Abstract
Oil and gas field water not only contains low concentrations of lithium but also a lot of suspended matter, inorganic salt, and organic matter. Both inorganic ions and organic substances influence the extraction of lithium. To improve the extraction efficiency of low-concentration lithium in oil and gas field water, the effects of Na+, K+, Ca2+, Mg2+, Cl−, Br−, SO42−, NO3−, and organic substances on the extraction efficiency of lithium were studied. The results showed that Na+ can promote the extraction of lithium to a certain extent, and lithium ions competed with K+ for extraction; however, the separation coefficient remained more than 13. Ca2+ and Mg2+ have a significant influence on the extraction of lithium and should be removed prior to extraction. Cl−, SO42−, and NO3− have little influence on the extraction solution of lithium. Among the organic components, a high concentration of long-chain alkane has a certain effect on the extraction efficiency of lithium, while other substances have little effect. On this basis, the first step for precipitating impurity ions and the second step for solvent extraction of lithium were established. After removing the impurity ions, the extraction efficiency of lithium can reach over 90%. Taking 15L of oil and gas field water as the research object, after extraction, back extraction, concentration, depth impurities removal by extraction, and precipitation drying, the purity of the lithium carbonate product can be achieved at 99.28%. This study can provide technical support for the efficient extraction of low-concentration lithium from oil and gas field water.
1. Introduction
Water in oil and gas fields refers to the groundwater adjacent to and associated with oil and gas in natural water systems, including reservoir water and non-reservoir water [1]. During the stage of oil and gas accumulation, the water in the oil and gas field undergoes a long evolutionary process, together with the oil and gas, and dissolves lithium in the formation through complex physical and chemical reactions, which has potential exploitation and utilization value [2]. Currently, the primary methods for extracting lithium from oil and gas field water include the adsorption method [3], the membrane method [4], and the extraction method [5,6]. The adsorption method is generally used to extract lithium by filling the adsorbent into an adsorption column and then passing the Hanli solution through it, utilizing the larger surface area of the adsorbent to achieve the adsorption separation of lithium [7]. The commonly used adsorbents for lithium extraction are metal-based. Metal-based adsorbents can be broadly categorized into aluminum (Al)-based, manganese (Mn)-based, and titanium (Ti)-based. At the current stage, Al-based adsorbents exhibit the highest potential for industrial applications due to their high technological maturity; however, the selective uptake performance of Li+ requires further improvement. Mn-based adsorbents exhibit excellent Li+ adsorption performance but lack the necessary stability and robustness for long-term applications due to the gradual dissolution of Mn from their structure. Ti-based adsorbents are the newest entry and are found not to exhibit the limitations of Al- or Mn-based adsorbents. Ti-based adsorbents are currently being investigated for the extraction of lithium from salt lake brines [8]. MGX Minerals, a Canadian company, utilizes a new technology of nanofiltration to extract lithium from water in the Sturgeon Lake oil field, achieving lithium extraction in just a few hours. The company has applied for patents on this technology, but its core technology is not publicly available. Chen et al. [9] evaluated the performance of three kinds of lithium adsorbents in the brine of oil and gas fields. The results showed that under optimal conditions, the saturated adsorption capacities of the three adsorbents were approximately 7.9 mg/g, 12.5 mg/g, and 15.3 mg/g, respectively. The service life of the aluminum series was higher than that of the titanium and manganese series, with an average adsorption rate of 79.2% for lithium. Li et al. [10] used a manganese molecular sieve to adsorb lithium from oil and gas field water. The mass concentration of lithium-ion in the oil and gas field water was 65.2 mg/L, and the concentration of lithium-ion in the water after adsorption was 7.8 mg/L. The recovery efficiency of lithium-ion reached 88.0%, and the adsorption capacity of lithium was 24.5 mg/g. Tang et al. [11] researched lithium extraction technology in complex oil and gas fields with water containing sulfur, oil, and suspended matter. The process of air flotation, desulfurization, catalytic oxidation, flocculation, sedimentation, and two-stage filtration was selected for preconditioning oil and gas field water.
Membrane-based processes have been one of the most widely studied technologies for recovering lithium from aqueous sources due to their better energetics and lower footprint compared to chemical exchange or stripping methods [7]. Here, the membrane either acts as a high-precision sieve, allowing the selective permeation of Li+ over other ions and resulting in a Li+-enriched permeate solution, or as a membrane adsorber capable of sequestering Li+ ions. The first approach to selective permeation relies on the single-ion species selectivity of the membrane and is used in the extraction of lithium from salt lake brines or geothermal waters, where the initial concentration of lithium is typically hundreds of ppm. Nanofiltration (NF) membranes are typically used to enrich the concentration of lithium (500–7000 ppm) prior to the precipitation of solid lithium compounds. The desired properties for NF membranes are high water permeability and high Li+/Mg2+ selectivity, ensuring the process can be operated at low applied pressures (<8 bar) [12]. However, due to the large amount of dissolved oil and organic matter in the oil and gas field water, there are often problems such as membrane channel blockage and adsorbent pollution, which seriously affect the adsorption effect of lithium, resulting in the adsorption method and membrane method have not been industrialized in the extraction of lithium from oil and gas fields water [13].
Liquid–liquid extraction involves the selective removal of compounds or metal complexes from a mixture using a solvent, provided that the two phases are immiscible [7]. The solvent extraction method has been successfully applied in the fields of lithium extraction from salt lakes and lithium recovery from waste batteries, yielding promising results. The extractants used in solvent extraction for lithium extraction primarily include neutral organophosphorus systems and β-dione systems [14]. Shi et al. [15] extracted lithium from low-grade salt lake brine with an ultra-high Mg/Li ratio using a TBP–kerosene–FeCl3 system.
The effects of various factors (such as TBP concentration, Fe/Li molar ratio, and acidity of brine on Li+ extraction) were studied, and the optimum conditions of single-stage extraction were identified as follows: TBP concertation was 75%, Fe/Li molar ratio was 1.3, and the acidity of brine was 0.01 mol/L. Hu et al. [16] used TOP to replace TBP, and the separation capacity and extraction mechanism of the TOP/FeCl3 system were studied. Density functional theory (DFT) calculations were employed to model the cationic complexes of the TOP and TBP systems. The results showed that in the presence of FeCl3, the extraction efficiency of Li+ (ELi) reached 91%, and the Li/Mg separation factor (beta Li/Mg) was 5688. However, the use of neutral organophosphorus TBP to extract lithium not only requires FeCl3 to be used as a synergistic agent but also necessitates operation under acidic conditions, which can lead to emulsification and has a corrosive effect on equipment [17,18]. The β-dione system does not require FeCl3 and can be operated under medium alkaline conditions, which is suitable for extracting lithium from the produced water of neutral oil and gas fields. Dang et al. [19] studied the extraction of lithium from smackover oilfield water in the southern United States using a diketo-based (ditrimethylacetyl methane) chelating agent. The system has a strong selectivity for lithium ions in the presence of other metal ions. Seo et al. [20] extracted and recovered lithium by PC88A and HBTA. The results indicated a sixfold difference in efficiency when these extractants were used in the counter-current solvent-extraction process. HBTA’s striking superiority over PC88A in terms of efficiency. Future studies should include evaluations of equipment and extractant costs, as well as overall efficiency. Zhang et al. [21] investigated the extraction process of lithium using a co-extraction system of benzoyltrifluoroacetone/trioctylphosphine oxide (HBTA/TOPO) in a submerged lithium mother liquor containing approximately 1.8 g/L of lithium, which is a byproduct of the lithium carbonate production process. The results show that the organic phase composition is 0.4 mol/L HBTA+ 0.4 mol/L TOPO + kerosene. The extraction efficiency of lithium is up to 98% by three-stage counter-current extraction under the condition of O/A 1:1. However, the composition of oil and gas field water is more complex than that from salt lake brine and waste battery leaching liquid phase, so it is necessary to deeply explore the influence mechanism of organic and inorganic substances on solvent extraction of lithium, determine the key influencing factors, and lay the foundation for the future industrial production of lithium extraction from of oil and gas fields water.
In recent years, considerable work has been conducted on the impact of coexisting substances in water on the lithium extraction effect [22]. Zhang et al. [23] used solvent extraction to extract lithium from salt lake brines with high Na+/Li+ and studied the extraction sequence of cations in salt lake brines. The results showed that the order of cation extraction from brine by diketone extractants was Mg2+ > Ca2+ > Li+ > Na+ > K+. Jiang et al. [24] used the solvent extraction method to extract lithium from the shale gas fields’ water in two steps. To weaken the influence of cations on the extraction of lithium ions, Di (2-ethylhexyl) phosphate (D2EHPA) was initially used as an extractant to remove impurity ions, such as Ca2+, Mg2+, Sr2+, and Ba2+. Then, Di (2-ethylhexyl) phosphate (D2EHPA) and tributyl phosphate (TBP) were used to extract the Li+. Mg2+ > Ca2+ > Li+ > Na+ > K+. Gan et al. [25] investigated the influence of anions on the lithium extraction effect. In order to improve the efficiency of lithium extraction, firstly introduce (NH4)2S2O8 to remove excess Cl−, then introduce Ca (OH)2 to adjust the aqueous pH value, and finally add hydrogen peroxide to eliminate C2O42− impurity ions to improve the purity of the product. Lee et al. [26] used TOC as the organic component to investigate the effects of TOC and the length of the alkane carbon chain on lithium extraction. The results show that the extraction efficiency of lithium decreases with increasing total organic carbon (TOC) concentration and the length of the alkane carbon chain. In summary, the influence of related coexisting substances on lithium extraction has achieved some results. However, the oil and gas field water has certain particularities; the research results cannot be directly applied to the extraction of low-concentration lithium from oil and gas field water.
In this study, benzoyltrifluoroacetone (HBTA) was used as the extractant, and trin-octylphosphine oxide (TOPO) was used as a synergist. Sulfonated kerosene was used as diluent, the influence mechanism of cations (K+, Na+, Ca2+, Mg2+, etc.), anions (Cl−, Br−, SO42−, NO3−, etc.) and organic components (xylene, phenol, naphthalene, n-hexane acetate and n-hexadecane, etc.) on the extraction of low concentration lithium from oil and gas field water were investigated in detail. The low concentration of lithium in oil and gas field water was extracted by a two-step precipitation method and solvent extraction. The preparation route of lithium carbonate was determined by taking the actual oil and gas field water as the research object. This study provides technical support for the efficient extraction of lithium from oil and gas field water.
2. Materials and Methods
2.1. Materials and Reagents
The oil and gas field water was used as the research object, and simulated water samples were prepared. The composition is shown in Table 1. Extractant HBTA and TOPO were analytically pure grade (Shanghai Maclin Biochemical Technology Co., Ltd., Shanghai, China), the diluent was sulfonated kerosene (Jinan Hengrui Chemical Co., Ltd., Jinan, China), and other reagents were purchased from Sinopsin Chemical Reagent Co., Ltd., Shanghai, China.

Table 1.
Components in Simulated oil gas field produced water.
2.2. Experimental Methods
2.2.1. Extraction
A certain quality of HBTA and TOPO was dissolved in sulfonated kerosene and then placed on a magnetic stirrer to ensure even stirring. A volume of 2 mol/L NaOH solution was gradually added for saponification. The prepared organic phase was mixed with a specified volume of simulated water, as per the comparison, and the magnetic stirrer was used to stir at a constant speed for 6 min, thereby clarifying and separating the two phases. Take a certain volume of the water phase, determine the content of metal ions, and take three parallel samples each time. According to the analysis and determination results, the extraction efficiency, separation coefficient, and partition ratio were calculated.
2.2.2. Preparation of Lithium Carbonate
Take 15 L of actual oil and gas field water, filter it, and remove the suspended matter. After precipitation and impurity removal, divide the solution into five groups, each of 3 L, for use. The 1.5 L organic phase was prepared with 0.15 mol/L HBTA, 0.15 mol/L TOPO. The organic phase was saponified by a 2 mol/L NaOH solution. The saponification degree was 100%. The loaded lithium organic phase was obtained by mixing 1.5 L of extracted organic phase with 3 L of oil and gas field water for oscillatory extraction. Back extraction was performed by mixing 75 mL of 6 mol/L hydrochloric acid with 1.5 L of the loaded lithium organic phase. The organic phase regeneration was completed using a 20% Na2CO3 solution and a 2 mol/L NaOH solution, and the organic phase was recycled 5 times to complete the extraction of 15 L of oil and gas field water. The 5 times back-extracted Li-rich liquid was mixed, and after evaporation and concentration, a 0.1 mol/L NaOH solution was added to adjust the pH to 4. P507 organic phase with a saponification degree of 50% and 1.5 mol/L was used to remove impurities at the extraction depth under the condition of a 1:1 oil–water ratio. The pH value of the Li-rich solution was adjusted to 10 after impurity removal. The Li-rich solution was heated to 80 °C, and 10 g of Na2CO3 was added. The Li2CO3 was then precipitated and separated. The precipitate of Li2CO3 was washed 3 times and then dried in the oven to obtain the Li2CO3 product. The concentration of Li+ in the solution was measured before and after extraction, after back extraction, after deep impurity removal, and after precipitation. The extraction efficiency, back extraction efficiency, and transfer efficiency of lithium were calculated.
2.3. Calculation Method
2.3.1. Extraction Efficiency
The extraction efficiency (E) is the proportion of the metal ions extracted into the organic phase after the extraction equilibrium, to the total metal ions in the two phases. The formula is listed in (1).
In the formula, C0 and Ce are respectively the initial mass concentration and equilibrium mass concentration of metal ions in aqueous phase, mg/L. V0 and Va, respectively, refers to the volume of the organic phase and water phase, L.
2.3.2. Back Extraction Efficiency
The back extraction efficiency (R) refers to the proportion of the lithium ions amount of liquid entering the back extraction liquid after back extraction, to the total lithium ions in the amount of two phases in the back extraction process. The formula is listed in (2).
In the formula, C0HCI and CeHCI represent, respectively, the initial mass concentration and equilibrium mass concentration of lithium ions in the back extraction liquid, expressed in mg/L. C0 refers to the initial mass concentration of lithium ions in the aqueous phase, mg/L. V0 and VHCI, respectively, refer to the volume of the organic phase and the back extraction liquid, L.
2.3.3. Transfer Efficiency
Transfer efficiency (T) refers to the proportion of lithium ions in the back extraction liquid to the amount of lithium ions in the initial water phase. The formula is listed in (3).
2.3.4. Distribution Ratio
The distribution ratio (D) refers to the ratio of lithium ion concentration in the organic phase to the lithium ion concentration in the raffinates at extraction equilibrium. The formula is listed in (4).
In the formula, CO and CR represent, respectively, the mass concentrations of metal ions in the organic phase and the raffinates at extraction equilibrium, expressed in mg/L.
2.3.5. Separation Coefficient
The separation coefficient (β) refers to the ratio of the distribution ratio (D) of two substances in two phases. The formula is listed (5).
In the formula, DA is the distribution ratio of substance A. DB is the distribution ratio of substance B. The higher the separation coefficient, the higher the separation selectivity of the extractant for substance A.
2.4. Analysis Method
The initial pH values of the aqueous feed solutions were determined by a pH meter (pH211, HANNA, Villafranca Padovana, Italy). The concentrations of metal ions in the water phase were analyzed by an ICP-MS Agilent 7200 (Agilent, San Clara, CA, USA). The surficial topography was determined by the atomic force microscope (BY3000, Semilab, Hilton Head Island, SC, USA). Infrared (IR) analysis was performed on Thermo Scientific L1280080, Waltham, MA, USA. The TOC content was determined using the combustion oxidation method with a TOC-L CPN total organic carbon analyzer manufactured by Shimadzu, Kyoto, Japan.
3. Results and Discussion
3.1. Extraction Mechanism
In the experiment, the organic extract phase consisted of HBTA, TOPO, and sulfonated kerosene. A low concentration of lithium was extracted from water in oil and gas fields. The extraction mechanism is as follows: under alkaline conditions, the hydroxyl H+ in the enol form of HBTA can be exchanged with Na+ in the saponification agent NaOH to generate Na·BTA. Moreover, the higher the saponification degree, the greater the conversion of the diketo form to the enol form in HBTA. Because Li+, as a hard acid, is difficult to chelate with a single extractant, it is necessary to cooperate with a synergist. With the addition of the synergist TOPO, which has an electron-donating ability, and Na•BTA, which has an electron-absorbing ability, a stable extract, Na·BTA·TOPO, was formed. Na+ in Na·BTA·TOPO can be replaced by Li+ to form Li·BTA·TOPO extractant. The specific reaction process is as follows:
HBTA(org) + NaOH(aq) ⇌ NaBTA(org) + H2O(aq)
NaBTA(org) + TOPO(org) ⇌ NaBTA·TOPO(org)
NaBTA·TOPO(org) + Li+(aq) ⇌ LiBTA·TOPO(org) + Na+(aq)
The HBTA, HBTA+TOPO mixture, and saponified HBTA+TOPO mixture were analyzed and characterized by their infrared spectra, as shown in Figure 1.
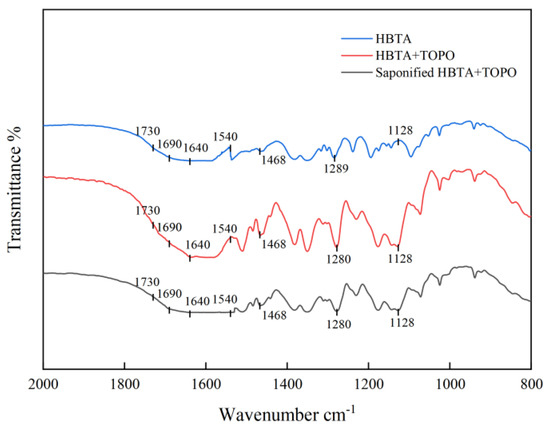
Figure 1.
Infrared spectrogram.
As shown in Figure 1, after TOPO is added to HBTA, strong peak lines appear at wave numbers of 1468 cm−1 and 1128 cm−1, representing the functional groups -CH2P and -P=O in TOPO, respectively. The -C=O peak of HBTA appears at wave number 1289 cm−1, and the redshift occurs after TOPO is added, indicating the interaction between HBTA and TOPO. After saponification, the part of HBTA -C=O was replaced by -C-O-NA, which also showed a redshift. In addition, after saponification, the absorption peak near 1730–1690 cm−1 was weakened, while the absorption peak at 1640–1540 cm−1 was widened, indicating that the enol form of β-diketone organic matter HBTA was converted to the enol form.
In order to further clarify the interaction between Li+ and HBTA+TOPO, 1H NMR analysis was conducted. Figure 2a presents the 1H NMR spectrum of n-hexane and TOPO. No significant chemical shift is observed in the hydrogen signals after saponification, likely due to n-hexane masking the TOPO peak. After the extraction of Li, a new peak appeared at 1.19 ppm. This new peak is attributed to the complexation between Li+ and TOPO, which increases the electron cloud density around the hydrogen atoms in TOPO. This also indicates that TOPO does indeed have a synergistic effect.
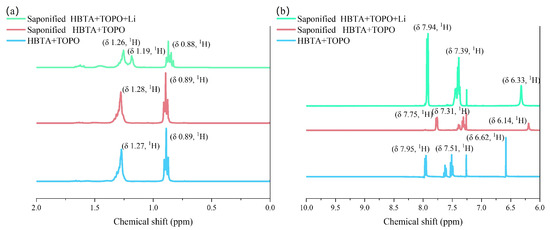
Figure 2.
Stacked 1H NMR spectra (a) 0–2 ppm and (b) 6–10 ppm.
In Figure 2b, after saponification, the hydrogen signals of HBTA are shifted to the right. That indicates that the enol form of β-diketone organic matter HBTA was converted to the enol form. Additionally, a shielding effect is observed due to the complexity between HBTA and Na+. After extracting lithium ions, the chemical shift direction of the hydrogen in the double bond shifted to the high field. It can also be inferred that the coordinated water molecules have produced a certain degree of electron-pushing effect during the coordination process. This increases the electron cloud density of the diketone structure and shifts the chemical displacement toward the high field. These results suggest that Li+ extraction is driven by a synergistic effect between HBTA and TOPO functionalities.
3.2. Influence of Cation on Lithium Extraction
3.2.1. Influence of Na+ on Lithium Extraction
In this section, the concentrations of the extractant HBTA and the synergist TOPO in the organic phase are 0.015 mol/L and 0.015 mol/L, respectively. The saponification degree of HBTA is 100%. The concentration of lithium-ion in the water phase is 60 mg/L, the concentration of K+, Ca2+, and Mg2+ in the water phase is 0 mg/L, and the pH value is 6. Under the condition that the oil–water ratio (VO:VA) was 1:1, extraction was carried out for 6 min. The sodium ion concentration in aqueous solution was changed to 60 mg/L, 2000 mg/L, 4000 mg/L, 6000 mg/L, 8000 mg/L, 10,900 mg/L, respectively. The influence of Na+ concentration changes on the lithium extraction effect was explored. The results are shown in Figure 3.
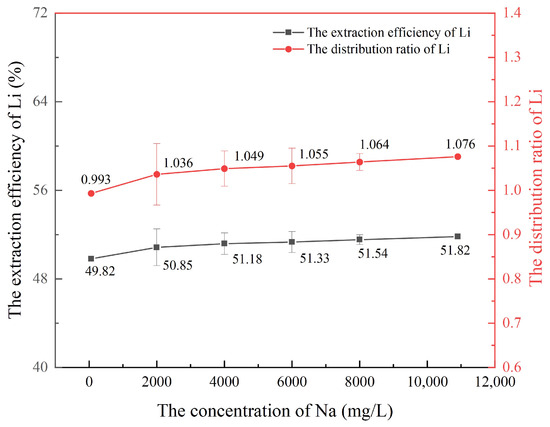
Figure 3.
Effect of Na+ concentration on lithium extraction.
As shown in Figure 3, the extraction efficiency of lithium increases gradually with an increase in Na+ concentration in the aqueous phase. When the concentration of Na+ is 0 mg/L, the extraction efficiency of lithium is 49.82%, and when the concentration of Na+ is 10,900 mg/L, the extraction efficiency of lithium is 51.82%. According to Pearson’s soft and hard acid-base theory, the Pearson hardness of lithium-ion (Ha = 35.1 eV) is higher than that of sodium ion (ηA = 21.1 eV), and Li+ is more easily chelated with the extraction agent, which can replace Na+ in the organic phase [27,28]. In addition, the higher Na+ concentration in the aqueous solution produces a salt-out effect, allowing the organic phase and water phase to be quickly separated upon completion of the extraction, thereby reducing emulsification and further promoting lithium extraction. Therefore, the separation coefficient also increases with the increase in Na+ concentration in aqueous solution. It can be seen that the HBTA/TOPO extraction system has a certain promoting effect on the extraction of low-concentration lithium from oil and gas field water.
3.2.2. Influence of K+ on Lithium Extraction
K+ is similar to Na+ as a monovalent cation, so it is significant to investigate its influence on the extraction of lithium. Under the same experimental conditions as Section 3.2.1, the concentration of lithium ions in the water phase is 60 mg/L, the concentration of Na+, Ca2+, and Mg2+ in the water phase is 0 mg/L, and the pH value is 6. The concentration of K+ in aqueous solution was changed to 60 mg/L, 300 mg/L, 400 mg/L, 560 mg/L and 650 mg/L, respectively. The influence of K+ concentration changes on the separation and extraction effect of lithium was investigated, as shown in Figure 4.
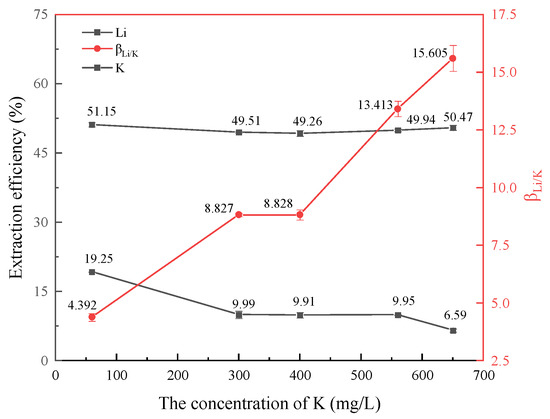
Figure 4.
Effect of K+ concentration on lithium extraction.
As shown in Figure 4, with an increase in K+ concentration, the extraction efficiency of lithium remains essentially unchanged, and the loaded lithium ion concentration in the organic phase remains approximately 30 mg/L. At the same time, it was found that the HBTA/TOPO extraction system could extract a certain amount of K+. When the concentration of K+ is 60 mg/L, the extraction efficiency is 19.25%. When the concentration of K+ is 400 mg/L, the extraction efficiency of K+ is 9.91%. With the increase in K+ concentration, the separation coefficient increases gradually, and the extraction selectivity for Li+ and K+ also increases gradually. When the concentration of K+ reaches 560 mg/L, the separation coefficient is 13.413, indicating that the extraction system can effectively separate lithium and potassium in oil and gas field water.
3.2.3. Influence of Ca2+ on Lithium Extraction
Ca2+ has a certain influence on the extraction of lithium, so it is necessary to study the extraction effect of the diketone extraction system under different Ca2+ concentrations. The experimental conditions in this section are the same as those in Section 3.2.1. The concentration of lithium-ion in the water phase is 60 mg/L, the concentration of K+, Na+, and Mg2+ in the water phase is 0 mg/L, and the pH value is 6. The concentration of Ca2+ in aqueous solution is changed to 15 mg/L, 30 mg/L, 60 mg/L, 90 mg/L, and 120 mg/L, respectively. The influence of Ca2+ concentration changes on the separation and extraction effect of lithium was investigated. The results are listed in Figure 5.
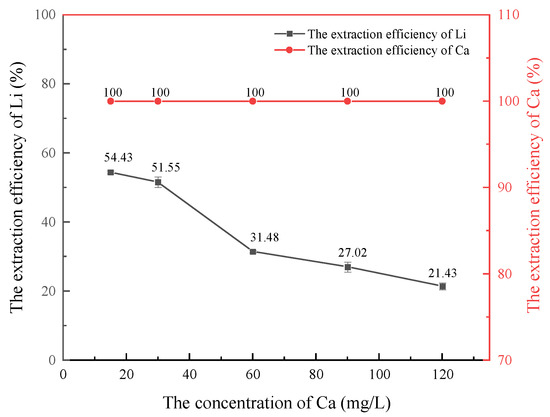
Figure 5.
Effect of Ca2+ concentration on lithium extraction.
As shown in Figure 5, with an increase in Ca2+ concentration, the extraction efficiency of lithium decreased gradually, while the extraction efficiency of Ca2+ essentially remained at 100% and almost completely entered the organic phase. This indicates that the extraction ability of the HBTA/TOPO extraction system for Ca2+ is significantly higher than that of lithium. The preferential extraction of Ca2+ occupied the exchange site in the extractant, resulting in a reduction in the extraction efficiency of lithium ions and inhibiting the extraction of lithium. Therefore, in order to ensure the extraction efficiency of lithium, Ca2+ must be removed from the oil and gas field water in advance.
3.2.4. Influence of Mg2+ on Lithium Extraction
Lithium and magnesium co-extraction has always been a bottleneck problem restricting the extraction of lithium from brine. It is crucial to investigate the impact of Mg2+ on the extraction of lithium using the diketone extraction system. In this section, the experimental conditions are the same as those in Section 3.2.1. The concentration of lithium ions in the water phase is 60 mg/L, while the concentrations of K+, Na+, and Ca2+ in the water phase are 0 mg/L. The pH value is 6. The concentration of Mg2+ in aqueous solution is changed to 4 mg/L, 15 mg/L, 30 mg/L, 60 mg/L and 90 mg/L, respectively. The influence of Mg2+ concentration changes on the separation and extraction of lithium was investigated; the results are shown in Figure 6.
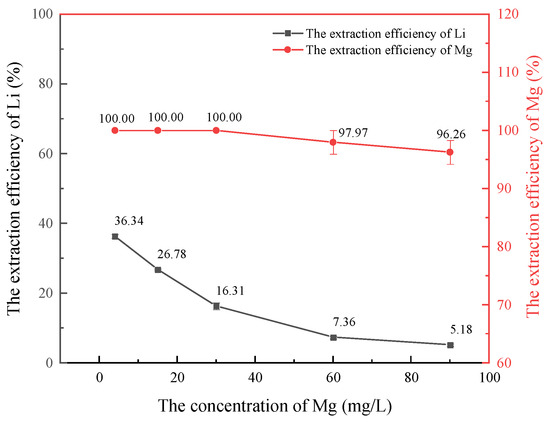
Figure 6.
Effect of Mg2+ concentration on lithium extraction.
As shown in Figure 6, the extraction efficiency of lithium follows a similar trend to that of Ca2+, decreasing with increasing Mg2+ concentration. Notably, the extraction efficiency of Mg2+ is significantly higher than that of lithium. The HBTA/TOPO extraction system has a higher extraction order for magnesium than lithium, and the separation coefficient is very small. Therefore, the extraction of lithium from oil and gas field water requires a pre-treatment to remove Mg2+.
To further explore the extraction sequence of Li+, Ca2+, and Mg2+ in the HBTA/TOPO extraction system, the concentrations of Li+, Ca2+, and Mg2+ are all set at 60 mg/L, and the pH of the aqueous phases is 6. The results are shown in Table 2.

Table 2.
Extraction results of Li+, Ca2+, and Mg2+ at the same concentration.
As shown in Table 2, under the same concentration of Li+, Ca2+, and Mg2+, the order of extraction efficiency of the extracted organic relative to the three is EMg > ECa > ELi, indicating that the extraction order is Mg2+ > Ca2+ > Li+. According to Pearson’s soft and hard acid-base theory, the Lewis acid hardness of the magnesium ion is 80.1 eV, and the Lewis acid hardness of lithium is 75.6 eV. The coordination stability of Mg2+ and the hydroxyl oxygen atom on the diketone extractant is stronger. At the same time, the radius of Mg2+ is also slightly larger than that of Li+, and the steric hindrance generated between the extractant molecules when complexes with extractants and synergies are small, allowing a more stable complex to be formed.
Atomic Force Microscopy was used to analyze the surface morphology of the organic phases after the extraction of Li+, Ca2+, and Mg2+, and the results are shown in Figure 7.
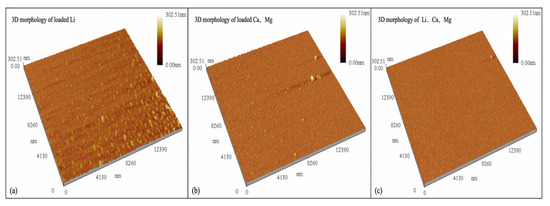
Figure 7.
AFM of the organic phase after loading metal ions.
As can be seen from Figure 7a, when the organic phase only extracts Li+, there are more bumps in the three-dimensional structure of the organic phase, and the generated lithium extractant is aggregated, exhibiting an agglomeration phenomenon at the interface and uneven aggregate distribution. It can be seen from Figure 7b,c that when a certain amount of Ca2+ and Mg2+ is extracted from the organic phase, the agglomeration phenomenon is weakened. This also demonstrates that the coordination force between Ca2+ and Mg2+ and the hydroxyl oxygen atoms on the diketone extractant is stronger. A more stable complex is formed, the molecular conformation and orientation change, and the distribution of interfacial molecules becomes more uniform, which promotes the extraction of Ca2+ and Mg2+.
As shown above, Na+ and K+ have no specific effects on Li+ extraction, although Na+ can promote the extraction of Li+ to a certain extent. The divalent ions, such as Ca2+ and Mg2+, have a significant influence on lithium extraction. It can be extracted competitively with Li+, and the extraction sequence is Mg2+ > Ca2+ > Li+. Therefore, it is necessary to pretreat the Mg2+ and Ca2+ before extracting Li+ from the oil and gas field water.
3.3. Influence of Anion on Lithium Extraction
Because of the solvation effect, anions also have a certain influence on the extraction of lithium. In this section, a lithium solution with a concentration of 60 mg/L was configured, and then NaCl, KCl, NaBr, KBr, NaNO3, KNO3 and Na2SO4 and K2SO4 were added to the four parts of solution containing lithium, respectively, to maintain the Na+ concentration of 10,900 mg/L and K+ concentration of 560 mg/L in solution, and the pH value was 6. The configured organic phase (with the same composition as Section 2.2.1) was extracted in contact with four lithium solutions containing Cl−, Br−, NO3−, and SO42−, respectively. Compared with the VO:VA ratio of 1:1, extraction was conducted for 6 min to explore the influence of four anions, Cl−, Br−, NO3−, and SO42−, on the extraction effect of lithium, as shown in Figure 8.
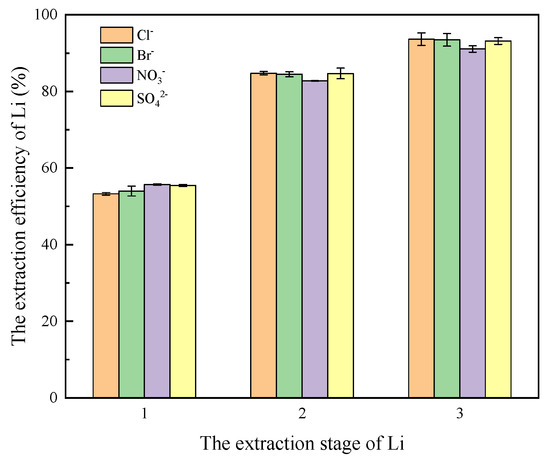
Figure 8.
Effect of anion on lithium extraction.
As shown in Figure 8, after the three-stage extraction, the extraction efficiency of Li exceeds 91%, and the four anions (Cl−, Br−, NO3−, and SO42−) have a negligible influence on the extraction effect of lithium. From the extraction principle, the extraction of lithium by the HBTA/TOPO system belongs to ion chelation extraction, and the anion itself does not participate in the extraction process. During the extraction process, it is ensured that the anions do not alter the pH value of the aqueous phase, allowing lithium to be extracted efficiently from solutions containing various types of anions, which is consistent with the research results of Zhang et al. [29].
3.4. Influence of Organic Matter on Lithium Extraction
Relevant studies have shown that the presence of organic matter in water significantly impacts the extraction of metal ions. The oil and gas field water contains a relatively high concentration of organic components. The TOC of the actual oil and gas field water is 2160 mg/L, mainly containing benzene series, phenol compounds, polycyclic aromatic hydrocarbons, organic acids, and long-chain alkane, among which the content of benzene series and phenol compounds is relatively high. The contents of acetic acid and propionic acid in lower fatty acids are higher and exist in an anion form. In this section, xylene, phenol, naphthalene, n-hexane acetate, and n-hexadecane were selected as representative organic compounds, and their concentrations were adjusted to 200 mg/L, 1000 mg/L, and 2000 mg/L, respectively, to investigate their effects on lithium extraction. The results are shown in Figure 9.
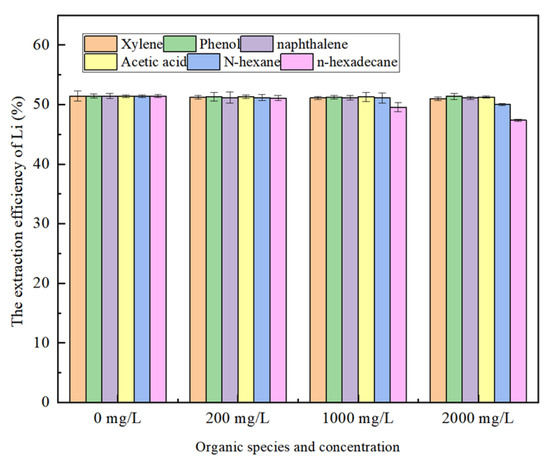
Figure 9.
Effect of organic species and concentration in aqueous phase on lithium.
As can be seen from Figure 9, xylene, phenol, and naphthalene have essentially no effect on the extraction efficiency of lithium, while the extraction efficiency of lithium gradually decreases with the increase in the amount of n-hexane and n-octane added to the aqueous phase. In particular, when the addition of n-cetane is 2000 mg/L, the extraction efficiency of lithium decreases to 46.37%. In addition, the test results show that the longer the alkane chain length, the greater the impact on the extraction effect of lithium. This is mainly because long-chain alkanes can form super-large aggregates with extractant molecules and their extracts under the action of intermolecular forces. With the increase in alkane concentration, more large aggregates are generated and coated on the surface of the extractant oil droplets, which slows down the interfacial renewal rate of the oil droplets and increases steric hindrance, thereby hindering the exchange reaction between lithium and extractant molecules and reducing the extraction rate of lithium [30].
To investigate the influence of organic phase type and concentration on total organic carbon (TOC) in the aqueous phase, the raffinate from the above experiment was analyzed to determine the TOC concentration. The change in TOC concentration in the aqueous phase after extraction was also analyzed. The results are presented in Figure 10. As shown in Figure 10, xylene, phenol, and naphthalene have a minimal impact on the total organic carbon (TOC) in the aqueous phase, whereas n-hexane and n-octane have a more significant influence on the TOC in the aqueous phase. The concentration of TOC in the aqueous phase gradually increases with the increase in the chain length and the additional amount of alkane. Analyzing the reasons, the properties of long-chain alkanes are similar to those of surfactants, and they are oleophilic and hydrophobic. High-concentration alkanes, especially long-chain alkanes, are easily soluble in the organic phase and form polymers with it. After the polymers are aggregated into supramolecular aggregates, they can disperse into the aqueous phase and form emulsions, thus increasing the content of TOC in the aqueous phase. In particular, long-chain alkanes are easily soluble in the organic phase and form polymers with it. After the polymers are aggregated into supramolecular aggregates, they can disperse into the aqueous phase and form emulsions, thus increasing the content of TOC in the aqueous phase. Therefore, it is suggested that advanced oxidation technology be added to the pre-treatment stage of oil and gas field-produced water to decompose long-chain alkane into small-molecular organic matter or mineralization, thereby reducing interference with subsequent extraction.
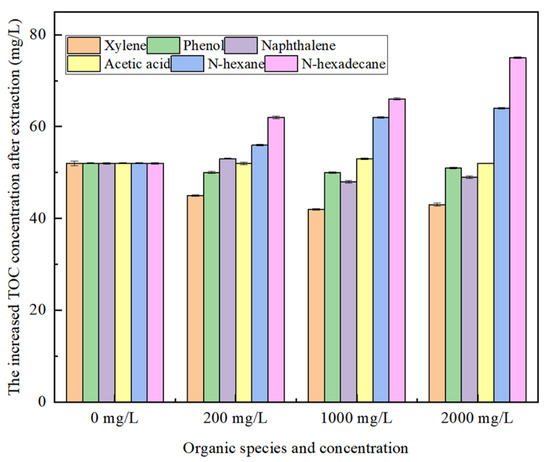
Figure 10.
Effect of organic species and concentration in aqueous phase on TOC.
3.5. Two-Step Lithium Extraction
According to the above experimental results, the HBTA-TOPO extraction system can effectively separate Li+ from K+ and Na+, but it is not easy to separate Li+ from Ca2+, Mg2+, and other bivalent metal ions. Therefore, to improve the extraction efficiency of Li+, it is necessary to pretreat the Ca2+, Mg2+, and Ba2+ ions. In this study, Ca2+, Mg2+, and Ba2+ were removed from the aqueous phase by chemical precipitation. After precipitation is complete, a three-stage extraction process is used to extract Li+ from the aqueous phase.
3.5.1. Impurity Removal of Precipitation
The mechanism of precipitation removal of Ca2+, Mg2+ and Ba2+ is as follows:
Mg2+(aq) + 2NaOH(aq) ⇌ 2Na+(aq) + Mg(OH)2↓
Ca2+(aq) + Na2CO3(aq) ⇌ 2Na+(aq) + CaCO3↓
Ba2+(aq) + Na2CO3(aq) ⇌ 2Na+(aq) + BaCO3↓
The dosage of precipitant NaOH and Na2CO3 was calculated according to the Ksp constant in Table 3.

Table 3.
Ksp of insoluble precipitate.
As shown in Figure 11, MgOH precipitation was generated after the addition of NaOH, with a removal efficiency of approximately 93%. The purity of the MgOH precipitate was achieved through filtration, making it suitable for refining magnesium salts. After the addition of Na2CO3, CO32+ preferentially binds to Ca2+ and Ba2+, and the removal efficiency of Ca2+ and Ba2+ is 99% and 92%, respectively. The filtered, mixed sediment contains CaCO3 and BaCO3, with a CaCO3 content of approximately 96.6%. The remaining portion is primarily BaCO3 and MgCO3, which can be used for refining CaCO3 products. In summary, the precipitation removal method can effectively remove impurity ions while keeping the content of lithium ions unchanged.
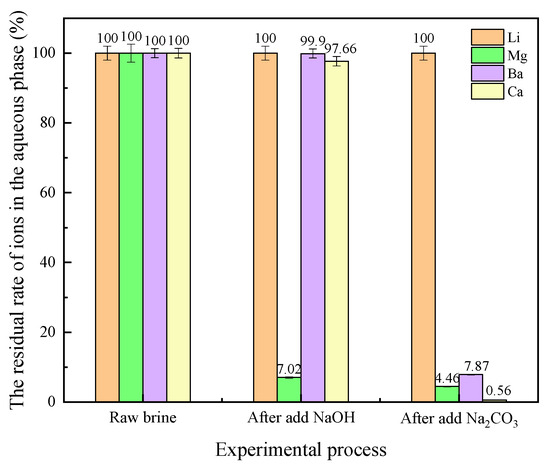
Figure 11.
Residue number of ions in the solution after precipitation.
3.5.2. Solvent Extraction of Lithium
Based on the above, Li was extracted using 0.015 mol/L HBTA as the extractant, 0.015 mol/L TOPO as the synergist, and sulfonated kerosene as the diluent. The saponification degree of the organic phase was 100%. Under the conditions that VO:VA is 1:1 and extraction time is 6 min, three-stage cross-flow extraction is carried out to extract lithium from the aqueous phase after impurity removal in Section 3.5.1. The results are shown in Table 4.

Table 4.
Extraction efficiency of lithium by a three-stage process after impurity removal.
As shown in Table 4, the extracted organic phase can effectively extract lithium ions from the residual liquid through a three-stage cross-flow extraction process, with primary extraction efficiencies of 54.96%, secondary extraction efficiencies of 85.60%, and tertiary extraction efficiencies of 97.17%. Therefore, the precipitation process can eliminate the influence of Mg2+, Ca2+, and Ba2+ in the aqueous phase on lithium extraction. In addition, the concentration of Mg2+, Ca2+, Ba2+, and other metal ions in the aqueous phase after extraction is significantly reduced, which improves the water quality for reinjection and solves the problem of hard ions in the water during the reinjection process, making it easier to precipitate and block the reinjection pipeline.
3.6. Lithium Carbonate Preparation
Taking the actual oil and gas field water, 15 L, as the research object, 1.5 L of organic phase was recycled five times to complete the lithium extraction from 15 L of oil and gas field water. Then, hydrochloric acid was used for back extraction to obtain a Li-rich solution. For specific experimental methods, see Section 2.2.2. The extraction efficiency, back-extraction efficiency, and lithium transfer efficiency of Li+ are shown in Figure 12.
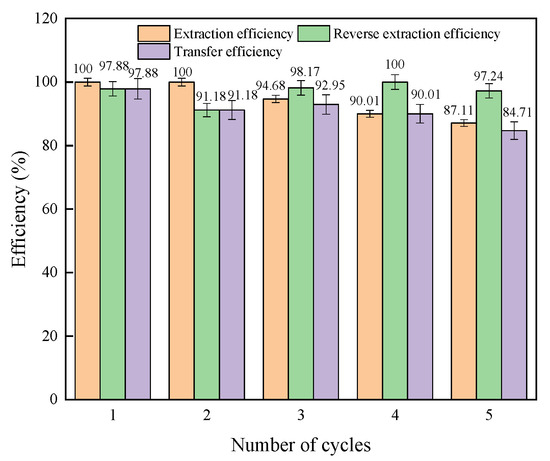
Figure 12.
Extraction efficiency, back extraction efficiency, and transfer efficiency of lithium.
As shown in Figure 12, the extraction efficiency of lithium decreases with increasing number of cycles in the organic phase, and all Li+ can be extracted in the first and second extraction processes. When the organic phase is recycled for the fifth time, the extraction efficiency of lithium is 87.11%. This is mainly because the alkaline Na2CO3 and NaOH solutions used in the regeneration cannot be quickly and evenly dispersed, which destroys part of the diketone structure of the extractant and results in a reduction in lithium extraction efficiency. To eliminate the above effects, the extractant used in the process is recycled to extract lithium five times and then quickly stirred to minimize the influence of the washing liquid. The extraction effect of the tenth lithium extraction was measured, and it was found that the extraction efficiency remained at 81%. The extraction efficiency was higher than 90%. The average transfer efficiency of lithium in 15 L oil and gas field water is 91.35%. The HBTA/TOPO extraction system has good stability.
The main ion concentration changes during the preparation of Li2CO3 are shown in Table 5. The precipitation obtained after drying is 4.80 g, of which 4.77 g is Li2CO3. The purity of Li2CO3 is about 99.28%.

Table 5.
Metal ions concentration in solution during the preparation of lithium carbonate.
The XRD spectroscopy of the collected Li2CO3 is listed in Figure 13. As shown in Figure 13, the diffraction pattern of the Li2CO3 product closely matches that of pure Li2CO3. The quantitative analysis reveals that the collected Li2CO3 has a purity of 99.28% ± 0.04%, with a magnesium content of 0.56%, and the remainder is primarily sodium salts.
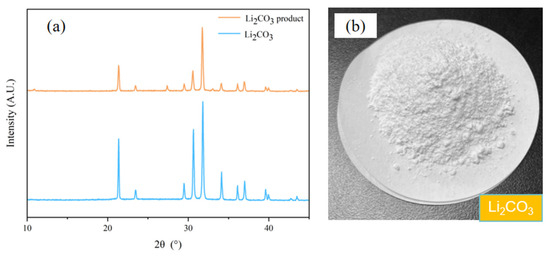
Figure 13.
The Li2CO3 product precipitated from enrichment solution. (a) The XRD spectroscopy of the collected and pure Li2CO3. (b) A photographic image of collected Li2CO3.
The premise of obtaining industrial-grade lithium carbonate by extracting lithium and precipitating lithium carbonate is to remove impurity ions, such as calcium and magnesium, from the produced water. The primary method for removing impurity ions in produced water is to add sodium hydroxide to remove magnesium, followed by the addition of sodium carbonate to remove calcium. However, the high cost of the drug may become one of the difficult problems restricting its industrialization.
4. Conclusions
The oil and gas field water contains low concentrations of lithium, which has a certain industrial exploitation value. In this paper, HBTA was used as an extractant, TOPO as a synergist, and sulfonated kerosene as a diluent. The effects of inorganic cations, such as Na+, K+, Ca2+, and Mg2+, and inorganic anions, including Cl−, Br−, NO3−, and SO42−, as well as organic substances, on the extraction of lithium with low concentration were studied, and the influence mechanism was analyzed. The technological routes for removing impurities through chemical precipitation, extracting lithium via solvent extraction, and preparing lithium carbonate were established. The results show that Univalent ions such as Na+ and K+ have specific effects on lithium extraction, and Na+ can promote the extraction of lithium. The divalent ions, such as Ca2+ and Mg2+, have a significant influence on lithium extraction. It can be extracted competitively with lithium, and the extraction sequence is Mg2+ > Ca2+ > Li+. Therefore, it is necessary to pretreat the divalent ions before extracting lithium from the oil and gas field water. The four anions Cl−, Br−, NO3−, and SO42− have a negligible influence on the extraction efficiency of lithium. Xylene, phenol, naphthalene, and other organic components do not affect the extraction efficiency of lithium. N-hexane and n-cetane have adverse effects on the extraction efficiency of lithium with increasing amounts in the aqueous phase, especially n-cetane. When the addition amount is 2000 mg/L, the extraction efficiency of lithium decreases to 46.37%. NaOH and Ca2+ precipitated Na2CO3, and precipitated Mg2+. The total precipitation efficiency of Mg2+ was over 95%, and that of Ca2+ was 99%. The extraction efficiency of lithium is greater than 97% using a three-stage cross-flow extraction method. Taking the actual oil and gas water, 15L in volume, as the research object, after extraction, back extraction, concentration, depth impurity removal by extraction, and precipitation drying, a lithium carbonate product with a purity of 99.28% was obtained. Although this method can efficiently extract Li+ and prepare Li2CO3, it requires a large number of precipitating agents to remove calcium and magnesium ions in advance. The organic phase has a certain solubility, resulting in a certain extent of organic phase loss, which will pollute the water phase. Therefore, the future development of green precipitating agents and extractants is a key direction for future development.
Author Contributions
Conceptualization, G.Z.; validation, P.F.; formal analysis, M.L.; investigation, X.Z.; resources, J.L.; data curation, G.W.; writing—original draft preparation, Q.S.; writing—review and editing, J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the National Natural Science Foundation of China (52174336), the Natural Science Foundation of Shandong Province in China (ZR2021MB051), and the National Engineering Laboratory for Exploration and Development of Low Permeability Oil and Gas Fields (KFKT2023-18).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Authors Qiaoli Shan, Guocheng Zhu and Pengjun Fan were employed by the company Changqing Engineering Design Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Gao, J.; Wang, D.; Wang, W.; Feng, Y.; Yang, Y. Current status and prospects of lithium extraction in major domestic and foreign oil (gas) field waters. Acta Geol. Sin. 2019, 93, 1489–1500. [Google Scholar]
- Yue, J.C.; Cen, C.Y.; Bai, S.C.; Wang, X.Q.; Yang, R.; Wen, W.; Liu, D.; Xiao, G.Q. A novel HTO@PAN/CS membrane for efficient Li plus recovery from gas field water. Sep. Purif. Technol. 2025, 362. [Google Scholar] [CrossRef]
- Zhang, Q.; Hai, C.; Sun, Y.; Gao, Y.; Dong, S.; Ma, L.; He, X.; Xu, Q.; Zhou, Y. Formation and working mechanisms of layered TiO2·H2O adsorbents for effectively recovering Li+ from oil-gas field brines. Sep. Purif. Technol. 2025, 354, 128998. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, P.; Tu, W.; Sun, H.; Li, S.; Zhang, Y. Lithium recovery from oil and gas produced water: Opportunities, challenges, and future outlook. J. Water Process Eng. 2023, 55, 104148. [Google Scholar] [CrossRef]
- Zante, G.; Trebouet, D.; Boltoeva, M. Solvent extraction of lithium from simulated shale gas produced water with a bifunctional ionic liquid. Appl. Geochem. 2020, 123, 104783. [Google Scholar] [CrossRef]
- Wang, X.; Numedahl, N.; Jiang, C. Direct lithium extraction from Canadian oil and gas produced water using functional ionic liquids-A preliminary study. Appl. Geochem. 2024, 172, 106126. [Google Scholar] [CrossRef]
- Lim, Y.J.; Goh, K.; Goto, A.; Zhao, Y.; Wang, R. Uranium and lithium extraction from seawater: Challenges and opportunities for a sustainable energy future. J. Mater. Chem. A 2023, 11, 22551–22589. [Google Scholar] [CrossRef]
- Li, H.; Chen, T.; Jiang, J.; Gao, G.; Wang, C.; Lu, Z. Prelithiation-derived hierarchical TiO2 sieve with metal-organic framework gate for selective lithium recovery. Chem. Eng. J. 2023, 451, 138662. [Google Scholar] [CrossRef]
- Chen, L.; Ma, J.; Gao, Y. Performance evaluation of lithium-based adsorbents in oil and gas fields brine. Chem. Eng. Oil Gas 2024, 53, 111–118. [Google Scholar]
- Gao, L.; Ling, N.; Jin, Y. Key technology and application of lithium extraction from produced water in high sulfur and high hardness gas fields. Inorg. Chem. Ind. 2023, 55, 74–80. [Google Scholar]
- Tang, G.J.; Zhang, H.J.; He, H. Research on technology for lithium extraction from oil and gas field produced water. Nat. Gas Oil 2023, 41, 67–72. [Google Scholar]
- Wang, H.; Yang, Y.; Yuan, X.Z.; Liang Teo, W.; Wu, Y.; Tang, L.; Zhao, Y. Structure–performance correlation guided applications of covalent organic frameworks. Mater. Today 2022, 53, 106–133. [Google Scholar] [CrossRef]
- Ochromowicz, K.; Zablocka-Malicka, M.; Chojnacka, I.; Worsa-Kozak, M. Assessing the viability of integrating evaporation and solvent extraction systems for lithium recovery from low-grade brines. Processes 2024, 12, 1453. [Google Scholar] [CrossRef]
- Li, H.F.; Li, J.L.; Dong, L.M. Extraction of lithium from salt lake brine with high Mg/Li mass ratio by n523-dibk extraction system. J. Sustain. Metall. 2023, 9, 1456–1465. [Google Scholar] [CrossRef]
- Shi, D.; Cui, B.; Li, L.J.; Peng, X.W.; Zhang, L.C.; Zhang, Y.Z. Lithium extraction from low-grade salt lake brine with ultrahigh Mg/Li ratio using TBP-kerosene-FeCl3 system. Sep. Purif. Technol. 2019, 211, 303–309. [Google Scholar] [CrossRef]
- Hu, Y.; Su, H.; Zhu, Z.; Zhou, M.; Qi, T. Mechanisms for the separation of Li+ and mg2+ from salt lake brines using TBP and TOP systems. Desalination 2024, 583, 117698. [Google Scholar] [CrossRef]
- Disu, B.; Rafati, R.; Haddad, A.S.; Roca, J.A.M.; Clar, M.I.I.; Bakhtiari, S.S.E. Review of recent advances in lithium extraction from subsurface brines. Geoenergy Sci. Eng. 2024, 241, 213189. [Google Scholar] [CrossRef]
- Rebekka, R.; Klemens, S.; Jochen, E.K. Lithium extraction techniques and the application potential of different sorbents for lithium recovery from brines. Miner. Process. Extr. Metall. Rev. 2023, 44, 261–280. [Google Scholar]
- Dang, V.D.; Steinberg, M. Preliminary design and analysis of recovery of lithium from brine with the use of a selective extractant. Energy 1977, 3, 325–336. [Google Scholar] [CrossRef]
- Seo, J.; Vu, T.T.; Cho, S.; Cha, J.; Choi, Y.; Song, D. Qualitative Assessment of PC88A and HBTA Extractants in Lithium Recovery Processes Using Solvent Extraction. Korean J. Chem. Eng. 2025, 42, 323–328. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Rui, H.; Shi, D.; Song, X. Lithium recovery from effluent of spent lithium battery recycling process using solvent extraction. J. Hazard. Mater. 2020, 398, 122840. [Google Scholar] [CrossRef]
- Knapik, E.; Rotko, G.; Marszalek, M. Recovery of lithium from oilfield brines-current achievements and future perspectives: A mini review. Energies 2023, 16, 6628. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Liu, R.; Zhou, Y.; Zhang, Y.; Ji, L.; Li, L. Recovery of lithium from salt lake brine with high Na/Li ratio using solvent extraction. J. Mol. Liq. 2022, 362, 119667. [Google Scholar] [CrossRef]
- Jang, E.; Jang, Y.; Chung, E. Lithium recovery from shale gas produced water using solvent extraction. Appl. Geochem. 2017, 78, 343–350. [Google Scholar] [CrossRef]
- Gan, G.F.; Liao, L.F. Separation of lithium from oilfield brine by coprecipitation method. J. Jianghan Pet. Inst. 1996, 18, 65–68. [Google Scholar]
- Lee, J.; Chung, E. Lithium recovery by solvent extraction from simulated shale gas produced water-Impact of organic compounds. Appl. Geochem. 2020, 116, 104571. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases, HSAB, part 1: Fundamental principles. J. Chem. Educ. 1968, 45, 581. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases, HSAB, part II: Underlying theories. J. Chem. Educ. 1968, 45, 643. [Google Scholar] [CrossRef]
- Zhang, L.C. Study on the Mechanism and Technology of Extracting Lithium from Alkaline Solution by HBTA/TOPO Extraction System. Ph.D. Thesis, University of Chinese Academy of Sciences, Beijing, China, 2019. [Google Scholar]
- Liu, J.; Zhang, X.; Zhang, N.; Wang, Z.R.; Jia, M.H.; Huang, K.; Xia, W.X. Preventing emulsification during rare earths solvent extraction by the addition of Span 80 to enhance the interfacial stability of the P507 oil droplets. Sep. Purif. Technol. 2025, 354, 128755. [Google Scholar] [CrossRef]
- Peng, C.H.; Feng, J.Z.; Zhang, X.Y. Analytical Chemistry: A Concise Course in Quantitative Chemical Analysis; Peking University Press: Beijing, China, 2009. [Google Scholar]
- Sun, Y.Q.; Hu, Y.Z. Analytical Chemistry; Science Press: Beijing, China, 2006; pp. 238–243. [Google Scholar]
- Yan, H.; Zhu, X.; Liu, Z.; Jin, S.; Liu, J.; Han, Z.; Woo, J.; Meng, L.; Chi, X.; Han, C. Co-removal and recycling of Ba2+ and Ca2+ in hypersaline wastewater based on the microbially induced carbonate precipitation technique: Overlooked Ba2+ in extracellular and intracellular vaterite. J. Hazard. Mater. 2024, 475, 134923. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).