Abstract
The widespread discharge of synthetic dyes such as Eriochrome Black T (EBT) into water bodies poses significant environmental and health concerns due to their toxicity, persistence, and resistance to degradation. In response to this issue, the removal of EBT dye from aqueous solutions using nickel-modified orange peel biochar (MOPB) was investigated in this study at various experimental conditions such as adsorbent dose, pH, concentration of dye, temperature, and contact time. Biochar was prepared from orange peels via pyrolysis, and structural characterization was performed using FTIR, XRD, and SEM to assess morphological changes, pore structure, and functional groups post-modification. MOPB exhibited significantly enhanced adsorption capacity compared to unmodified biochar. Optimal removal (at 0.1 g adsorbent dose, 25 ppm dye concentration, 90 min contact time, 35 °C, and pH 4) resulted in maximum EBT elimination. The equilibrium dataset was evaluated using Langmuir and Freundlich isotherm models. The Langmuir model (R2 = 0.99) best described the uptake of EBT dye, which implies that the adsorption of EBT dye onto MOPB was monolayered. The kinetic data were also analyzed using pseudo-first-order and pseudo-second-order models. The pseudo-second-order kinetic model was found to be the best fit (R2 = 0.99), indicating that it governs the rate-limiting step of the reaction. Thermodynamic parameters confirmed that the adsorption process is spontaneous and exothermic. These findings demonstrate the potential of MOPB as a low-cost, sustainable adsorbent for the efficient removal of EBT from industrial wastewater.
1. Introduction
Environmental pollution, primarily of water sources, has become a global issue, with the agricultural and industrial sectors being major sources of water pollution in developing countries [1,2]. Waterborne infections and diseases account for 80% of all illnesses according to the WHO, and 3.1% of fatalities are attributed to unhygienic and low-quality water [3]. The imbalance of water supply and demand further exacerbates these challenges, standing as one of the critical issues societies will face globally in this century [4]. The growth of industrial activities is increasingly contributing to environmental pollution, with various pollutants including plastics [5], heavy metals, and dyes contaminating water sources. The textile industry, in particular, is a major consumer of water and a significant contributor to water pollution, as it releases large volumes of wastewater containing harmful chemicals, including dyes. This industry produces more than 735 tons of dyes, and the World Bank estimates that textile treatment and dyeing account for roughly 17 percent to 20 percent of global industrial water emissions [6]. Eriochrome Black T is one of the crucial azo dyes which are used for the purpose of coloring multi fibers, nylon, wool, and silk. It is also used as a complexometric titrant for determining metal cation concentrations [7]. Its presence in clean water is toxic to health because of its extremely hazardous nature and carcinogenic agents [8]. Eriochrome black T has a chemically complex structure that makes it more resistant to photocatalytic activity, and water, along with some chemical substances, prevent its color from being eliminated or reduced during the treatment of dirty wastewater [9].
Traditional treatment methods, such as coagulation and chemical oxidation, often prove inefficient or cost-prohibitive, making the search for sustainable and cost-effective alternatives critical. Biochar, derived from renewable biomass, has emerged as a promising adsorbent due to its high surface area, stability, and ability to capture pollutants. However, challenges remain in optimizing biochar’s adsorption efficiency, particularly for dyes such as EBT. Moreover, many existing methods of biochar modification—while effective—require careful consideration of factors such as surface area, charge distribution, and temperature to enhance their performance. Among various wastewater treatment methods, adsorption is frequently preferred due to its cost-effectiveness and high performance. Adsorption is the accumulation or transfer of molecules of pollutant onto the surface of adsorbent (modified biochar) via physical or chemical bonds. The key and challenging attribute to attaining determined pollution removal is the selection of an appropriate adsorbent that successfully fulfills the requirements of the adsorption mechanism. Adsorption of toxic dyes from textile discharges by simple, cost-effective, and effective adsorbents is urgently needed [10]. Hence, researchers are looking for new adsorbent materials that have a strong adsorption potential with good stability and reusability (long-lasting) [7].
Biomass of agricultural wastes such as grass, rice husk, banana pith, plum kernels, garlic peel, walnut shell, corn stalk, corn straw, corn cob, hen feathers, orange peel, bagasse, hazelnut shell, and neem sawdust are pyrolyzed at low or high temperatures with little or no oxygen to create biochar, a carbon-rich solid [11,12]. Different natural adsorbents for the removal of pollutants are presented in Table 1. Pore size, specific surface area, molecular size, polarity, surface binding sites, and adsorbent weight can all influence the adsorption process. Moreover, the operational conditions such as pH, ionic strength and temperature also affect the process [13]. The modification of biochar by the binding of different materials to its surface not only improves the porous structure of the adsorbent but also reduces the amount of time it takes to eliminate adsorbent from wastewater after it has been treated [14]. Modification methods significantly increase surface area and pore volume and allow for more dye molecules to be adsorbed onto the surface and into micropores.
Citrus sinensis, or orange, is one of the most well-known subtropical fruits in the world. In the citrus production industry, orange fruit is mainly consumed fresh and used as a food complement in cakes, desserts, food colorings, cranberry jelly, honey, or juice combinations. Global orange production is estimated at 60 million tons annually, with approximately 32 million tons of orange peel waste generated each year.. In reality, orange peels are renewable, environmentally friendly, and low-cost components of carbonaceous materials that can be used to remove a variety of water contaminants while also reducing contamination. Since orange peel is a renewable resource, it can be used to make good-quality activated charcoal that can be used in environmental technologies, such as treating water [15].

Table 1.
Different natural adsorbents for the removal of pollutants.
Table 1.
Different natural adsorbents for the removal of pollutants.
| Adsorbent | Dye | Reference |
|---|---|---|
| Bentonite clay & sweet sorghum bagasse | Malachite green & toluidine blue | [10] |
| Oleaster stones | Eriochrome Black T (EBT) dye | [11] |
| Pongamia glabra seed cover | Methylene blue & thodamine | [16] |
| Banana peel | Direct Blue 86 (DB86) | [17] |
| Mangosteen peel | Methylene blue | [14] |
| Wheat straw | Methylene blue (MB) | [18] |
| Peanut stalks | Orange G dye | [19] |
| Spirulina platensis algae | Congo red dye | [20] |
| Empty fruit bunch | Methylene blue and orange-G | [6] |
| Corn stalks | Crystal violet (CV) | [21] |
| Bamboo | Metal complex dye acid black 172 | [22] |
| Rice straw and charcoal/MgO nanocomposite | Reactive dye blue 221 (RB-221) | [23] |
| Cistus ladanifer seeds (CLS) | Reactive red 23 (RR-23) | [24] |
| Rice husk | Reactive dyes yellow (RY145), red (RR195), and blue (RB19) | [25] |
| Wakame (undaria) | Methylene blue dye | [26] |
| Hazelnut shell | Methylene blue (MB) | [27] |
The aim of this study was to synthesize and characterize nickel-modified biochar and to assess the EBT adsorption on nickel-modified orange peel and adsorption of dye from synthetic wastewater through magnetic orange peel biochar for the treatment of wastewater. The results may provide insights into overcoming the limitations of conventional adsorbents, thus advancing the development of more sustainable wastewater treatment solutions.
2. Materials and Methods
2.1. Reagents and Material
The oranges, purchased from a nearby market, were peeled, thoroughly washed, and air dried. The Eriochrome Black T (EBT) dye (purity of EBT was 90%) was procured from Micko Industrial Chemical Co. For the modification of orange-derived biochar, nickel chloride (Sigma Aldrich, St. Louis, MO, USA) was used. Both chemicals were analytical grade reagents that had not been purified further.
2.2. Synthesis of Biochar and Modified Biochar
Approximately 200 g of orange peel was placed in Petri dishes, which were then covered with aluminum foil. The Petri dishes were then placed in a muffle furnace for 30 min. The pyrolysis temperature of the muffle furnace was 350 °C. The sample of biochar was allowed to cool at room temperature inside the furnace after pyrolysis, and its yield was 32%.
For the preparation of modified biochar, 129.6 g of nickel chloride was dissolved with 1000 mL of double-distilled water to make 1 M solution. After that, the mixture was stirred for 15 min with a magnetic stirrer. After that, 150 g of biochar was added in solution and followed by further stirring for 20 min. The above-mentioned solution was dissolved in a beaker. The mixture was allowed to heat in an oven for 24 h at 100 °C. The modified biochar sample was put into airtight bottles. In modified orange peel biochar, nickel provides new surface sites for the ionization process, in which significant electrostatic contact is established between the anionic dye and cationic biochar surface (adsorbent).
2.3. Preparation of EBT Solution
The stock solution of EBT was prepared by accurately dissolving the required amount of the dye in 1 L of double-distilled water to achieve an initial concentration of 200 ppm. The dye was carefully weighed, and the water was added slowly while being stirred to ensure complete dissolution. Once the EBT was fully dissolved, the solution was transferred to a volumetric flask and diluted to the final volume of 1 L. The initial pH of the stock solution was measured to be 8.5, and adjustments were made as required for the experiment by adding 1 M HCl or 1 M NaOH to achieve the desired pH values for the adsorption studies. The pH of the solution was monitored and maintained at the required levels throughout the experiments
2.4. Characterization Techniques
Through several characterization investigations, the form and characteristics of magnetic biochar made from orange peels were examined. The functional groups that developed on the surface of the modified orange peel biochar were examined using Fourier transform infrared (FTIR) (PerkinElmer, Springfield, IL, USA; Spectrum IR Version 10.6.2.). A KYKY- EM 6900 scanning electron microscope (SEM) was used to obtain surface images of orange peel before and after the modification and adsorption procedure. The crystallinity of the biochar and modified biochar was examined via X-ray diffractometry (XRD), (X’Pert3 Panalytical Turkey).
2.5. Batch Adsorption Experiment
The effect of process parameters such as initial dye concentration, temperature, contact time, adsorbent dose, and pH on the efficiency of EBT dye removal by MOPB was examined. Table 2 shows three levels of each parameter of the batch adsorption study.

Table 2.
Experimental design for the batch adsorption study.
Depending on the required pH value, either 1.0 M of hydrochloric acid or 1.0 M of sodium hydroxide was used to change the pH values of the dye stock solutions. The factors employed in this study are displayed in Table 2.
The amount of adsorbed EBT was measured at 530 nm with a UV spectrometer (Specord 205 Analytik Jena, Germany) to study the adsorption kinetics [28]. The removal efficiency of BC and MBC was measured as follows:
where represents the initial dye concentration (ppm), represents the final dye concentration (ppm), V represents the volume of dye solution used (mL), and m represents the weight of the MOPB (g).
2.6. Adsorption Isotherm Model Studies
The distribution and adsorption mechanism of EBT molecules on nickel-modified biochar was identified by equilibrium isotherm modeling. The adsorption studies were carried out using the optimum level of initial dye concentration (25 ppm in 50 mL), pH (4.0), adsorbent dose (0.1 g), contact time (90 min), and temperature (35 °C). After agitation at 300 rpm, the sample was filtered, and the final dye concentration was analyzed using a UV–vis spectrophotometer. The adsorption equilibrium capacity was determined using the following equation:
where represents the initial dye concentration (ppm), represents the equilibrium dye concentration (ppm), V represents the volume of dye solution used (mL), and m represents the mass of the MOPB (g). In order to describe how much EBT was adsorbed by a given amount of MOPB, the equilibrium data were analyzed using Langmuir and Freundlich isotherms. The Langmuir isotherm assumes that monolayer adsorption occurs at binding sites with homogenous energy levels, with no interactions between adsorbed molecules and no transmigration of adsorbed molecules on the adsorption surface [29]. The Langmuir equation is given as Equation (3):
where Ce is the equilibrium concentration of the EBT solution mg/L), Qe is the adsorption capacity at equilibrium (mg/g), KL is the constant related to free energy of adsorption (L/mg), and Qm is the maximum adsorption capacity at monolayer coverage (mg/g) [30].
The Freundlich isotherm is an empirical equation that assumes the heterogeneity of the adsorbent surface with its adsorption sites at varying energy levels [31]. The Freundlich isotherm is shown in Equation (4):
where Kf is the Freundlich constant (mg/g), and n is the adsorption intensity constant [28]
2.7. Adsorption Kinetic Model Studies
Adsorption kinetics is essential in process design since it determines the rate of adsorption of the adsorbent. In addition, adsorption kinetic studies could help examine the mechanism that controls the adsorption onto the MOPB and determine the rate-limiting step. Two adsorption kinetic models including pseudo-first-order and pseudo-second-order equations were employed to evaluate the experimental data.
The pseudo-first-order equation, which is based on solid capacity, is shown in Equation (5):
where Qe is the equilibrium adsorption capacity (mg/g), Qt is the adsorption capacity at time t (mg/g), and k1 is the pseudo-first-order rate constant (min−1) [32].
The pseudo-second-order model is represented by Equation (6):
where k2 is the rate constant of pseudo-second-order adsorption (g/mg min) [33].
2.8. Thermodynamics of Adsorption
The study of the influence of temperature on the adsorption of the two dyes on the substrate studied could make it possible to determine thermodynamic parameters such as the variation of the standard entropy ΔS°, the variation of standard enthalpy ΔH°, and Gibbs free energy variation of ΔG°. These standard thermodynamic adsorption quantities can be related to the dissolved body distribution coefficient (Kd) via the following equations [34]:
where R is the perfect gas constant (R = 8.314 J mol−1 K−1), T is the absolute temperature of the solution (K), Kd is the partition coefficient, Qe is the amount of adsorbate in the adsorbent at equilibrium (mg g−1), and Ce is the equilibrium concentration (mg L−1).
2.9. Statistical Analysis
Origin Pro 22 software was used to conduct the statistical analysis of the data. All results are the mean of three replications with the standard deviation displayed.
3. Results and Discussion
3.1. Characterization of Biochar and Magnetic Biochar Derived from Orange Peel
3.1.1. FTIR Analysis
The FTIR plots for orange peel biochar and modified orange peel biochar are shown in Figure 1, respectively. On the adsorbent surface, the chemical adsorption of adsorbent-adsorbate was strengthened by the development of different functional groups. The production of many functional groups was triggered by the disintegration of the cellulose structure of orange peel in the presence of nickel. A strong peak was observed in the range of 3000–3500 , which can be attributed to the -OH stretch from carboxylic groups (-COOH and -COH). Meanwhile, the C = O stretching of carboxylic acids showed a peak at 1638 for OPB and 1637 for MOPB. A peak at 1341 for OPB and 1318 for MOPB explained the C-O, while a peak at 1044 and 1051 explained C-Cl. Nickel modified orange peel biochar, as compared to orange peel biochar, enhanced the development of pore size. The existence of Ni ions may be rationalized at a peak of 826 . The broadness of peak indicates the crystallinity of Ni [35]. All peaks being prominent indicates the presence of certain minerals such as oxygen, hydrogen, and carbon on the surface of biochar [32].
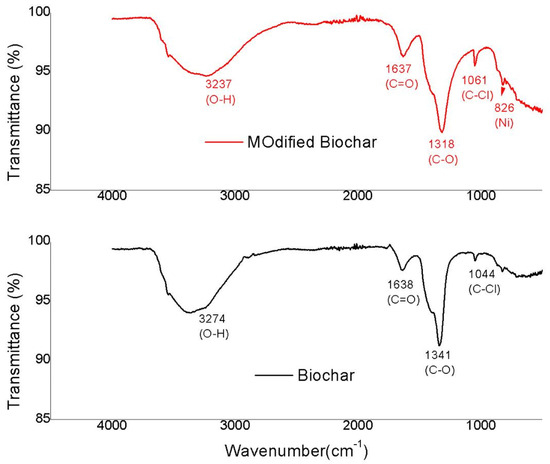
Figure 1.
FTIR analysis of OPB and MOPB.
3.1.2. SEM Analysis
For the SEM analysis, both samples (OPB and MOPB) were investigated, and SEM images exhibited notable alterations on the surface morphology of the OPB and MOPB before and after dye adsorption. The SEM image of orange peel showed a relatively clear, grid pattern with jagged spots prior to alteration. It was dominated by large pores, which were about 50 μm in size. On the other hand, the modified orange peel surface morphology was altered, resulting in a rough surface with distinct patterns. This is because the composition of the orange peel broke down following alteration, and the irregular surface structure increased its contact area and added structural features to it since the pores provided adequate adsorption sites. The pore size reduced to 30 μm after modification. The surface of the bio-sorbent became shiny and whitish after the integration of Ni ions [36], as shown in Figure 2.
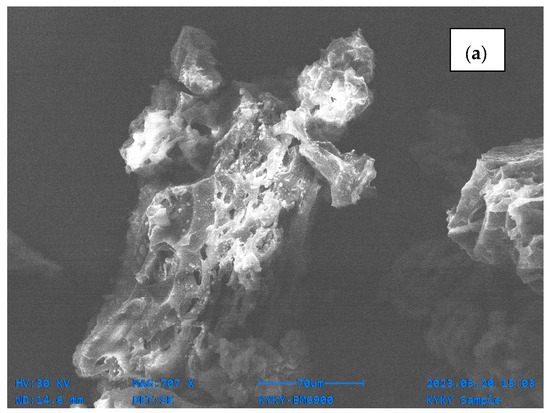
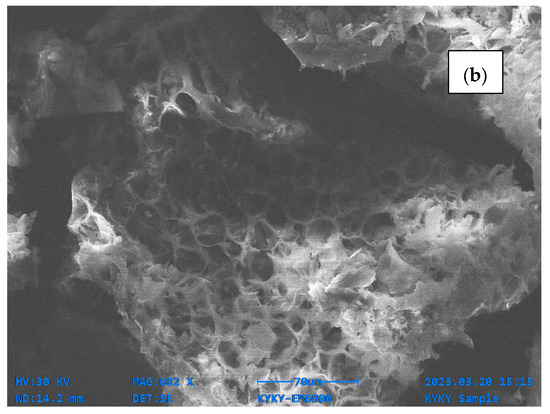
Figure 2.
SEM analysis of orange peel biochar (a) and modified orange peel biochar (b).
3.1.3. XRD Analysis
XRD analysis provides information about the structure, phase, crystal orientation, lattice parameters, crystallite size, strain, and crystal defects [37]. The observed XRD patterns are shown in Figure 3. The results of the orange peel biochar and modified orange peel biochar showed a minor shift in peaks. The XRD peak pattern of orange peel biochar at 2θ° was 19.19, 22, 26.6, 33.4, 46.2, and 61.5, whereas, for the modified orange peel biochar, the peaks obtained at 2θ° were 16.8, 22.9, 25, 25.5, 44.63, 52.5, 58.8, and 76.96. Three strong peaks of nickel can be easily seen (44.63°, 52.5°, 76.96°). Presence of Ni provides new surface sites and enhances the interactions. Ni has a face-centered cubic crystal structure in which atoms are arranged in a specific pattern within the crystal lattice which helps in the adsorption mechanism. The sharpness of the peaks indicates the crystallite size; sharper peaks indicate a larger crystallite size, whereas broader peaks indicate smaller crystallite size.
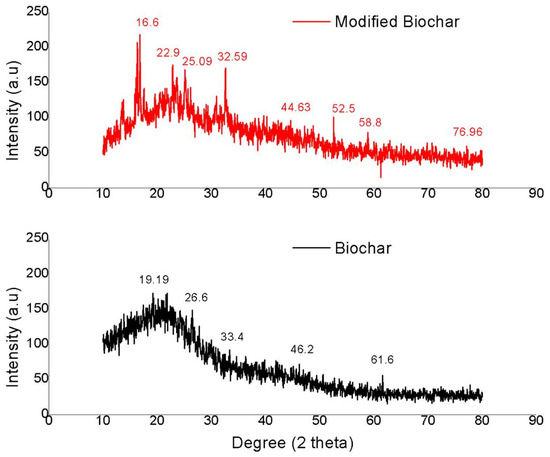
Figure 3.
XRD analysis of orange peel biochar and modified orange peel biochar.
3.2. Optimization of Adsorption Parameters
The effect of different adsorption parameters, the pH of the dye stock solution, the dose of the magnetic biochar adsorbent, and the contact time of the removal of orange peel biochar and modified orange peel biochar were examined through a series of experiments. The results of each process parameter are discussed in the following section.
3.2.1. Removal Efficiency of OPB and MOPB for EBT at Different Doses
Adsorbent dose substantially impacts the adsorption process by altering the adsorption capability of the adsorbent [38]. The removal efficiency of orange peel biochar and modified orange peel biochar synthesized at 350 °C using different adsorbent doses (0.01, 0.025, 0.05, 0.075, 0.1) is shown in Figure 4a. In general, removal efficiency of biochar increased with increasing weight of adsorbent from 0.01 g to 0.1 g. The weight dose of 0.01 gm of orange peel biochar showed minimum sorption of EBT (22%), and maximum sorption was recorded for 0.1 g of biochar (58%). The adsorption of EBT was 92% and 98% at 0.01 g and 0.1 g of modified orange peel biochar, respectively (Figure 4a). Altering the raw orange peel biochar considerably improved the elimination of EBT. This was due to the fact that the number of adsorption sites increased in direct proportion to the quantity of MOPB particles [27]. At 0.1 g/L of MOPB, the highest amount of 98% EBT was found to be adsorbed.
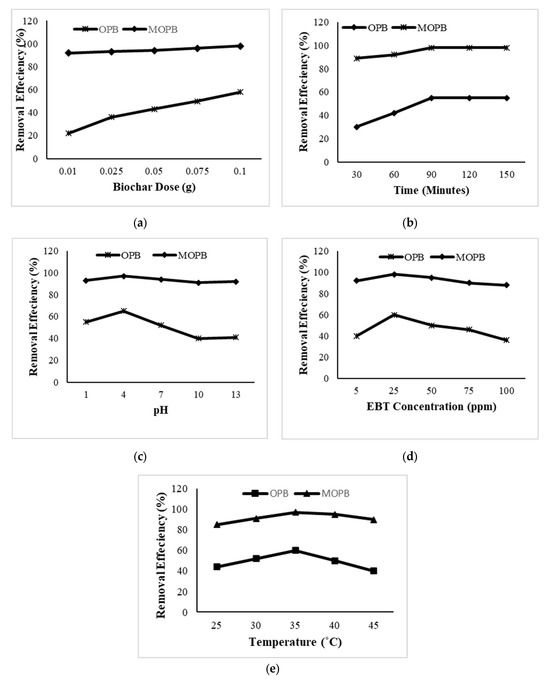
Figure 4.
Effect of OPB and MOPB doses (a), contact time (b), different concentrations of dye (c), pH (d), and temperature (e) on the removal efficiency of EBT.
3.2.2. Removal Efficiency of OPB and MOPB for EBT at Different Contact Times
The effect of contact time on removal efficiency of raw orange peel biochar was studied, and the results (Figure 4b) showed that the minimum adsorption of EBT at 30 min (30%) and maximum adsorption of EBT at 90 min (55%) at the point equilibrium were attained. Whereas, the adsorption of EBT by the nickel-modified biochar was 89% at 30 min, 92% at 60 min. and 98% at 90 min. After this, no change was observed at 120 or 150 min, indicating that equilibrium was attained after 90 min. Various investigations have shown that modified biochar has a substantially higher capacity for dye adsorption than does untreated biochar. Previous studies have found that as contact length increases, the rate of adsorption decreases due to a decrease in the total surface area and the attainment of equilibrium. Once the equilibrium is attained, the amount of dye adsorbed does not change with longer contact time [1,39].
3.2.3. Removal Efficiency of OPB and MOPB for EBT at Different Concentration of Dye
For an effective adsorption process, the concentration of the adsorbate is considered as an important factor. Figure 4c. illustrates the effect of the concentration of dyes on the efficiency of EBT removal. Different concentrations (5 ppm, 25 ppm, 50 ppm, 75 ppm, and 100 pm) were studied to determine the adsorption capacity for EBT of the unmodified and modified orange peel biochar. The orange peel biochar showed a maximum adsorption of EBT at a 25 ppm concentration (60%) and a minimum adsorption capacity at 100 pm (29%). Whereas, in the modified orange peel biochar, the removal efficiency was 98% at 25 ppm, while the minimum adsorption (88%)occurred at a concentration of 100 ppm. According to previous investigations, when the dye concentrations increase, the percent of dye eliminated decreases. Because the adsorbent’s active binding sites reach saturation at a specific concentration, none of dye molecules in the solution interact with the adsorbent’s binding sites at higher concentrations [40,41].
3.2.4. Removal Efficiency of OPB and MOPB for EBT at Different pH Values
The pH of a solution plays a key regulatory role in the adsorption process by affecting the solubility of different dyes [42]. Studies were carried out at pH 1, 4, 7, 10, and 13 (Figure 4d). For the orange peel biochar, the maximum dye adsorption occurred at pH 4 (65%) and minimum adsorption of dye at pH 10 (40%). The results for modified orange peel biochar revealed that adsorption was minimal at pH 10 (91%) and greatest at pH 4 (97%). At pH 4, due to the ionization process, significant electrostatic contact is established between the anionic dye and the adsorbent’s positive charge surface. The quantity of negatively charged sites increases as the pH of the system rises. A negatively charged surface location on the adsorbent does not promote the adsorption of dye anions due to electrostatic repulsion. However, at an effective pH value of 4, the modified orange peel biochar performed successfully. Similar patterns were observed for the adsorption of Congo red and Acid Yellow 36 on activated carbon and sawdust and rice husk carbon, respectively [43].
3.2.5. Removal Efficiency of OPB and MOPB for EBT at Different Temperatures
The removal efficiency of orange peel biochar at various temperatures (25 °C, 30 °C, 35 °C, 40 °C, and 45 °C) is shown in Figure 4e. In general, as the temperature increased, the removal efficiency of the biochar also increased. In this study, the results showed maximum adsorption at 35 °C (60%) and minimum adsorption of EBT at 25 °C (40%) for orange peel biochar. Whereas, in the modified orange peel biochar, the maximum EBT adsorption of 97% occurred at 35 °C, and the minimum adsorption of EBT of 85% occurred at 25 °C. The modified orange peel biochar showed better results and removal efficiency as compared to the orange peel biochar. As the temperature rose, the percentage of EBT dye removal increased. This might be because when the temperature rises, the dye molecules in the solution diffuse more quickly [26]. From the bulk to the surface, adsorption is controlled by the diffusion process. By enhancing the mobility of dye molecules and possibly exposing additional binding sites on the adsorbent, raising the kinetic energy of dye molecules at higher temperatures can accelerate the rate of adsorption. This endothermic process indicates that adsorption mechanism is favored at higher temperature [37].
3.3. Adsorption Isotherm Model Study
The Langmuir adsorption isotherm, which was first devised to characterize the gas–solid-phase adsorption of biochar, has long been used to compare and measure adsorption performance. Normal adsorption is indicated by a Freundlich constant less than one. As pressure grows without limitation, the function reaches an asymptotic maximum. Furthermore, the higher the value is, the more heterogeneity is predicted. A result between 1 and 10, on the other hand, indicates that this isotherm model is desirable. The R2 correlation value of 0.98 in this study illustrates that the EBT dye adsorption was favorable and that a good adsorption model was achieved (Figure 5). The adsorption amount of modified orange peel biochar was larger than that of the orange peel biochar, indicating that the modified biochar has a greater adsorption capability. Under the same concentration, the amount of EBT adsorbed by the orange peel biochar and modified orange peel biochar increased with increasing temperature. This shows that the EBT adsorption by the orange peel biochar and modified orange peel biochar is facilitated by an increase in temperature.
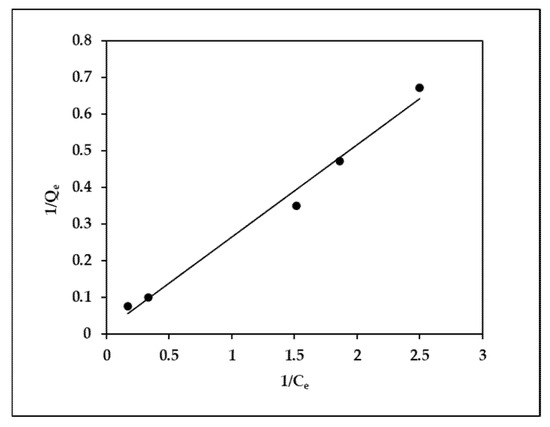
Figure 5.
Linear plot of the Langmuir isotherm model.
The findings in Table 3 show that Langmuir’s isotherm was the perfect fit because it confirmed the monolayer adsorption of EBT onto MOPB, and its linear regression coefficient (R2 > 0.98 for all cases) was greater than that of Freundlich’s isotherm (R2 > 0.96 for all cases) (Figure 6). MOPB’s maximal EBT adsorption compressive strength was found to be 71.94 mg/g. Because the MOPB removal rate (RL) ratios are less than one, the adsorption phenomena is more likely to occur.

Table 3.
Isothermic, thermodynamic, and kinetic parameters for adsorption of MOPB onto EBT.
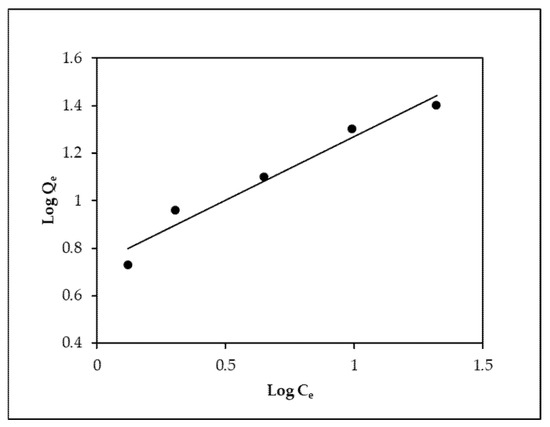
Figure 6.
Linear plot of the Freundlich isotherm model.
3.4. Kinetic Isotherm Model Study
Figure 7 and Figure 8 depict the adsorption kinetic curves for EBT onto MOPB. To further assess the kinetic characteristics of EBT adsorption on MOPB, the pseudo-first-order and pseudo-second-order rate equations were both used in the current work. The pseudo-second-order kinetic model was found to have a greater frequency of the linear regression coefficient (R2 > 0.98, in all cases) than that of the pseudo-first-order model (R2 0.97, in all cases), making it the model that best describes the kinetics of EBT adsorption on MOPB. The computed and observed adsorption capacity (qe) values coincide rationally, favoring the pseudo-second-order kinetic model. The data presented above demonstrate that the percentages of both the adsorbents and the type of EBT predominantly define the phase percentages of the process.
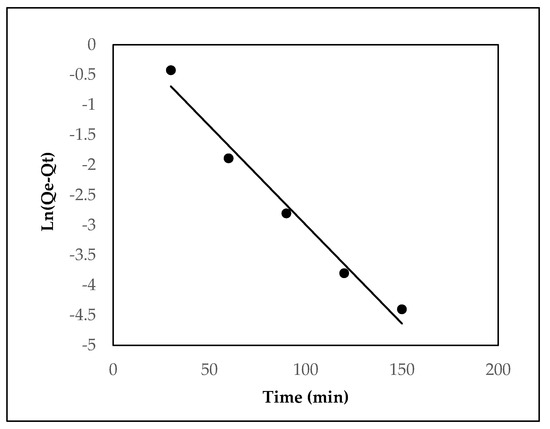
Figure 7.
Pseudo-first-order model for the adsorption of EBT onto MOPB.
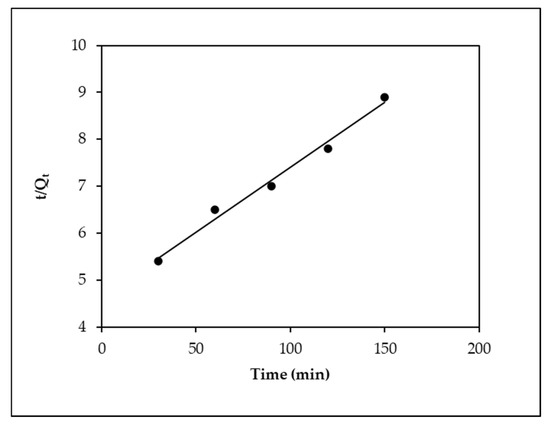
Figure 8.
Pseudo-second-order model for the adsorption of EBT onto MOPB.
3.5. Thermodynamic Isotherm Model Study
Numerous thermodynamic characteristics are linked to the adsorption mechanism’s temperature dependency. To determine whether an adsorption process is spontaneous or not, thermodynamic factors are required. It is possible to determine whether adsorption is endothermic or exothermic using the thermodynamic properties, including the enthalpy H, entropy S, and Gibbs free-energy G values (Figure 9). The mechanism is spontaneous, thermodynamically possible, and corresponds to a physical reaction according to the negative G values. The exothermic character of the adsorption mechanism is shown by the negative value of H (Table 3). The enhanced unpredictability at the solid–solution interface after the adsorption of EBT ions on amplified orange peel biochar is shown by the positive value of S°.
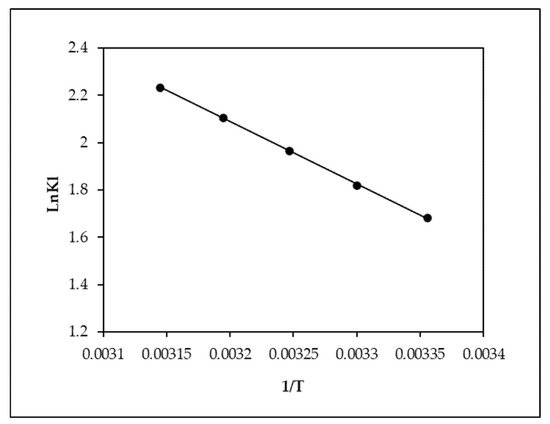
Figure 9.
Thermodynamic isotherm model for the adsorption of EBT onto MOPB.
3.6. Comparative Analysis with Existing Literature
In recent years, various adsorbents have been evaluated for the removal of toxic dyes from wastewater. The performance of nickel-modified orange peel biochar (MOPB) in adsorbing Eriochrome Black T (EBT) dye stands out due to several distinguishing features compared to other reported materials. For instance, studies have shown that biochars derived from agricultural waste, such as orange peel, are low cost, biodegradable, and effective in pollutant removal, with MOPB further enhanced by nickel modification, which introduces new surface sites and increases adsorption efficiency.
In comparison to other natural adsorbents, MOPB shows superior removal efficiency. For example, activated carbon, widely regarded as a high-performance adsorbent, achieves around 85–90% removal of similar dyes under optimal conditions [44,45]. In contrast, MOPB reaches up to 98% removal efficiency for EBT at an adsorbent dose of 0.1 g, significantly outperforming activated carbon in terms of efficiency at lower doses, suggesting its potential for cost-effective large-scale applications.
Biochars derived from rice husk and other agricultural wastes have demonstrated promising results in the removal of dyes from wastewater, which is in line with the findings of the current study. For example, rice husk biochar has been reported to achieve removal efficiencies of 66.8–96.9% for Congo red dye [46] and 71.0–99.0% for methylene blue [47]. Similarly, the current study highlights the superior adsorption capacity of nickel-modified orange peel biochar (MOPB) in removing Eriochrome Black T, demonstrating its potential for effective dye removal from wastewater. Additionally, the modification of biochar using nickel ions not only enhances surface area and pore volume but also accelerates the adsorption kinetics, as demonstrated by the pseudo-second-order kinetic model that best describes EBT uptake on MOPB. This is in line with previous studies where metal-modified biochars exhibited faster adsorption rates compared to unmodified biochars [48,49].
The performance of MOPB, in terms of both adsorption capacity (71.94 mg/g) and thermodynamic stability, further sets it apart from conventional adsorbents, offering a more sustainable and efficient alternative for the treatment of wastewater containing toxic dyes. The exothermic nature of the adsorption process indicates that MOPB can effectively treat wastewater at ambient temperatures, reducing energy costs and enhancing its applicability in real-world scenarios.
Thus, the work presented in this study not only adds to the growing body of literature on biochar-based adsorbents but also provides a novel approach for improving the performance of these materials through simple, cost-effective modification techniques. The enhanced removal efficiency, along with the renewability and low environmental impact of MOPB, underscores its potential as a viable solution for large-scale wastewater treatment applications.
4. Conclusions
Nickel-modified orange peel biochar (MOPB), synthesized by a simple pyrolysis method, was used as an adsorbent for the removal of Eriochrome Black T dye (EBT). Batch experiments were applied on both simple peel biochar (OPB) and nickel-modified orange peel biochar (MOPB) and revealed that nickel-modified orange peel biochar had the best results at the optimum conditions of 0.1 g of adsorbent dose, 25 ppm of initial dye concentration, 90 min of contact time, 35 °C, and pH 4 for the maximum removal of EBT dye. The equilibrium data were evaluated using Langmuir and Freundlich isotherms models. The Langmuir model (R2 = 0.98) best describes the uptake of EBT dye. which indicated homogenous adsorption of EBT dye. The kinetic studies were also completed by using pseudo-first-order and pseudo-second-order studies to calculate the rate-limiting step of the reaction. Pseudo-second order was found to be the best fit (R2 = 0.99), which indicated that the chemisorption process took place through the exchange of electrons between the adsorbate and adsorbent. Thermodynamic studies revealed that the adsorption reaction is exothermic in nature. The present study will provide a favorable platform for the synthesis of metal-mixed biochars based on organic waste (orange peel) that can be used for the remediation of various environmental pollutants. In the present study, nickel provided new surface sites and enhanced the interactions between biochar and EBT, which shows that OP-derived biochar may be used as an EBT adsorbent in an aqueous solution and is efficient, low-cost, and environmentally safe.
Author Contributions
A.K.: conceptualization, methodology, investigation, software, and writing—original draft. J.A.: conceptualization and writing—review. S.G.: investigation, software, and writing—review, M.N.: methodology, data curation, and writing—review and editing. A.M.I.: resources and writing—review and editing. H.U.: software, investigation, supervision, funding acquisition, and writing—review and editing. W.-U.-N.: investigation, validation, conceptualization, supervision, project administration, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through the Large Groups Project under grant number RGP.2/25/46.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
Authors wish to thank the Centre For Advanced Electronics & Photovoltaic Engineering (CAEPE) for providing facility of biochar characterization through SEM and XRD.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ahmad, R. Studies on adsorption of crystal violet dye from aqueous solution onto coniferous pinus bark powder (CPBP). J. Hazard. Mater. 2009, 171, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Liu, J.; Ge, J. Dynamic game in agriculture and industry cross-sectoral water pollution governance in developing countries. Agric. Water Manag. 2021, 243, 106417. [Google Scholar] [CrossRef]
- Dou, G.; Jiang, Z. Preparation of Sodium Humate-Modified Biochar Absorbents for Water Treatment. ACS Omega 2019, 4, 16536–16542. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Liang, H.; Chen, T.; Yang, W.; Ding, C. Influence of long-term irrigation with treated papermaking wastewater on soil ecosystem of a full-scale managed reed wetland. J. Soils Sediments 2016, 16, 1352–1359. [Google Scholar] [CrossRef]
- Lakhiar, I.A.; Yan, H.; Zhang, J.; Wang, G.; Deng, S.; Bao, R.; Zhang, C.; Syed, T.N.; Wang, B.; Zhou, R.; et al. Plastic Pollution in Agriculture as a Threat to Food Security, the Ecosystem, and the Environment: An Overview. Agronomy 2024, 14, 548. [Google Scholar] [CrossRef]
- Mubarak, N.M.; Fo, Y.T.; Al-Salim, H.S.; Sahu, J.N.; Abdullah, E.C.; Nizamuddin, S.; Ganesan, P. Removal of Methylene Blue and Orange-G from Waste Water Using Magnetic Biochar. Int. J. Nanosci. 2015, 14, 1550009. [Google Scholar] [CrossRef]
- Kansal, S.K.; Swati Sood, S.; Umar, A.; Mehta, S.K. Photocatalytic degradation of Eriochrome Black T dye using well crystalline anatase TiO2 nanoparticles. J. Alloys Compd. 2013, 581, 392–397. [Google Scholar] [CrossRef]
- Inyang, M.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.R.; Pullammanappallil, P.; Cao, X. Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass. Bioresour. Technol. 2012, 110, 50–56. [Google Scholar] [CrossRef]
- Barka, N.; Abdennouri, M.; El Makhfouk, M. Removal of Methylene Blue and Eriochrome Black T from aqueous solutions by biosorption on Scolymus hispanicus L.: Kinetics, equilibrium and thermodynamics. J. Taiwan Inst. Chem. Eng. 2011, 42, 320. [Google Scholar] [CrossRef]
- Fosso-Kankeu, E.; Potgieter, J.; Waanders, F.B. Removal of malachite green and toluidine blue dyes from aqueous solution using a clay-biochar composite of bentonite and sweet sorghum bagasse. Int. J. Appl. Eng. Res. 2019, 14, 1324–1333. [Google Scholar]
- Akbarnezhad, A.A.; Safa, F. Biochar-Based Magnetic Nanocomposite for Dye Removal from Aqueous Solutions: Response Surface Modeling and Kinetic Study. Russ. J. Appl. Chem. 2018, 91, 1856–1866. [Google Scholar] [CrossRef]
- Jing, Z.; Ding, J.; Zhang, T.; Yang, D.; Qiu, F.; Chen, Q.; Xu, J. Flexible, versatility and superhydrophobic biomass carbon aerogels derived from corn bracts for efficient oil/water separation. Food Bioprod. Process. 2019, 115, 134–142. [Google Scholar] [CrossRef]
- Syeda, S.R.; Ferdousi, S.A.; Ahmmed, K.M.T. Decolorization of textile wastewater by adsorption in a fluidized bed of locally available activated carbon. J. Environ. Sci. Health A 2012, 47, 210–220. [Google Scholar] [CrossRef]
- Ruthiraan, M.; Abdullah, E.C.; Mubarak, N.M.; Nizamuddin, S. Adsorptive removal of methylene blue using magnetic biochar derived from agricultural waste biomass: Equilibrium, Isotherm, Kinetic study. Int. J. Nanosci. 2018, 17, 1850002. [Google Scholar] [CrossRef]
- Michael-Igolima, U.; Abbey, S.J.; Ifelebuegu, A.O.; Eyo, E.U. Modified Orange Peel Waste as a Sustainable Material for Adsorption of Contaminants. Materials 2023, 16, 1092. [Google Scholar] [CrossRef]
- Bordoloi, N.; Dey, M.D.; Mukhopadhyay, R.; Kataki, R. Adsorption of Methylene blue and Rhodamine B by using biochar derived from Pongamia glabra seed cover. Water Sci. Technol. 2017, 77, 638–646. [Google Scholar] [CrossRef]
- Ding, F.; Luo, Z.; Xiao, Z.; Wu, T.; Zhong, H. Degradation of printing and dyeing wastewater by modified biochar catalyzed persulfate. E3S Web Conf. 2019, 118, 04040. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Li, J.; Ma, D.; Han, R. Characterization of bio-char from pyrolysis of wheat straw and its evaluation on methylene blue adsorption. Desalin. Water Treat. 2012, 46, 115–123. [Google Scholar] [CrossRef]
- Tan, W.; Wang, L.; Yu, H.; Zhang, H.; Zhang, X.; Jia, Y.; Li, T.; Dang, Q.; Cui, D.; Xi, B. Accelerated Microbial Reduction of Azo Dye by Using Biochar from Iron-Rich-Biomass Pyrolysis. Materials 2019, 12, 1079. [Google Scholar] [CrossRef]
- Nautiyal, P.; Subramanian, K.A.; Dastidar, M.G. Adsorptive removal of dye using biochar derived from residual algae after in-situ transesterification: Alternate use of waste of biodiesel industry. J. Environ. Manag. 2016, 182, 187–197. [Google Scholar] [CrossRef]
- Sun, P.; Hui, C.; Azim Khan, R.; Du, J.; Zhang, Q.; Zhao, Y.H. Efficient removal of crystal violet using Fe3O4-coated biochar: The role of the Fe3O4 nanoparticles and modeling study their adsorption behavior. Sci. Rep. 2015, 5, 12638. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lin, X.; Wei, B.; Zhao, Y.; Wang, J. Evaluation of adsorption potential of bamboo biochar for metal-complex dye: Equilibrium, kinetics and artificial neural network modeling. Int. J. Environ. Sci. Technol. 2014, 11, 1093–1100. [Google Scholar] [CrossRef]
- Moazzam, A.; Jamil, N.; Nadeem, F.; Qadir, A.; Ahsan, N.; Zameer, M. Reactive dye removal by a novel biochar/MgO nanocomposite. J. Chem. Soc. Pak. 2017, 39, 26–34. [Google Scholar]
- El Farissi, H.; Lakhmiri, R.; Albourine, A.; Safi, M.; Cherkaoui, O. Removal of RR-23 dye from industrial textile wastewater by adsorption on Cistus ladaniferus seeds and their biochar. J. Environ. Earth Sci. 2017, 7, 105–118. [Google Scholar]
- Trinh, B.; Le, P.; Werner, D.; Nguyen, P.; Luu, T. Rice Husk Biochars Modified with Magnetized Iron Oxides and Nano Zero Valent Iron for Decolorization of Dyeing Wastewater. Processes 2019, 7, 660. [Google Scholar] [CrossRef]
- Yao, X.; Ji, L.; Guo, J.; Ge, S.; Lu, W.; Cai, L.; Wang, Y.; Song, W.; Zhang, H. Magnetic Activated Biochar Nanocomposites Derived from Wakame and its Application in Methylene Blue Adsorption. Bioresour. Technol. 2020, 302, 122842. [Google Scholar] [CrossRef]
- Kaya, N.; Yıldız, Z.; Ceylan, S. Preparation and characterisation of biochar from hazelnut shell and its adsorption properties for methylene blue dye. J. Polytechnic. 2018, 21, 765–776. [Google Scholar] [CrossRef]
- Alamzeb, M.; Tullah, M.; Ali, S.; Ihsanullah; Khan, B.; Setzer, W.N.; Al-Zaqri, N.; Ibrahim, M.N.M. Kinetic, Thermodynamic and Adsorption Isotherm Studies of Detoxification of Eriochrome Black T Dye from Wastewater by Native and Washed Garlic Peel. Water 2022, 14, 3713. [Google Scholar] [CrossRef]
- Hanafiah, M.A.K.M.; Wan, N.W.S.; Zolkafly, S.H.; Teong, L.C.; Majid, Z.A.A. Acid blue25 adsorption on base treated Shorea dasyohylla sawdust: Kinetic, isotherm, thermodynamics and spectroscopic analysis. J. Environ. Sci. 2012, 24, 261. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica, and platinum. J. Am. Chem. Soc. 1918, 40, 1361. [Google Scholar] [CrossRef]
- Freundlich, H. Uber die adsorption in Losungen. J. Phys. Chem. 1907, 57, 385. [Google Scholar] [CrossRef]
- Ho, Y. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451. [Google Scholar] [CrossRef]
- Badraoui, A.E.; Miyah, Y.; Nahali, L.; Zerrouq, F. Fast adsorption for removal of methylene blue from aqueuous solutions using of local clay. Mor. J. Chem. 2019, 7, 416–423. [Google Scholar]
- Subhash, D.K.; Vinod, S.S. Facile synthesis of nickel oxide nanoparticles for the degradation of Methylene blue and Rhodamine B dye: A comparative study. J. Taibah. Univ. Sci. 2019, 13, 1108–1118. [Google Scholar] [CrossRef]
- Felicia, O.A.; Paul, M. Synthesis, Characterization, and Biosorption of Cu2+ and Pb2+ Ions from an Aqueous Solution Using Biochar Derived from Orange Peels. Molecules 2023, 28, 7050. [Google Scholar] [CrossRef]
- Dey, S.; Basha, S.R.; Babu, G.V.; Nagendra, T. Characteristic and biosorption capacities of orange peels biosorbents for removal of ammonia and nitrate from contaminated water. Clean. Mater. 2021, 1, 100001. [Google Scholar] [CrossRef]
- Luk, C.J.; Yip, J.; Yuen, C.M.; Kan, C.; Lam, K. A comprehensive study on adsorption behaviour of direct, reactive and acid dyes on crosslinked and non-crosslinked chitosan beads. J. Fiber Bioeng. Inform. 2014, 7, 35–52. [Google Scholar]
- Chakraborty, S.; Chowdhury, S.; Saha, P. Adsorption of crystal violet from aqueous solution onto NaOH modified rice husk. Carbohydr. Polym. 2011, 86, 1533–1541. [Google Scholar] [CrossRef]
- Saha, P.; Chowdhury, S.; Gupta, S.; Kumar, I. Insight into adsorption equilibrium, kinetics and thermodynamics of malachite green onto clayey soil of Indian origin. Chem. Eng. J. 2010, 165, 874–882. [Google Scholar] [CrossRef]
- Fu, Y.; Viraraghavan, T. Fungal decolorization of dye wastewaters: A review. Bioresour. Technol. 2001, 79, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Waranusantigul, P.; Pokethitiyook, P.; Kruatrachue, M.; Upatham, E.S. Kinetics of basic dye (methylene blue) biosorption by giant duckweed (Spirodela polyrrhiza). Environ. Pollut. 2013, 125, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Namasivayam, C.; Kavitha, D. Removal of Congo Red from water by adsorption onto activated carbon prepared from coir pith, an agricultural solid waste. Dye Pigment. 2002, 54, 47–58. [Google Scholar] [CrossRef]
- Saleem, J.; Moghal, Z.K.B.; Pradhan, S.; McKay, G. High-performance activated carbon from coconut shells for dye removal: Study of isotherm and thermodynamics. RSC Adv. 2024, 14, 33797–33808. [Google Scholar] [CrossRef]
- Jain, R.; Shrivastava, M. Adsorptive studies of hazardous dye Tropaeoline 000 from an aqueous phase on to coconut-husk. J Hazard Mater. 2008, 158, 549–556. [Google Scholar] [CrossRef]
- Khan, N.; Chowdhary, P.; Ahmad, A.; Shekher Giri, B.; Chaturvedi, P. Hydrothermal liquefaction of rice husk and cow dung in Mixed-Bed-Rotating Pyrolyzer and application of biochar for dye removal. Bioresour. Technol. 2020, 309, 123294. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, N.; Giri, B.S.; Chowdhary, P.; Chaturvedi, P. Removal of methylene blue dye using rice husk, cow dung and sludge biochar: Characterization, application, and kinetic studies. Bioresour. Technol. 2020, 306, 123202. [Google Scholar] [CrossRef]
- Hama Aziz, K.H.; Fatah, N.M.; Muhammad, K.T. Advancements in application of modified biochar as a green and low-cost adsorbent for wastewater remediation from organic dyes. R. Soc. Open Sci. 2024, 11, 232033. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nguyen, D.L.T.; Binh, Q.A.; Nguyen, X.C.; Huyen Nguyen, T.T.; Vo, Q.N.; Nguyen, T.D.; Phuong Tran, T.C.; Hang Nguyen, T.A.; Kim, S.Y.; Nguyen, T.P.; et al. Metal salt-modified biochars derived from agro-waste for effective congo red dye removal. Environ. Res. 2021, 200, 111492. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).