Photoelectrocatalysis as an Effective Treatment for Removing Perfluoroalkyl Substances from Contaminated Groundwaters: The Real Case of the Veneto Region (Italy)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up
2.2. Test Monitoring and Analytical Methods
2.3. Data Processing
3. Results and Discussion
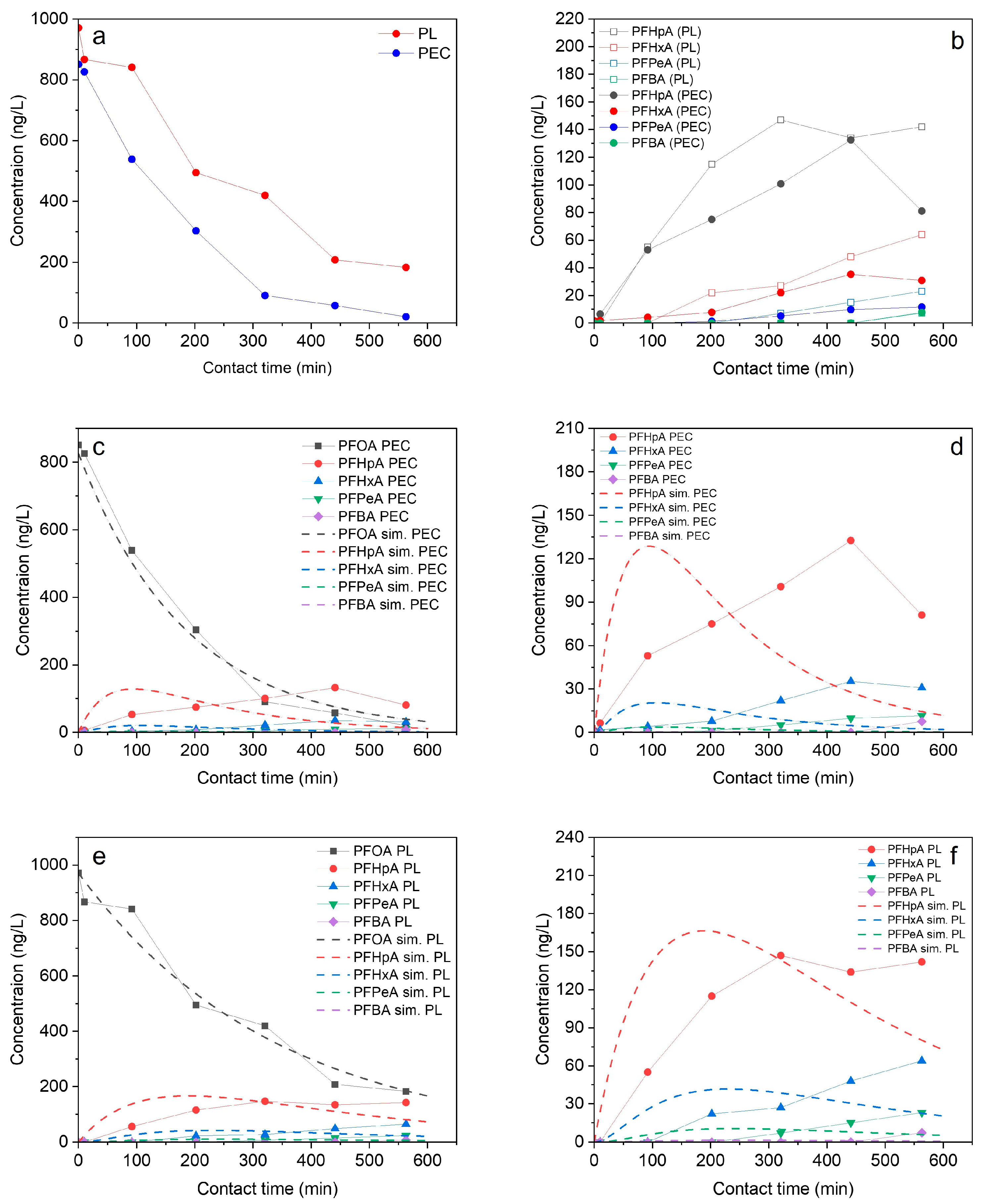
3.1. Degradation of PFOA
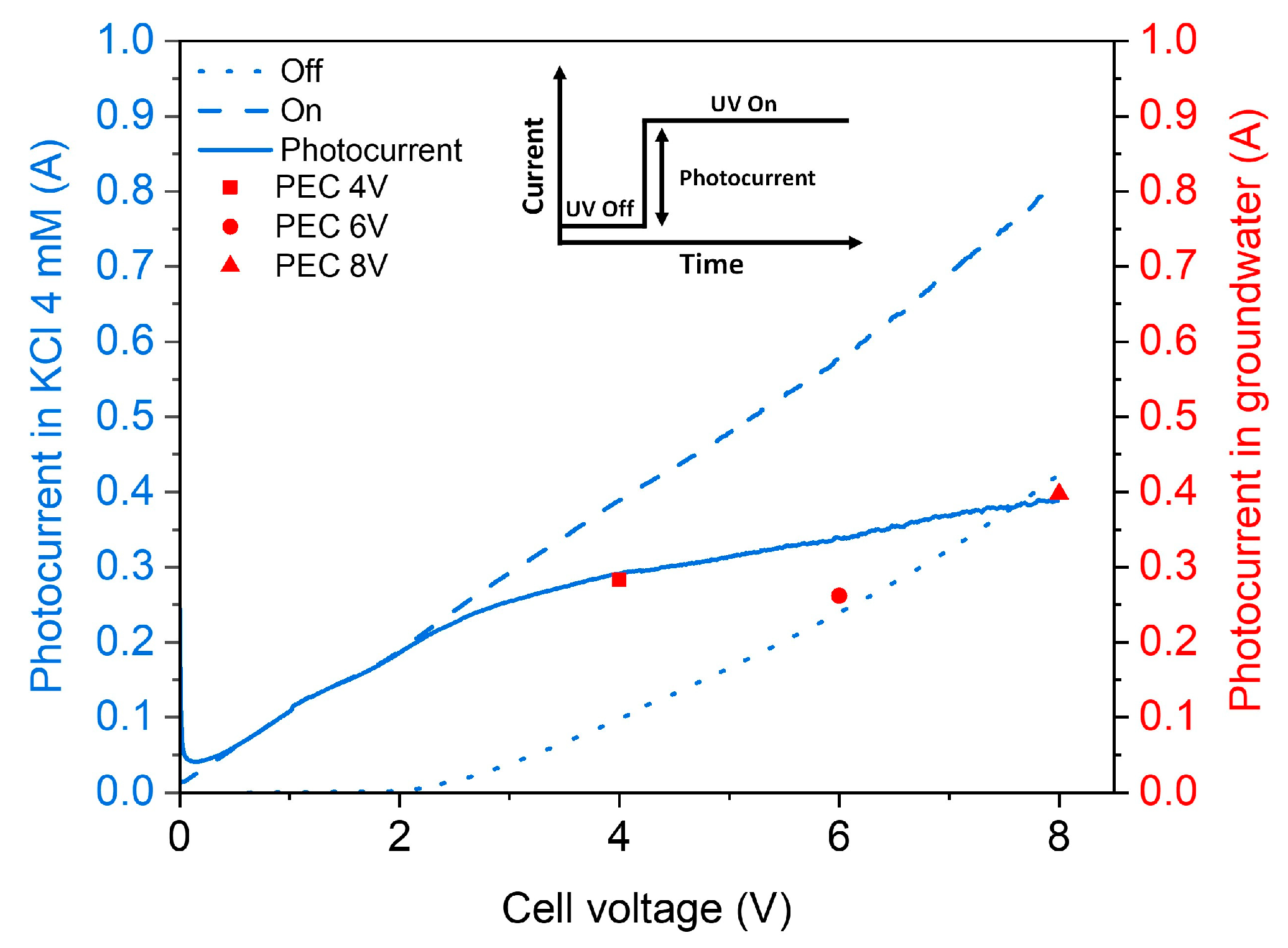
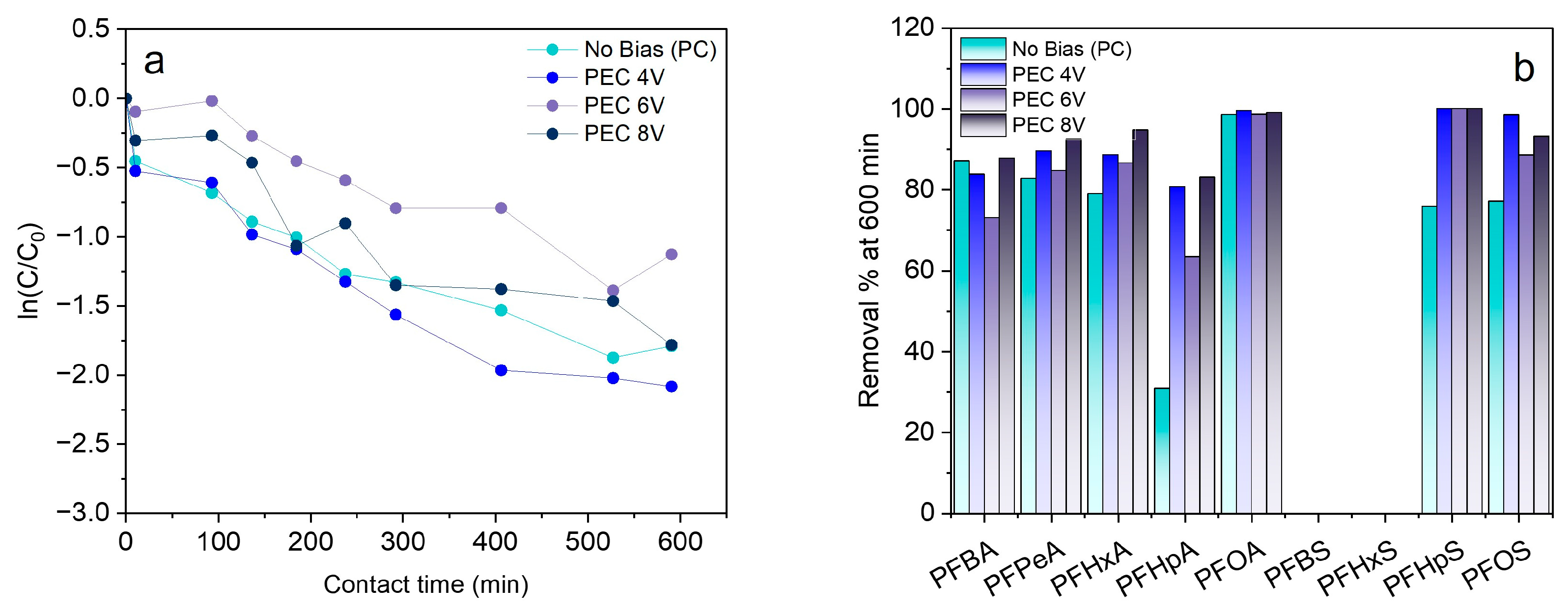
3.2. Effect of Electric Bias
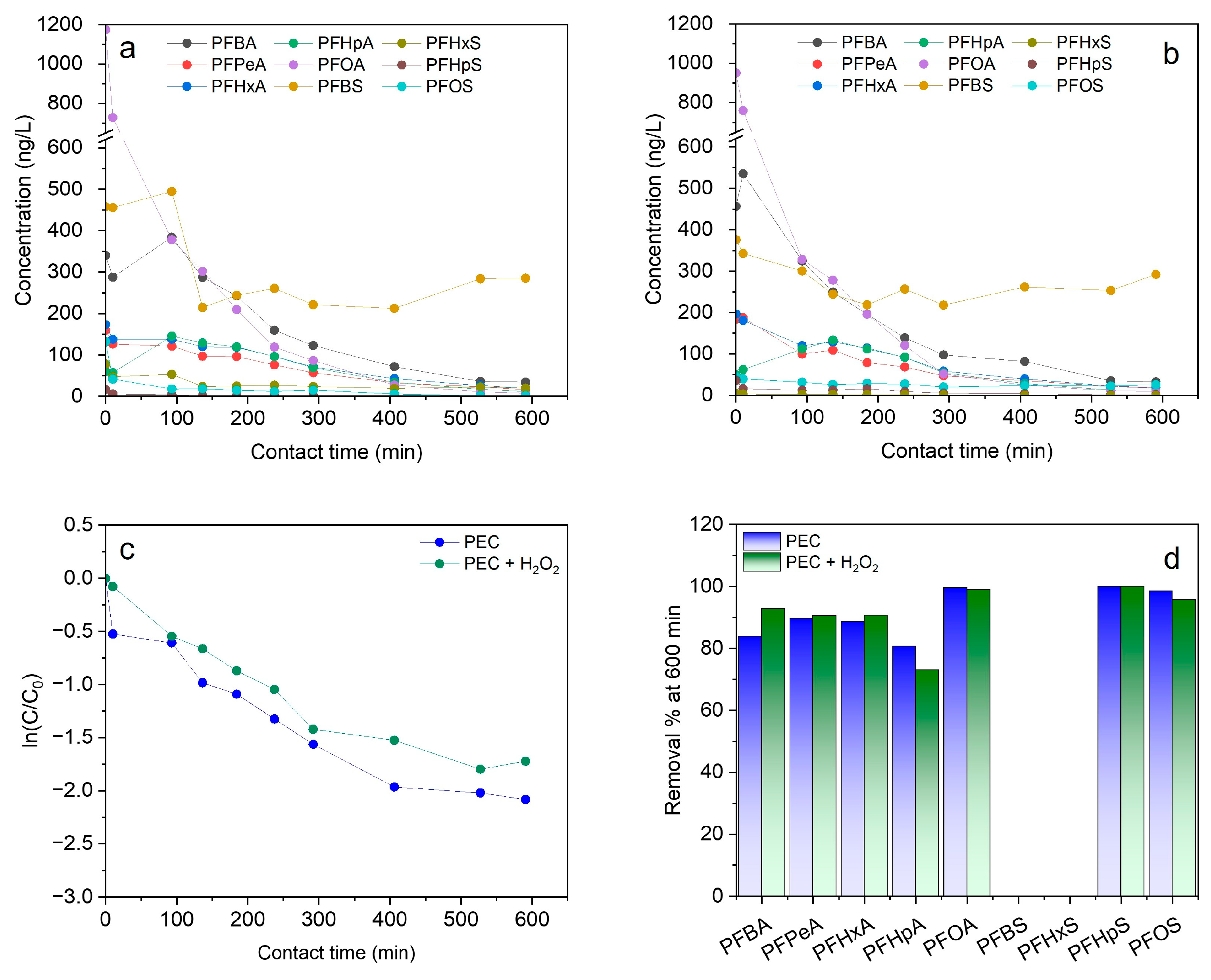
3.3. Effect of H2O2
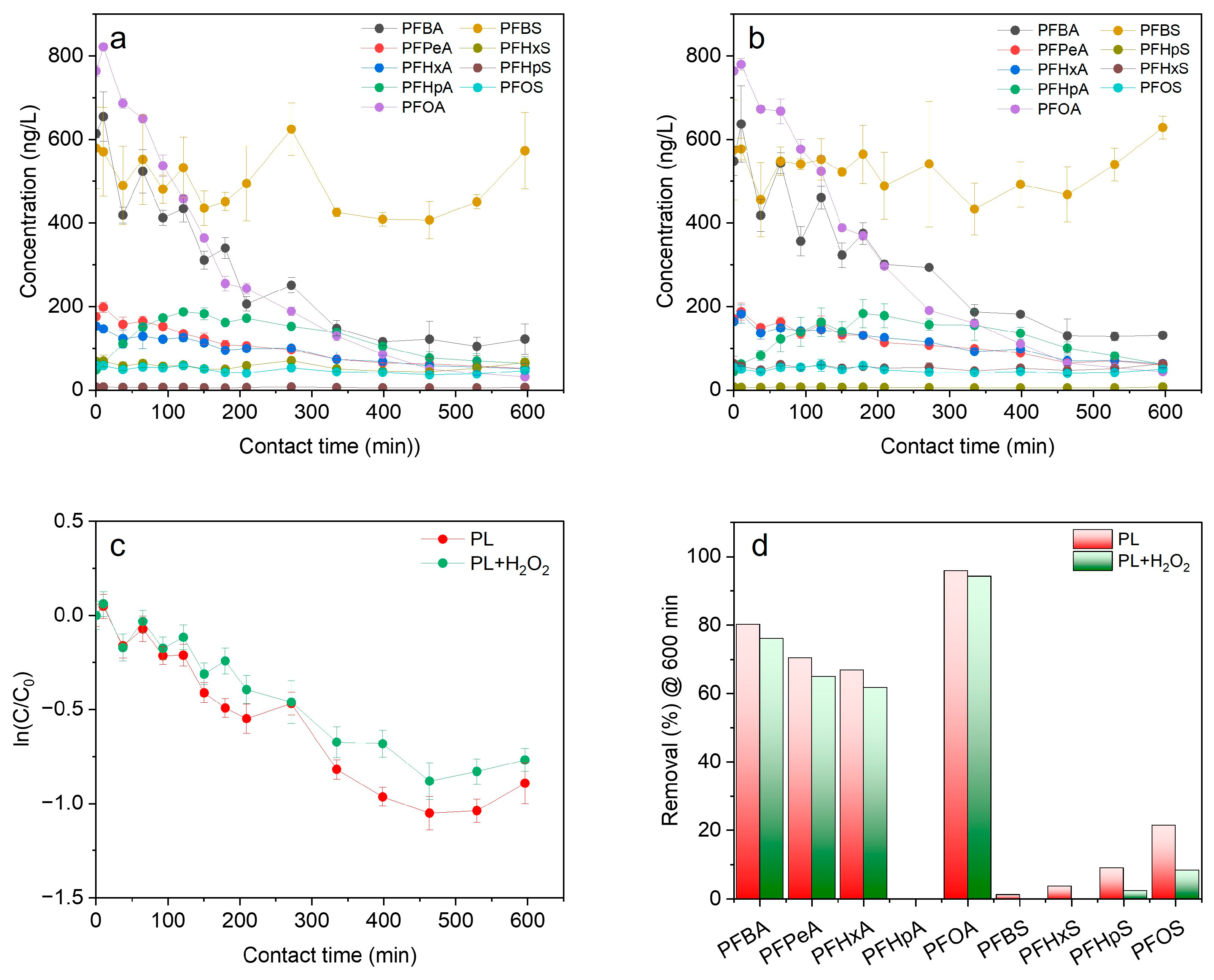
3.3.1. Photolysis
3.3.2. Photoelectrocatalysis
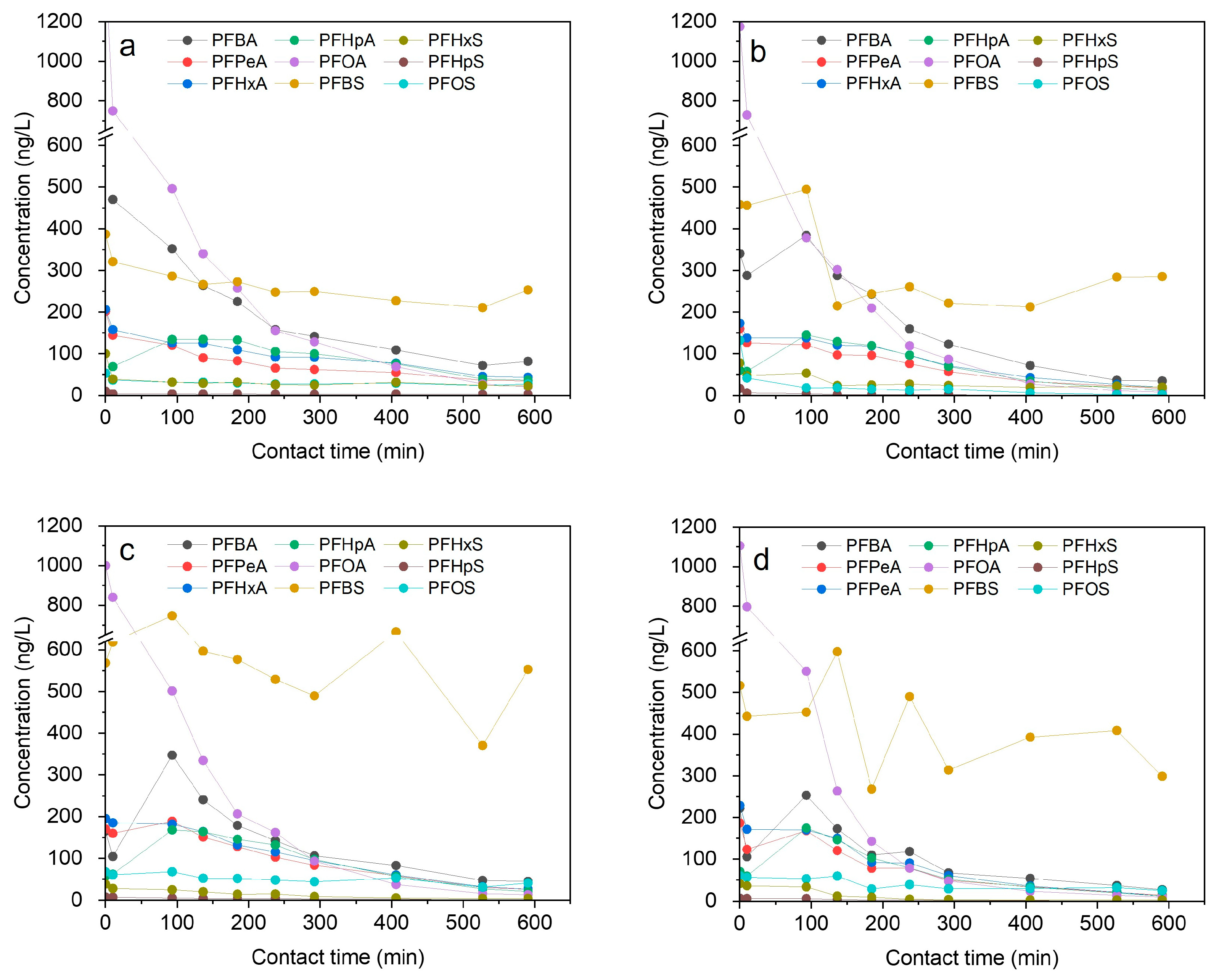
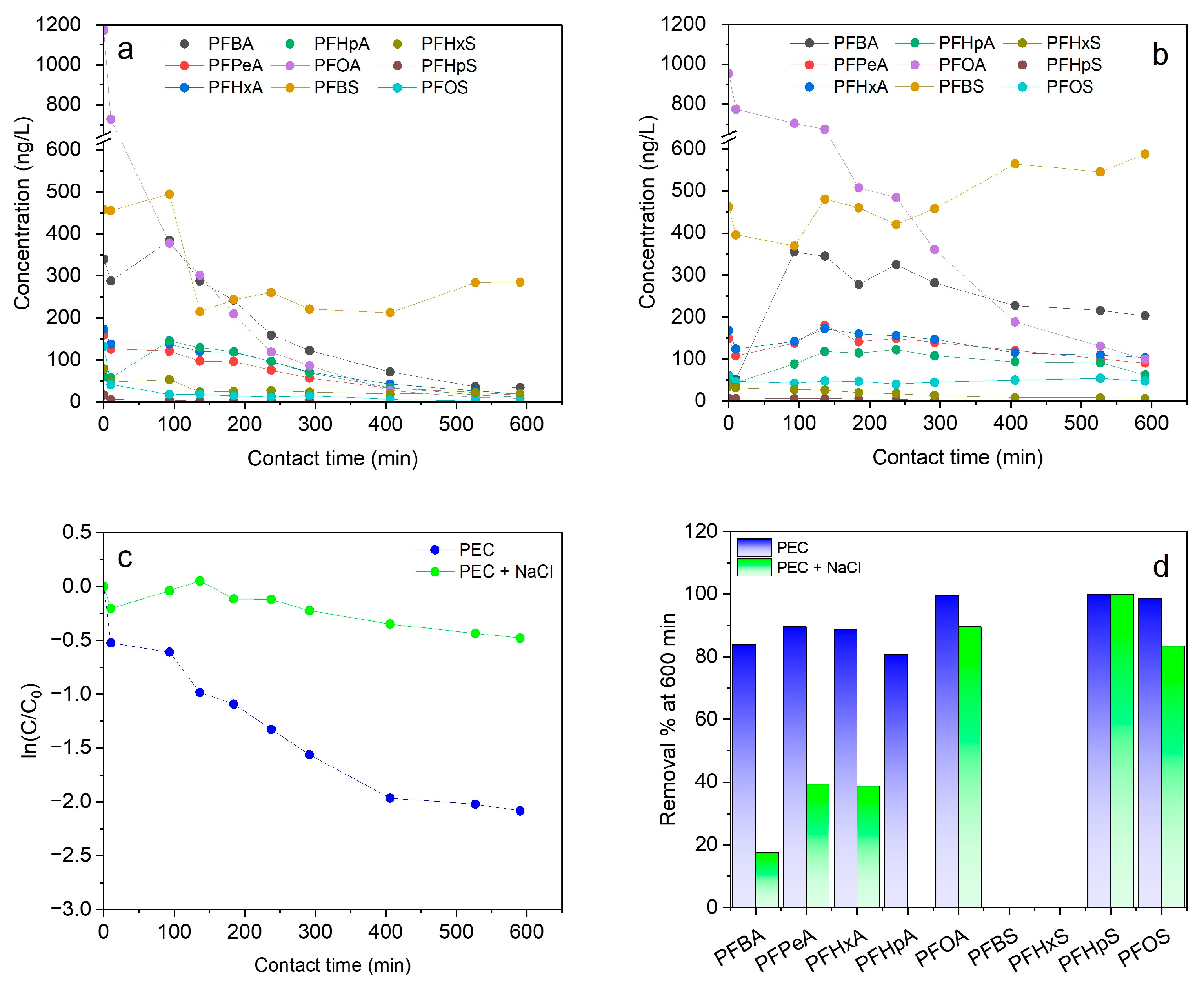
3.4. Effect of NaCl
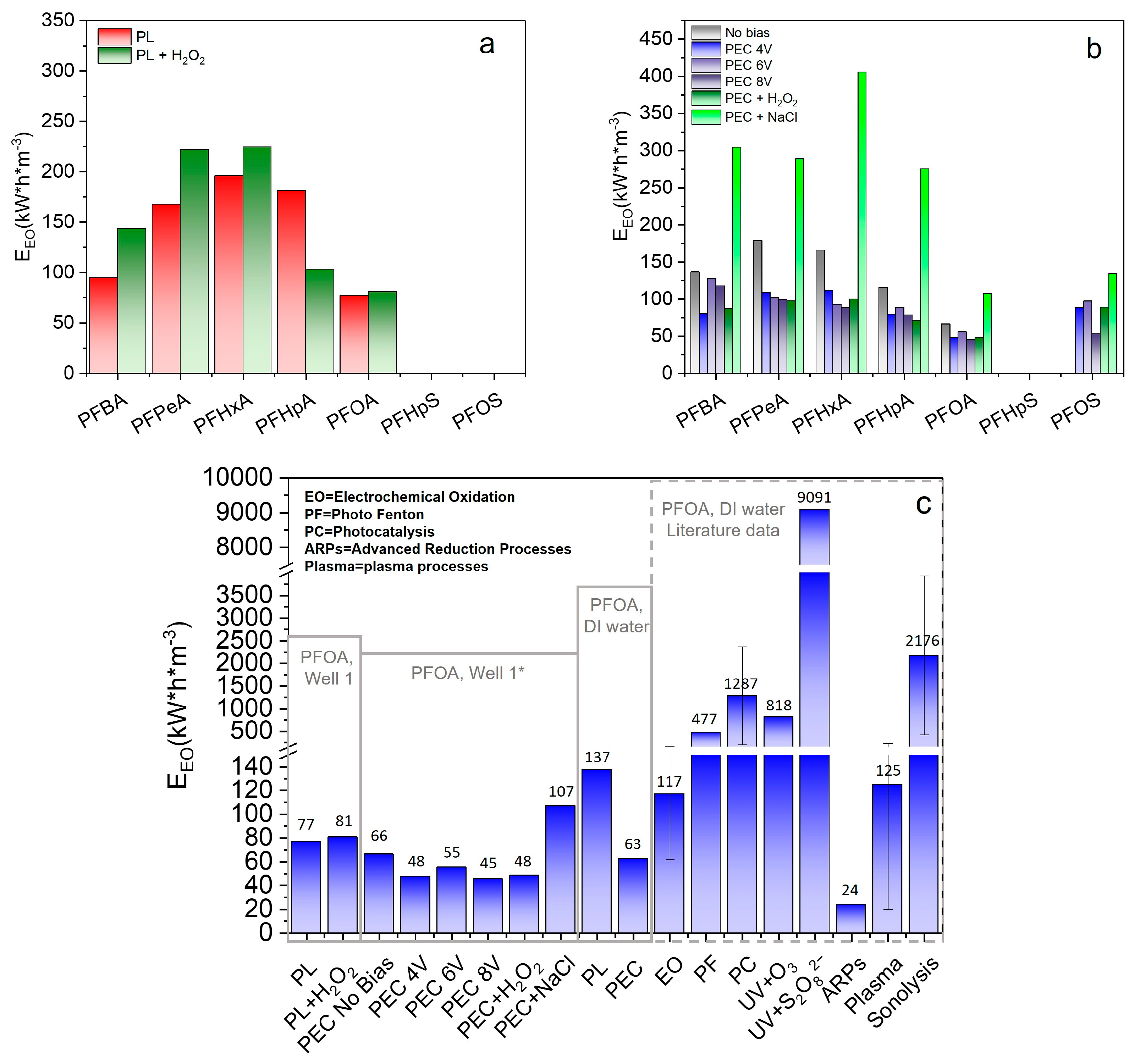
3.5. Energy Consumption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schymanski, E.L.; Zhang, J.; Thiessen, P.A.; Chirsir, P.; Kondic, T.; Bolton, E.E. Per- and Polyfluoroalkyl Substances (PFAS) in PubChem: 7 Million and Growing. Environ. Sci. Technol. 2023, 57, 16918–16928. [Google Scholar] [CrossRef]
- Bentel, M.J.; Yu, Y.; Xu, L.; Li, Z.; Wong, B.M.; Men, Y.; Liu, J. Defluorination of Per- and Polyfluoroalkyl Substances (PFASs) with Hydrated Electrons: Structural Dependence and Implications to PFAS Remediation and Management. Environ. Sci. Technol. 2019, 53, 3718–3728. [Google Scholar] [CrossRef]
- Xiao, F.; Sasi, P.C.; Yao, B.; Kubátová, A.; Golovko, S.A.; Golovko, M.Y.; Soli, D. Thermal Stability and Decomposition of Perfluoroalkyl Substances on Spent Granular Activated Carbon. Environ. Sci. Technol. Lett 2020, 7, 343–350. [Google Scholar] [CrossRef]
- Gaines, L.G.T. Historical and Current Usage of Per- and Polyfluoroalkyl Substances (PFAS): A Literature Review. Am. J. Ind. Med. 2022, 66, 353–378. [Google Scholar] [CrossRef]
- Brunn, H.; Arnold, G.; Körner, W.; Rippen, G.; Steinhäuser, K.G.; Valentin, I. PFAS: Forever Chemicals—Persistent, Bioaccumulative and Mobile. Reviewing the Status and the Need for Their Phase out and Remediation of Contaminated Sites. Environ. Sci. Eur. 2023, 35, 20. [Google Scholar] [CrossRef]
- World Health Organization; Regional Office for Europe. Keeping Our Water Clean: The Case of Water Contamination in the Veneto Region, Italy; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Perfluoroalkyls; Federal Register: Washington, DC, USA, 2021. [Google Scholar]
- Post, G.B.; Cohn, P.D.; Cooper, K.R. Perfluorooctanoic Acid (PFOA), an Emerging Drinking Water Contaminant: A Critical Review of Recent Literature. Environ. Res. 2012, 116, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Reducing PFAS in Drinking Water with Treatment Technologies|US EPA. Available online: https://www.epa.gov/sciencematters/reducing-pfas-drinking-water-treatment-technologies (accessed on 17 May 2023).
- Rahman, M.F.; Peldszus, S.; Anderson, W.B. Behaviour and Fate of Perfluoroalkyl and Polyfluoroalkyl Substances (PFASs) in Drinking Water Treatment: A Review. Water Res. 2014, 50, 318–340. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, E.; Falciglia, P.P.; Zaker, Y.; Birben, N.C.; Karanfil, T.; Roccaro, P. State of the Research on Regeneration and Reactivation Techniques for Per- and Polyfluoroalkyl Substances (PFAS)-Laden Granular Activated Carbons (GACs). Curr. Opin. Chem. Eng. 2023, 42, 100955. [Google Scholar] [CrossRef]
- Vakili, M.; Cagnetta, G.; Deng, S.; Wang, W.; Gholami, Z.; Gholami, F.; Dastyar, W.; Mojiri, A.; Blaney, L. Regeneration of Exhausted Adsorbents after PFAS Adsorption: A Critical Review. J. Hazard. Mater. 2024, 471, 134429. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Gar Alalm, M.; Boffito, D.C. Mechanisms and Pathways of PFAS Degradation by Advanced Oxidation and Reduction Processes: A Critical Review. Chem. Eng. J. 2022, 450, 138352. [Google Scholar] [CrossRef]
- James Wood, R.; Sidnell, T.; Ross, I.; McDonough, J.; Lee, J.; Bussemaker, M.J. Ultrasonic Degradation of Perfluorooctane Sulfonic Acid (PFOS) Correlated with Sonochemical and Sonoluminescence Characterisation. Ultrason. Sonochem. 2020, 68, 105196. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Arotiba, O.A.; Brillas, E. The Pathway towards Photoelectrocatalytic Water Disinfection: Review and Prospects of a Powerful Sustainable Tool. Catalysts 2021, 11, 921. [Google Scholar] [CrossRef]
- Murgolo, S.; Franz, S.; Arab, H.; Bestetti, M.; Falletta, E.; Mascolo, G. Degradation of Emerging Organic Pollutants in Wastewater Effluents by Electrochemical Photocatalysis on Nanostructured TiO2 Meshes. Water Res. 2019, 164, 114920. [Google Scholar] [CrossRef] [PubMed]
- Collivignarelli, M.C.; Abbà, A.; Carnevale Miino, M.; Arab, H.; Bestetti, M.; Franz, S. Decolorization and Biodegradability of a Real Pharmaceutical Wastewater Treated by H2O2-Assisted Photoelectrocatalysis on TiO2 Meshes. J. Hazard. Mater. 2020, 387, 121668. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, S.; Tang, Y.; Li, X.; Wang, J.; Li, L. Highly Efficient Degradation of Perfluorooctanoic Acid: An Integrated Photo-Electrocatalytic Ozonation and Mechanism Study. Chem. Eng. J. 2020, 391, 123533. [Google Scholar] [CrossRef]
- Zhou, Y.; Lee, Y.; Ren, Y.; Cui, M.; Khim, J. Quantification of Perfluorooctanoic Acid Decomposition Mechanism Applying Negative Voltage to Anode during Photoelectrochemical Process. Chemosphere 2021, 284, 131311. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, W.; Zhang, J.; Dong, L.; Chen, X.; Guan, Y.; Wang, Z.; Li, Y. Flower-like CN Layer-Doped WO3/W Photoanode as an Efficient Sun-Light Photoelectrocatalyst for PFOA Degradation and Electricity Generation. Chem. Eng. J. 2024, 480, 147910. [Google Scholar] [CrossRef]
- Thind, S.S.; Ryane, B.A.; Hayden, J.B.; Chagunda, I.; Paul, M.; Hayden, J.A.; Scott McIndoe, J. Bias Enhanced Electro-Photocatalysis on TiO2 Nanoporous Materials for Decomposition of Forever Chemicals in Saltwater. Environ. Sci. Adv. 2024, 4, 1024–1034. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Y.; Chen, X.; Li, C.; Dong, L.; Wang, Z. Wide Spectra-Responsive Polypyrrole-Ag3PO4/BiPO4 Co-Coupled TiO2 Nanotube Arrays for Intensified Photoelectrocatalysis Degradation of PFOA. Sep. Purif. Technol. 2022, 287, 120521. [Google Scholar] [CrossRef]
- Peng, Y.P.; Chen, H.; Huang, C.P. The Synergistic Effect of Photoelectrochemical (PEC) Reactions Exemplified by Concurrent Perfluorooctanoic Acid (PFOA) Degradation and Hydrogen Generation over Carbon and Nitrogen Codoped TiO2 Nanotube Arrays (C-N-TNTAs) Photoelectrode. Appl. Catal. B 2017, 209, 437–446. [Google Scholar] [CrossRef]
- Tucci, A.P.; Murgolo, S.; De Ceglie, C.; Mascolo, G.; Carmagnani, M.; Ronco, P.; Bestetti, M.; Franz, S. Photoelectrocatalytic Advanced Oxidation of Perfluoroalkyl Substances in Groundwaters of the Veneto Region, Italy. Catal. Today 2025, 450, 115205. [Google Scholar] [CrossRef]
- Verma, S.; Mezgebe, B.; Hejase, C.A.; Sahle-Demessie, E.; Nadagouda, M.N. Photodegradation and Photocatalysis of Per- and Polyfluoroalkyl Substances (PFAS): A Review of Recent Progress. Next Mater. 2024, 2, 100077. [Google Scholar] [CrossRef] [PubMed]
- Baxendale, J.H.; Wilson, J.A. The Photolysis of Hydrogen Peroxide at High Light Intensities. Trans. Faraday Soc. 1957, 53, 344–356. [Google Scholar] [CrossRef]
- da Rosa, A.P.P.; Cavalcante, R.P.; da Silva, D.A.; da Silva, L.d.M.; da Silva, T.F.; Gozzi, F.; McGlynn, E.; Brady-Boyd, A.; Casagrande, G.A.; Wender, H.; et al. H2O2-Assisted Photoelectrocatalytic Degradation of Mitoxantrone Using CuO Nanostructured Films: Identification of by-Products and Toxicity. Sci. Total Environ. 2019, 651, 2845–2856. [Google Scholar] [CrossRef]
- Chen, G. Electrochemical Technologies in Wastewater Treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, Q.; Su, Y.; Tang, Y.; Zhou, M. A Novel UV Based Advanced Oxidation Process with Electrochemical Co-Generation of Chlorine and H2O2 for Carbamazepine Abatement: Better Performance, Lower Energy Consumption and Less DBPs Formation. Chem. Eng. J. 2021, 425, 131857. [Google Scholar] [CrossRef]
- Zainal, Z.; Lee, C.Y.; Hussein, M.Z.; Kassim, A.; Yusof, N.A. Effect of Supporting Electrolytes in Electrochemically-Assisted Photodegradation of an Azo Dye. J. Photochem. Photobiol. A Chem. 2005, 172, 316–321. [Google Scholar] [CrossRef]
- Yang, J.S.; Lai, W.W.P.; Panchangam, S.C.; Lin, A.Y.C. Photoelectrochemical Degradation of Perfluorooctanoic Acid (PFOA) with GOP25/FTO Anodes: Intermediates and Reaction Pathways. J. Hazard. Mater. 2020, 391, 122247. [Google Scholar] [CrossRef]
- Franz, S.; Arab, H.; Lucotti, A.; Castiglioni, C.; Vicenzo, A.; Morini, F.; Bestetti, M. Exploiting Direct Current Plasma Electrolytic Oxidation to Boost Photoelectrocatalysis. Catalysts 2020, 10, 325. [Google Scholar] [CrossRef]
- Jing, C.; Zhang, P.-Y.; Liu, J. Photodegradation of Perfluorooctanoic Acid by 185 Nm Vacuum Ultraviolet Light. J. Environ. Sci. 2007, 19, 387–390. [Google Scholar] [CrossRef]
- Giri, R.R.; Ozaki, H.; Okada, T.; Taniguchi, S.; Takanami, R. Factors Influencing UV Photodecomposition of Perfluorooctanoic Acid in Water. Chem. Eng. J. 2012, 180, 197–203. [Google Scholar] [CrossRef]
- Park, K.; Ali, I.; Kim, J.O. Photodegradation of Perfluorooctanoic Acid by Graphene Oxide-Deposited TiO2 Nanotube Arrays in Aqueous Phase. J Environ. Manag. 2018, 218, 333–339. [Google Scholar] [CrossRef]
- Liang, R.; Li, S.; Zhang, X.; Hu, Z.; Wu, H.; Sun, J.; Song, G.; Liu, J.; Chai, Y.; Sirés, I.; et al. Highly Efficient Singlet Oxygen Production in a Cascade Photoelectrocatalytic System for Water Decontamination. Angew. Chem. Int. Ed. 2025, 2025, e202508548. [Google Scholar] [CrossRef]
- Javed, H.; Lyu, C.; Sun, R.; Zhang, D.; Alvarez, P.J.J. Discerning the Inefficacy of Hydroxyl Radicals during Perfluorooctanoic Acid Degradation. Chemosphere 2020, 247, 125883. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, Y.; Vo, P.H.N.; Shukla, P.; Ge, L.; Zhao, C.X. Electrochemical Advanced Oxidation of Per- and Polyfluoroalkyl Substances (PFASs): Development, Challenges and Perspectives. Chem. Eng. J. 2024, 500, 157222. [Google Scholar] [CrossRef]
- Shi, H.; Wang, Y.; Li, C.; Pierce, R.; Gao, S.; Huang, Q. Degraation of Perfluorooctanesulfonate by Reactive Electrochemical Membrane Compose of Magnéli Phase Titanium Suboxie. Environ. Sci. Technol. 2019, 53, 14528–14537. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, J.; Li, L.; Wang, Y.; Huang, Q. Effects of Chloride on Electrochemical Degradation of Perfluorooctanesulfonate by Magnéli Phase Ti4O7 and Boron Doped Diamond Anodes. Water Res. 2020, 170, 115254. [Google Scholar] [CrossRef]
- Nzeribe, B.N.; Crimi, M.; Mededovic Thagard, S.; Holsen, T.M. Physico-Chemical Processes for the Treatment of Per- And Polyfluoroalkyl Substances (PFAS): A Review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 866–915. [Google Scholar] [CrossRef]
- Papalexopoulou, K.; Huang, X.; Ronen, A.; Aggelopoulos, C.A. Reactive Species and Mechanisms of Perfluorooctanoic Acid (PFOA) Degradation in Water by Cold Plasma: The Role of HV Waveform, Reactor Design, Water Matrix and Plasma Gas. Sep. Purif. Technol. 2024, 342, 126955. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, J.; Wang, S.; Xu, Y.; Wang, J.; Liu, B.; Yu, C.; Yu, J. Strong Hydrophobic Affinity and Enhanced •OH Generation Boost Energy-Efficient Electrochemical Destruction of Perfluorooctanoic Acid on Robust Ceramic/PbO2-PTFE Anode. Sep. Purif. Technol. 2022, 280, 119919. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tucci, A.P.; Murgolo, S.; De Ceglie, C.; Mascolo, G.; Carmagnani, M.; Lucchini Huspek, A.; Bestetti, M.; Franz, S. Photoelectrocatalysis as an Effective Treatment for Removing Perfluoroalkyl Substances from Contaminated Groundwaters: The Real Case of the Veneto Region (Italy). Water 2025, 17, 2790. https://doi.org/10.3390/w17182790
Tucci AP, Murgolo S, De Ceglie C, Mascolo G, Carmagnani M, Lucchini Huspek A, Bestetti M, Franz S. Photoelectrocatalysis as an Effective Treatment for Removing Perfluoroalkyl Substances from Contaminated Groundwaters: The Real Case of the Veneto Region (Italy). Water. 2025; 17(18):2790. https://doi.org/10.3390/w17182790
Chicago/Turabian StyleTucci, Alessandro Pietro, Sapia Murgolo, Cristina De Ceglie, Giuseppe Mascolo, Massimo Carmagnani, Andrea Lucchini Huspek, Massimiliano Bestetti, and Silvia Franz. 2025. "Photoelectrocatalysis as an Effective Treatment for Removing Perfluoroalkyl Substances from Contaminated Groundwaters: The Real Case of the Veneto Region (Italy)" Water 17, no. 18: 2790. https://doi.org/10.3390/w17182790
APA StyleTucci, A. P., Murgolo, S., De Ceglie, C., Mascolo, G., Carmagnani, M., Lucchini Huspek, A., Bestetti, M., & Franz, S. (2025). Photoelectrocatalysis as an Effective Treatment for Removing Perfluoroalkyl Substances from Contaminated Groundwaters: The Real Case of the Veneto Region (Italy). Water, 17(18), 2790. https://doi.org/10.3390/w17182790






