Abstract
Disinfection by ultrasound and carbon nanotubes (CNTs) provides attractive alternatives to conventional methods for water and wastewater treatment. This study explored the inactivation of Escherichia coli (E. coli) by 5 mg/L pristine short and long multi-walled CNTs (MWCNTs) and 20 kHz ultrasound individually or in combinations in DI water, Suwannee River natural organic matter (SRNOM), and sodium dodecyl sulfate (SDS) solution, respectively. The results indicated that the dispersity of MWCNTs was the single most important factor determining the inactivation rate of E. coli. The dispersity of short MWCNTs in solutions increased in the order of DI water <10 mgC/L SRNOM < 2 mM SDS. Correspondingly, the greatest log inactivation of E. coli was achieved in SDS when short MWCNTs were used alone (0.67 ± 0.12) and combined with ultrasound (1.80 ± 0.02) for 10 min. Short MWCNTs alone had a slightly greater inactivation (0.29 ± 0.07) in SRNOM solution than in DI water (0.18 ± 0.05). However, long MWCNTs had a slightly higher inactivation in DI water (0.24 ± 0.03) than short ones (0.18 ± 0.05), because of better dispersity in DI. The observed synergistic inactivation when ultrasound and short MWCNTs were used together in 2 mM SDS shows that ultrasound energized the MWCNTs more effectively when they were well dispersed, although SDS and MWCNTs can occupy the reaction sites at the cavitational bubble–water interfacial regions and scavenge •OH radicals. The results suggest that sonophysical effects are more important to inactivate E. coli than sonochemical effects. Ultrasound inactivates E. coli and/or energizes MWCNTs through the mechanisms of acoustic streaming, microstreaming, microstreamers, transient cavitation collapse-generated shock waves and microjets (transitional forms), and localized hot temperatures. The results of this study indicate that the cytotoxicity of CNTs includes impinging bacterial cells and/or direct contact with the bacteria.
1. Introduction
Water disinfection is of critical importance for inactivating pathogenic microorganisms and protecting public health and safety. However, because of concerns regarding halogenated disinfection byproducts (e.g., trihalomethanes (THMs) and haloacetic acids (HAAs)) from chlorine and chloromines, exploring alternative disinfection technologies is of great importance, especially for disinfectant-resistant pathogenic microorganisms. Among various alternative disinfectants and emerging technologies, ultrasound and carbon nanotubes (CNTs) are attractive ones. Ultrasound is a longitudinal wave with a typical frequency between 16 kHz and 500 MHz [1,2]. Ultrasonic waves create periodic compression and expansion cycles in water. Cavitational bubbles are formed when the acoustic pressure exceeds the tensile strength of water during the rarefaction of ultrasonic waves. Ultrasonic waves bring acoustic streaming, microstreaming, and microstreamers in water solutions. The transient collapse of cavitational bubbles occurs during the compression cycle of ultrasonic waves [3]. Localized hot spots are formed by ultrasonic waves that reach typical temperatures and pressures of 4000–6000 K [4] and 100–500 bar [5] in water, respectively. The violence of cavitation collapse depends on factors such as ultrasonic power, frequency, hydrostatic pressure, temperature, solvent property, and dissolved gas [3]. Under such high temperatures, water vapor is pyrolyzed into •OH and •H radicals:
H2O → •H + •OH
In the meantime, cavitational collapse produces microjets and shock waves, which bring extreme velocity and pressure gradients in water. Several mechanisms have been proposed for the ultrasonic inactivation of bacteria, including the impingement/disruption of cells and cell membranes by a high velocity jet [6,7], shock waves [8,9,10], fluid eddies and shear stress from transient cavitation collapse [11,12], microstreaming [13], localized hot temperature [12], and free-radicals (e.g., •OH, HO2•, and O•) [12,14]. It was previously reported that hydroxyl radicals (•OH) are less important than shock waves [9], which is evidenced by cell wall burst through sonication [10]. However, free-radicals and hydrogen peroxide (H2O2) can enter the cell and attack internal structures or degrade released vital structures in solution [15]. In addition, sonication may break the agglomeration of microorganism clusters and flocs in solution, thus making them more susceptible to other disinfectants [15,16]. Microorganisms can be “weak spots” in water and act as nuclei to induce cavitation in an ultrasonic field due to different inertia and bonding to neighboring water molecules compared to a pure water system. This effect may enhance disinfection efficiency. It was reported that the inactivation rate of microorganisms depends on the duration of sonication, the ultrasonic power level, frequency, dissolved gas, and the properties of the microorganisms, including the size and the shape of the cell, stage of growth, and species [17,18,19]. Although sonication alone can inactivate microorganisms, it is usually combined with other disinfection techniques to obtain synergistic effects [20]. The advantages of ultrasonic disinfection include being less sensitive to particulate and UV-absorbance materials in water and being barely affected by disinfection byproducts.
Carbon nanotubes (CNTs), first discovered by Iijima in 1991 [21], can also serve as antimicrobial agents. A CNT that contains one layer of a rolled graphite sheet is called a single-walled CNT (SWCNT), while several SWCNTs with different diameters concentrically nested together are called a multi-walled CNT (MWCNT) [22]. The cytotoxicity of CNTs has been reported by several studies [23,24,25,26,27,28,29], in which the proposed toxicity mechanisms of CNTs include direct contact/impinging with bacterial cells, the interruption of transmembrane electron transfer, and the oxidation of cell components. It was found that the diameter of CNTs was a key factor governing their antimicrobial activity, and SWCNTs had a stronger antimicrobial effect than MWCNTs [30]. In another study, the same research group showed that short MWCNTs were more cytotoxic when they were uncapped, debundled, and dispersed in solutions [31]. Yang et al. [26] indicated that longer SWCNTs aggregated with bacterial cells and exhibited stronger antimicrobial activity than shorter ones, which were more likely to be self-aggregated. Consistently, Liu et al. [24] reported that well-dispersed, shaper, and faster SWCNTs acting as nanodarts attacked bacteria more effectively, while the mechanisms for inhibiting microbial cell growth and oxidative stress were not important for the antimicrobial effect. Although their cytotoxicity is less than SWCNTs, MWCNTs are much cheaper than SWCNTs and should be considered a viable alternative to SWCNTs for disinfection.
After disinfection, CNTs can be readily separated from water by membrane filtration without fear of contamination. This technology can serve as a point-of-use treatment to provide safe drinking water. Therefore, the hypothesis of this study was that in addition to disinfection alone, ultrasound can energize MWCNTs and enhance their dispersion and contact with pathogenic bacteria, thus resulting in synergistic disinfection. Although CNTs scavenge •OH radicals [32] produced by ultrasound, this effect might be insignificant for low-frequency ultrasound, like the 20 kHz ultrasound used in this study. This is because low-frequency ultrasound has stronger sonophysical effects than sonochemical effects compared to high-frequency ultrasound [33]. This hypothesis was also consistent with our previous study [34], which found that much fewer •OH radicals are produced at 20 kHz than 354 kHz at the same power density. The main objectives of this study were to (i) investigate the disinfection effects of ultrasound and MWCNTs individually and in combination, (ii) explore the effect of solution chemistry on the efficiency of disinfection, and (iii) provide a mechanistic explanation and understanding of the disinfection results. This is a pioneering study on the combined use of carbon nanotubes (CNTs) and sonication, focusing on the mechanisms involved and the synergistic disinfection efficiency. To the best of our knowledge, few studies on this topic have been reported to date.
2. Materials and Methods
2.1. Materials and Equipment
All chemical reagents were of certified grade. The solutions in this study were prepared with sterile DI water (R = 18.2 MΩ·cm). Pristine short and long MWCNTs were procured from Sigma Aldrich (St. Louise, MI, USA). According to the supplier, the short MWCNTs (Product #755117) have less than 5% metal oxide with an outside diameter of about 9.5 nm and a length of about 1 µm, while the long MWCNTs (Product #698849) have a >98% carbon basis with an outside diameter of 6–13 nm and a length of 2.5–20 µm. E. coli strain ATCC 11229 was obtained from the American Type Culture Collection. Suwannee River natural organic matter (SRNOM), obtained by RO isolation, was procured from the International Humic Substances Society (IHSS). SRNOM serves as a model NOM ubiquitously present in natural waters and wastewaters.
A 20 kHz ultrasonic probe system with a 3.2 mm diameter micro-tip from Fisher Scientific (Sonic Dismembrator model FB120, Thermo Fisher Scientific, Waltham, MA, USA) was employed for ultrasonic disinfection. Sonication was operated at 40% of the power level. Calorimetry [2] was performed to measure the power output of ultrasound, which was 2.4 ± 0.1 W for all experiments. The corresponding ultrasonic power intensity and density were 29.8 ± 1.2 W/cm2 and 0.69 ± 0.03 W/mL, respectively.
2.2. Cultivation of E. coli
E. coli was cultivated by using a 2 mL overnight culture to inoculate a 50 mL nutrient broth in a 125 mL flask. This E. coli culture was grown on a batch shaker (200 rpm, pH 7, temperature 37 °C) until the stationary phase was reached. Growth was monitored by measuring the optical density at 600 nm (OD600) every hour. At the end of the growth period, the bacteria were plated on nutrient agar in triplicate and incubated overnight at 37 °C before counting the colony-forming units per mL (CFU/mL). The microbial concentration was around 109 CFU/mL. The plating media were prepared by dissolving 8 g of nutrient broth (5.0 g/L of peptone from meat, 3.0 g/L of meat extract, EMD) and 10 g of agar powder in 1.0 L of DI water and autoclaved at 121 °C and a 20 min cycle.
2.3. Procedures of Inactivating E. coli
To test the chemical solution effects on the inactivation of E. coli, batch cultures were purified with DI water by centrifugation in a 50 mL Falcon tube at 5000 rpm for 5 min to remove residual growth media. After centrifugation, the supernatant was discarded by decanting. The cells were then washed four times by resuspending the pellet in 25 mL of DI water and vortexing completely, followed by centrifugation as described above. Finally, the purified cells were resuspended in DI water and transferred to a sterile 125 mL flask, where they were diluted with sterile DI water to a final volume of 50 mL. The target concentration of the purified microbe was 105–106 CFU/mL.
The bulk solutions of MWCNTs, sodium dodecyl sulfate (SDS), and SRNOM were prepared before mixing with the purified E. coli suspension accordingly. SDS was chosen because it is a commonly used biodegradable surfactant and less toxic to bacteria [35,36]. In total, 2 mM of SDS was used in this study, which is below its critical micelle concentration of between 9 and 10 mM at pH 4–10 and room temperature [37]. The solutions of MWCNTs alone were sonicated in an ultrasonic bath for 5 min to obtain well-mixed solutions before adding them to the microbial suspension or chemical solutions. The final volume of each sample containing E. coli was 3.5 mL for all of the control and inactivation tests.
Sonication was performed in 10 mL KIMAX® glass test tubes (13 mm OD × 100 mm L). The ultrasonic probe tip was inserted to the mid-depth of the solution in the glass tube and sonicated for 10 min. The glass tube was placed in an ice/water mixture (0 °C) during sonication to maintain the temperature of the culture solution at 23 ± 4 °C during sonication. For control tests without ultrasound, the microbial solution was shaken at 200 rpm at room temperature for 10 min.
2.4. Sample Analyses
Control and inactivated samples were taken for counting CFUs via bacterial plating counting with a 10−4–10−9 final dilution in triplicate. The plates were cultivated at 37 °C for 24 h before determining CFU/mL using the base CLIQS software (Totallab Ltd., Gosforth, Newcastle, UK). To quantify the inactivation efficiency, log inactivation was calculated as –log (N/N0), with N being the concentration (CFU/mL) of E. coli after inactivation treatment with MWCNTs and/or ultrasound and N0 being the control CFU/mL concentration in the same background chemical solution but without MWCNTs or ultrasound. Table 1 lists the N0 and pH values in different chemical solutions. Compared to DI water, N0 had a moderate increase in SRNOM and a slight decrease in SDS solution. SRNOM is a natural organic compound and may support microbial growth.

Table 1.
The initial E. coli concentration and pH values of the solutions.
The size and morphology of the MWCNTs were examined by transmission electron microscopy (TEM) (Tecnai G2T20, FEI, Hillsboro, OR, USA) operated at 100 kV. The determination of the hydrogen peroxide concentration was performed by the triiodide method [38]. UV and visible absorbance was conducted using a UV-Vis spectrophotometer (Lambda 25, PerkinElmer, Shelton, CT, USA) with a 10 mm quartz cuvette. The ultrasonic degradation of SDS was evaluated through examining its concentration following the methylene blue active substance analysis, according to the standard methods for the examination of water and wastewater [39].
3. Results
3.1. Morphology and Dispersion of MWCNTs in Different Solutions
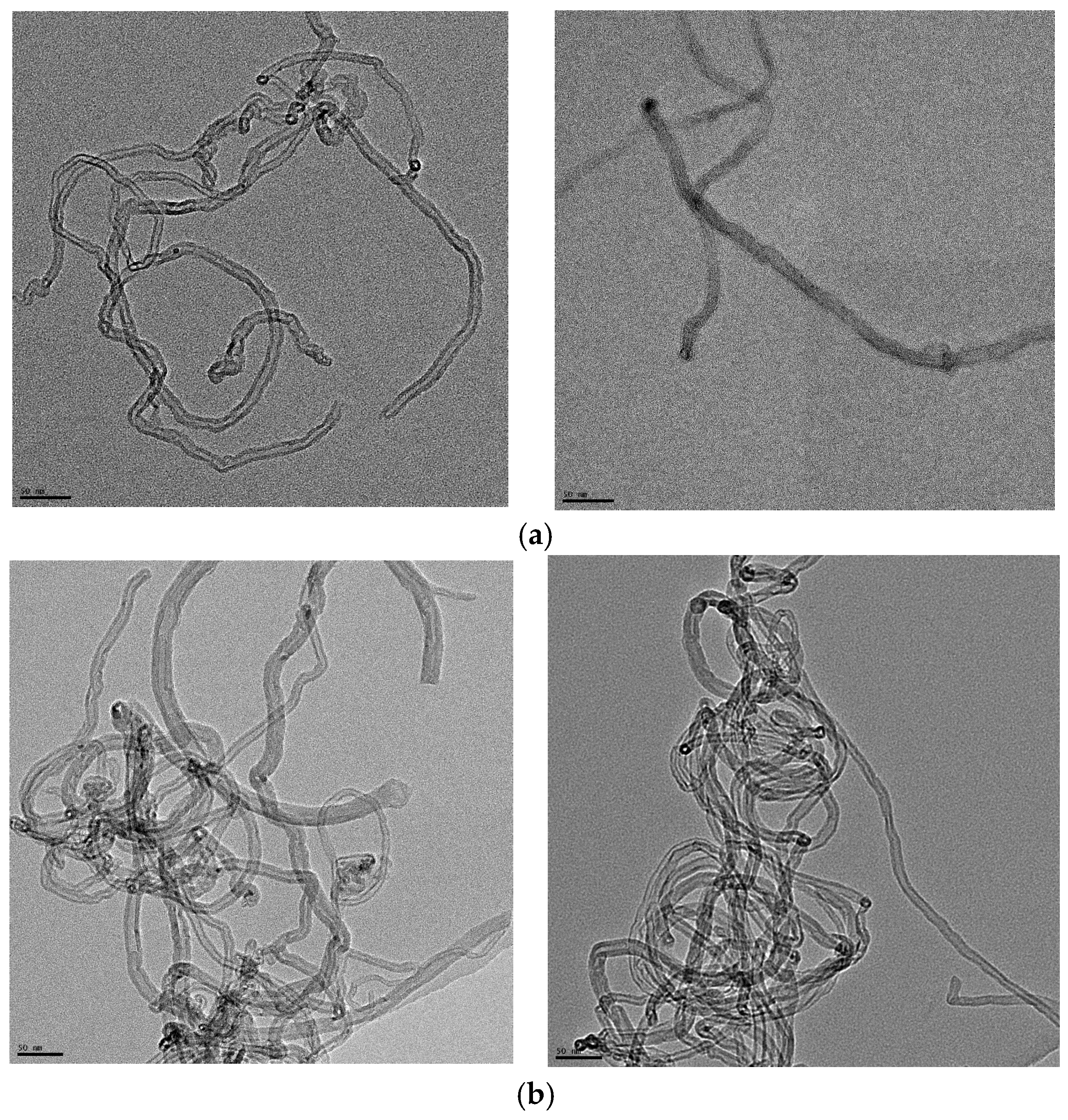
Figure 1a,b show the TEM images of the pristine short and long MWCNTs, respectively. In Figure 1a, the short MWCNTs have a measured outer diameter between 5 and 15 nm. According to the supplier, the average outer diameter and length were 9.5 nm and 1 µm, respectively. The average length in Figure 1a is significantly shorter than the long MWCNTs in Figure 1b: the outside diameter is between 7 and 22 nm and the length is between 2.5 and 20 µm based on information from the supplier. Therefore, the major difference between these two types of MWCNTs is the length, although short MWCNTs are also slightly thinner than long MWCNTs.
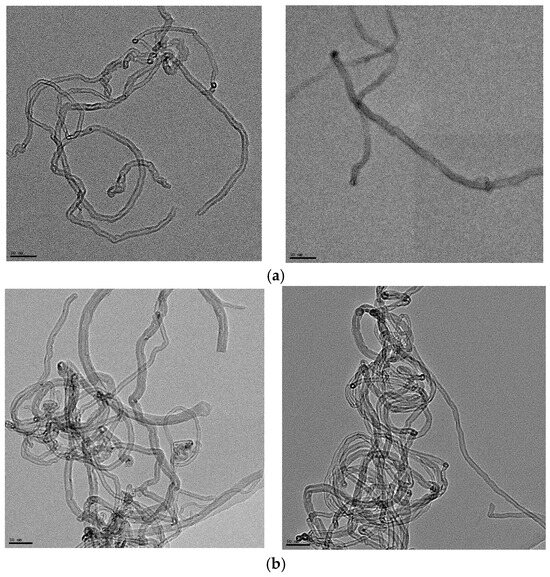
Figure 1.
(a) TEM images of the short MWCNTs. The length of the scale bar is 50 nm. According to the images, the outer diameter of the MWCNTs is between 5 and 15 nm. (b) TEM images of the long MWCNTs. The length of the scale bar is 50 nm. According to the images, the outer diameter of the MWCNTs is about 7–22 nm.
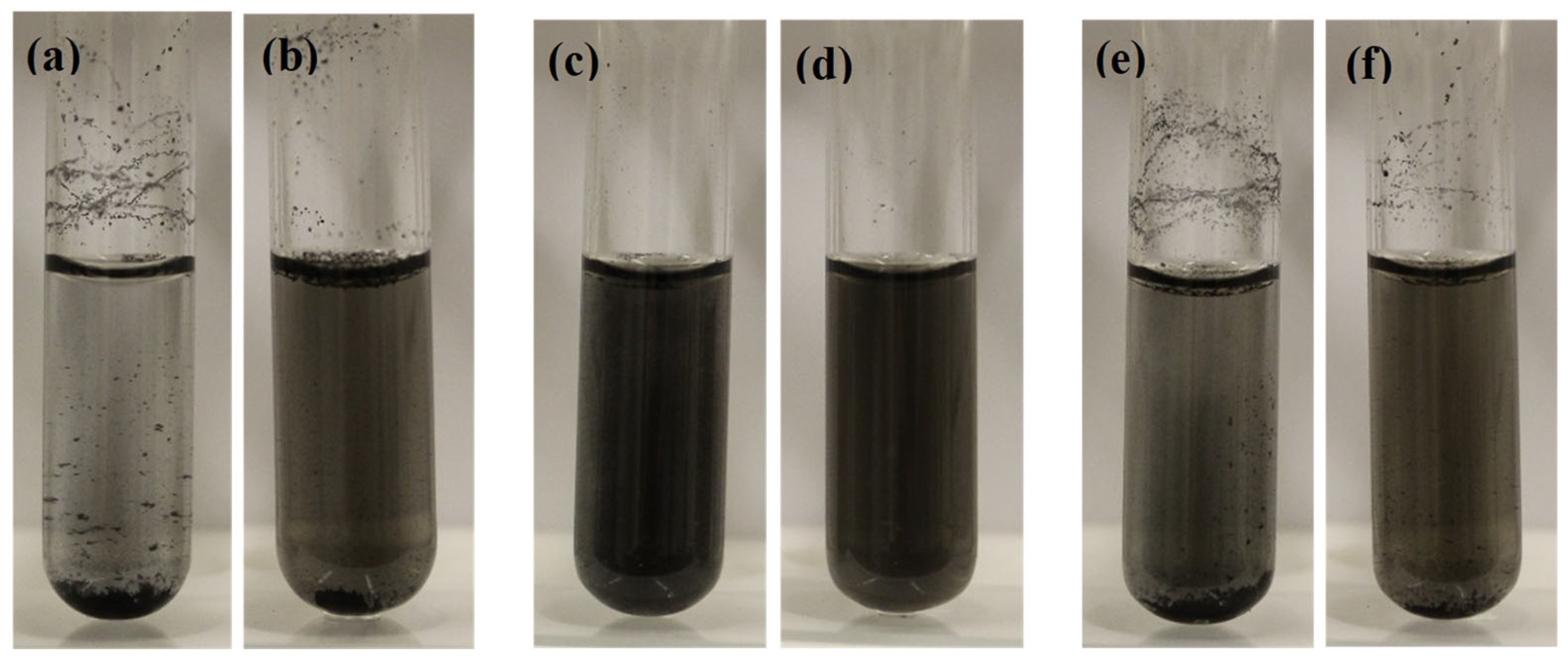
Figure 2 shows the dispersion of 50 mg/L MWCNTs in different background solutions, i.e., DI water, 2 mM of SDS, and 10 mgC/L of SRNOM. As seen in the images, short MWCNTs mostly aggregated and accumulated at the DI water’s bottom and surface, and they had poorer dispersion than long MWCNTs in DI water. Other than size, it was reported that CNTs might contain impurities such as amorphous carbon, metal, graphite, and functionalized groups [24,26], which might affect their aggregation and dispersion in water. However, moderate and significant improvements in the dispersion of short MWCNTs were found in 10 mgC/mL of SRNOM and the 2 mM SDS solution, respectively. For long MWCNTs, slight and moderate improvements in the dispersion were observed in 10 mgC/mL of SRNOM and the 2 mM SDS solution, respectively. The results suggest that the dispersity of MWCNTs in solution generally increased in the order of DI water < 10 mgC/L SRNOM < 2 mM SDS.
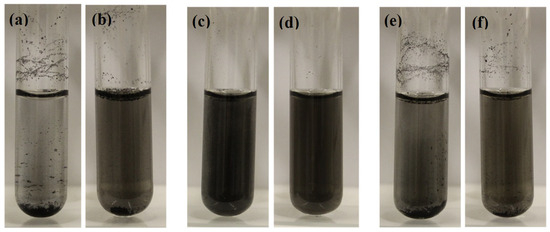
Figure 2.
Dispersion of 50 mg/L MWCNTs in different solutions: (a) short MWCNTs in DI water; (b) long MWCNTs in DI water; (c) short MWCNTs in 2 mM of SDS; (d) long MWCNTs in 2 mM of SDS; (e) short MWCNTs in 10 mgC/L of SRNOM; (f) long MWCNTs in 10 mgC/L of SRNOM.
SDS is a strong anionic surfactant with hydrophilic heads and hydrophobic tails. Hydrophobic tails have high affinity to CNTs due to hydrophobic interactions, because CNTs are mostly hydrophobic [40,41]. On the other hand, the hydrophilic heads of the surfactant help the complexed CNTs disperse in water [42], as they would otherwise mostly aggregate and accumulate at the water surface or bottom. However, NOM has a much more complicated chemical structure, which varies depending on the source and the degree of degradation but typically contains aromatic, aliphatic, carboxylic, and phenolic functional groups [43], NOM contains both hydrophilic (mainly carboxylic (3 ≤ pKa ≤ 6) and phenolic groups (8 ≤ pKa ≤ 12)) [44] and hydrophobic functional groups (non-ionized groups), and it behaves like a weak anionic surfactant [45]. Similarly to SDS, the hydrophobic functional groups of NOM may attach and complex with CNTs. In contrast, the deprotonated acidic functional groups (mainly carboxylic at neutral pH range) are hydrophilic and, thus, facilitate the dispersion of CNTs in water. In agreement, the stabilization of CNTs in NOM solutions was also reported by Hyung et al. [46,47].
3.2. Inactivation of E. coli in DI Water
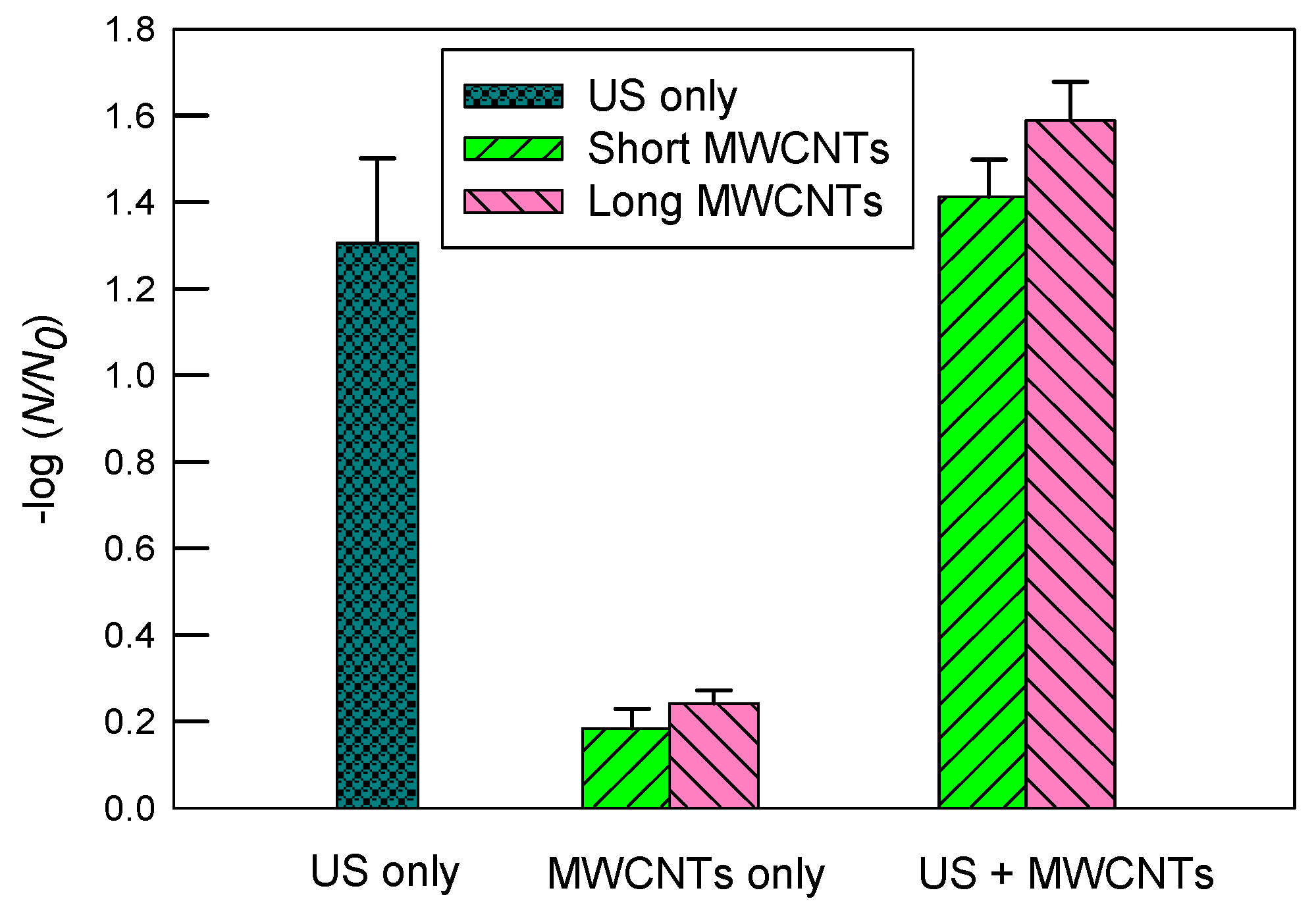
Figure 3 indicates inactivation of E. coli in DI water by ultrasound, MWCNTs, and ultrasound with MWCNTs, respectively. First, ultrasound resulted in a much greater inactivation of E. coli than MWCNTs alone. Ultrasound alone had a log inactivation of 1.31 ± 0.20, while short and long MWCNTs achieved log inactivation of 0.18 ± 0.05 and 0.24 ± 0.03, respectively. When both ultrasound and MWCNTs worked together, however, the log inactivation rates achieved were 1.41 ± 0.09 and 1.59 ± 0.09 for short and long MWCNTs, respectively. The results indicate that long MWCNTs had a slightly better inactivation than short MWCNTs with or without ultrasound. Since short MWCNTs are more likely to impinge bacterial cells [24] and, thus, it was expected to have a greater inactivation rate than long MWCNTs, it is likely that the poorer dispersion of short than long MWCNTs in DI water significantly reduced their contact with E. coli. Therefore, this result is in agreement with Figure 2 regarding the dispersion of MWCNTs. Consistently, Yang et al. [26] reported that short SWCNTs tended to aggregate themselves without involving many bacterial cells, while at the same concentration, longer SWCNTs exhibited stronger antimicrobial activity because they had more contact with more bacterial cells. Although ultrasound can facilitate the dispersion of CNTs in water, it was observed that significant amounts of short MWCNTs still floated on the water surface through sonication. Typically, an immersed ultrasonic probe (or horn) transmits sound waves downwards into the water, which is less effective for dispersing floating particles on the water surface, especially when a rosette reactor is not used.
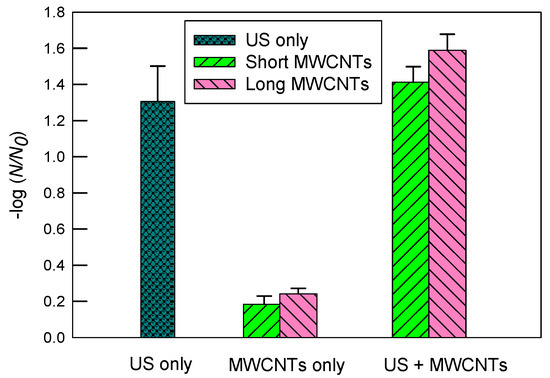
Figure 3.
The inactivation of E. coli in DI water by ultrasound (US), MWCNTs, and ultrasound with MWCNTs, respectively. The sonication time was 10 min. The concentration of MWCNTs was 5 mg/L. For MWCNTs only, the mixtures of E. coli solutions were shaken at 200 rpm for 10 min. The error bars represent standard deviations.
3.3. Inactivation of E. coli in 10 mgC/mL SRNOM
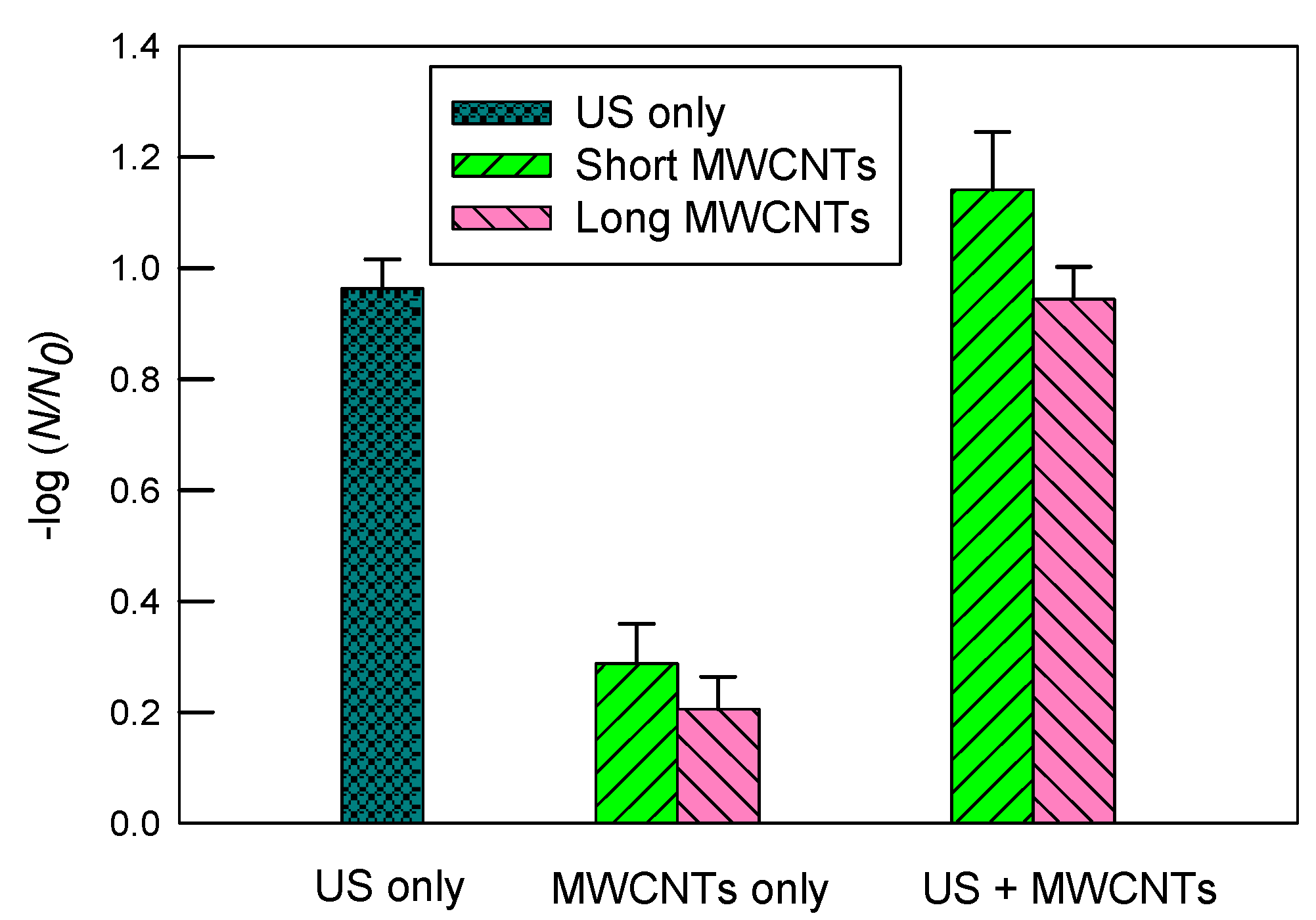
Figure 4 shows the inactivation of E. coli in a 10 mgC/L SRNOM solution by ultrasound, MWCNTs, and ultrasound with MWCNTs, respectively. The log inactivation by ultrasound alone was 0.96 ± 0.05. The short MWCNTs had a log inactivation of 1.14 ± 0.10 with ultrasound and 0.29 ± 0.07 without ultrasound. In contrast, the log inactivation values of long MWCNTs were 0.94 ± 0.06 when combined with ultrasound and 0.21 ± 0.06 alone.
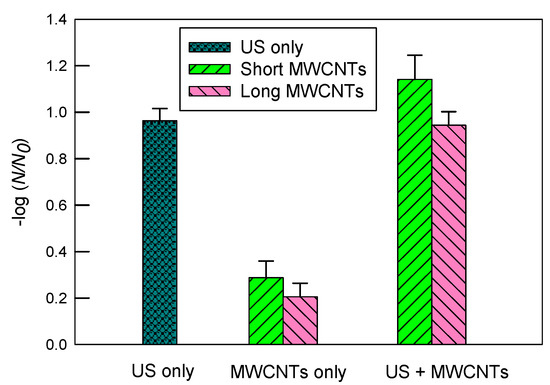
Figure 4.
The inactivation of E. coli in a 10 mgC/L SRNOM solution by ultrasound (US), MWCNTs, and ultrasound with MWCNTs, respectively. The sonication time was 10 min. The concentration of MWCNTs was 5 mg/L. For MWCNTs only, the mixtures of E. coli solutions were shaken at 200 rpm for 10 min. The error bars represent standard deviations.
Compared to the results shown in Figure 3, the log inactivation by ultrasound alone in SRNOM solution is lower than that in DI water (1.31 ± 0.20). The hydrophobic fractions (non-ionized groups) of SRNOM may accumulate in the cavitational bubble–water interfacial regions and be degraded via attack by •OH radicals and shock waves, according to our prior study [34]. The original SRNOM is a non-volatile macromolecular organic. Therefore, it is unlikely to be thermalized in the vapor phase of cavitational bubbles. As a result, SRNOM may occupy the space of the cavitational bubble–water interfacial regions and scavenge free-radicals including •OH, both of which would otherwise contribute to inactivating E. coli. Therefore, this is likely the reason why a lower inactivation rate was observed in SRNOM than in DI water. The potential inactivation mechanisms in the cavitational bubble–water interfacial regions include shock waves, fluid eddies and shear stress from microjets and microstreaming, and localized hot temperatures. Microjets are generated when a bubble collapses near a solid surface (including an ultrasonic probe or reactor walls) several orders of magnitude larger than the cavitation bubble, with symmetric cavitation being hindered and collapse occurring asymmetrically [2,48]. As the bubble collapses, microjets of solvent are formed perpendicular to the solid surface. The diameter of a cavitation bubble is normally between 1 and 100 µm, depending on the acoustic pressure and frequency [49]. Because the rod shaped E. coli has an average length of 2–4 µm and an average diameter of 1 µm [50], compared to a cavitational bubble, it is too small to induce or be impacted by microjets directly. However, the transition of microjets (e.g., microfluid eddies and velocity gradient du/dy) may shear and inactivate E. coli. The shearing stress τ = μ (du/dy), where μ is the dynamic fluid viscosity of water.
Short MWCNTs yielded a slightly greater inactivation (0.29 ± 0.07) in the SRNOM solution than in DI water (0.18 ± 0.05). Also, unlike DI water, short MWCNTs had greater inactivation than long MWCNTs in the SRNOM solution. As shown in Figure 2, SRNOM moderately improved the dispersity of short MWCNTs in water. Consequently, well-dispersed short MWCNTs had better contact with and, therefore, caused more damage to microbial cells. However, the inactivation of long MWCNTs was almost the same between DI water (0.24 ± 0.03) and SRNOM (0.21 ± 0.06). Figure 2 shows that the presence of SRNOM only slightly enhanced the dispersion of long MWCNTs, which were relatively well dispersed in DI water already. Other than the effect of SRNOM facilitating the dispersion of MWCNTs, the complexation between SRNOM and MWCNTs through hydrophobic interactions might decrease the mobility or diffusivity (DL) of MWCNTs, which declines with an increase in molecular weight (MW) (DL ∝ MW−0.2) [51]. This side effect might reduce the inactivation of E. coli.
When ultrasound and MWCNTs worked together, the presence of SRNOM decreased the log inactivation rates of both types of MWCNTs compared to DI water. A greater decline was observed for the long MWCNTs (decrease by 0.65) than for the short MWCNTs (0.27 decrease). As mentioned before, the occupancy of the cavitational bubble–water interfacial regions and the scavenging effect of •OH radicals by SRNOM molecules may have reduced the inactivation efficiency caused by ultrasound. In other words, the slightly (long MWCNTs) to moderately (short MWCNTs) improved dispersion of MWCNTs by SRNOM did not compensate for the loss of reaction sites and •OH radicals in the sonication process, especially for the long MWCNTs.
3.4. Inactivation of E. coli in 2 mM SDS Solution
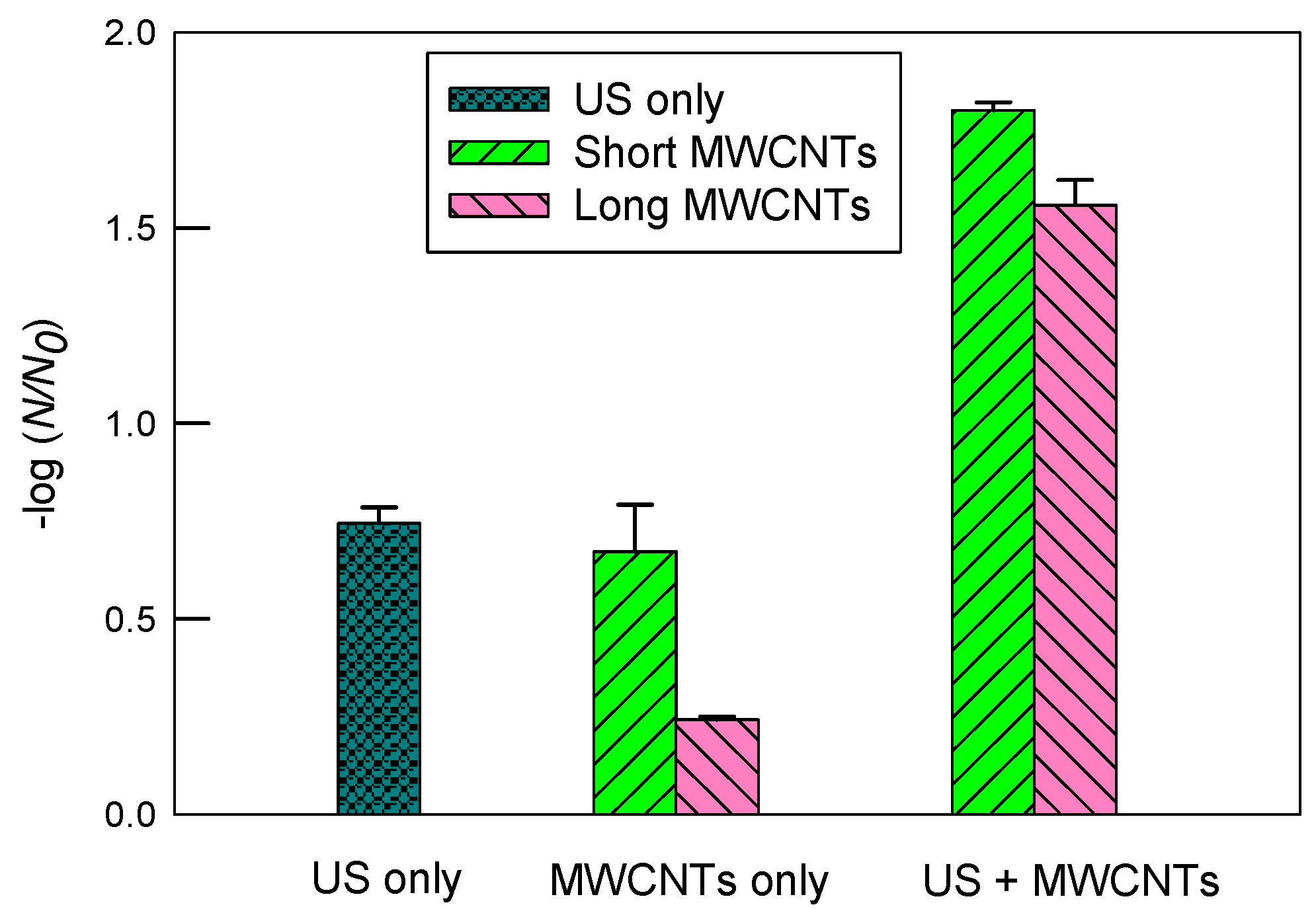
Figure 5 illustrates inactivation of E. coli in the 2 mM SDS solution. There was a moderate decrease in inactivation with ultrasound alone compared to DI water. More specifically, the log inactivation decreased from 1.31 ± 0.20 (DI water) to 0.75 ± 0.04 (SDS). In contrast, the log inactivation using MWCNTs apparently increased compared to DI water. For short MWCNTs, the log inactivation values were 1.80 ± 0.02 and 0.67 ± 0.12 with and without ultrasound, respectively. The corresponding values were 1.56 ± 0.06 and 0.24 ± 0.01 for long MWCNTs, respectively. The results suggest that SDS boosted the inactivation of E. coli by short MWCNTs considerably compared to DI water.
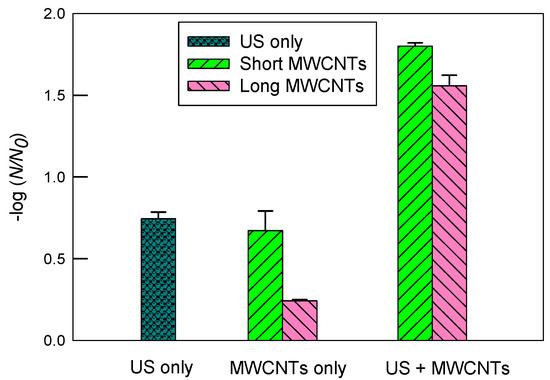
Figure 5.
The inactivation of E. coli in 2 mM SDS solution by ultrasound (US), MWCNTs, and ultrasound with MWCNTs, respectively. The sonication time was 10 min. The concentration of MWCNTs was 5 mg/L. For MWCNTs only, the mixtures of E. coli solutions were shaken at 200 rpm for 10 min. The error bars represent standard deviations.
Consistent with SRNOM, short MWCNTs had superior inactivation over long MWCNTs in the 2 mM SDS solution. Among the three types of chemical solutions tested in this study, the greatest log inactivation (i.e., 1.80 ± 0.02) was observed with short MWCNTs in 2 mM of SDS with ultrasound. SDS is an anionic surfactant, and it improved the dispersion of CNTs, as evidenced in Figure 2. In other words, when short MWCNTs were well dispersed in water, they had a greater inactivation than long MWCNTs. In contrast, SDS brought an insignificant change in inactivation for long MWCNTs compared to DI, and the enhancement of short MWCNTs was more pronounced. It is likely that long MWCNTs had a low potential for inactivation and were relatively well dispersed in DI water already, so the moderate improvement in their dispersion in 2 mM of SDS barely compensated the loss of ultrasonic disinfection capacity (e.g., scavenging free-radicals including •OH and the occupation of the cavitational bubble–water interfacial regions by SDS). Table 2 summarizes the log inactivation rates of E. coli by ultrasound and MWCNTs in different chemical solutions.

Table 2.
Log inactivation rates of E coli in different chemical solutions.
Statistical analysis was performed to compare the inactivation rates between the MWCNTs by the R program. With 2 mM of SDS without ultrasound, the t- and p-values of the inactivation rates between the two types of MWCNTs were 6.193 and 0.025, respectively, while with ultrasound, the t- and p-values were 6.251 and 0.016, respectively. A lower p-value means a more significant difference, suggesting that SDS improved the disinfection of short MWCNTs more significantly than long MWCNTs.
3.5. The Antimicrobial Mechanisms of Ultrasound and MWCNTs
Ultrasonically generated •OH radicals can contribute to inactive microorganisms. The measurement of H2O2 formation is used to estimate the intensity of ultrasonic cavitation and the relative quantities of •OH radicals generated during the sonication process [34,52]. Hydrogen peroxide is formed by the •OH radical’s self-combination or the scavenging of •H by O2, followed by subsequent self-combination [53]:
2 •OH → H2O2
•H + O2 → •HO2
2 •HO2 → H2O2 + O2
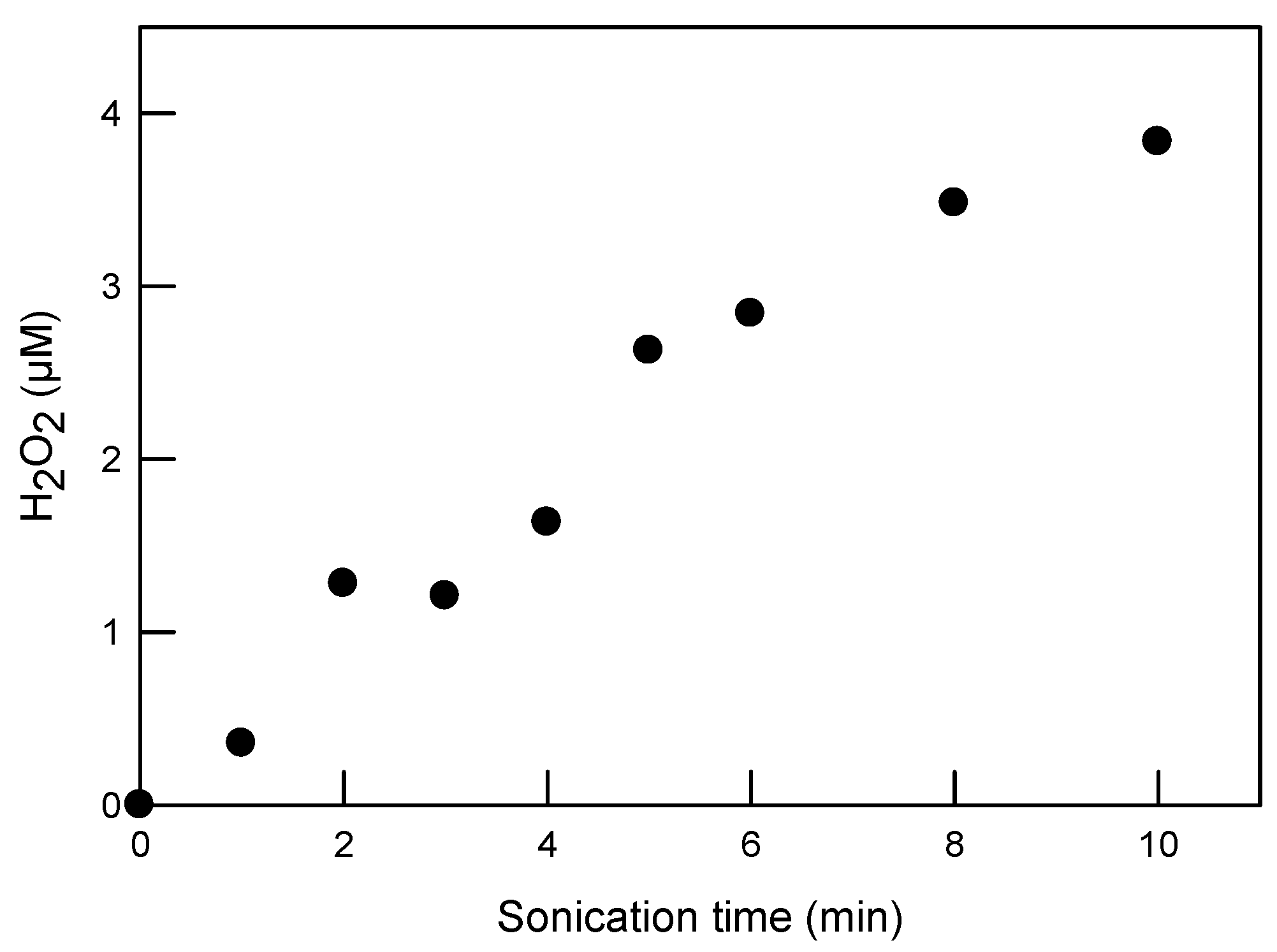
In reaction (2), the recombination rate constant measured in water under ambient conditions is 5.5 × 109 L/(mol·s) [54]. Figure 6 shows the concentration of H2O2 formed with sonication in DI water. The highest recorded concentration of H2O2 is 3.8 µM after 10 min of sonication at 20 kHz with an energy density of 0.69 ± 0.03 W/mL. Control tests showed that the ultrasonic degradation of SRNOM (by UV absorbance at 280 nm) and SDS was less than 4%, suggesting that their property alteration through sonication could be neglected in this study. This result is also consistent with our prior study [34], where NOMs were difficult to degrade using 20 kHz ultrasound in a short time period.
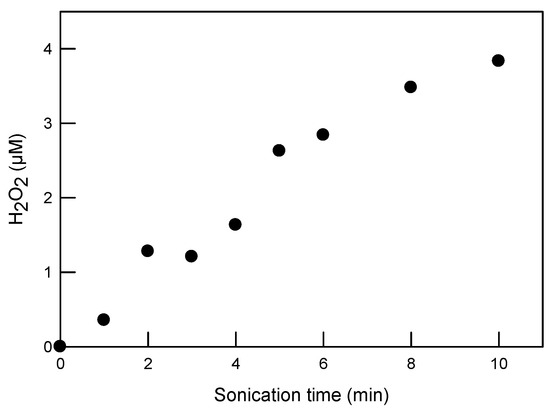
Figure 6.
H2O2 formation during sonication in DI water, with 20 kHz ultrasound at a power intensity of 29.8 ± 1.2 W/cm2 and power density of 0.69 ± 0.03 W/mL.
For sonication alone in different chemical solutions, DI water yielded the highest log inactivation (1.31 ± 0.20), followed by 10 mgC/L of SRNOM (0.96 ± 0.05), and the lowest was for 2 mM of SDS (0.75 ± 0.04). Compared to sonication in DI water, the presence of SDS and SRNOM likely decreased the antimicrobial efficiency of ultrasound through (i) occupying cavitational bubble–water interfacial regions and (ii) scavenging ultrasonically generated free-radicals including •OH. These two mechanisms would otherwise lead to the inactivation of E. coli. The weight-averaged MW of SRNOM is between 2200 and 2320 Da [55]. The carbon content of SRNOM is 50.70% according to IHSS. As a result, the molar concentration of 10 mgC/L SRNOM is less than 9 × 10−6 M, which is much smaller than 2 mM, the SDS concentration used in this study. In addition, SDS has a much smaller MW (288 Da) than SRNOM. Diffusivity (DL) increases with a smaller MW (DL ∝ MW−0.2) [51]. The greater amount of SDS and its increased diffusivity over SRNOM result in a greater accumulation of SDS in the cavitational bubble–water interfacial regions. In contrast, the E. coli concentration was around ~2 × 108/mL, or ~3.3 × 10−13 M, which is about 10 orders of magnitude less than the SDS concentration. As mentioned before, the size of E. coli cells is about 1–4 µm [50], which is much bigger than the sizes of SDS or SRNOM. The radius of SRNOM is about 28 nm [56]. Consequently, SDS is much more likely to accumulate in the cavitational bubble–water interfacial regions, decreasing access for microbial cells. In other words, SDS shields E. coli from the attacks from shock waves, fluid eddies and shear stress from microjets and microstreaming, localized hot temperatures, and •OH radicals. This may explain why SDS reduced the log inactivation of ultrasound in DI water to a greater extent than SRNOM.
However, in the presence of MWCNTs, SDS improved their dispersion in the solution. Consequently, the log inactivation of short MWCNTs increased from 0.18 ± 0.05 in DI water to 0.67 ± 0.12 in SDS, although insignificant change was observed for long MWCNTs. When combined with ultrasound, short MWCNTs in SDS yield the greatest log inactivation (1.80 ± 0.02) in this study. Since the presence of SDS reduced the antimicrobial efficiency of ultrasound, the enhancement of inactivation must stem from the short MWCNTs, which were in close contact with E. coli in SDS and energized through mechanisms including acoustic streaming [57], microstreaming [49,58], microstreamers [57,59], and cavitation collapse-produced shock waves [58,60,61] and microjets [57,62] (the transitional forms). Since CNTs also scavenge •OH radicals [32], these results suggest that •OH is less important for inactivation. In other words, the enhanced antimicrobial effects from the well-dispersed short MWCNTs and ultrasound outweighed the loss of ultrasonic disinfection (•OH and reaction sites at the cavitational bubble–water interface) by SDS and CNTs. The results of this study are in agreement with the toxicity mechanisms of CNTs, i.e., impinging bacterial cells [24] and/or direct contact with the bacteria [27].
In addition, Ince and Belen [63] showed that activated carbon particles may act as adsorbents for E. coli via hydrophobic interactions and also serve as nucleation sites to induce cavitation. Similarly to activated carbon particles, CNTs may also have these effects. In agreement, Thacker [19] compared the ultrasonic inactivation of yeast cells in saline or yeast extract–peptone–dextrose medium (an effective •OH radical scavenger) and found no significant difference. Therefore, it was suggested that the inactivation was mainly due to cavitationally produced mechanical stresses. Moreover, Hua and Thompson [15] found that 205 kHz ultrasound had a greater inactivation rate coefficient for E. coli than 358 kHz ultrasound, although the formation rate of H2O2 at 358 kHz was higher than that for the 205 kHz ultrasound. The result suggests that •OH radicals and H2O2 were not the sole mechanisms of disinfection, because 205 kHz ultrasound is supposed to produce more violent collapse and stronger sonophysical effects than 358 kHz ultrasound [33].
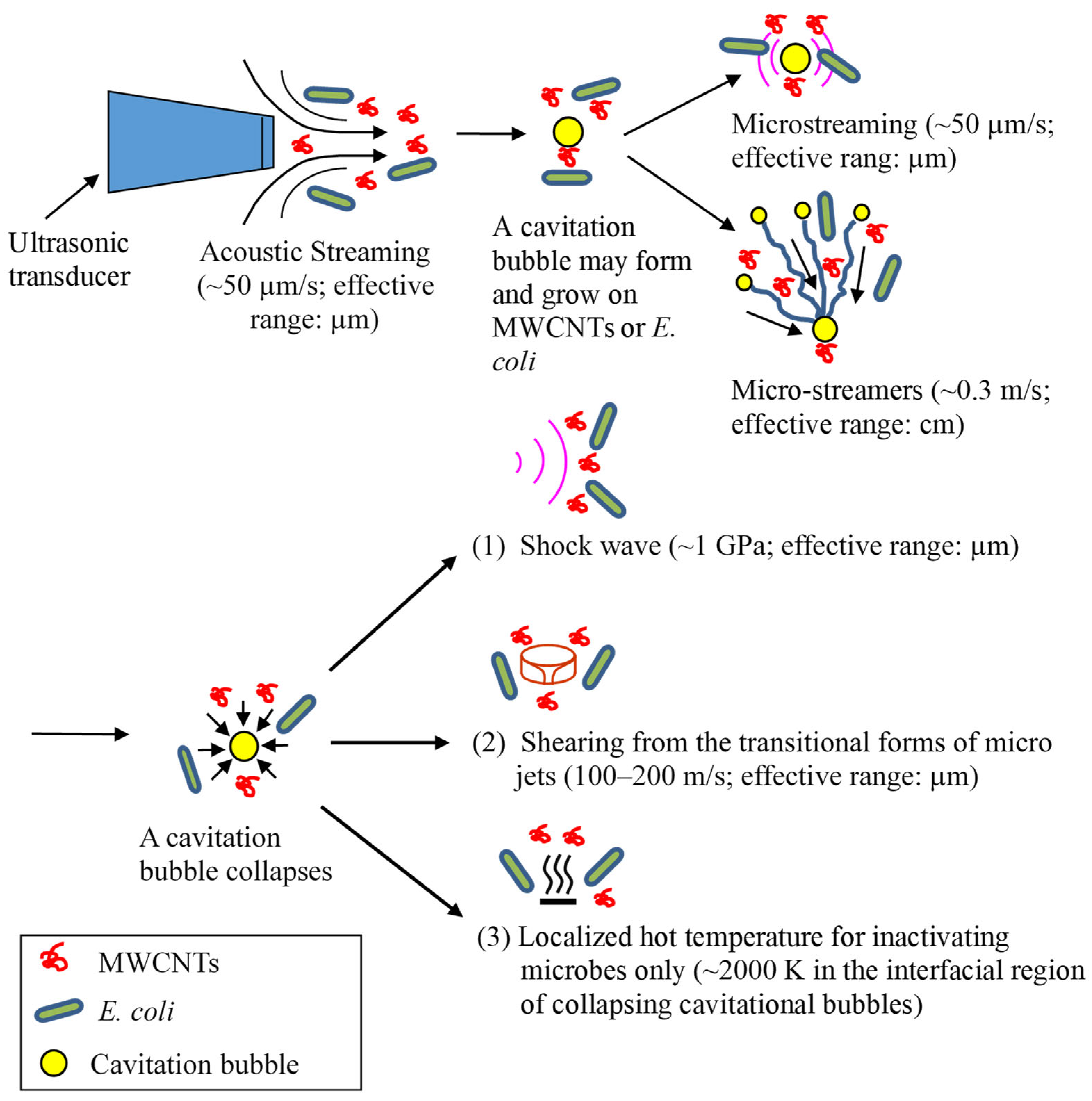
The sonophysical mechanisms used to inactivate bacteria and energize MWCNTs are illustrated in Figure 7. Acoustic streaming is the result of the absorbance of an acoustic wave by water when it travels and propagates through the medium. Consequently, water flows in the direction of the sound wave field with a velocity of up to about 10 cm/s [57]. Microstreaming forms when a bubble pulsates, as it moves fluid back and forth around the bubble. The fluid motion eventually manifests itself as a steady flow called cavitation microstreaming [58]. Microstreamers are caused when a stably oscillating bubble experiences translational radiation forces in a traveling-wave field. A bubble travels from the nucleation site towards the pressure anti-node caused by Bjerknes forces. The translating microbubbles may follow a single pathway or stream, which is referred to as a cavitation microstreamer [57,59]. An acoustic cavitation in liquids generates implosive bubble collapse and associated shock waves. Similarly to acoustic streaming, shock waves are absorbed by liquid, and as a result, liquid moves in the propagation direction of shock waves. Shock waves may also shear macromolecules in liquid [64]. At the same time, the localized hot temperature of cavitational collapse in the bubble–water interfacial region (~2000 K [3]) may inactivate microorganisms directly rather than energizing MWCNTs. Like E. coli cells, MWCNTs are too small to induce or be impacted by microjets directly. However, micro-eddies and the velocity gradient from the transition of microjets may energize MWCNTs and shear microbial cells. Overall, these sonophysical mechanisms may shear and disrupt microbial cells and/or energize MWCNTs in water to impinge microbial cells.
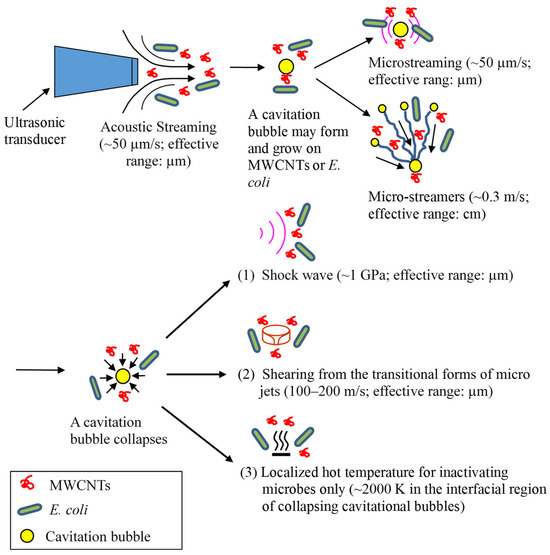
Figure 7.
The proposed sonophysical mechanisms through which ultrasound damages microbial cells and energizes MWCNTs for the inactivation of E. coli [3,49,57,58,59,62]. The sonochemical mechanisms not shown here are mainly of free-radical species, including •OH radicals.
Figure 7 shows the velocity or pressure magnitude of these phenomena from a single bubble event. Due to the differences in mass, size, and shape between E. coli cells and MWCNTs, they have different momentums, velocities, and accelerations in an ultrasonic field. Consequently, MWCNTs may contact and collide with E. coli cells and cause inactivation. It should be noted that the velocity or pressure magnitude in Figure 7 is from a single bubble event. When numerous bubbles form, grow, and collapse simultaneously and continuously, the effective working range from the transformation of these phenomena should be greater [65].
4. Conclusions
Two types of MWCNTs (short and long), ultrasound, and their combinations were investigated for the inactivation of E. coli in solutions of DI water, 10 mgC/L of SRNOM, and 2 mM of SDS. The major findings and conclusions are as follows:
- (1)
- The results showed that the inactivation rate of E. coli by MWCNTs was determined primarily by their dispersity in water solutions.
- (2)
- Overall, 2 mM SDS had the greatest improvement, while 10 mgC/L SRNOM had a moderate improvement in the dispersity of short MWCNTs in DI water. Correspondingly, the log inactivation of E. coli without ultrasound was 0.67 ± 0.12 in SDS, 0.29 ± 0.07 in SRNOM, and 0.18 ± 0.05 in DI.
- (3)
- However, long MWCNTs used alone had better dispersity in DI water and slightly greater log inactivation (0.24 ± 0.03) than short MWCNTs.
- (4)
- When combined with ultrasound, the greatest synergistic log inactivation of E. coli (1.80 ± 0.02) was observed with short MWCNTs in 2 mM of SDS, although SDS and MWCNTs could occupy the cavitational bubble–water interfacial regions and scavenge •OH radicals.
- (5)
- The results suggest that the sonophysical effects of ultrasound are more important than the sonochemical effects for the inactivation of E. coli. The sonophysical mechanisms for inactivating E. coli and/or energizing MWCNTs include acoustic streaming, microstreaming, microstreamers, shock waves, transitional forms of microjets, and localized hot temperatures from the transient collapse of cavitation bubbles. The results of this study are in agreement with the toxicity mechanisms of CNTs, i.e., impinging bacterial cells and/or direct contact with the bacteria.
- (6)
- This study aims to understand the mechanisms of key chemicals present in background solutions and their influence on disinfection using ultrasound and MWCNTs. In the future, we plan to expand the scope of our research to include chloride, soluble extracellular polymeric substances, and different microbial species in water, as well as to investigate the effects of different ultrasonic frequencies, power levels, and MWCNT concentrations.
Author Contributions
Investigation, D.C.; writing—original draft preparation, D.C.; data curation, D.C. and E.I.C.; writing—review and editing, D.C. and E.I.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Purdue University Fort Wayne Senior Faculty Summer Research Grant.
Data Availability Statement
Data are contained within the article.
Acknowledgments
This article is a revised and expanded version of a paper (ref. [66]), which was presented in the World Environmental and Water Resources Congress 2025, held in Anchorage, Alaska, USA.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mlosek, R.K.; Migda, B.; Migda, M. High-frequency ultrasound in the 21st century. J. Ultrason. 2021, 20, e233–e241. [Google Scholar] [CrossRef]
- Thompson, L.; Doraiswamy, L. Sonochemistry: Science and engineering. Ind. Eng. Chem. Res. 1999, 38, 1215–1249. [Google Scholar] [CrossRef]
- Chen, D. Applications of ultrasound in water and wastewater treatment. In Handbook on Applications of Ultrasound: Sonochemistry for Sustainability; Chen, D., Sharma, S.K., Mudhoo, A., Eds.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Pflieger, R.; Lejeune, M.; Noel, C.; Belmonte, T.; Nikitenko, S.I.; Draye, M. Diagnosing the plasma formed during acoustic cavitation in [BEPip][NTf2] ionic liquid. Phys. Chem. Chem. Phys. 2019, 21, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Merouani, S.; Dehane, A.; Hamdaoui, O.; Yasui, K.; Ashokkumar, M. Review on the impacts of external pressure on sonochemistry. Ultrason. Sonochem. 2024, 106, 106893. [Google Scholar] [CrossRef]
- Engler, C.; Robinson, C. Effects of organism type and growth conditions on cell disruption by impingement. Biotechnol. Lett. 1981, 3, 83–88. [Google Scholar] [CrossRef]
- Keshavarz, T.; Eglin, R.; Walker, E.; Bucke, C.; Holt, G.; Bull, A.; Lilly, M. The large-scale immobilization of Penicillium chrysogenum: Batch and continuous operation in an air-lift reactor. Biotechnol. Bioeng. 1990, 36, 763–770. [Google Scholar] [CrossRef]
- Sundaram, J.; Mellein, B.R.; Mitragotri, S. An experimental and theoretical analysis of ultrasound-induced permeabilization of cell membranes. Biophys. J. 2003, 84, 3087–3101. [Google Scholar] [CrossRef]
- Furuta, M.; Yamaguchi, M.; Tsukamoto, T.; Yim, B.; Stavarache, C.; Hasiba, K.; Maeda, Y. Inactivation of Escherichia coli by ultrasonic irradiation. Ultrason. Sonochem. 2004, 11, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Oyane, I.; Furuta, M.; Stavarache, C.; Hashiba, K.; Mukai, S.; Nakanishi, M.; Kimata, I.; Maeda, Y. Inactivation of Cryptosporidium parvum by ultrasonic irradiation. Environ. Sci. Technol. 2005, 39, 7294–7298. [Google Scholar] [CrossRef]
- Mahulkar, A.; Riedel, C.; Gogate, P.; Neis, U.; Pandit, A. Effect of dissolved gas on efficacy of sonochemical reactors for microbial cell disruption: Experimental and numerical analysis. Ultrason. Sonochem. 2009, 16, 635–643. [Google Scholar] [CrossRef]
- Scherba, G.; Weigel, R.; O’brien, W. Quantitative assessment of the germicidal efficacy of ultrasonic energy. Appl. Environ. Microbiol. 1991, 57, 2079–2084. [Google Scholar] [CrossRef]
- Bakhtiari, A.; Schumm, B.; Schönfelder, M.; Kähler, C.J. Investigating cell viability under shear stress in complex microstreaming flows generated by ultrasound-driven actuated microbubbles. Flow 2025, 5, E7. [Google Scholar] [CrossRef]
- Yang, Y.; Nie, G.; Bi, J.; Zhang, P.; Huo, P. Agitation-intensified sonochemistry for water disinfection. Environ. Surf. Interfaces 2025, 3, 176–182. [Google Scholar] [CrossRef]
- Hua, I.; Thompson, J.E. Inactivation of Escherichia coli by sonication at discrete ultrasonic frequencies. Water Res. 2000, 34, 3888–3893. [Google Scholar] [CrossRef]
- Joyce, E.; Phull, S.; Lorimer, J.; Mason, T. The development and evaluation of ultrasound for the treatment of bacterial suspensions. A study of frequency, power and sonication time on cultured Bacillus species. Ultrason. Sonochem. 2003, 10, 315–318. [Google Scholar] [CrossRef]
- Gogate, P.R. Application of cavitational reactors for water disinfection: Current status and path forward. J. Environ. Manag. 2007, 85, 801–815. [Google Scholar] [CrossRef]
- Blume, T.; Neis, U. Improved wastewater disinfection by ultrasonic pre-treatment. Ultrason. Sonochem. 2004, 11, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Thacker, J. An approach to the mechanism of killing of cells in suspension by ultrasound. BBA-Gen. Subj. 1973, 304, 240–248. [Google Scholar] [CrossRef]
- Broekman, S.; Pohlmann, O.; Beardwood, E.; de Meulenaer, E.C. Ultrasonic treatment for microbiological control of water systems. Ultrason. Sonochem. 2010, 17, 1041–1048. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Pan, B.; Xing, B. Adsorption mechanisms of organic chemicals on carbon nanotubes. Environ. Sci. Technol. 2008, 42, 9005–9013. [Google Scholar] [CrossRef]
- Brady-Estevez, A.S.; Nguyen, T.H.; Gutierrez, L.; Elimelech, M. Impact of solution chemistry on viral removal by a single-walled carbon nanotube filter. Water Res. 2010, 44, 3773–3780. [Google Scholar] [CrossRef]
- Liu, S.; Wei, L.; Hao, L.; Fang, N.; Chang, M.W.; Xu, R.; Yang, Y.; Chen, Y. Sharper and faster “nano darts” kill more bacteria: A study of antibacterial activity of individually dispersed pristine single-walled carbon nanotube. ACS Nano 2009, 3, 3891–3902. [Google Scholar] [CrossRef]
- Li, A.; Yao, J.; Li, N.; Shi, C.; Bai, M.; Wang, Z.; Hrynsphan, D.; Savitskayac, T.; Chen, J. Effect of biochar, graphene, carbon nanotubes, and nanoparticles on microbial denitrification: A review. Crit. Rev. Environ. Sci. Technol. 2024, 55, 99–122. [Google Scholar] [CrossRef]
- Yang, C.; Mamouni, J.; Tang, Y.; Yang, L. Antimicrobial activity of single-walled carbon nanotubes: Length effect. Langmuir 2010, 26, 16013–16019. [Google Scholar] [CrossRef]
- Kang, S.; Pinault, M.; Pfefferle, L.D.; Elimelech, M. Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir 2007, 23, 8670–8673. [Google Scholar] [CrossRef]
- Maksimova, Y.; Zorina, A.; Nesterova, L. Oxidative stress response and E. coli biofilm formation under the effect of pristine and modified carbon nanotubes. Microorganisms 2023, 11, 1221. [Google Scholar] [CrossRef]
- Moskvitina, E.; Kuznetsov, V.; Moseenkov, S.; Serkova, A.; Zavorin, A. Antibacterial effect of carbon nanomaterials: Nanotubes, carbon nanofibers, nanodiamonds, and onion-like carbon. Materials 2023, 16, 957. [Google Scholar] [CrossRef]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial effects of carbon nanotubes: Size does matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef]
- Kang, S.; Mauter, M.S.; Elimelech, M. Microbial cytotoxicity of carbon-based nanomaterials: Implications for river water and wastewater effluent. Environ. Sci. Technol. 2009, 43, 2648–2653. [Google Scholar] [CrossRef]
- Fenoglio, I.; Tomatis, M.; Lison, D.; Muller, J.; Fonseca, A.; Nagy, J.B.; Fubini, B. Reactivity of carbon nanotubes: Free radical generation or scavenging activity? Free Radic. Biol. Med. 2006, 40, 1227–1233. [Google Scholar] [CrossRef]
- Crum, L.A. Comments on the evolving field of sonochemistry by a cavitation physicist. Ultrason. Sonochem. 1995, 2, S147–S152. [Google Scholar] [CrossRef]
- Chen, D.; He, Z.; Weavers, L.K.; Chin, Y.-P.; Walker, H.W.; Hatcher, P.G. Sonochemical reactions of dissolved organic matter. Res. Chem. Intermed. 2004, 30, 735–753. [Google Scholar] [CrossRef]
- Margesin, R.; Schinner, F. Biodegradation of the anionic surfactant sodium dodecyl sulfate at low temperatures. Int. Biodeterior. Biodegrad. 1998, 41, 139–143. [Google Scholar] [CrossRef]
- Deschenes, L.; Lafrance, P.; Villeneuve, J.-P.; Samson, R. Adding sodium dodecyl sulfate and Pseudomonas aeruginosa UG2 biosurfactants inhibits polycyclic aromatic hydrocarbon biodegradation in a weathered creosote-contaminated soil. Appl. Microbiol. Biotechnol. 1996, 46, 638–646. [Google Scholar] [CrossRef]
- Rahman, A.; Brown, C. Effect of pH on the critical micelle concentration of sodium dodecyl sulphate. J. Appl. Polym. Sci. 1983, 28, 1331–1334. [Google Scholar] [CrossRef]
- Klassen, N.V.; Marchington, D.; McGowan, H.C. H2O2 determination by the I3-method and by KMnO4 titration. Anal. Chem. 1994, 66, 2921–2925. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for The Examination of Water and Wastewater, 24th ed.; American Public Health Association (APHA): Washington, DC, USA, 2023. [Google Scholar]
- Vaisman, L.; Wagner, H.D.; Marom, G. The role of surfactants in dispersion of carbon nanotubes. Adv. Colloid Interface Sci. 2006, 128, 37–46. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, J.; Wang, R.; Lu, H.; Li, L.; Kong, Y.; Qi, K.; Xin, J. Artificial lotus leaf structures from assembling carbon nanotubes and their applications in hydrophobic textiles. J. Mater. Chem. 2007, 17, 1071–1078. [Google Scholar] [CrossRef]
- Rastogi, R.; Kaushal, R.; Tripathi, S.; Sharma, A.L.; Kaur, I.; Bharadwaj, L.M. Comparative study of carbon nanotube dispersion using surfactants. J. Colloid Interface Sci. 2008, 328, 421–428. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions; John Wiley & Sons: Hoboken, NJ, USA, 1994. [Google Scholar]
- Edwards, M.; Benjamin, M.M.; Ryan, J.N. Role of organic acidity in sorption of natural organic matter (NOM) to oxide surfaces. Colloids Surf. A 1996, 107, 297–307. [Google Scholar] [CrossRef]
- Conte, P.; Agretto, A.; Spaccini, R.; Piccolo, A. Soil remediation: Humic acids as natural surfactants in the washings of highly contaminated soils. Environ. Pollut. 2005, 135, 515–522. [Google Scholar] [CrossRef]
- Hyung, H.; Fortner, J.D.; Hughes, J.B.; Kim, J.-H. Natural organic matter stabilizes carbon nanotubes in the aqueous phase. Environ. Sci. Technol. 2007, 41, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.; Yang, J.; Bitter, J.L.; Ball, W.P.; Fairbrother, D.H. Influence of surface oxygen on the interactions of carbon nanotubes with natural organic matter. Environ. Sci. Technol. 2012, 46, 12839–12847. [Google Scholar] [CrossRef]
- Neppiras, E.A. Acoustic cavitation. Phys. Rep. 1980, 61, 159–251. [Google Scholar] [CrossRef]
- Verraes, T.; Lepoint-Mullie, F.; Lepoint, T.; Longuet-Higgins, M.S. Experimental study of the liquid flow near a single sonoluminescent bubble. J. Acoust. Soc. Am. 2000, 108, 117–125. [Google Scholar] [CrossRef]
- Matthess, G.; Bedbur, E.; Gundermann, K.-O.; Loof, M.; Peters, D. Vergleichende Untersuchung zum Filtrationsverhalten von Bakterien und organischen Partikeln in Porengrundwasserleitern. I, Grundlagen und Methoden. Zentralblatt Hyg. Umweltmed. 1991, 191, 53–97. [Google Scholar]
- Cornel, P.K.; Summers, R.S.; Roberts, P.V. Diffusion of humic acid in dilute aqueous solution. J. Colloid Interface Sci. 1986, 110, 149–164. [Google Scholar] [CrossRef]
- Hua, I.; Hoffmann, M.R. Optimization of ultrasonic irradiation as an advanced oxidation technology. Environ. Sci. Technol. 1997, 31, 2237–2243. [Google Scholar] [CrossRef]
- Henglein, A. Contributions to various aspects of cavitation chemistry. In Advances in Sonochemistry; Mason, T., Ed.; JAI Press Ltd.: Bingley, UK, 1993; pp. 17–83. [Google Scholar]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms, hydroxyl radicals in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Meier, M.; Namjesnik-Dejanovic, K.; Maurice, P.A.; Chin, Y.-P.; Aiken, G.R. Fractionation of aquatic natural organic matter upon sorption to goethite and kaolinite. Chem. Geol. 1999, 157, 275–284. [Google Scholar] [CrossRef]
- Wagoner, D.B.; Christman, R.F.; Cauchon, G.; Paulson, R. Molar mass and size of Suwannee River natural organic matter using multi-angle laser light scattering. Environ. Sci. Technol. 1997, 31, 937–941. [Google Scholar] [CrossRef]
- Leighton, T. The Acoustic Bubble; Academic Press, Inc.: London, UK, 1994. [Google Scholar]
- Roy, R.A. Cavitation sonophysics. In Sonochemisty and Sonolymninescence; Crum, L.A., Mason, T.J., Reisse, J.L., Suslick, K.S., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 1999; pp. 25–38. [Google Scholar]
- Luther, S.; Mettin, R.; Koch, P.; Lauterborn, W. Observation of acoustic cavitation bubbles at 2250 frames per second. Ultrason. Sonochem. 2001, 8, 159–162. [Google Scholar] [CrossRef]
- Holzfuss, J.; Rüggeberg, M.; Billo, A. Shock wave emissions of a sonoluminescing bubble. Phys. Rev. Lett. 1998, 81, 5434. [Google Scholar] [CrossRef]
- Pecha, R.; Gompf, B. Microimplosions: Cavitation collapse and shock wave emission on a nanosecond time scale. Phys. Rev. Lett. 2000, 84, 1328. [Google Scholar] [CrossRef] [PubMed]
- Suslick, K.S. Sonochemistry. Science 1990, 247, 1439–1445. [Google Scholar] [CrossRef]
- Ince, N.H.; Belen, R. Aqueous phase disinfection with power ultrasound: Process kinetics and effect of solid catalysts. Environ. Sci. Technol. 2001, 35, 1885–1888. [Google Scholar] [CrossRef]
- Price, G.J.; Smith, P.F. Ultrasonic degradation of polymer solutions: 2. The effect of temperature, ultrasound intensity and dissolved gases on polystyrene in toluene. Polymer 1993, 34, 4111–4117. [Google Scholar] [CrossRef]
- Chen, D.; Weavers, L.K.; Walker, H.W. Ultrasonic control of ceramic membrane fouling: Effect of particle characteristics. Water Res. 2006, 40, 840–850. [Google Scholar] [CrossRef]
- Chen, D. Ultrasonic inactivation of Escherichia coli with multi-walled carbon nanotubes: The effect of solution chemistry. In Proceedings of the World Environmental and Water Resources Congress, Anchorage, AK, USA, 18–21 May 2025; pp. 1218–1229. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).