1. Introduction
LD is considered being the fourth most common cause of community-acquired pneumonia (3–15%). It is a disease with a global distribution. Approximately 0.5–5% of community acquired pneumonias are due to legionella, while epidemic outbreaks are thought to affect 0.1–5% of the population.
Legionnaires’ disease causes acute illness characterized by high fever and accompanying chills, abdominal pain and diarrheal episodes. In half of the cases, it manifests as pneumonia with bilateral localization in the lungs. It belongs to the so-called atypical pneumonias and occurs mainly in immunocompromised individuals. Legionnaires’ disease is a notifiable disease. Outbreaks have been associated with contaminated water sources, such as showers, faucets and cooling towers in air conditioners.
Legionella pneumophila, the primary disease-causing species of
Legionella, lives in rivers, sea bays, lakes and water reservoirs and can survive and grow optimally at temperatures of 32–42 °C. Although cooling towers represent a significant source of
Legionella, the truth is that their use has long been abandoned in the touristic buildings in Crete. It is transmitted to humans when aerosolized droplets of contaminated water are inhaled or aspirated. As a result, Legionellosis can occur, which primarily results in either Legionnaires’ disease or Pontiac fever. Although Legionnaires’ disease is a severe life-threatening pneumonia usually leading to hospitalization with an approximate mortality of 10%, Pontiac fever is a flu-like syndrome with only mild symptoms. The disease onset is usually 2–14 days after infection with the bacteria and may cause serious morbidity with pneumonia, fever, nausea, abdominal pain, diarrhea, vomiting and bradycardia. Neurological symptoms, although rare, include headache, lethargy and encephalopathy [
1,
2].
Legionella bacteria live as intracellular pathogens of freshwater protozoa and replicate within amoebae, making water and soil ideal environmental reservoirs. The bacteria grow optimally in temperatures of 25–42 °C, and these temperatures have been associated with increased risk of exposure and infection [
1,
3].
Contemporary published studies examining the association between Legionnaires’ disease and weather events have produced varying conclusions, primarily focused on outbreaks. Rainfall, temperature and humidity were the main factors associated with increased Legionnaires’ disease (LD) incidence, as reported in these studies. The contribution of the different factors varied across studies, which could be explained by different approaches or genuine differences due to local conditions [
4,
5]. Moreover, Fishman et al. suggested that humidity and rainfall appeared as better predictors than temperature [
4].
The current study examined the associations between climatic parameters and LD on a precise ecological and geographic scale using Travel-associated Legionnaires’ disease (TALD) case reports and weather stations from across Crete Island, to capture the variability. TALD cases can occur on cruise ships, in hotels, and at resorts. A common feature among these settings is the presence of a large, often complex, water system that can be challenging to maintain properly. The importance of our research is established in the re-emergence of TALD cases that were hospitalized in intensive care units and received significant publicity at a national level, as well as in the enormous importance that tourism has for the socio-economic well-being of Crete. It seems that Legionnaires’ disease re-emerged after the COVID-19 pandemic, and this fact may have to do with the focusing of attention by public health experts on the COVID-19 pandemic and also with the probable relaxation of epidemiological surveillance and Legionnaires’ disease prevention measures.
By disclosing critical information, progress is expected to be achieved in prevention, thus strengthening the protection of public health and the safety of residents and tourists on the island.
2. Materials and Methods
2.1. Case Ascertainment
Case data processing involved the collection of annual reports from local representatives of the National Public Health Organization, Athens, Greece and the Laboratory of Clinical Microbiology and Microbial Pathogenesis, School of Medicine, University of Crete, Heraklion, Crete.
TALD cases from hotels across Crete were collected between 2000 and 2022. The data used in this analysis originated from the national surveillance database and include every traveler recorded with LD between January 2000 and December 2022. All the cases were first diagnosed in the travelers’ countries of origin.
A confirmed case was considered a patient with a positive
Legionella urinary antigen test, isolation of the pathogen by culture, paired serology or positive Polymerase Chain Reaction (PCR) method. Since there was no proven correlation among cases, and they could not be considered as clusters, all cases were considered as sporadic [
3].
2.2. Meteorological Data
Weather and environmental data were collected from the National Weather Service (
www.emy.gr). Data for each variable were downloaded from all available weather stations (
Figure 1).
The meteorological variables included in this analysis were weekly maximum temperature, weekly cloudiness, weekly wind speed, and weekly relative humidity.
The data from the meteorological stations of each prefecture were georeferenced to each spatial unit. Practically, each meteorological station corresponds to each prefecture. In Rethymnon Prefecture, significant deficiencies were detected in the meteorological data from the Rethymnon station for the years 2009–2022. We therefore calculated the meteorological variables for Rethymnon Prefecture for these years, taking into account the values from the nearby stations of Heraklion and Souda through the formula: R = 0.614 × S + 0.386 × H, where S are the meteorological variables from the Souda station, H the variables from the Heraklion station and R the corresponding values for Rethymnon.
Heraklion is located at the center of the island of Crete and is the largest city and the administrative capital of the island. It is the fourth-largest city in Greece with a municipal population of ~180,000 inhabitants. Heraklion was Europe’s fastest-growing tourism destination for 2017, according to Euromonitor, with 11% growth in international arrivals. According to the ranking, Heraklion was ranked as the 20th most-visited region in Europe, 66th on the planet and 2nd in Greece for the year 2017, with 3.2 million visitors, and 19th in Europe for 2018, with 3.4 million visitors. The industrial area of Heraklion is located south of the city but very near to it, just a couple of kilometers. It houses several enterprises such as internet service providers, kitchen furniture manufacturers, liquor stores, livestock breeders, logistics companies, machine shops, metal fabricators, and more. Chania is the capital of the Chania regional unit. It lies along the northwest coast of the island of Crete, about 70 km west of Rethymnon and 145 km west of Heraklion. The municipality has ~110,000 inhabitants. The Industry Park is located 7 km east from the city of Chania and mainly consists of small industries. Rethymnon is the capital of Rethymnon regional unit and has a population of more than 35,000 inhabitants. It is located between the cities of Heraklion and Chania. All three cities are located on the northern part of the island of Crete, and they account for the highest percentage of tourists who visit the island annually.
The Industrial Area of Rethymnon is located on the outskirts of the city. The area houses a variety of businesses, including manufacturing, warehousing, and other commercial activities [
6].
Unfortunately, Lasithi Prefecture meteorological station lacked over 75% of data and could not be used in our research.
2.3. Statistical Methodology
Weekly periods were used as reference time points in discrete time analysis, since weekly observations avoid zero counts and reduce rounding errors. The hotels were designated as “spatial units”. Meteorological variables were collected in the range (lag) of a weekly period, and their average values for these weeks were used [
7,
8,
9]. Regression-type models were fitted for modeling the response counts, corresponding to suitable distributions for count data, such as Poisson and the Negative Binomial (NB).
We employed Poisson and Negative Binomial (NB) regression models as they are well-suited for analyzing count data such as the weekly number of reported TALD cases. The Poisson model is a standard starting point for modeling count data due to its simplicity and interpretability, assuming the mean and variance of the outcome variable are equal. The Negative Binomial model is an alternative approach to the Poisson regression, since it introduces an additional dispersion parameter allowing it to better accommodate overdispersion in count data. This model was preferred over Poisson based on comparative goodness-of-fit metrics (e.g., deviance, Pearson chi-square, AIC, BIC), which consistently indicated superior fit for the NB model. This methodological choice aligns with best practices in epidemiological modeling where overdispersed count outcomes are present.
2.4. Poisson Regression Model
Let
denote the weekly count of TALD cases in spatial unit
i. Assuming
, the model specifies the mean incidence rate
as
where
are covariates (e.g., temperature, humidity, seasonal and spatial indicators) and
are the regression coefficients that are estimated from a set of data. Note that
is called the intercept.
The regression coefficients are estimated using the method of maximum likelihood. The logarithm of the likelihood function is
2.5. Negative Binomial Regression Model
The Negative Binomial is often used as an overdispersed alternative to the Poisson model. It is similar to the Poisson model but incorporates an additional term to account for the excess variance count data (integers), where the majority of data points are clustered toward lower values of a variable. Here, the response
follows:
with mean:
and variance:
where α > 0 is the dispersion parameter. When α = 0, the Negative Binomial model reduces to the Poisson model.
The regression coefficients are estimated using the method of maximum likelihood [
10]. The log-likelihood function for the NB model is
In the above equation, Γ denotes the Gamma function.
To evaluate model performance and select the most appropriate regression framework for the count data for both Poisson and Negative Binomial regression models, we assessed model fit using several goodness-of-fit statistics. Specifically, we calculated the deviance and Pearson chi-square statistics, both of which compare the observed and expected counts under the fitted model. Lower values of these statistics indicate a better model fit. In addition, we used information criteria—including the Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC), and Consistent AIC (CAIC)—which balance model fit with complexity by penalizing the inclusion of additional parameters. Models with lower AIC, BIC, and CAIC values are considered more parsimonious and better suited to the data. Finally, we compared the log-likelihood values, with higher values (closer to zero) indicating better explanatory power. These metrics collectively guided the selection of the Negative Binomial model over the Poisson model due to evidence of over dispersion in the data.
In this research, we did not utilize continuous sampling distributions—as in Choi et al. [
11], where a Gaussian and a square root transform model were used—due to the issues arising when using continuous sampling distributions for datasets consisting of point observations [
12].
The models involve the weekly cases of Legionnaires’ disease in regions of the Greek island of Crete as a response variable and utilize both environmental variables (max temperature, cloudiness, humidity and wind speed) as well as temporal information in the form of a seasonal variable and the year effects as independent variables. In addition, a spatial categorical variable was included, indicating three different regions in Crete (namely the Chania, Heraklion and Rethymnon regions).
3. Results
A total of 64.2% of the 214 TALD cases reported in Crete during 2000–2022 were male, and the mean age was 62.4 years (SD = 10.9 years). Probably the increased incidence of Legionnaires’ disease in men depends mostly on the harmful habit of smoking, which is more common in men than in women and also the fact that men seem to suffer more frequently from lung diseases, like chronic obstructive pulmonary disease or lung cancer, than women, which seem to predispose to severe pneumonia [
2]. The most common countries of origin of the TALD cases recorded were France (8.8%), the Netherlands (8.8%), and Germany (4.4%).
The number of cases per year is given in
Table 1, both for the island of Crete and by region. Most cases occurred in the years 2017 and 2018, with the percentages being 12.1% and 12.6%, respectively. Specifically, in Heraklion, most cases were recorded in 2018 (11.5%) and 2002 (10.3%), in Rethymnon in 2018 (11.5%), and in Chania in 2017 (15.5%), 2018 (10.7%) and 2015 (9.7%).
The Poisson regression and negative binomial regression analysis (
Table 2) involved the weekly cases of Legionnaires’ disease as a response variable in Crete, utilizing both environmental variables (i.e., max temperature, cloudiness, humidity and wind speed) as independent variables as well as temporal information in the form of a seasonal variable and the year effects. In addition, a spatial categorical variable was included, indicating three different regions in Crete (Chania, Heraklion and Rethymnon).
In our regression models, we selected 2022, autumn, and Chania as reference categories for interpretative clarity and statistical relevance. Specifically, the year 2022 was chosen as the reference year because it is the most recent year in the dataset, representing the baseline against which historical changes in TALD incidence can be compared. The “Autumn” category was selected as the seasonal reference because it exhibited the highest number of TALD cases, serving as a logical comparator for identifying reductions in other seasons. Finally, “Chania” was used as the regional reference category since it recorded the highest number of cases among the three regions studied, making it a suitable baseline for regional comparisons.
Based on the results provided, the Negative Binomial model appears to fit the data better than the Poisson model. More specifically, according to the Deviance and Pearson Chi-Square goodness-of-fit statistics, the Deviance and Pearson Chi-Square values for the Negative Binomial model (832.411 and 3643.888, respectively) are lower compared to the Poisson model (1005.863 and 3935.567, respectively) (
Table 2), with lower values indicating a better fit to the data.
According to rest information criteria, the Akaike’s Information Criterion (AIC) is lower for the Negative Binomial model (1418.287) compared to the Poisson model (1440.650). Similarly, the Bayesian Information Criterion (BIC) and Consistent AIC (CAIC) are lower for the Negative Binomial model (1603.655 and 1633.655) than the Poisson model (1626.017 and 1656.017). Lower values for these criteria indicate a model that better balances goodness-of-fit with model complexity.
Finally, the log-likelihood value is higher (less negative) for the Negative Binomial model (−679.143) compared to the Poisson model (−690.325), suggesting that the Negative Binomial model better explains the observed data.
The lower information criteria values and improved deviance and chi-square values strongly favor the Negative Binomial model over the Poisson model. Overall, the Negative Binomial model provides a better fit to the data.
Upon selecting the Negative Binomial model, a stepwise selection procedure was performed to derive the best model that involved statistically significant independent variables only (
Table 3). To do so, a backward elimination stepwise technique was followed.
Based on the results of the application of the Negative Binomial regression results, we reached the following conclusions:
3.1. Year Effects
The reference year is 2022, and each coefficient for the YEAR variable represents the difference in the log count of cases compared to 2022. The significance levels (p-values) highlight whether these differences are statistically meaningful. Many of the year effects have negative coefficients, particularly for earlier years (e.g., YEAR = 2000, YEAR = 2001, YEAR = 2003). This indicates that, compared to 2022 (reference year), the expected count of cases was generally lower in those years, at the 5% significance level. For example, in 2000, the coefficient is −1.790 (p-value = 0.023), meaning a significantly lower count of cases compared to 2022. In 2017 and 2018, the coefficients are positive (e.g., 0.869, p-value = 0.028; 0.935, p-value = 0.017), suggesting a higher count compared to 2022.
Non-significant results for other years (e.g., 2019, 2021) imply no substantial difference in cases compared to 2022. Specifically, in 2000, the log count of cases was 1.790 lower than in 2022. In raw counts, this corresponds to an approximate reduction by a factor of e 1.790 ≈ 0.167, meaning there were about 83% fewer cases in 2000 compared to 2022. Similarly, there were significantly fewer cases in 2001, with the count reduced by a factor of e 2.410 ≈ 0.09, or about 91% fewer cases (YEAR = 2001: Coefficient = −2.410, p-value = 0.023).
As regards 2003, there were about e 2.425 ≈ 0.089 times as many cases in that year compared to 2022, an approximately 91% reduction (YEAR = 2003: Coefficient = −2.425, p-value = 0.022). In 2017, the log count of cases was 0.869 higher than in 2022. This translates to e 0.869≈2.38, meaning there were about 138% more cases in 2017 than in 2022 (YEAR = 2017: Coefficient = 0.869, p-value = 0.028). Similarly, in 2018, there were e 0.935≈2.55 times as many cases compared to 2022, a 155% increase (YEAR = 2018: Coefficient = 0.935, p-value = 0.017).
3.2. Seasonal Effects
The reference season in our modeling is autumn, and the coefficients for other seasons indicate differences in the log count of cases relative to autumn.
All seasons (winter, spring, summer) have negative coefficients compared to the reference category (autumn), and these effects are highly significant (p < 0.001). This means that the count of cases is significantly lower in these seasons than in autumn. For instance, the largest drop in expected cases (β: −1.891; p < 0.001) is observed in winter. A moderate decrease in cases during summer is also observed (β: −0.900; p < 0.001). Hence, the log count of cases in winter is 1.891 lower than in autumn. This corresponds to e 1.891 ≈ 0.15, meaning about 85% fewer cases occur in winter compared to autumn. Similarly, for autumn, the log count is reduced by e 1.262 ≈ 0.28, or about 72% fewer cases occur in spring compared to autumn. Also, the log count for the summer indicator is reduced by e 0.900 ≈ 0.41, or 59% fewer cases occur in summer compared to autumn. Overall, cases are significantly more frequent in autumn, making it the riskiest season for Legionnaires’ disease on Crete.
3.3. Regional Effects
The analysis used Chania Prefecture as the reference region to compare the log count of cases across regions. Results showed a significant reduction in cases in Rethymnon, with a coefficient of −0.981 (p< 0.001), corresponding to approximately 62.5% fewer cases compared to Chania. In contrast, the difference between Heraklion and Chania was not statistically significant, with a coefficient of 0.049 (p = 0.798), indicating no meaningful variation in case counts between these two regions.
3.4. Environmental Variables
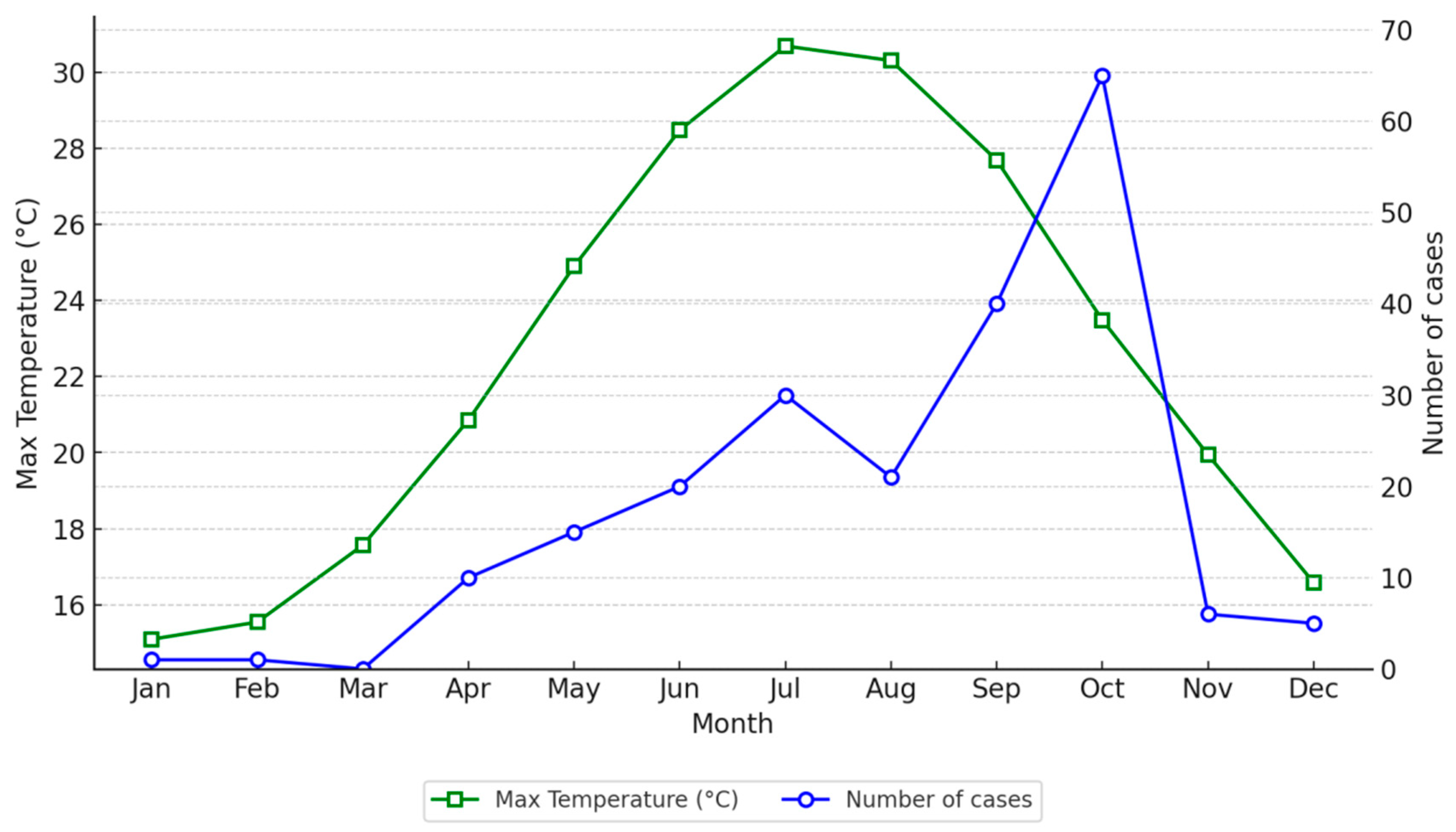
The analysis of environmental variables, modeled as continuous predictors, revealed their impact on the log count of Legionnaires’ disease cases. Maximum temperature (Tmax) (
Figure 2) showed a positive association, with a coefficient of 0.093 (
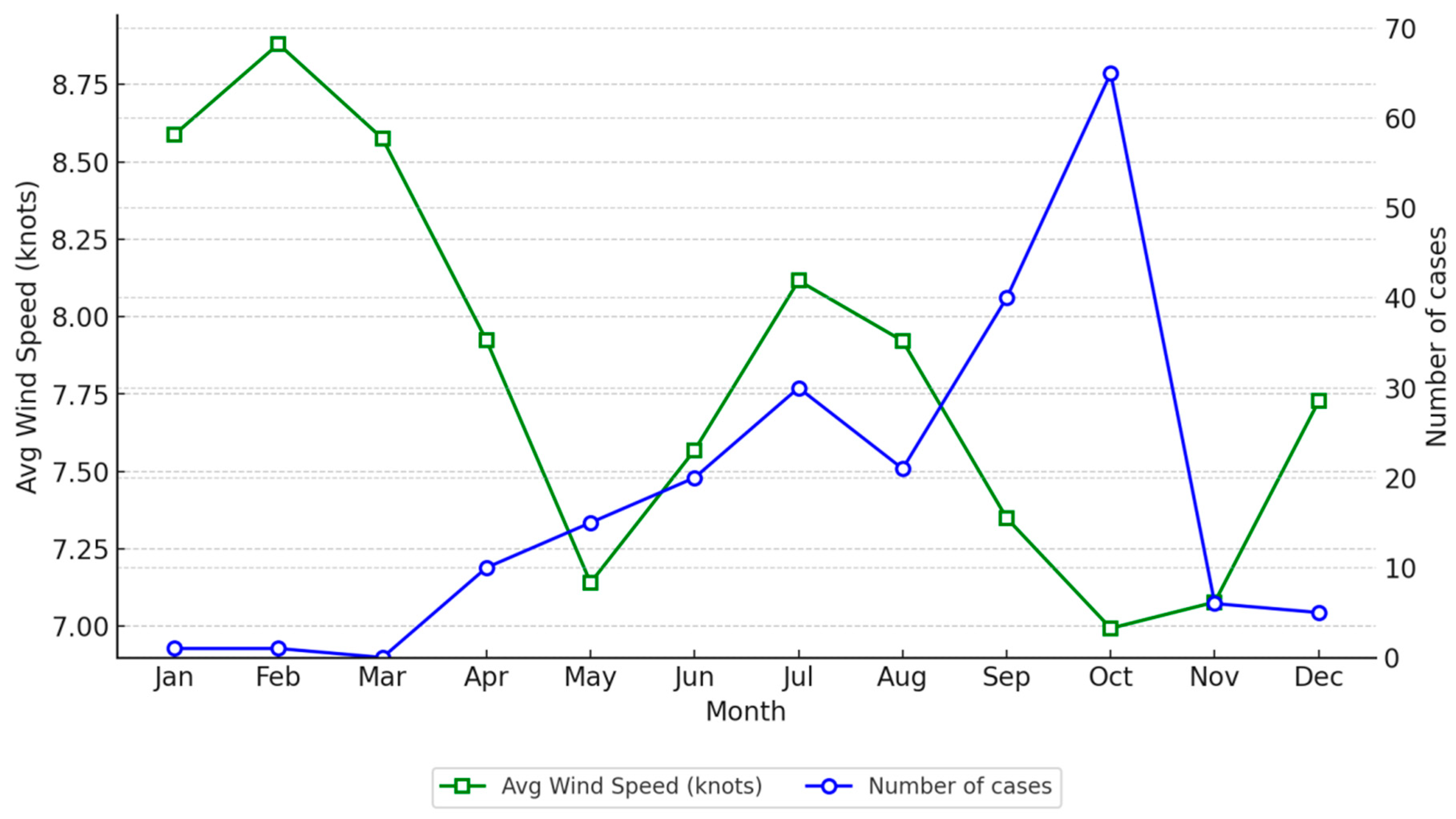
p< 0.001), indicating that a 1 °C increase in Tmax results in a 0.093 increase in the log count of cases. This corresponds to an approximate 9.8% increase in the expected case count. Conversely, wind speed (
Figure 3) demonstrated a negative association, with a coefficient of −0.081 (
p = 0.030), suggesting that a one-unit increase in wind speed reduces the log count of cases by 0.081, equivalent to about an 8% decrease in the expected count.
4. Discussion
Although the first Legionnaires’ disease (LD) cluster was recorded in 1976, it was practically only after 2000, and particularly after 2010, that the scientific community tried to establish possible correlations between incidence of the disease and environmental/geographical factors [
13,
14,
15].
In 2006, a sudden unexpected increase in the number of sporadic cases was recorded in Northern Europe. Scientists started to hypothesize that weather conditions might have been responsible [
16]. Temperature, rainfall, and atmospheric pressure are associated with LD risk.
According to the Karagiannis et al. (2009) study in the Netherlands regarding precipitation and RH, warm and wet weather patterns, but not the hottest ones, are associated with more LD cases [
14]. Dunn et al. [
17] found an association between LD, RH, and wind speed, but the association did not remain after controlling for season and year, see also [
18]. A prospective study of a large cohort of hospitalized patients with CAP clearly showed that the frequency of LD correlates directly with rainfall and has a distinct seasonal pattern compared to pneumonia caused by other etiologies [
19]. The work by Halsby et al. (2014) provided evidence of association between the risk of LD in England and Wales and temperature, with an apparently long lag of 14 days, possibly modified by RH, and also between LD and rainfall [
13]. The largest study was conducted in the Netherlands and included around 800 LD cases over four (4) years. It showed that the 4-week mean temperature, 2-week rainfall intensity and 2-week rainfall duration were the best predictors of higher LD incidence [
20].
Seasons and local weather conditions also affect disease incidence, as indicated by other studies. Community-acquired LD cases occurring during wintertime may be associated with sources less influenced by meteorological conditions. Unusually high temperatures were not associated with higher risk for LD in the study by Beaute et al. [
21]. The results of another survey in the United States suggest that the incidence of LD varies considerably by season and local weather patterns. Specifically, LD is more likely to occur in moderate warm (15.5–26.7 °C) and very humid (>80.0%) months [
22].
Villanueva1 & Schepanski (2019) found that the occurrence of fog was related to four LD outbreaks across Europe between 2004 and 2009, suggesting that the presence of fog droplets and/or the thermal inversions associated with fog may play a role in the disease spreading [
23].
Braeye et al. (2020) applied distributed lag nonlinear models to avoid temporal autocorrelation over different days. Although this research has not delivered consistent results on short-term associations, given seasonality, a sequence of precipitation, followed by high relative humidity and low wind speed, showed a statistically significant association with the number of cases four (4) to six (6) days later [
24].
More recently, several similar studies have been published with interesting results. The Han study [
25] analyzed legionellosis incidence in the US during the 20-year period between 1999 and 2018, and correlated with concurrent temperature, precipitation, solar ultraviolet B (UVB) radiation, and vehicle mileage data. These results suggest that warm temperature and precipitation surplus have likely elevated the density of Legionella bacteria in the environment [
25].
The findings of another survey carried out by Passer et al. [
15] in Minnesota between 2011 and 2018 show that there is an increased risk of sporadic LD with increased precipitation. Higher precipitation can lead to more saturated soil, which increases the risk of contamination with Legionella [
15].
Our study provides evidence of an association between TALD cases of LD and meteorological conditions, seasonality, and probably local weather conditions. We suggest that temperature and wind speed may be associated with the risk of the disease. More specifically, there seems to be an increased risk of LD in Crete with increased (maximum) temperature and lower wind speed.
In fact, the incidence and the severity of Legionnaires’ disease depend on the virulence of Legionella and the vulnerability of the infected persons. Moreover, the virulence has to do with the number of microbes that the contaminated aerosols contain, their serogroup and the infectious agents they express. The effect of temperature on the survival of
Legionella pneumophila is significant. It can survive for over a year in tap water at room temperature. It has been isolated in hot water systems at temperatures up to 66 °C. As the temperature drops below 37 °C, the rate of bacterial reproduction decreases, and below 20 °C, there is little or no bacterial growth.
L. pneumophila survives and grows at temperatures between 25 °C and 45 °C with the optimal growth temperature between 32 and 42 °C [
1]. Possible hypotheses to explain the effect of temperature on the virulence of
Legionella are as follows:
1. Increased expression of virulence genes contained in the Legionella genome.
2. Consequent increased synthesis of surface proteins that facilitate the attachment and invasion of the microbes into alveolar macrophages. (ex. Thermic shock proteins).
The above, as a consequence, facilitate the long-term intracellular survival and proliferation of Legionella in the alveolar macrophages, thus increasing infectiveness, while bypassing the patient’s immune system.
On the other hand, high temperatures (along with increased relative humidity) predispose vulnerable persons (ex. those who suffer from chronic obstructive lung disease) to pneumonia.
Finally low wind speed favors the presence of contaminated aerosols for a longer time in the atmosphere, resulting in an increased likelihood of infection.
These results are in line with studies from foreign countries, as mentioned above. Indeed, the relationship between (max) temperature and TALD incidence has shown variability across studies. In our research, maximum temperature emerged as a statistically significant predictor of TALD cases, with higher temperatures being associated with increased risk. This aligns with known biological behavior of
Legionella pneumophila, which thrives in warm water environments typically between 25 and 42 °C. However, differences in findings across studies may stem from multiple factors: geographic location, temporal resolution of data (daily vs. weekly vs. monthly), methodological approaches, and lag structures used in the analysis. For instance, in regions where temperatures routinely exceed the optimal range for Legionella growth, or where indoor water systems are tightly regulated, temperature may not exhibit a strong association. Additionally, the effect of temperature may interact with other variables such as humidity or precipitation, which can either amplify or attenuate its impact. In general, temperature and relative humidity (RH) seem to have statistically significant correlations with the incidence of Legionnaires’ disease in the majority of the surveys. Wet, humid weather predisposes to the increased incidence of the disease. And this is the situation “from West to East”, in the USA and also in European countries, the United Kingdom, the Netherlands and Italy [
26]. Although we did not provide statistically significant correlation between relative humidity and LD incidence in our survey, this is probably due to the fact that the prefectures studied maintain a relatively stable pattern of relative humidity values throughout the year and especially during the months of epidemic peaks of the disease, so it is difficult to establish a statistically significant correlation due to the low variation in the RH variables.
Overall, the analysis reveals that Legionnaires’ disease (LD) cases are influenced by temporal, spatial, and environmental factors. Temporally, cases are lower in earlier years and during winter, spring, and summer, possibly due to improved public health measures, reporting practices, or seasonal transmission patterns. Additionally, cases are significantly more frequent in autumn, making it the riskiest season for LD on the island. Spatially, Rethymnon has significantly fewer cases than Chania, while Heraklion shows no significant difference, likely reflecting variations in population density, water systems, or environmental conditions. Environmentally, higher maximum temperatures increase LD risk, aligning with the bacterium’s preference for warm environments, while higher wind speeds may reduce transmission by dispersing contaminated aerosols. We also found that in 2017–2018, there was an inexplicable spike in the number of TALD cases in Crete, which needs further investigation.
Actionable insights include targeted interventions that focus on the autumn months and the Chania and Heraklion regions, where cases are higher. Surveillance during periods of elevated temperatures is critical to managing outbreaks. Investigating local water management systems in Chania and Heraklion prefectures could reveal factors contributing to the higher case counts there. Preventive measures include proper maintenance of the water supply network and all potential sources of contamination in terms of temperature, chlorination and acidity and also informing the public about the severity of the disease and ways to avoid exposure. More specifically, the European Union’s Community directives EN 15975-2, as well as 98/83/EC, 2015/1787 of the corresponding European Council, define the framework for the quality of drinking water, but also more generally the quality of water intended for use by the population. As part of preventive controls and quantitative detection of the Legionella pneumophila bacterium, sample checks of hotel units should be carried out. The surveillance should be carried out in accordance with what was ratified by the European Parliament with Directive No 2119/98/EC. The definition of the number of sample points to be inspected depends on the area and facilities of the hotel unit (National Action Plan for Public Health 2008–2012). Systematic monitoring of the hotel unit is essential and must include, as a priority, microbiological analysis to check for the presence of Legionella pneumophila. The preventive measures involve sufficient chlorination—[Cl] > 0.2–1 mg/dl is prohibitive for the growth of Legionella,—disinfection of the water pipes at temperatures > 60 °C and finally maintenance of the water pH in the range of 7–7.6. Also UV irradiation and filtration of the water systems are of extreme significance. All these measures are effective although with limitations [
27].
All the above findings of our survey emphasize the significance of spatial epidemiological approaches in identifying high-risk areas and guiding public health interventions to prevent and control Legionella outbreaks in Crete and similar regions worldwide.
Limitations
One of the limitations of our study was the use of an ecological study design. The data included foreign tourists who were diagnosed with LD, but not a comparison population. In addition, the small number of cases (approximately 200 TALDs) was an adverse factor in the statistical processing of data. Another limitation was the fact that the vast majority of cases were recorded on the northern coast of Crete, where the majority of the cities and hotels are located. This factor narrows the geographical limits of the study.
Finally, the data collected by the meteorological stations of the national meteorological service had significant deficiencies, which was a fact that created significant difficulties in calculating weekly values and limited the data used to a small number of weather stations, practically one station per prefecture. However, the fact that these stations were located on the northern coast of the island provided reliable meteorological data for the regions investigated.
In our research, we gained insights into the spread and occurrence of LD in Crete, Greece. Crete—as the largest island of Greece, with significant tourism development—is an area with particular prominence in tourism, with many visitors from various parts of the world. LD, as an airborne disease transmitted through micro droplets, can pose a major threat to public health and tourism sustainability on the island.
This is a pioneering study that will contribute to improving our knowledge of the epidemiological characteristics of the disease in a specific geographical area. The results of this research will have a significant impact on public health and provide valuable information for the development of preventive and disease control measures on the island of Crete.
Our study is unique in Greece and the first one to be conducted on a Greek island. Although similar studies have been conducted in Central Europe and the USA, this is the only one that involves a Mediterranean island, considering the specific meteorological conditions of the southeastern Mediterranean Sea. The study results are a significant addition to the already existing bibliography but also offer precious knowledge as far as the management of the island’s tourism industry is concerned. This research aspires to contribute to the safety and protection of tourists and the population of Crete.