio). Environ. Toxicol. Chem. 2009, 28, 1455–1461. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Xavier, J.B.; Picioreanu, C.; Abdul Rani, S.; van Loosdrecht, M.C.M.; Stewart, P.S. Biofilm-control strategies based on enzymic disruption of the extracellular polymeric substance matrix—A modelling study. Microbiology 2005, 151, 3817–3832. [Google Scholar] [CrossRef]
- Harrison, J.J.; Ceri, H.; Turner, R.J. Multimetal resistance and tolerance in microbial biofilms. Nat. Rev. Microbiol. 2007, 5, 928–938. [Google Scholar] [CrossRef]
- Lin, W.; Yu, Z.; Chen, X.; Liu, R.; Zhang, H.; Lin, J.; Gao, W. Bacterial community dynamics in a full-scale drinking water treatment plant. Front. Microbiol. 2016, 7, 246. [Google Scholar] [CrossRef]
- Mikolay, A.; Huggett, S.; Tikana, L.; Grass, G.; Braun, J.; Nies, D.H. Survival of bacteria on metallic copper surfaces in a hospital trial. Appl. Microbiol. Biotechnol. 2010, 87, 1875–1879. [Google Scholar] [CrossRef]
- Sharan, R.; Chhibber, S.; Reed, R.H. Inactivation and potential mechanisms of Escherichia coli by copper in drinking water. Water Sci. Technol. 2010, 61, 865–871. [Google Scholar] [CrossRef]
- Pamp, S.J.; Sternberg, C.; Tolker-Nielsen, T. Insight into the microbial multicellular lifestyle via flow-cell technology and confocal microscopy. Cytom. A 2008, 75, 90–103. [Google Scholar] [CrossRef]
- Tiwari, N.; Santhiya, D.; Sharma, J.G. Significance of landfill microbial communities in biodegradation of polyethylene and nylon 6,6 microplastics. J. Hazard. Mater. 2024, 462, 132786. [Google Scholar] [CrossRef]
- Mathews, S.; Hans, M.; Mücklich, F.; Solioz, M. Contact killing of bacteria on copper is suppressed if bacterial-metal contact is prevented and is induced on iron by copper ions. Appl. Environ. Microbiol. 2015, 81, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Haas, C.N.; Karra, S.B. Kinetics of microbial inactivation by chlorine—I. Review of results in demand-free systems. Water Res. 1984, 18, 1443–1449. [Google Scholar] [CrossRef]
- Rosenblatt, A.A.; Margalit, E.; Nejidat, A.; Ronen, Z. Monitoring biofilm bacterial communities in a pilot drinking water distribution system using flow cytometry and high-throughput sequencing. Water 2018, 10, 166. [Google Scholar] [CrossRef]
- Teitzel, G.M.; Parsek, M.R. Heavy metal resistance of biofilm and planktonic Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2003, 69, 2313–2320. [Google Scholar] [CrossRef]
- Harrison, J.J.; Turner, R.J.; Ceri, H. High-throughput metal susceptibility testing of microbial biofilms. BMC Microbiol. 2005, 5, 53. [Google Scholar] [CrossRef]
- Merchel Piovesan Pereira, B.; Tagkopoulos, I. Benzalkonium chlorides: Uses, regulatory status, and microbial resistance. Appl. Environ. Microbiol. 2019, 85, e00377-19. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Dupont, C.L.; Grass, G.; Rensing, C. Copper toxicity and the origin of bacterial resistance—New insights and applications. Metallomics 2011, 3, 1109–1118. [Google Scholar] [CrossRef]
- Ji, G.; Beavis, R.; Novick, R.P. Bacterial interference caused by autoinducing peptide variants. Science 1997, 276, 2027–2030. [Google Scholar] [CrossRef]
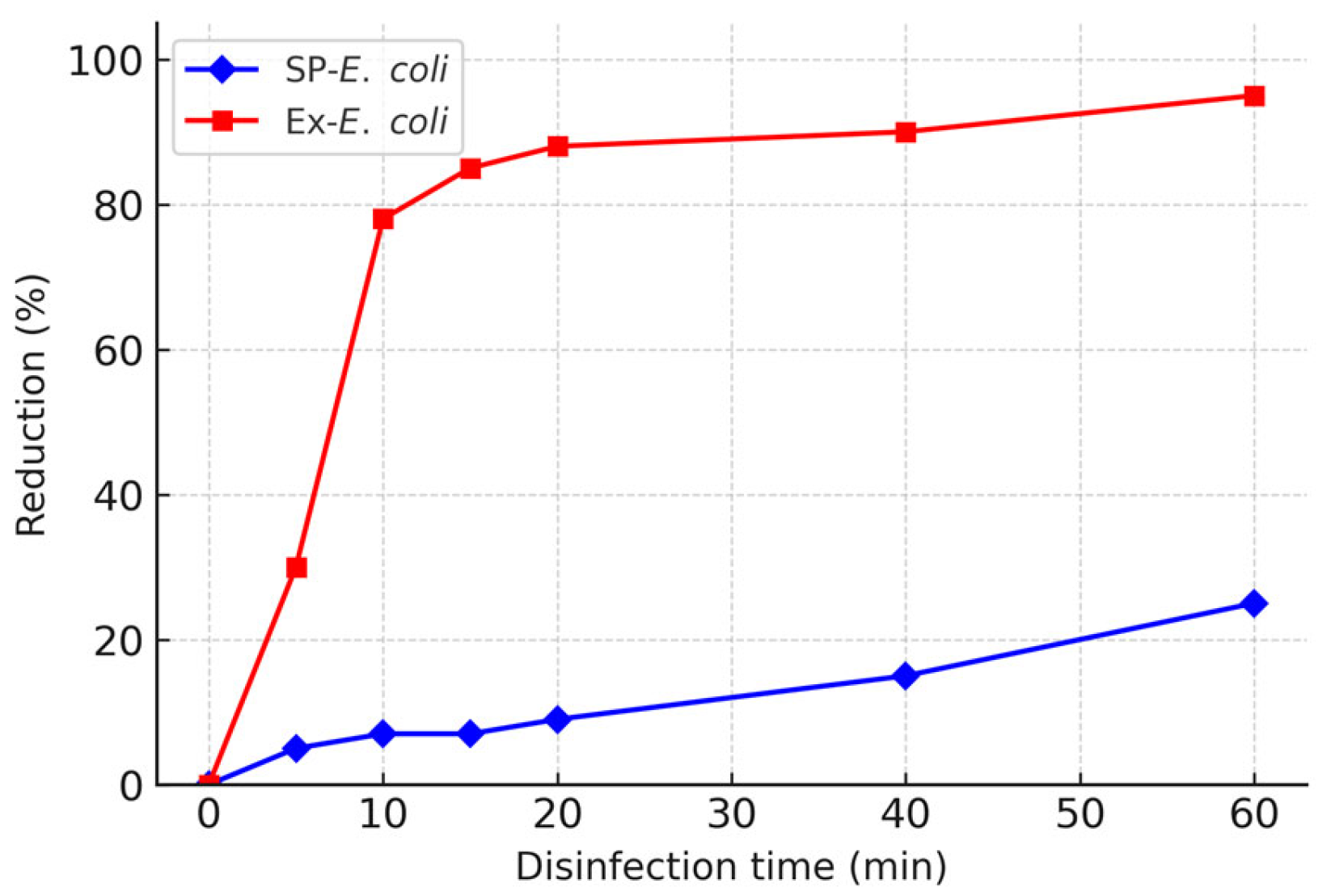
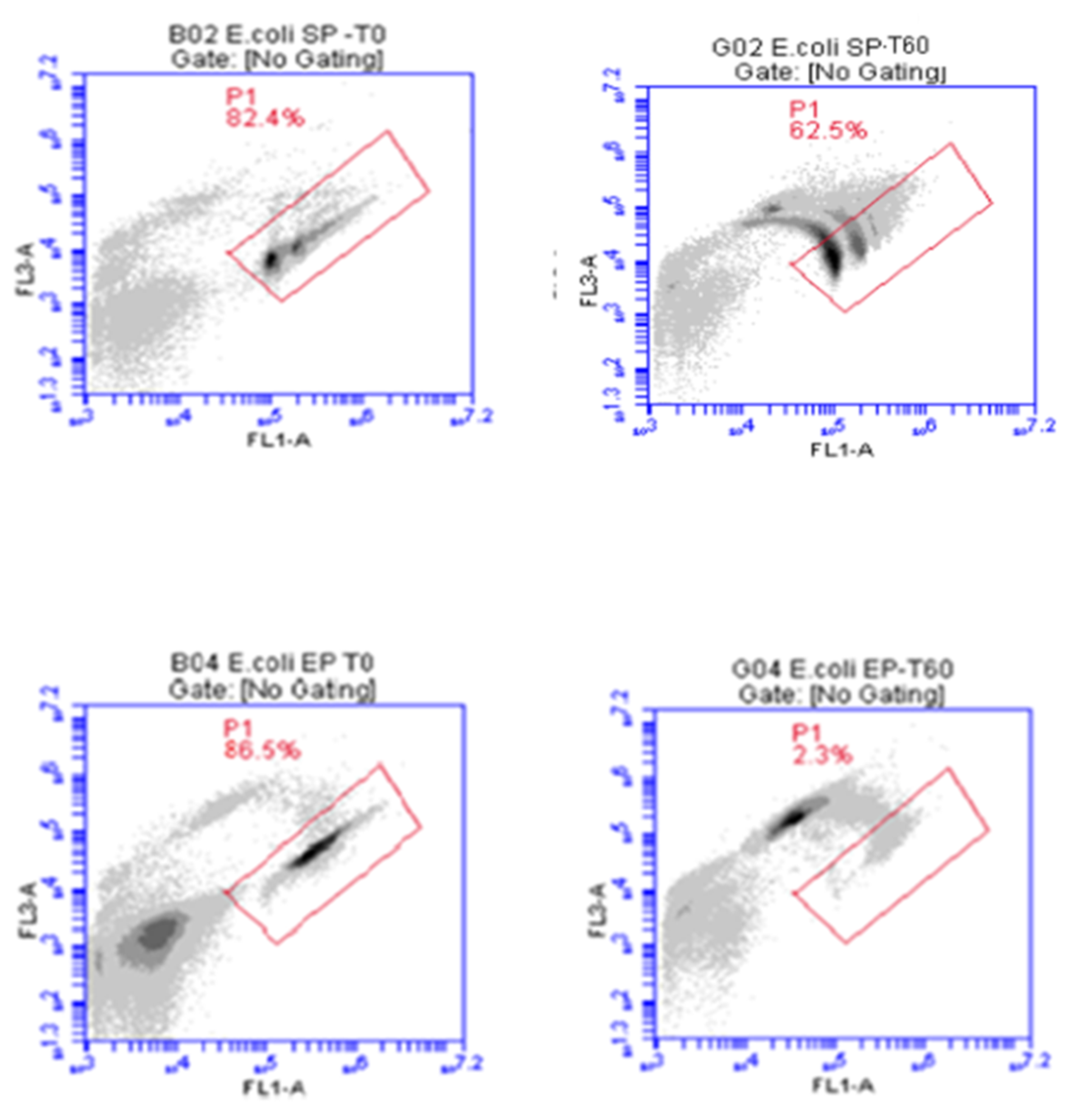
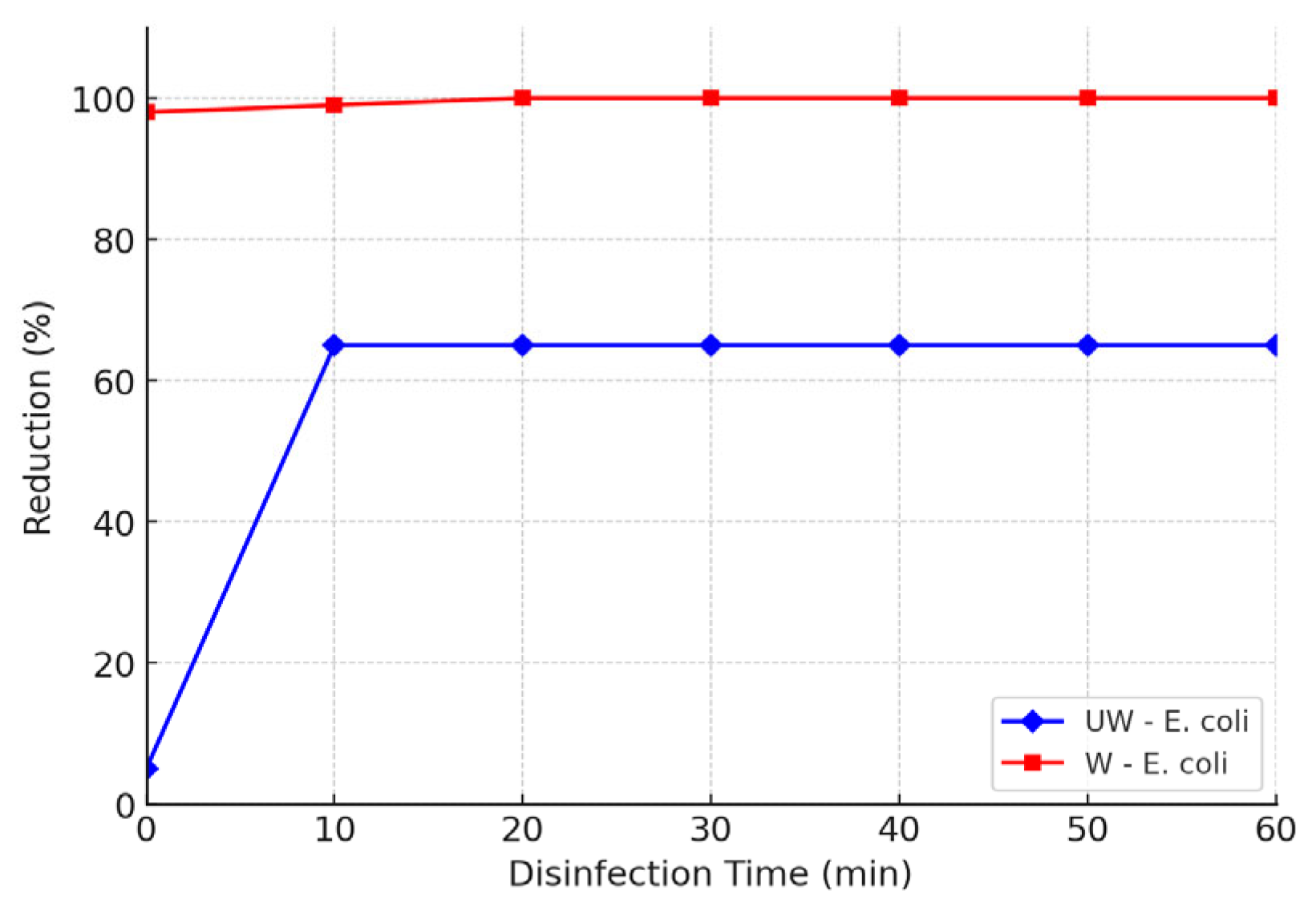
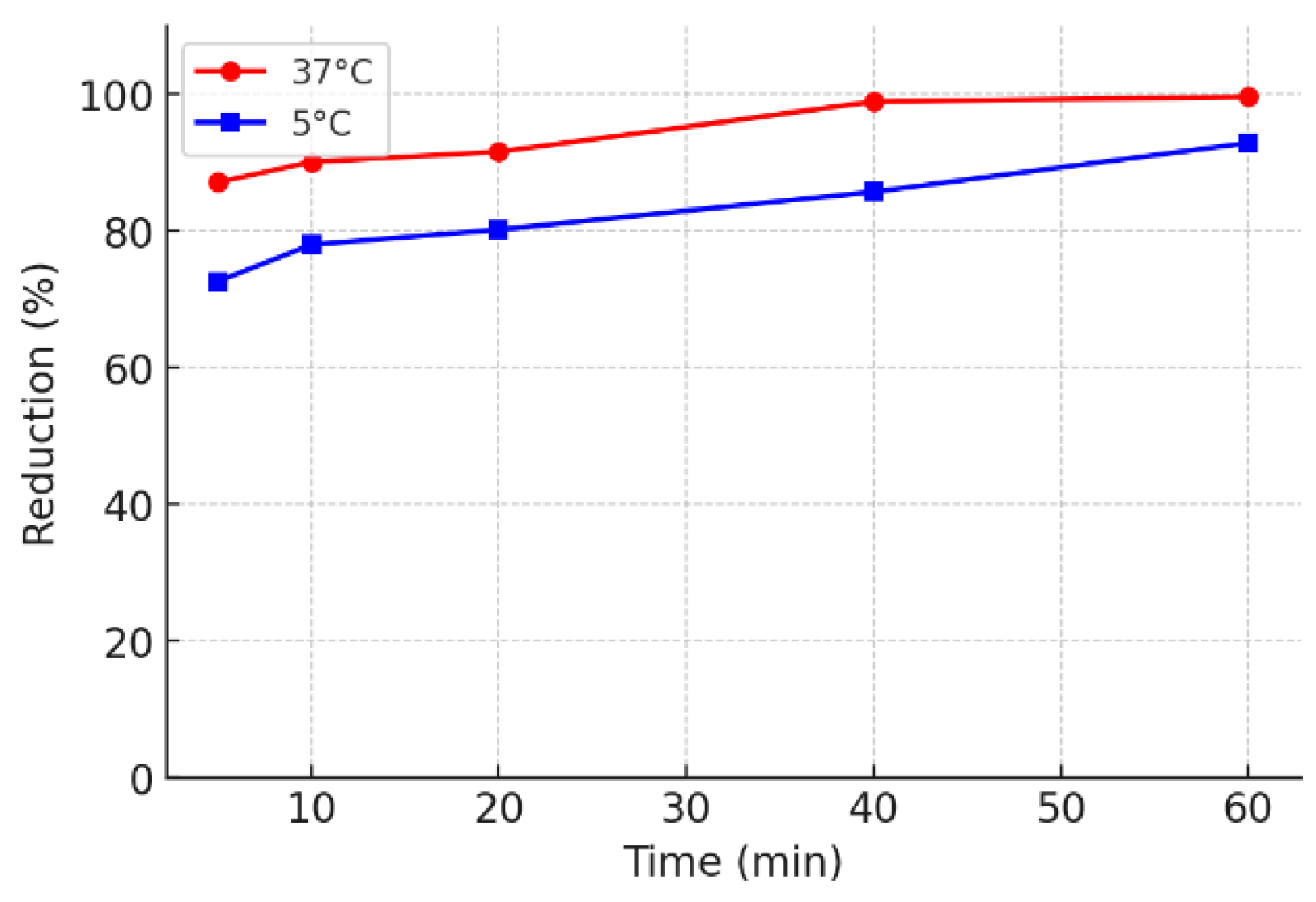
| Source | Statistic | E. coli | P. aeruginosa | S. epidermidis |
|---|---|---|---|---|
| Cu concentration | F | 448.13 | 74.84 | 243.19 |
| p-value | <0.001 | <0.001 | <0.001 | |
| Partial η2 | 0.94 | 0.74 | 0.90 | |
| Time | F | 504.16 | 637.44 | 1045.0 |
| p-value | <0.001 | <0.001 | <0.001 | |
| Partial η2 | 0.99 | 0.99 | 0.99 | |
| Interaction (Conc × Time) | F | 11.67 | 8.23 | 13.92 |
| p-value | <0.001 | <0.001 | <0.001 | |
| Partial η2 | 0.78 | 0.71 | 0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teksoy, A.; Özyiğit, M.E. A Study on the Use of Copper Ions for Bacterial Inactivation in Water. Water 2025, 17, 2797. https://doi.org/10.3390/w17192797
Teksoy A, Özyiğit ME. A Study on the Use of Copper Ions for Bacterial Inactivation in Water. Water. 2025; 17(19):2797. https://doi.org/10.3390/w17192797
Chicago/Turabian StyleTeksoy, Arzu, and Melis Ece Özyiğit. 2025. "A Study on the Use of Copper Ions for Bacterial Inactivation in Water" Water 17, no. 19: 2797. https://doi.org/10.3390/w17192797
APA StyleTeksoy, A., & Özyiğit, M. E. (2025). A Study on the Use of Copper Ions for Bacterial Inactivation in Water. Water, 17(19), 2797. https://doi.org/10.3390/w17192797






