Coastal Wetland Management and Restoration: Importance of Abiotic Factors and Vegetation for Healthy Fish Communities in the Laurentian Great Lakes
Abstract
1. Introduction
2. Materials and Methods
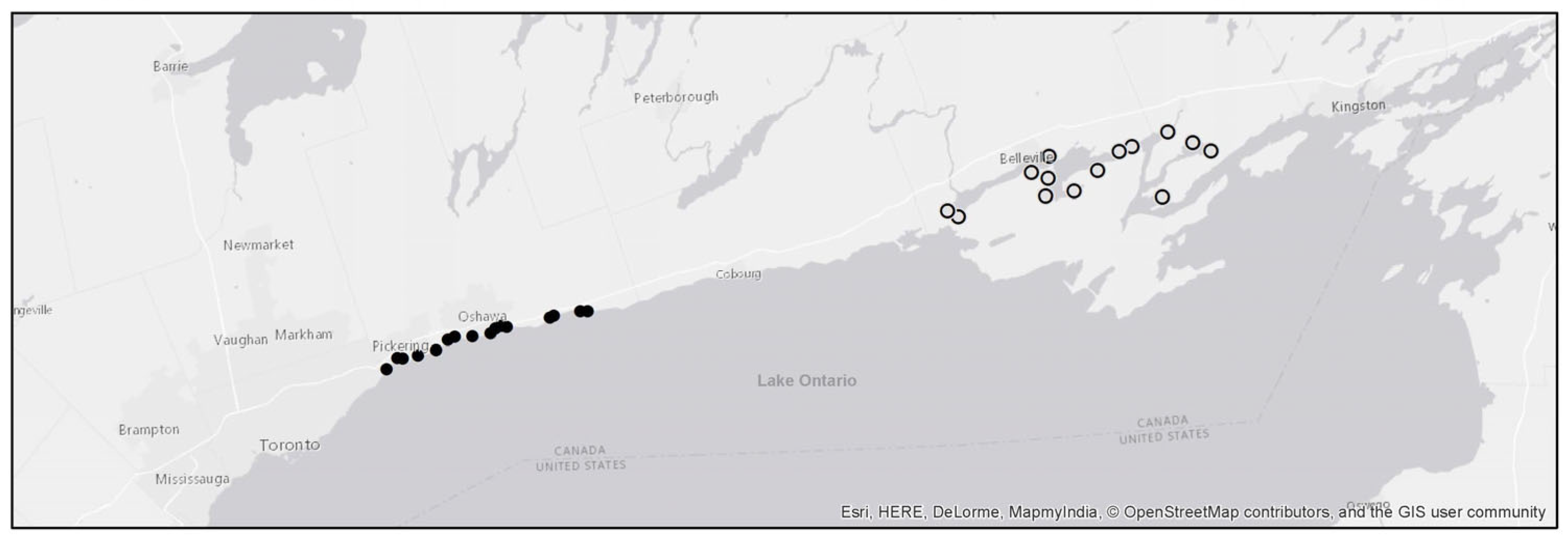
2.1. Study Area
2.2. Data Collection
2.3. Fisheries Sampling
2.4. Environmental Data
2.5. Data Analysis
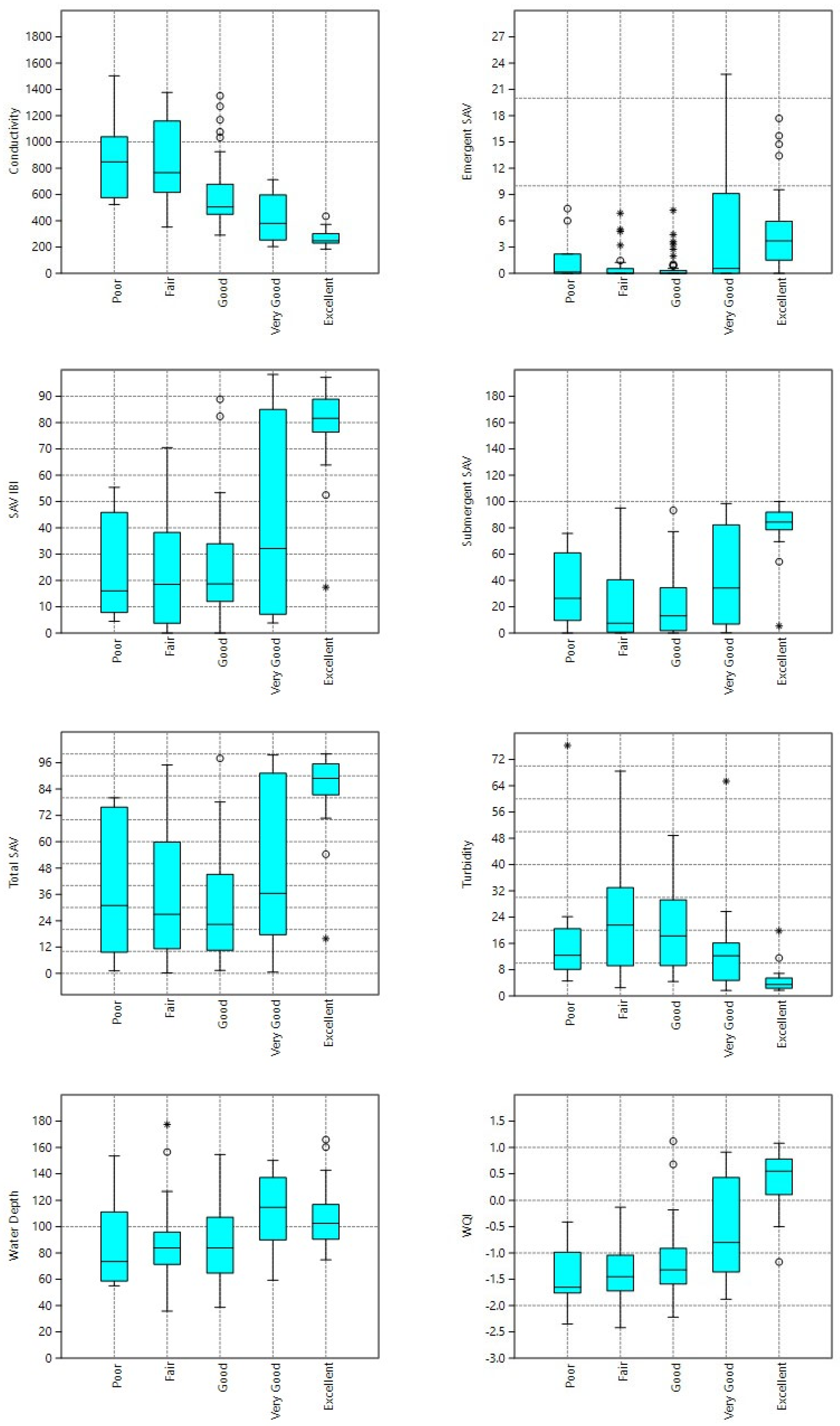
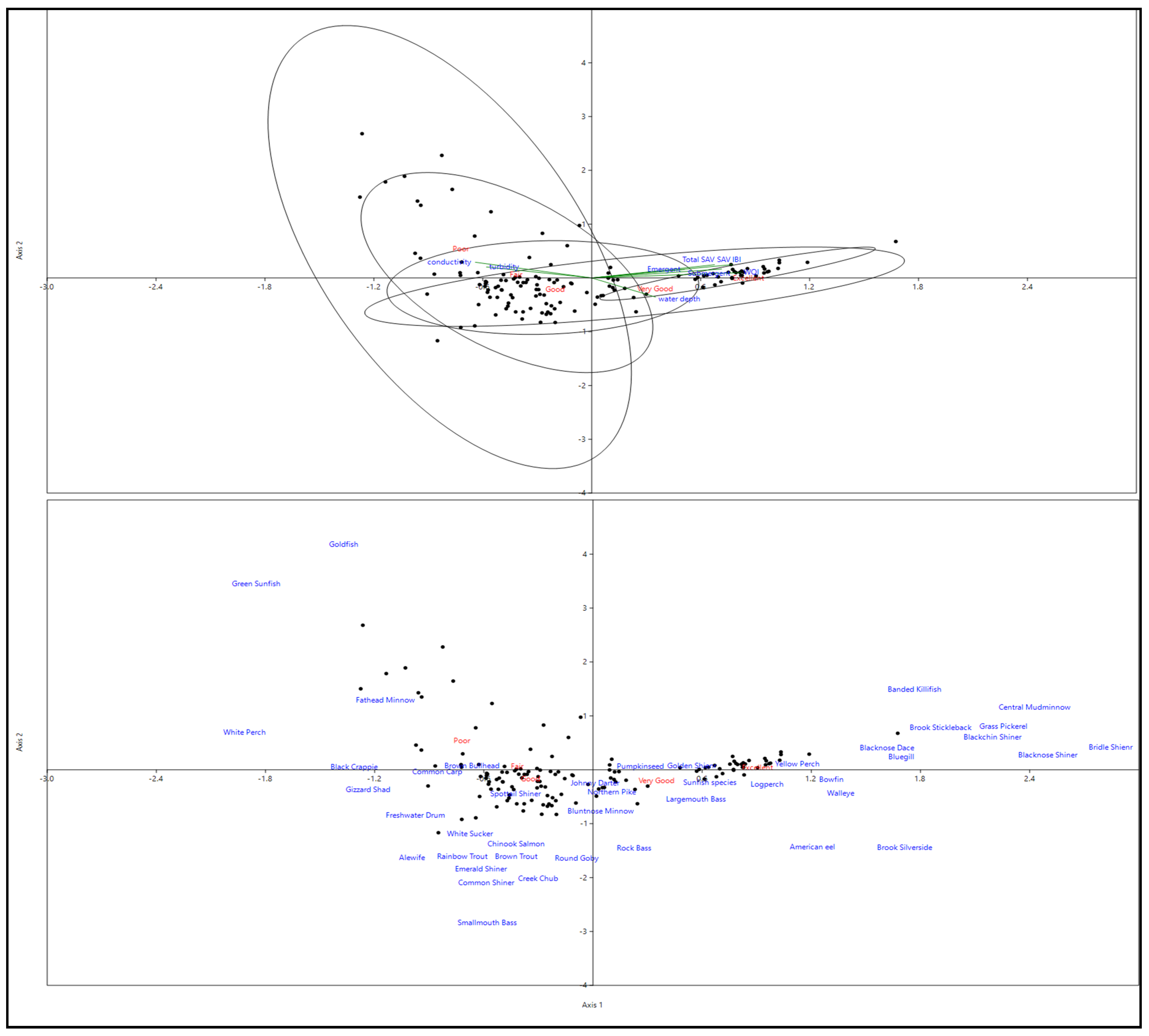
3. Results
Fish Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOSIM | Analysis of Similarities |
| ANOVA | Analysis of Variance |
| AOC | Area of Concern |
| BoQ | Bay of Quinte |
| BUI | Beneficial Use Impairment |
| CCA | Canonical Correspondence Analysis |
| DRCWMP | Durham Region Coastal Wetland Monitoring Program |
| GTA | Greater Toronto Area |
| IBI | Index of Biotic Integrity |
| Kg/ha | Kilograms per hectare |
| NTU | Nephelometric Turbidity Units |
| PAST | Paleontological Statistics Software |
| RAP | Remedial Action Plan |
| SAV | Submerged Aquatic Vegetation |
| SIMPER | Similarity Percentage Analysis |
| µS cm−1 | Microsiemens per centimeter |
| WQI | Water Quality Index |
Appendix A
| Family | Common Name | Scientific Name | Sensitive/Tolerant | Vegetation |
|---|---|---|---|---|
| Clupeidae | Alewife | Alosa pseudoharengus | Tolerant | |
| Anguillidae | American eel | Anguilla rostrata | Sensitive | |
| Fundulidae | Banded Killifish | Fundulus diaphanus | Tolerant | H |
| Centrarchidae | Black Crappie | Pomoxis nigromaculatus | Tolerant | H |
| Cyprinidae | Blackchin Shiner | Miniellus heterodon | Sensitive | H |
| Cyprinidae | Blacknose Dace | Rhinichthys atratulus | Tolerant | |
| Cyprinidae | Blacknose Shiner | Notropis heterolepis | Sensitive | H |
| Centrarchidae | Bluegill | Lepomis macrochirus | Tolerant | H |
| Cyprinidae | Bluntnose Minnow | Pimephales notatus | Tolerant | |
| Amiidae | Emerald Bowfin | Amia ocellicauda | Tolerant | H |
| Cyprinidae | Bridle Shiner | Notropis bifrenatus | Sensitive | H |
| Atherinopsidae | Brook Silverside | Labidesthes sicculus | Tolerant | M |
| Gasterosteidae | Brook Stickleback | Culaea inconstans | Tolerant | H |
| Ictaluridae | Brown Bullhead | Ameiurus nebulosus | Tolerant | M |
| Salmonidae | Brown Trout | Salmo trutta | Sensitive | |
| Umbridae | Central Mudminnow | Umbra limi | Tolerant | H |
| Salmonidae | Chinook Salmon | Oncorhynchus tshawytscha | Sensitive | |
| Salmonidae | Coho Salmon | Oncorhynchus kisutch | Sensitive | |
| Cyprinidae | Common Carp | Cyprinus carpio | Tolerant | |
| Cyprinidae | Common Shiner | Luxilus cornutus | Tolerant | |
| Cyprinidae | Creek Chub | Semotilus atromaculatus | Tolerant | H |
| Cyprinidae | Emerald Shiner | Notropis atherinoides | Tolerant | |
| Cyprinidae | Fathead Minnow | Pimephales promelas | Tolerant | |
| Sciaenidae | Freshwater Drum | Aplodinotus grunniens | Tolerant | |
| Clupeidae | Gizzard Shad | Dorosoma cepedianum | Tolerant | L |
| Cyprinidae | Golden Shiner | Notemigonus crysoleucas | Tolerant | H |
| Cyprinidae | Goldfish | Carassius auratus | Tolerant | H |
| Esocidae | Grass Pickerel | Esox americanus vermiculatus | Sensitive | H |
| Centrarchidae | Green Sunfish | Lepomis cyanellus | Tolerant | H |
| Percidae | Johnny Darter | Etheostoma nigrum | M | |
| Centrarchidae | Largemouth Bass | Micropterus nigricans | Tolerant | H |
| Percidae | Logperch | Percina caprodes | Tolerant | H |
| Cyprinidae | Longnose Dace | Rhinichthys cataractae | Tolerant | |
| Esocidae | Northern Pike | Esox lucius | Tolerant | H |
| Centrarchidae | Pumpkinseed | Lepomis gibbosus | Tolerant | H |
| Salmonidae | Rainbow Trout | Oncorhynchus mykiss | Sensitive | |
| Centrarchidae | Rock Bass | Ambloplites rupestris | Tolerant | M |
| Gobiidae | Round Goby | Neogobius melanostomus | Tolerant | |
| Catostomidae | Silver Redhorse | Moxostoma anisurum | Tolerant | |
| Centrarchidae | Smallmouth Bass | Micropterus dolomieu | Tolerant | |
| Cyprinidae | Spotfin Shiner | Cyprinella spiloptera | Tolerant | H |
| Cyprinidae | Spottail Shiner | Hudsonius hudsonius | Tolerant | H |
| Centrarchidae | Sunfish species | Lepomis sp. | ||
| Percidae | Tessellated darter | Etheostoma olmstedi | Tolerant | |
| Percidae | Walleye | Sander vitreus | Tolerant | H |
| Moronidae | White Bass | Morone chrysops | Tolerant | |
| Moronidae | White Perch | Morone americana | Tolerant | |
| Catostomidae | White Sucker | Catostomus commersonii | Tolerant | M |
| Percidae | Yellow Perch | Perca flavescens | Tolerant | H |
| Wetland Name | Region | Sampling Years Included in study | Latitude | Longitude |
|---|---|---|---|---|
| Airport Creek Marsh | Quinte | 2010, 2012, 2015 | 44.176117 | −77.096944 |
| Big Island East Marsh | Quinte | 2008, 2013 | 44.132709 | −77.191766 |
| Big Island West Marsh | Quinte | 2008, 2012, 2015 | 44.094032 | −77.258117 |
| Blessington Creek Marsh | Quinte | 2009, 2013 | 44.164071 | −77.321675 |
| Bowmanville Marsh | Durham | 2007–2015 | 43.889873 | −78.669071 |
| Carnachan Bay Marsh | Quinte | 2009, 2011, 2014 | 44.074796 | −77.021394 |
| Carruthers Creek Marsh | Durham | 2009, 2011, 2015 | 43.828947 | −78.986487 |
| Carrying Place Marsh | Quinte | 2009, 2014 | 44.054563 | −77.572057 |
| Corbett Creek Marsh | Durham | 2007, 2009–2010, 2012–2015 | 43.854242 | −78.889110 |
| Dead Creek Marsh | Quinte | 2010, 2012, 2015 | 44.067101 | −77.600465 |
| Duffins Creek Marsh | Durham | 2010, 2011, 2014–2014 | 43.820074 | −79.035843 |
| Frenchman’s Bay Marsh | Durham | 2007, 2009–2012, 2014–2015 | 43.815726 | −79.090901 |
| Hay Bay Marsh North | Quinte | 2008 | 44.177803 | −76.932037 |
| Hay Bay Marsh South | Quinte | 2008, 2014 | 44.160020 | −76.885154 |
| Hydro Marsh | Durham | 2007, 2011, 2012, 2014, 2015 | 43.814208 | −79.076567 |
| Lower Napanee Marsh | Quinte | 2010, 2011, 2014 | 44.200906 | −76.997626 |
| Lower Sucker Marsh | Quinte | 2010 | 44.167426 | −77.131410 |
| Lynde Creek Marsh | Durham | 2007–2011, 2013–2015 | 43.848623 | −78.954999 |
| McLaughlin Bay | Durham | 2008–2011, 2013–2015 | 43.870368 | −78.795776 |
| Oshawa Harbour Wetland Complex | Durham | 2009–2010, 2012–2013, 2015 | 43.868063 | −78.825765 |
| Oshawa Second Marsh | Durham | 2007–2011, 2013–2015 | 43.873219 | −78.811350 |
| Port Newcastle Marsh | Durham | 2007–2012, 2014–2015 | 43.897094 | −78.577324 |
| Pumphouse Marsh | Durham | 2011, 2013–2015 | 43.858643 | −78.840447 |
| Robinson Cove | Quinte | 2009, 2012, 2015 | ||
| Rouge River Marsh | Durham | 2007, 2009–2012, 2014–2015 | 43.793963 | −79.121589 |
| Sawguin Creek Central Marsh | Quinte | 2008, 2012, 2015 | 44.121685 | −77.326227 |
| Sawguin Creek Ditched Marsh | Quinte | 2009, 2013 | 44.086974 | −77.335135 |
| Sawguin Creek North Marsh | Quinte | 2010, 2011, 2014 | 44.133841 | −77.371001 |
| Westside Marsh | Durham | 2007–2010, 2012–2014 | 43.886768 | −78.678787 |
| Whitby Harbour Wetland Complex | Durham | 2009–2015 | 43.854437 | −78.936228 |
| Wilmot Creek Marsh | Durham | 2007–2008, 2010, 2012, 2014–2015 | 43.896958 | −78.597135 |
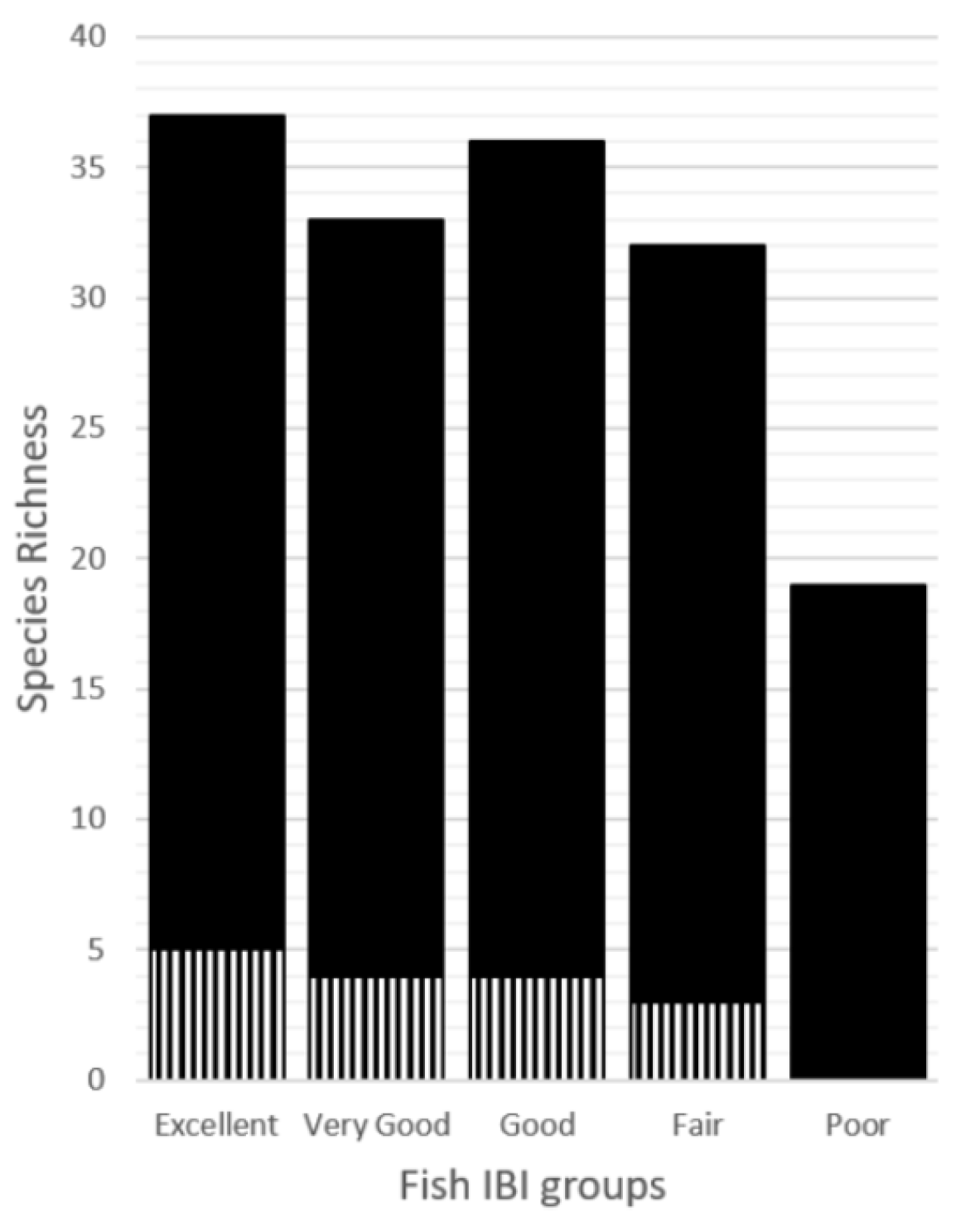
| Species List | Excellent | Very Good | Good | Fair | Poor |
|---|---|---|---|---|---|
| Alewife | X | X | X | X | X |
| American eel | X | X | X | ||
| Banded Killifish | X | X | X | X | X |
| Black Crappie | X | X | X | X | |
| Blackchin Shiner | X | X | |||
| Blacknose Dace | X | X | |||
| Blacknose Shiner | X | X | |||
| Bluegill | X | X | X | X | |
| Bluntnose Minnow | X | X | X | X | X |
| Bowfin | X | X | X | X | |
| Bridle Shiner | X | ||||
| Brook Silverside | X | X | |||
| Brook Stickleback | X | ||||
| Brown Bullhead | X | X | X | X | X |
| Brown Trout | X | ||||
| Central Mudminnow | X | X | X | X | |
| Chinook Salmon | X | X | X | ||
| Coho Salmon | X | ||||
| Common Carp | X | X | X | X | X |
| Common Shiner | X | X | X | X | X |
| Creek Chub | X | ||||
| Emerald Shiner | X | X | X | X | |
| Fathead Minnow | X | X | X | X | X |
| Freshwater Drum | X | X | X | ||
| Gizzard Shad | X | X | X | X | |
| Golden Shiner | X | X | X | X | |
| Goldfish | X | X | X | ||
| Grass Pickerel | X | ||||
| Green Sunfish | X | X | |||
| Johnny Darter | X | X | X | X | |
| Largemouth Bass | X | X | X | X | X |
| Logperch | X | X | X | X | X |
| Longnose Dace | X | ||||
| Northern Pike | X | X | X | X | |
| Pumpkinseed | X | X | X | X | X |
| Rainbow Trout | X | X | |||
| Rock Bass | X | X | X | X | X |
| Round Goby | X | X | X | X | X |
| Silver Redhorse | X | ||||
| Smallmouth Bass | X | X | X | ||
| Spotfin Shiner | X | ||||
| Spottail Shiner | X | X | X | X | |
| Tessellated darter | X | ||||
| Walleye | X | X | X | ||
| White Bass | X | ||||
| White Perch | X | X | X | ||
| White Sucker | X | X | X | X | X |
| Yellow Perch | X | X | X | X | X |
| Total | 37 | 33 | 36 | 32 | 19 |
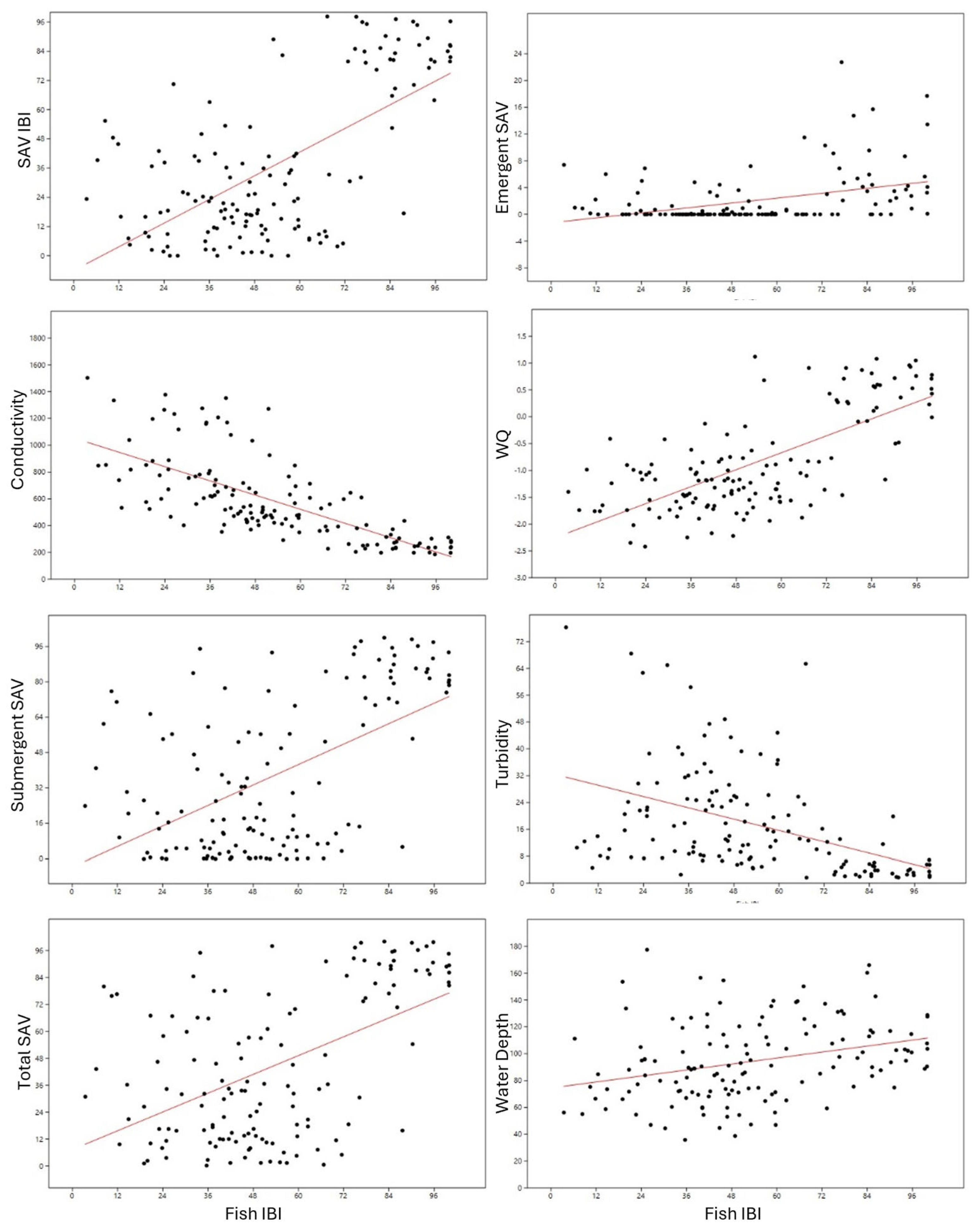
| Variable | Slope | Error | Intercept | Error | r | p |
|---|---|---|---|---|---|---|
| SAV IBI | 0.81027 | 0.083844 | −6.0457 | 4.9226 | 0.63807 | 3.86 × 10−17 |
| WQI | 0.026321 | 0.0022732 | −2.2502 | 0.13346 | 0.70458 | 5.29 × 10−22 |
| air temp | −0.0060553 | 0.010862 | 23.723 | 0.63771 | −0.047749 | 0.57811 |
| water temp | −0.0030268 | 0.010663 | 22.434 | 0.62606 | −0.024332 | 0.77696 |
| pH | 0.0014812 | 0.0011919 | 8.0303 | 0.069977 | 0.10596 | 0.21611 |
| conductivity | −8.839 | 0.7504 | 1052 | 44.057 | −0.71063 | 1.63 × 10−22 |
| DO | −0.017508 | 0.0093294 | 10.542 | 0.54774 | −0.15888 | 0.062707 |
| water depth | 0.37116 | 0.097458 | 74.499 | 5.7219 | 0.31044 | 0.00021087 |
| turbidity | −0.28075 | 0.048979 | 32.486 | 2.8756 | −0.44111 | 6.13 × 108 |
| Total SAV | 0.69776 | 0.098559 | 7.3543 | 5.7865 | 0.51894 | 7.01 × 10−11 |
| Emergent | 0.061331 | 0.012358 | −1.2546 | 0.72554 | 0.39159 | 2.04 × 10−6 |
| Submergent | 0.77046 | 0.10092 | −3.605 | 5.9254 | 0.5477 | 3.60 × 10−12 |
| Floating | −0.0019787 | 0.044218 | 10.632 | 2.5961 | −0.0038372 | 0.96437 |
| Tukey’s Pairwise | |||||
|---|---|---|---|---|---|
| SAV IBI | Excellent | Fair | Good | Poor | Very Good |
| Excellent | 1.72 × 10−5 | 1.72 × 10−5 | 1.72 × 10−5 | 2.44 × 10−5 | |
| Fair | 11.87 | 0.9996 | 0.9937 | 0.004927 | |
| Good | 11.59 | 0.284 | 0.9995 | 0.009873 | |
| Poor | 11.28 | 0.59 | 0.306 | 0.01988 | |
| Very Good | 6.982 | 4.893 | 4.609 | 4.303 | |
| WQI | Excellent | Fair | Good | Poor | Very Good |
| Excellent | 1.72 × 10−5 | 1.72 × 10−5 | 1.72 × 10−5 | 4.54 × 10−5 | |
| Fair | 13 | 0.9512 | 0.9861 | 7.53 × 10−5 | |
| Good | 11.97 | 1.023 | 0.7303 | 0.001386 | |
| Poor | 13.72 | 0.7253 | 1.748 | 2.15 × 10−5 | |
| Very Good | 6.603 | 6.393 | 5.37 | 7.118 | |
| Conductivity | Excellent | Fair | Good | Poor | Very Good |
| Excellent | 1.72 × 10−5 | 3.39 × 10−5 | 1.72 × 10−5 | 0.1579 | |
| Fair | 11.41 | 0.008695 | 0.9403 | 1.73 × 10−5 | |
| Good | 6.75 | 4.662 | 0.0004771 | 0.08784 | |
| Poor | 12.5 | 1.083 | 5.745 | 1.72 × 10−5 | |
| Very Good | 3.197 | 8.215 | 3.553 | 9.298 | |
| Water Depth | Excellent | Fair | Good | Poor | Very Good |
| Excellent | 0.1383 | 0.1104 | 0.06333 | 0.99 | |
| Fair | 3.282 | 1 | 0.9977 | 0.04201 | |
| Good | 3.42 | 0.1382 | 0.9995 | 0.03171 | |
| Poor | 3.734 | 0.4519 | 0.3136 | 0.01607 | |
| Very Good | 0.6645 | 3.946 | 4.085 | 4.398 | |
| Turbidity | Excellent | Fair | Good | Poor | Very Good |
| Excellent | 3.35 × 10−5 | 0.001237 | 0.009865 | 0.1915 | |
| Fair | 6.757 | 0.8765 | 0.55 | 0.06868 | |
| Good | 5.411 | 1.346 | 0.9798 | 0.4606 | |
| Poor | 4.609 | 2.148 | 0.8023 | 0.8118 | |
| Very Good | 3.067 | 3.69 | 2.344 | 1.542 | |
| Total SAV | Excellent | Fair | Good | Poor | Very Good |
| Excellent | 1.72 × 10−5 | 1.72 × 10−5 | 1.72 × 10−5 | 7.87 × 10−5 | |
| Fair | 8.988 | 0.9408 | 0.9992 | 0.3464 | |
| Good | 10.07 | 1.081 | 0.851 | 0.06826 | |
| Poor | 8.641 | 0.347 | 1.428 | 0.4962 | |
| Very Good | 6.376 | 2.612 | 3.693 | 2.265 | |
| Emergent | Excellent | Fair | Good | Poor | Very Good |
| Excellent | 0.0003546 | 0.0001727 | 0.008058 | 0.7195 | |
| Fair | 5.847 | 0.9998 | 0.9259 | 0.03242 | |
| Good | 6.094 | 0.2467 | 0.8599 | 0.01911 | |
| Poor | 4.693 | 1.154 | 1.4 | 0.2355 | |
| Very Good | 1.773 | 4.074 | 4.321 | 2.92 | |
| Submergent | Excellent | Fair | Good | Poor | Very Good |
| Excellent | 1.72 × 10−5 | 1.72 × 10−5 | 1.73 × 10−5 | 3.90 × 10−5 | |
| Fair | 10.48 | 1 | 0.6445 | 0.05587 | |
| Good | 10.42 | 0.05162 | 0.6677 | 0.0616 | |
| Poor | 8.533 | 1.943 | 1.891 | 0.6831 | |
| Very Good | 6.676 | 3.8 | 3.748 | 1.857 | |
References
- Federal, Provincial and Territorial Governments of Canada. Canadian Biodiversity: Ecosystem Status and Trends 2010; Canadian Councils of Resource Ministers: Ottawa, ON, Canada, 2010; pp. vi + 142.
- Sierszen, M.E.; Morrice, J.A.; Trebitz, A.S.; Hoffman, J.C. A review of selected ecosystem services provided by coastal wetlands of the Laurentian Great Lakes. Aquat. Ecosyst. Health Manag. 2012, 15, 92–106. [Google Scholar] [CrossRef]
- Zedler, J.B.; Kercher, S. WETLAND RESOURCES: Status, Trends, Ecosystem Services, and Restorability. Annu. Rev. Environ. Resour. 2005, 30, 39–74. [Google Scholar] [CrossRef]
- Diller, S.N.; Harrison, A.M.; Kowalski, K.P.; Brady, V.J.; Ciborowski, J.J.H.; Cooper, M.J.; Dumke, J.D.; Gathman, J.P.; Ruetz, C.R., III; Uzarski, D.G.; et al. Influences of seasonability and habitat quality on Great Lakes coastal wetland fish community composition and diets. Wet. Ecol. Man. 2022, 30, 439–460. [Google Scholar] [CrossRef]
- Jude, D.J.; Pappas, J. Fish Utilization of Great Lakes Coastal Wetlands. J. Great Lakes Res. 1992, 18, 651–672. [Google Scholar] [CrossRef]
- COSEWIC. COSEWIC Assessment and Status Report on the Pugnose shiner Notropis anogenus in Canada; Committee on the Status of Endangered Wildlife in Canada: Ottawa, ON, Canada, 2013; pp. x + 32. Available online: https://www.canada.ca/en/environment-climate-change/services/species-risk-public-registry/cosewic-assessments-status-reports/pugnose-shiner-2013.html (accessed on 10 February 2025).
- COSEWIC. COSEWIC Status Appraisal Summary on the Grass Pickerel Esox americanus vermiculatus in Canada; Committee on the Status of Endangered Wildlife in Canada: Ottawa, ON, Canada, 2014; p. xix. Available online: https://www.canada.ca/en/environment-climate-change/services/species-risk-public-registry/report-progress-recovery-document/grass-pickerel-2024.html (accessed on 10 February 2025).
- Holm, E.; Mandrak, N.E.; Burridge, B. The Royal Ontario Museum Field Guide to Freshwater Fishes of Ontario; Royal Ontario Museum: Toronto, ON, Canada, 2009. [Google Scholar]
- Trebitz, A.S.; Hoffman, J.C. Coastal Wetland Support of Great Lakes Fisheries: Progress from Concept to Quantification. Trans. Am. Fish. Soc. 2015, 144, 352–372. [Google Scholar] [CrossRef]
- Environment Canada. Canadian Federal Government Policy on Wetland Conservation; Environment Canada: Gatineau, QC, Canada, 1991; p. 15.
- Rasmar. The Rasmar Convention on Wetlands. 2015. Available online: www.rasmar.org (accessed on 10 August 2025).
- Hopfensperger, K.N.; Engelhardt, K.A.M.; Seagle, S.W. The Use of Case Studies in Establishing Feasibility for Wetland Restoration. Restor. Ecol. 2006, 14, 578–586. [Google Scholar] [CrossRef]
- Cui, B.; Yang, Q.; Yang, Z.; Zhang, K. Evaluating the ecological performance of wetland restoration in the Yellow River Delta, China. Ecol. Eng. 2009, 35, 1090–1103. [Google Scholar] [CrossRef]
- Marshall, E.E.M.; Larocque, S.M.; Reddick, D.T.; Midwood, J.D.; Doka, S.E. Temperature, Dissolved Oxygen, Fish, Vegetation, and Substrate Surveys in Lake Ontario Coastal Wetlands; Canadian Technical Report of Fisheries and Aquatic Sciences; Department of Fisheries and Oceans Canada: Ottawa, ON, Canada, 2021; pp. vii + 50.
- Millennium Ecosystem Assessment. Ecosystems Human Well-Being: Wetlands and Water–Synthesis; Millennium Eco-System Assessment Series; World Resources Institute: Washington, DC, USA, 2005; p. 80. [Google Scholar]
- Penfound, E.; Vaz, E. Analysis of 200 years of change in Ontario wetland systems. Appl. Geogr. 2022, 138, 102625. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Gosselink, J.G. Wetlands, 4th ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Whillans, T.H. Changes in Marsh Area Along the Canadian Shore of Lake Ontario. J. Great Lakes Res. 1982, 8, 570–577. [Google Scholar] [CrossRef]
- Brazner, J.; Beals, E. Patterns in fish assemblages from coastal wetland and beach habitats in Green Bay, Lake Michigan: A multivariate analysis of abiotic and biotic forcing factors. Can. J. Fish. Aquat. Sci. 1997, 54, 1743–1761. [Google Scholar] [CrossRef]
- Chow-Fraser, P. Development of the Wetland Water Quality Index (WQI) to assess effects of basin-wide land-use alteration on coastal marshes of the Laurentian Great Lakes. In Coastal Wetlands of the Laurentian Great Lakes: Health, Habitat, and Indicators; Simon, T.P., Stewart, P.M., Eds.; Indiana Biological Survey: Bloomington, IN, USA, 2006; pp. 137–166. [Google Scholar]
- Erwin, K.L. Wetlands and global climate change: The role of wetland restoration in a changing world. Wetl. Ecol. Manag. 2009, 17, 71–84. [Google Scholar] [CrossRef]
- Mortsch, L.D. Assessing the Impact of Climate Change on the Great Lakes Shoreline Wetlands. Clim. Change 1998, 40, 391–416. [Google Scholar] [CrossRef]
- Ontario Biodiversity Council (OBC). State of Ontario’s Biodiversity 2010. A Report of the Ontario Biodiversity Council, Peterborough, ON. 2010. Available online: https://ontariobiodiversitycouncil.ca/ (accessed on 13 December 2024).
- Wilcox, D.A.; Whillans, T.H. Techniques for restoration of disturbed coastal wetlands of the Great Lakes. Wetlands 1999, 19, 835–857. [Google Scholar] [CrossRef]
- Smith, P.G.R.; Glooschenko, V.; Hagen, D.A. Coastal wetland of three Canadian Great Lakes: Inventory, current conservation initiatives, and patterns of variation. Can. J. Fish. Aquat. Sci. 1991, 48, 1581–1594. [Google Scholar] [CrossRef]
- Coker, G.A.; Portt, C.B.; Minns, C.K. Morphological and Ecological Characteristics of Canadian Freshwater Fishes; Fisheries and Oceans Canada: Burlington, ON, Canada, 2001; Volume 2554.
- Tanner, D.K.; Brazner, J.C.; Brady, V.J.; Regal, R.R. Habitat Associations of Larval Fish in a Lake Superior Coastal Wetland. J. Great Lakes Res. 2004, 30, 349–359. [Google Scholar] [CrossRef]
- Brazner, J.; Danz, N.P.; Niemi, G.J.; Regal, R.R.; Trebitz, A.S.; Howe, R.W.; Hanowski, J.M.; Johnson, J.B.; Ciborowski, J.J.H.; Johnston, C.A.; et al. Evaluation of grographic, geomorphic and human influences on Great Lakes wetland indicators: A multi-assemblage approach. Ecol. Indic. 2007, 7, 610–635. [Google Scholar] [CrossRef]
- Lougheed, V.L.; Chow-Fraser, P. Spatial variability in the response of lower trophic levels after carp exclusion from a freshwater marsh. J. Aquat. Ecosyst. Stress Recover. 2001, 9, 21–34. [Google Scholar] [CrossRef]
- Trebitz, A.S.; Brazner, J.C.; Danz, N.P.; Pearson, M.S.; Peterson, G.S.; Tanner, D.K.; Taylor, D.L.; West, C.W.; Hollenhorst, T.P. Geographic, anthropogenic, and habitat influences on Great Lakes coastal wetland fish assemblages. Can. J. Fish. Aquat. Sci. 2009, 66, 1328–1342. [Google Scholar] [CrossRef]
- Uzarski, D.G.; Burton, T.M.; Cooper, M.J.; Ingram, J.W.; Timmermans, S.T. Fish Habitat Use Within and Across Wetland Classes in Coastal Wetlands of the Five Great Lakes: Development of a Fish-Based Index of Biotic Integrity. J. Great Lakes Res. 2005, 31, 171–187. [Google Scholar] [CrossRef]
- Karr, J.R. Assessment of Biotic Integrity Using Fish Communities. Fisheries 1981, 6, 21–27. [Google Scholar] [CrossRef]
- Wichert, G.A. Effects of Improved Sewage Effluent Management and Urbanization on Fish Associations of Toronto Streams. North Am. J. Fish. Manag. 1995, 15, 440–456. [Google Scholar] [CrossRef]
- Karr, J.R. Ecological integrity and ecological health are not the same. In Engineering Within Eco-Logical Constraints; Schulze, P., Ed.; National Academy Press: Washington, DC, USA, 1996; pp. 97–109. [Google Scholar]
- Environment Canada and Central Lake Ontario Conservation Authority. Durham Region Coastal Wetland Monitoring Project: Year 2; Technical Report; ECB-OR; Government of Canada Publications: Downsview, ON, Canada, 2004; p. 266.
- Brazner, J.C. Regional, Habitat, and Human Development Influences on Coastal Wetland and Beach Fish Assemblages in Green Bay, Lake Michigan. J. Great Lakes Res. 1997, 23, 36–51. [Google Scholar] [CrossRef]
- Langer, T.A.; Cooper, M.J.; Reisinger, L.S.; Reisinger, A.J.; Uzarski, D.G. Water depth and lake-wide water level fluctuation influence on α- and β-diversity of coastal wetland fish communities. J. Great Lakes Res. 2018, 44, 70–76. [Google Scholar] [CrossRef]
- Rook, N. Recolonization Trends of Fish Communities Following the Restoration of a Great Lakes Coastal Wetland. Master’s Thesis, University of Toronto, Toronto, ON, Canada, 2016. [Google Scholar]
- Trebitz, A.S.; Brazner, J.C.; Brady, V.J.; Axler, R.; Tanner, D.K. Turbidity Tolerances of Great Lakes Coastal Wetland Fishes. North Am. J. Fish. Manag. 2007, 27, 619–633. [Google Scholar] [CrossRef]
- Bowman, M.F.; Somers, K.M. Considerations when Using the Reference Condition Approach for Bioassessment of Freshwater Ecosystems. Water Qual. Res. J. Canada 2005, 40, 347–360. [Google Scholar] [CrossRef]
- Cooper, M.J.; Lamberti, G.A.; Moerke, A.H.; Ruetz, C.R.; Wilcox, D.A.; Brady, V.J.; Brown, T.N.; Ciborowski, J.J.H.; Gathman, J.P.; Grabas, G.P.; et al. An expanded fish-based index of biotic integrity for Great Lakes coastal wetlands. Environ. Monit. Assess. 2018, 190, 580. [Google Scholar] [CrossRef]
- Moore, D.M.; Mandrak, N.E. Evaluating the effect of Common Carp control on restoration of a coastal wetland in the Laurentian Greats Lakes. Water 2024, 16, 1929. [Google Scholar] [CrossRef]
- Zhang, Y.; Leung, J.Y.S.; Zhang, Y.; Cau, Y.; Zhang, Z.; Li, K. Agricultural activities compromise ecosystem health and functioning of rivers: Insights from multivariate and multimetric analysis of macroinvertebrate assemblages. Environ. Pol. 2021, 275, 116655. [Google Scholar] [CrossRef]
- Environment Canada and Central Lake Ontario Conservation Authority. Durham Region Coastal Wetland Monitoring Project: Methodology Handbook; March 2007–2008; Central Lake Ontario Conservation Authority: Oshawa, ON, Canada, 2007; p. 211. [Google Scholar]
- Bay of Quinte RAP Coordinating Committee. The Bay of Quinte Remedial Action Plan: Time to Act; Stage 2 Report; Bay of Quinte RAP Coordinating Committee: Trenton, ON, Canada, 1993; p. 271. [Google Scholar]
- Bailey, R.C.; Grapentine, L.; Stewart, T.J.; Schaner, T.; Chase, M.E.; Mitchell, J.S.; Coulas, R.A. Dreissenidae in Lake Ontario: Impact Assessment at the Whole Lake and Bay of Quinte Spatial Scales. J. Great Lakes Res. 1999, 25, 482–491. [Google Scholar] [CrossRef]
- Miehls, A.L.J.; Mason, D.M.; Frank, K.A.; Krause, A.E.; Peacor, S.D.; Taylor, W.W. Invasive species impacts on ecosystem structure and function: A comparison of Oneida Lake, New York, USA, before and after zebra mussel invasion. Ecol. Model. 2009, 220, 3182–3193. [Google Scholar] [CrossRef]
- Minns, C.K.; Munawar, M.; Koops, M.A.; Millard, E.S. Project Quinte 1972–2008 and Beyond: Long-term ecosystem studies in the Bay of Quinte, Lake Ontario: A prospectus. Aquat. Ecosyst. Health Manag. 2022, 14, 3–8. [Google Scholar] [CrossRef]
- Grabas, G.P.; Blukacz-Richards, E.A.; Pernanen, S. Development of a submerged aquatic vegetation community index of biotic integrity for use in Lake Ontario coastal wetlands. J. Great Lakes Res. 2012, 38, 243–250. [Google Scholar] [CrossRef]
- Mandrak, N.E.; Bouvier, L.D. Standardized Data Collection Methods in Support of a Classification Protocol for the Designation of Watercourses as Municipal Drains; Canadian Scientific Advisory Secretariat Research Document 2013/077; Fisheries and Oceans Canada: Ottawa, ON, Canada, 2014; pp. v + 26.
- Portt, C.B.; Coker, G.; Minns, C.K. Riverine Habitat Characteristics of Fishes of the Great Lakes Watershed; Canadian Manuscript Report of Fisheries and Aquatic Sciences, 2481; Fisheries and Oceans Canada: Ottawa, ON, Canada, 1999; pp. vi + 62.
- Judd, C.M.; McClelland, G.H. Data Analysis: A Model-Comparison Approach; Harcourt Brace Jovanovich: San Diego, CA, USA, 1989. [Google Scholar]
- Barnett, V.; Lewis, T. Outliers in Statistical Data, 3rd ed.; Elsevier Science: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1999. [Google Scholar]
- Leps, J.; Smilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; The Press Syndicate of the University of Cambridge: Cambridge, UK, 2003. [Google Scholar]
- ter Braak, C.J.F.; Smilauer, P. CANOCO Reference Manual and User’s Guide to CANOCO for Windows. Software for Canonical Community Ordination; Version 4; Centre for Biometry: Wageningen, The Netherlands; Microcomputer Power: Ithaca, NY, USA, 1998. [Google Scholar]
- Palmer, M.W. Putting things in even better order: The advantages of canonical correspondence analysis. Ecology 1993, 74, 2215–2230. [Google Scholar] [CrossRef]
- Sherry, A.; Henson, R.K. Conducting and Interpreting Canonical Correlation Analysis in Personality Research: A User-Friendly Primer. J. Pers. Assess. 2005, 84, 37–48. [Google Scholar] [CrossRef]
- Clarke, K.; Warwick, R. A further biodiversity index applicable to species lists: Variation in taxonomic distinctness. Mar. Ecol. Prog. Ser. 2001, 216, 265–278. [Google Scholar] [CrossRef]
- Trebitz, A.S.; Brazner, J.C.; Pearson, M.S.; Peterson, G.S.; Tanner, D.K.; Taylor, D.L. Patterns in habitat and fish assemblages within Great Lakes coastal wetlands and implications for sampling design. Can. J. Fish. Aquat. Sci. 2009, 66, 1343–1354. [Google Scholar] [CrossRef]
- Minns, C.K.; Cairns, V.W.; Randall, R.G.; Moore, J.E. An Index of Biotic Integrity (IBI) for fish assemblages in the littoral zone of Great Lakes’ Areas of Concern. Can. J. Fish. Aquat. Sci. 1994, 51, 1804–1822. [Google Scholar] [CrossRef]
- Seilheimer, T.S.; Chow-Fraser, P. Development and use of the Wetland Fish Index to assess the quality of coastal wetlands in the Laurentian Great Lakes. Can. J. Fish. Aquat. Sci. 2006, 63, 354–366. [Google Scholar] [CrossRef]
- Peterson, G.S.; Hoffman, J.C.; Trebitz, A.S.; West, C.W.; Kelly, J.R. Establishment patterns of non-native fishes: Lessons from the Duluth-Superior harbor and lower St. Louis, an invasion-prone Great Lakes coastal ecosystem. J. Great Lakes Res. 2011, 37, 349–358. [Google Scholar] [CrossRef]
- Lougheed, V.L.; Theÿsmeÿer, T.; Smith, T.; Chow-Fraser, P. Carp exclusion, foodweb interactions and the restoration of Cootes Paradise Marsh. J. Great Lakes Res. 2004, 30, 44–57. [Google Scholar] [CrossRef]
- Lyons, J.; Wang, L.; Simonson, T.D. Development and Validation of an Index of Biotic Integrity for Coldwater Streams in Wisconsin. North Am. J. Fish. Manag. 1996, 16, 241–256. [Google Scholar] [CrossRef]
- Grabas, G.P.; Rokitnicki-Wojcik, D. Characterizing daily water-level fluctuation intensity and water quality relationships with plant communities in Lake Ontario coastal wetlands. J. Great Lakes Res. 2015, 41, 136–144. [Google Scholar] [CrossRef]
- Sheldon, A.L. Species Diversity and Longitudinal Succession in Stream Fishes. Ecology 1968, 49, 193–198. [Google Scholar] [CrossRef]
- Chow-Fraser, P. A conceptual ecological model to aid restoration of Cootes Paradise Marsh, a degraded coastal wetland of Lake Ontario, Canada. Wetl. Ecol. Manag. 1998, 6, 43–57. [Google Scholar] [CrossRef]
- Harrington and Hoyle. Rattray Marsh Class Environmental Assessment for the Credit Valley Conservation; Final environmental study report; August 2009; Credit Valley Conservation: Mississauga, ON, Canada, 2009; p. 78. [Google Scholar]
- Haines, T.A. Acidic Precipitation and Its Consequences for Aquatic Ecosystems: A Review. Trans. Am. Fish. Soc. 1981, 110, 669–707. [Google Scholar] [CrossRef]
- Kramer, D.L. Dissolved oxygen and fish behaviour. Environ. Biol. Fish. 1987, 18, 81–92. [Google Scholar] [CrossRef]
- Scott, D.M.; Lucas, M.C.; Wilson, R.W. The effect of high pH on ion balance, nitrogen excretion and behaviour in freshwater fish from an eutrophic lake: A laboratory and field study. Aquat. Toxicol. 2005, 73, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Chow-Fraser, P. Seasonal, Interannual, and Spatial Variability in the Concentrations of Total Suspended Solids in a Degraded Coastal Wetland of Lake Ontario. J. Great Lakes Res. 1999, 25, 799–813. [Google Scholar] [CrossRef]
- Chow-Fraser, P.; Lougheed, V.; Le Thiec, V.; Crosbie, B.; Simser, L.; Lord, J. Long-term response of the biotic community to fluctuating water levels and changes in water quality in Cootes Paradise Marsh, a degraded coastal wetland of Lake Ontario. Wetl. Ecol. Manag. 1998, 6, 19–42. [Google Scholar] [CrossRef]
- Engel, S.; Nichols, S.A. Aquatic Macrophyte Growth in a Turbid Windswept Lake. J. Freshw. Ecol. 1994, 9, 97–109. [Google Scholar] [CrossRef]
- Novotny, E.V.; Murphy, D.; Stefan, H.G. Increase of urban lake salinity by road deicing salt. Sci. Total. Environ. 2008, 406, 131–144. [Google Scholar] [CrossRef]
- Gray, S.M.; Bieber, F.M.E.; Mcdonnell, L.H.; Chapman, L.J.; Mandrak, N.E. Experimental evidence for species-specific response to turbidity in imperilled fishes. Aquat. Conserv. Mar. Freshw. Ecosyst. 2014, 24, 546–560. [Google Scholar] [CrossRef]
- Lougheed, V.L.; Crosbie, B.; Chow-Fraser, P. Primary determinants of macrophyte community structure in 62 marshes across the Great Lakes basin: Latitude, land use, and water quality effects. Can. J. Fish. Aquat. Sci. 2001, 58, 1603–1612. [Google Scholar] [CrossRef]
- Lougheed, V.L.; Chow-Fraser, P. Factors that regulate the community structure of a turbid, hypereutrophic Great Lakes wetland. Can. J. Fish. Aquat. Sci. 1998, 55, 150–161. [Google Scholar] [CrossRef]
- Whillans, T.H. Historical and comparative perspectives on rehabilitation of marshes as habitat for fish in the lower Great Lakes basin. Can. J. Fish. Aquat. Sci. 1996, 53, 58–66. [Google Scholar] [CrossRef]
- Lowe, E.F.; Battoe, L.E.; Coveney, M.; Stites, D. Setting Water Quality Goals for Restoration of Lake Apopka: Inferring Past Conditions. Lake Reserv. Manag. 1999, 15, 103–120. [Google Scholar] [CrossRef]
- Thorpe, A.S.; Stanley, A.G. Determining appropriate goals for restoration of imperilled communities and species. J. Appl. Ecol. 2011, 48, 275–279. [Google Scholar] [CrossRef]
- Hamilton, J.G. Survey of Critical Fish Habitat Within International Joint Commission Designated Areas of Concern, August–November, 1986; Ontario Ministry of Natural Resources, Fisheries Branch: Toronto, ON, Canada, 1987; p. 119.
- Mack, J.J. Developing a wetland IBI with statewide application after multiple testing iterations. Ecol. Indic. 2007, 7, 864–881. [Google Scholar] [CrossRef]
- Adams, S.M.; Ham, K.D.; Greeley, M.S.; LeHew, R.F.; Hinton, D.E.; Saylor, C.F. Downstream gradients in bioindicator responses: Point source contaminant effects on fish health. Can. J. Fish. Aquat. Sci. 1996, 53, 2177–2187. [Google Scholar] [CrossRef]
- Paller, M.H.; Reichert, M.J.; Dean, J.M.; Seigle, J.C. Use of fish community data to evaluate restoration success of a riparian stream. Ecol. Eng. 2000, 15, S171–S187. [Google Scholar] [CrossRef]
- Trebitz, A.S.; Hill, B.H.; McCormick, F.H. Sensitivity of Indices of Biotic Integrity to Simulated Fish Assemblage Changes. Environ. Manag. 2003, 32, 499–515. [Google Scholar] [CrossRef]
- Granados, M. Detecting Changes in Fish Communities in Response to Habitat Rehabilitation: A Comparison of Multimetric and Multivariate Approaches. Master’s Thesis, University of Toronto, Toronto, ON, Canada, 2010. [Google Scholar]
- Matthews, J.W.; Spyreas, G. Convergence and divergence in plant community trajectories as a framework for monitoring wetland restoration progress. J. Appl. Ecol. 2010, 47, 1128–1136. [Google Scholar] [CrossRef]
- McKenna, J.E., Jr.; Riseng, C.; Wehrly, K. Decision support for aquatic restoration based on species-specific responses to disturbance. Ecol. Evol. 2022, 12, e9313. [Google Scholar] [CrossRef] [PubMed]
- Finigan, P.A. The Use of Historical Data to Describe Changes in Fish Communities Over Time. Master’s Thesis, Queen’s University, Kingston, ON, Canada, 2015. [Google Scholar]
- Scott, W.B.; Crossman, E.J. Freshwater Fishes of Canada. In Bulletin of the Fisheries Research Board of Canada; No. 184; Government of Canada Publications: Downsview, ON, Canada, 1973; p. 966. [Google Scholar]
- Grayson, J.; Chapman, M.; Underwood, A. The assessment of restoration of habitat in urban wetlands. Landsc. Urban Plan. 1999, 43, 227–236. [Google Scholar] [CrossRef]
- Hobbs, R.J. Setting Effective and Realistic Restoration Goals: Key Directions for Research. Restor. Ecol. 2007, 15, 354–357. [Google Scholar] [CrossRef]
- Sierszen, M.E.; Peterson, G.S.; Trebitz, A.S.; Brazner, J.C.; West, C.W. Hydrology and nutrient effects on food-web structure in ten lake superior coastal wetlands. Wetlands 2006, 26, 951–964. [Google Scholar] [CrossRef]
- Trebitz, A.S.; Morrice, J.A.; Cotter, A.M. Relative Role of Lake and Tributary in Hydrology of Lake Superior Coastal Wetlands. J. Great Lakes Res. 2002, 28, 212–227. [Google Scholar] [CrossRef]
- Quigley, J.T.; Harper, D.J. Effectiveness of Fish Habitat Compensation in Canada in Achieving No Net Loss. Environ. Manag. 2006, 37, 351–366. [Google Scholar] [CrossRef]
- Meli, P.; Ray Benayas, J.M.; Balvenera, P.; Ramos Martinez, M. Restoration Enhances Wetland Biodiversity and Eco-system Service Supply, but results are context-dependent: A meta-analysis. PLoS ONE 2014, 9, e93507. [Google Scholar] [CrossRef]
- Rubec, C.D.A.; Hanson, A.R. Wetland mitigation and compensation: Canadian experience. Wetl. Ecol. Manag. 2009, 17, 3–14. [Google Scholar] [CrossRef]
- Zedler, J.B.; Callaway, J.C. Tracking Wetland Restoration: Do Mitigation Sites Follow Desired Trajectories? Restor. Ecol. 1999, 7, 69–73. [Google Scholar] [CrossRef]
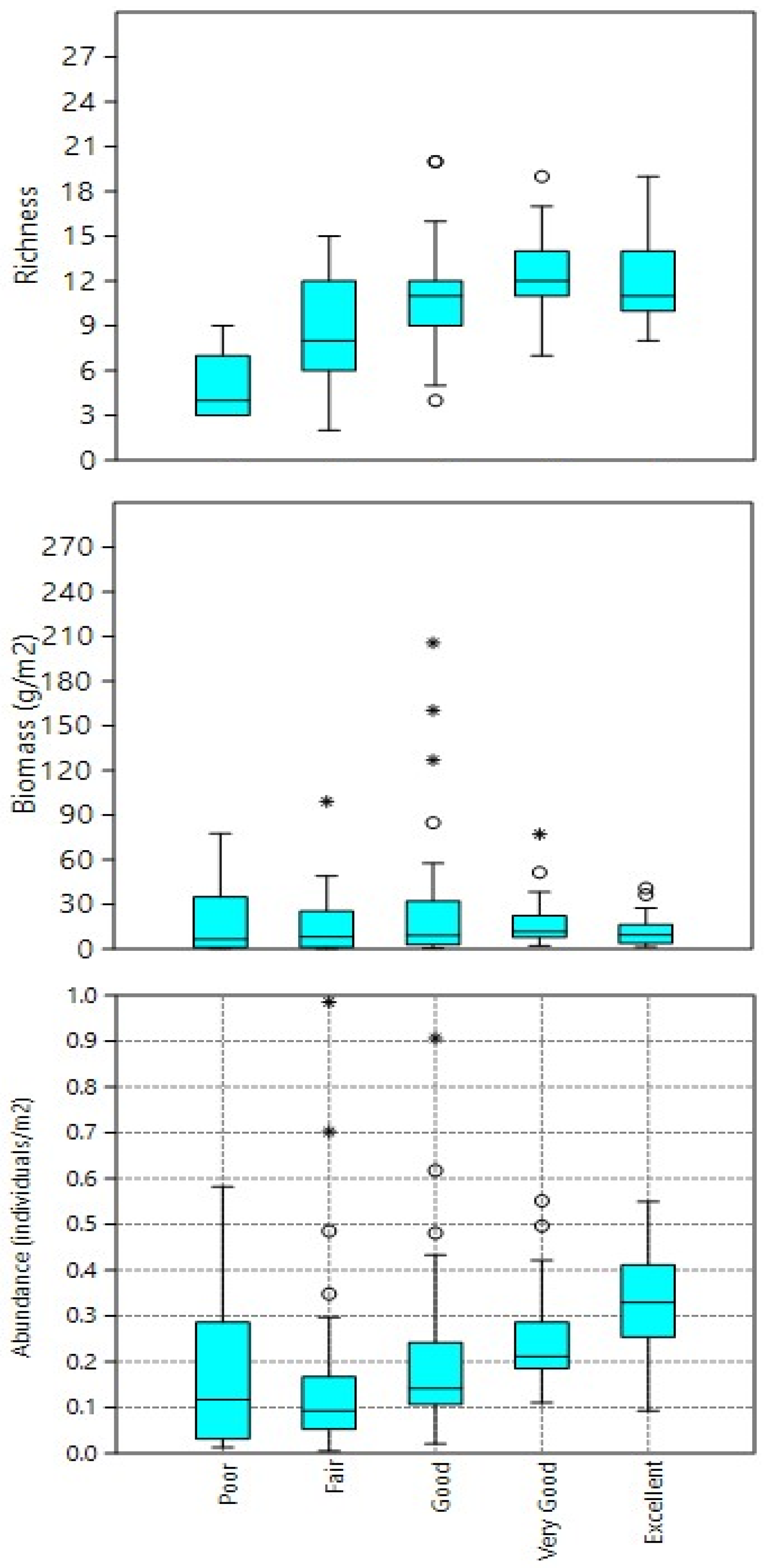
| IBI Grouping | IBI Score | Abundance (Individuals/m2) | Richness | Biomass (g/m2) |
|---|---|---|---|---|
| Poor | 0–20 | 0.1841 | 5.15 | 22.24 |
| Fair | 20–40 | 0.1511 | 8.45 | 15.31 |
| Good | 40–60 | 0.1967 | 10.79 | 25.25 |
| Very Good | 60–80 | 0.2479 | 12.13 | 17.61 |
| Excellent | 80–100 | 0.3343 | 11.96 | 12.05 |
| Group 1 | Group 2 | ANOSIM (p-Value) | Overall Dissimilarity | Three Species with the Largest Contribution (Species (% Contribution)) | ||
|---|---|---|---|---|---|---|
| Excellent | Very Good | 0.001 | 51.51 | Yellow Perch (13.8) | Bluegill (9.0) | Golden Shiner (7.3) |
| Excellent | Good | 0.001 | 66.25 | Yellow Perch (16.1) | Bluegill (10.8) | Brown Bullhead (7.1) |
| Excellent | Fair | 0.001 | 75.59 | Yellow Perch (18.3) | Bluegill (10.9) | Pumpkinseed (7.6) |
| Excellent | Poor | 0.001 | 87.06 | Yellow Perch (18.9) | Bluegill (10.9) | Pumpkinseed (9.9) |
| Very Good | Good | 0.165 | 59.48 | Brown Bullhead (9.5) | Yellow Perch (9.4) | Bluegill (7.8) |
| Very Good | Fair | 0.002 | 67.95 | Yellow Perch (11.4) | Brown Bullhead (8.9) | Pumpkinseed (8.3) |
| Very Good | Poor | 0.001 | 81.43 | Yellow Perch (12.1) | Pumpkinseed (10.9) | Brown Bullhead (7.8) |
| Good | Fair | 0.003 | 62.85 | Fathead Minnow (10.6) | Brown Bullhead (10.0) | Pumpkinseed (8.8) |
| Good | Poor | 0.001 | 74.60 | Pumpkinseed (11.5) | Brown Bullhead (9.8) | Goldfish (8.9) |
| Fair | Poor | 0.016 | 72.85 | Goldfish (12.77) | Brown Bullhead (11.1) | Fathead Minnow (11.1) |
| Pooled Groups | 67.09 | Yellow Perch (11.74) | Brown Bullhead (8.3) | Pumpkinseed (8.1) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moore, D.J.; Mandrak, N.E. Coastal Wetland Management and Restoration: Importance of Abiotic Factors and Vegetation for Healthy Fish Communities in the Laurentian Great Lakes. Water 2025, 17, 2470. https://doi.org/10.3390/w17162470
Moore DJ, Mandrak NE. Coastal Wetland Management and Restoration: Importance of Abiotic Factors and Vegetation for Healthy Fish Communities in the Laurentian Great Lakes. Water. 2025; 17(16):2470. https://doi.org/10.3390/w17162470
Chicago/Turabian StyleMoore, Daniel J., and Nicholas E. Mandrak. 2025. "Coastal Wetland Management and Restoration: Importance of Abiotic Factors and Vegetation for Healthy Fish Communities in the Laurentian Great Lakes" Water 17, no. 16: 2470. https://doi.org/10.3390/w17162470
APA StyleMoore, D. J., & Mandrak, N. E. (2025). Coastal Wetland Management and Restoration: Importance of Abiotic Factors and Vegetation for Healthy Fish Communities in the Laurentian Great Lakes. Water, 17(16), 2470. https://doi.org/10.3390/w17162470






