Removal of Octinoxate, a UV-filter Compound, from Aquatic Environment Using Polydimethylsiloxane Sponge
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
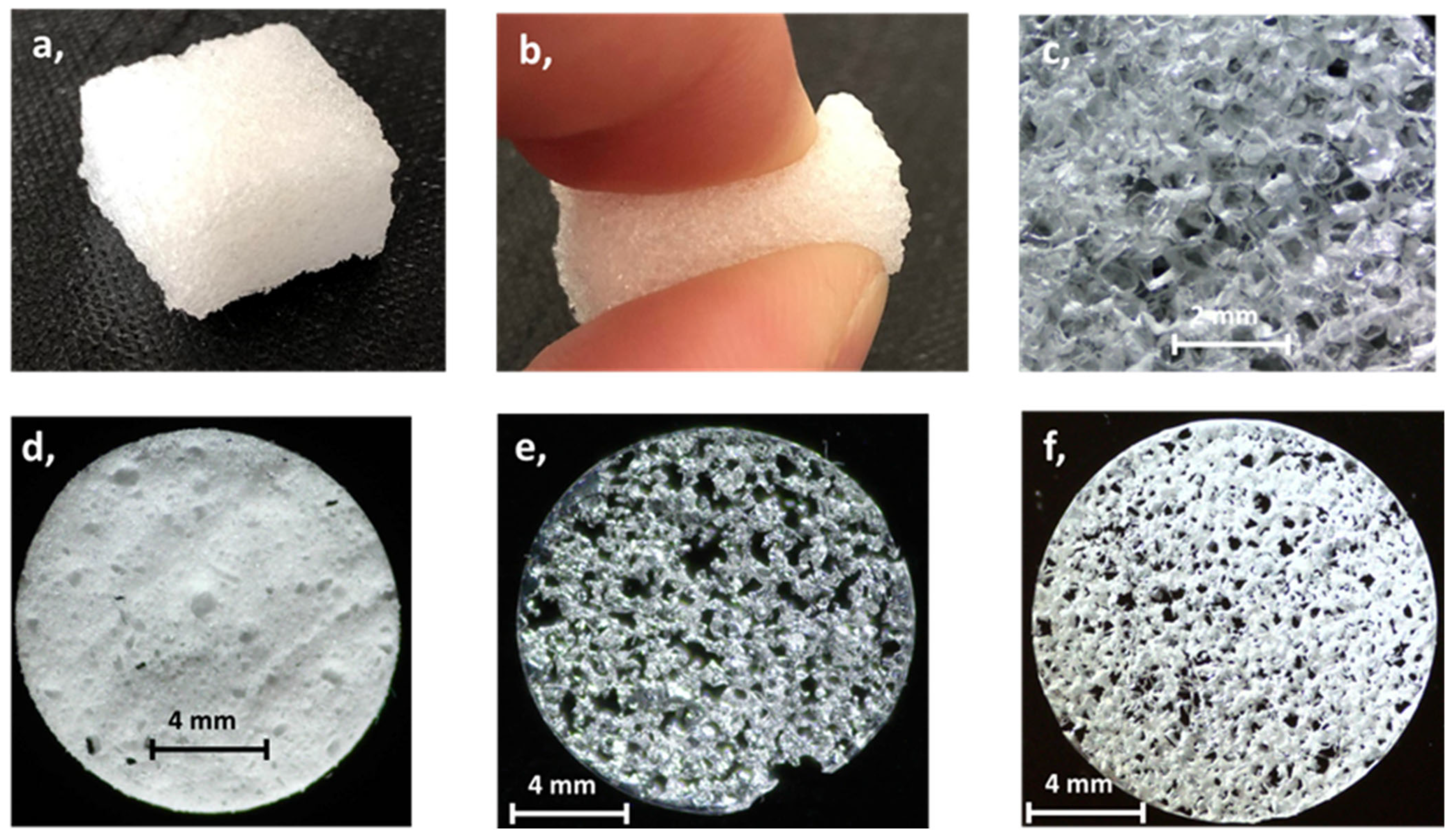
2.3. Fabrication of PDMS Sponge
2.4. Adsorption Studies
3. Results and Discussion
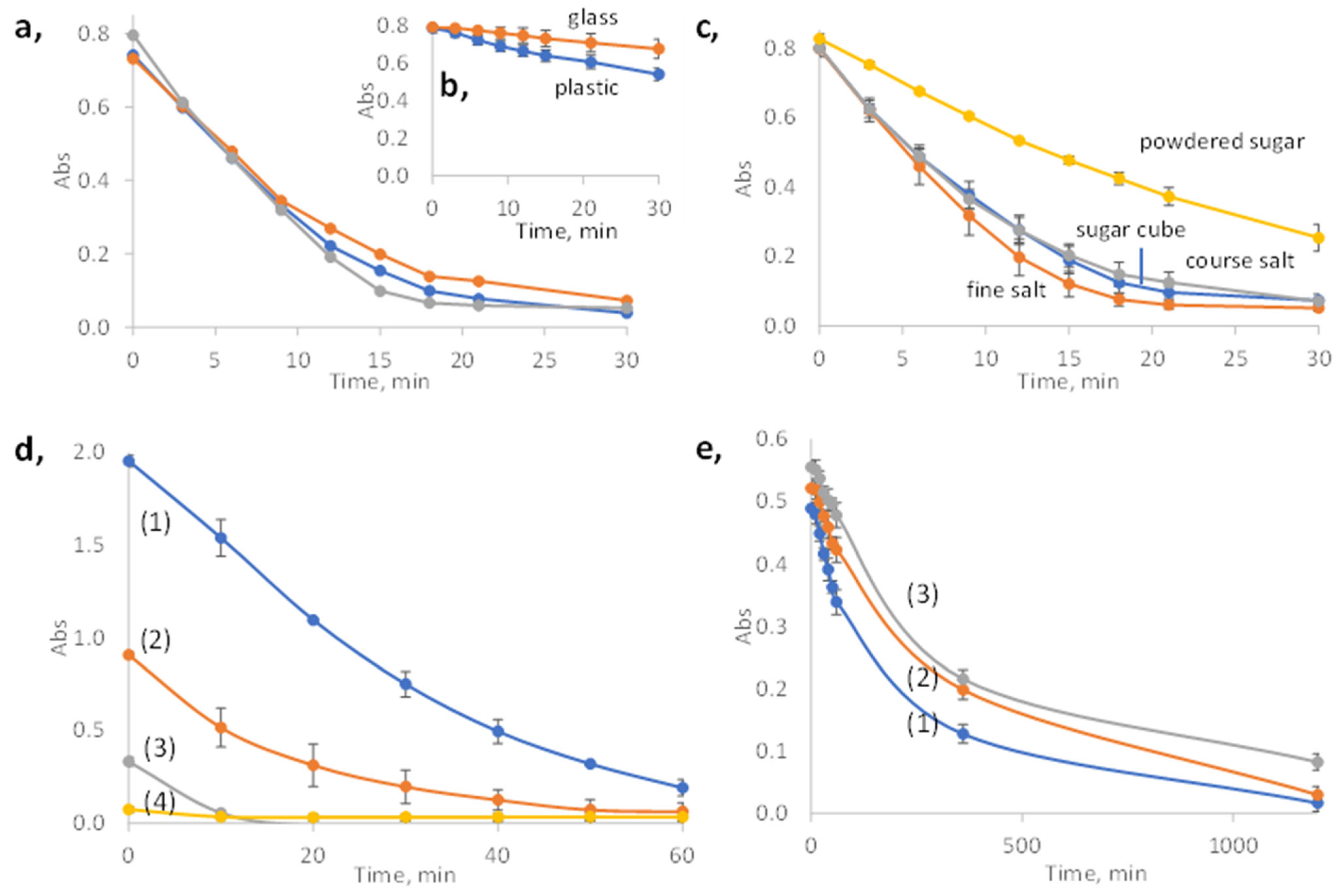
3.1. Adsorption of Octinoxate on Different PDMS Sponges
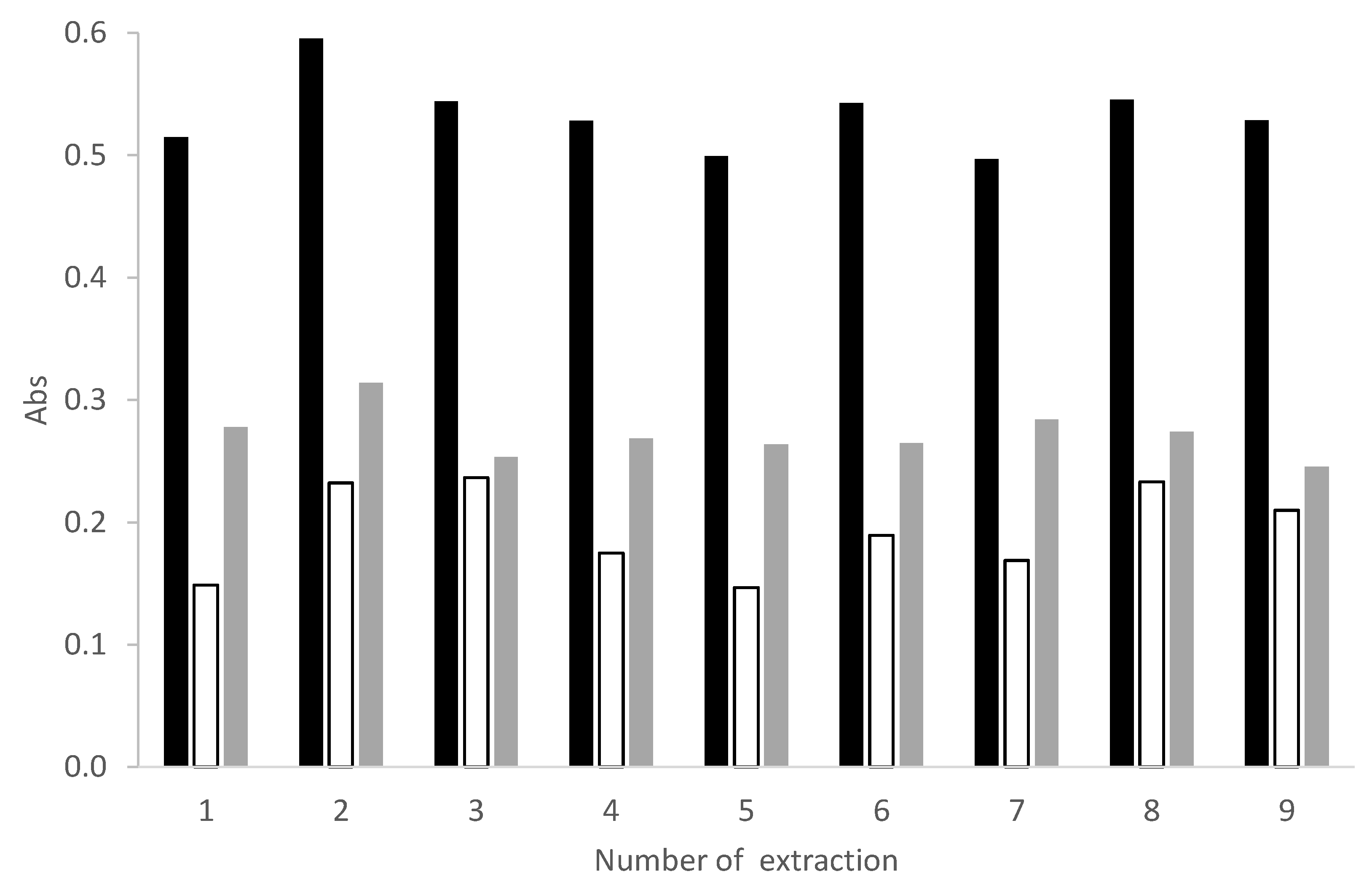
3.2. The Adsorption Capacity and Recyclability of the PDMS Sponge
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kameda, Y.; Kimura, K.; Miyazaki, M.; Tanaka, H. Occurrence and profiles of organic sun-blocking agents in surface waters and sediments in Japanese rivers and lakes. Environ. Sci. Proc. Imp. 2011, 13, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Ka, Y.; Ji, K. Waterborne exposure to avobenzone and octinoxate induces thyroid endocrine disruption in wild-type and thrαa−/− zebrafish larvae. Ecotoxicology 2022, 31, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Kwon, T.H.; Im, H.; Moon, D.I.; Baek, D.J.; Seol, M.L.; Duarte, J.P.; Choi, Y.K. A Polydimethylsiloxane (PDMS) Sponge for the Selective Absorption of Oil from Water. ACS Appl. Mater. Interfaces 2011, 3, 4552–4556. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, L.; Li, B.; Zhang, J.; Wang, A. Durable superhydrophobic/superoleophilic PDMS sponges and their applications in selective oil absorption and in plugging oil leakages. J. Mater. Chem. A 2014, 2, 18281–18287. [Google Scholar] [CrossRef]
- Duan, S.; Yang, K.; Wang, Z.; Chen, M.; Zhang, L.; Zhang, H.; Li, C. Fabrication of highly stretchable conductors based on 3D printed porous poly(dimethylsiloxane) and conductive carbon nanotubes/graphene network. ACS Appl. Mater. Interfaces 2016, 8, 2187–2192. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Duan, S.; Zhang, L.; Wang, Z.; Li, C. Three dimensional porous stretchable and conductive polymer composites based on graphene networks grown by chemical vapour deposition and PEDOT: PSS coating. Chem. Commun. 2015, 51, 3169–3172. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Kim, H.; Qaiser, N.; Baumli, P.; Hwang, B. A Review of Recent Progress in Fabrication Methods and Applications of Polydimethylsiloxane Sponge. J. Natur. Fib. 2023, 20, 2264497. [Google Scholar] [CrossRef]
- Keller, A.; Zainulabdeen, K.; Warren, H.; Panhuis, M. Fabrication of porous PDMS sponges using spontaneously self-removing sacrificial templates. MRS Adv. 2022, 7, 495–498. [Google Scholar] [CrossRef]
- Lord, H.; Pawliszyn, J. Evolution of solid-phase microextraction technology. J. Chromatogr. A 2000, 885, 153–193. [Google Scholar] [CrossRef] [PubMed]
- Boscaini, E.; Alexander, M.L.; Prazeller, P.; Mark, T.D. Investigation of fundamental physical properties of a polydimethylsiloxane (PDMS) membrane using a proton transfer reaction mass spectrometer (PTRMS). Int. Mass Spectrom. 2004, 239, 179–186. [Google Scholar] [CrossRef]
- Si, P.; Wang, J.; Zhao, C.; Xu, H.; Yang, K.; Wang, W. Preparation and morphology control of threedimensional interconnected microporous PDMS for oil sorption. Polym. Adv. Technol. 2015, 26, 1091–1096. [Google Scholar] [CrossRef]
- Tran, D.N.H.; Kabiri, S.; Sim, T.R.; Losic, D. Selective adsorption of oil–water mixtures using polydimethylsiloxane (PDMS)–graphene sponges. Environ. Sci. Water Res. Technol. 2015, 1, 298–305. [Google Scholar] [CrossRef]
- Zhang, A.; Chen, M.; Du, C.; Guo, H.; Bai, H.; Li, L. Poly(dimethylsiloxane) Oil Absorbent with a Three-Dimensionally Interconnected Porous Structure and Swellable Skeleton. ACS Appl. Mater. Interfaces 2013, 5, 10201–10206. [Google Scholar] [CrossRef] [PubMed]
- McNair, H.M.; Miller, J.M. Basic Gas Chromatography, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2009; ISBN 978-0-470-43954-8. [Google Scholar]
- Amiri, A.; Baghayeri, M.; Karimabadi, F.; Ghaemi, F.; Maleki, B. Graphene oxide/polydimethylsiloxane-coated stainless steel mesh for use in solid-phase extraction cartridges and extraction of polycyclic aromatic hydrocarbons. Microchim. Acta 2020, 187, 213. [Google Scholar] [CrossRef] [PubMed]
- Pawliszyn, J. Solid Phase Microextraction: Theory and Practice; Wiley: New York, NY, USA, 1997; ISBN 978-0-471-19034-9. [Google Scholar]
- Zhang, Y.; Idota, N.; Tsukahara, T. Surface-functionalized polydimethylsiloxane sponges for facile and selective recovery of molybdenum from aqueous/acidic solutions. J. Hazard. Mat. 2025, 488, 137485. [Google Scholar] [CrossRef] [PubMed]
- Giusto, L.A.R.; Pissetti, F.L. Polydimethylsiloxane amino functionalized sponge for adsorption of copper in water. J. Sol-Gel Sci. Technol. 2021, 99, 243–251. [Google Scholar] [CrossRef]
- Liu, L.; Chen, J.; Zhang, W.; Fan, M.; Gong, Z.; Zhang, J. Graphene oxide/polydimethylsiloxane composite sponge for removing Pb(II) from water. RSC Adv. 2020, 10, 22492. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Duan, T.; Shao, J.; Yu, H. Fabrication method for structured porous polydimethylsiloxane (PDMS). J. Mater. Sci. 2018, 53, 11873–11882. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, P.; Németh, Z.; Szabó, R.; Lázár, I.; Pirger, Z.; Gáspár, A. Removal of Octinoxate, a UV-filter Compound, from Aquatic Environment Using Polydimethylsiloxane Sponge. Water 2025, 17, 2306. https://doi.org/10.3390/w17152306
Szabó P, Németh Z, Szabó R, Lázár I, Pirger Z, Gáspár A. Removal of Octinoxate, a UV-filter Compound, from Aquatic Environment Using Polydimethylsiloxane Sponge. Water. 2025; 17(15):2306. https://doi.org/10.3390/w17152306
Chicago/Turabian StyleSzabó, Péter, Zoltán Németh, Ruben Szabó, István Lázár, Zsolt Pirger, and Attila Gáspár. 2025. "Removal of Octinoxate, a UV-filter Compound, from Aquatic Environment Using Polydimethylsiloxane Sponge" Water 17, no. 15: 2306. https://doi.org/10.3390/w17152306
APA StyleSzabó, P., Németh, Z., Szabó, R., Lázár, I., Pirger, Z., & Gáspár, A. (2025). Removal of Octinoxate, a UV-filter Compound, from Aquatic Environment Using Polydimethylsiloxane Sponge. Water, 17(15), 2306. https://doi.org/10.3390/w17152306






