The Mobility and Distribution of Lead and Cadmium in the Ecosystems of Two Lakes in Poland and Their Effect on Humans and the Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Materials
2.3. Methods
2.3.1. Sample Preparation for Pb and Cd Analysis
2.3.2. Determination of Moisture and Organic Matter Contents
2.3.3. Pb and Cd Determination
Total Metal Concentrations in Examined Materials
Sequential Extraction of Elements from Bottom Sediments
2.3.4. Analytical Quality Control
2.3.5. Pb and Cd Accumulation and Pollution in Bottom Sediments
Accumulation Factors (AFs)
Calculations of Sediment Pollution with Pb and Cd
2.3.6. Pb and Cd Bioaccumulation in Bottom Sediments
2.3.7. Consumer Risk Calculations
2.3.8. Statistical Methods
3. Results and Discussion
3.1. Pb and Cd in Water and Bottom Sediments
3.1.1. Pb and Cd Concentrations
3.1.2. Bottom Sediments of Lakes Ińsko and Wisola
Organic Matter Content and Bottom Sediments
Pb and Cd Contents in Bottom Sediments and Accumulation Factors
3.2. Distribution of Pb and Cd in Common Reed (Phragmites australis) and Bioaccumulation Factors
3.3. Distribution of Pb and Cd in the Organs of Fish—Pike (Esox lucius), Bream (Abramis brama) and Roach (Rutilus rutilus)—And Bioaccumulation Factors
3.4. Fish Safety Assessment for Consumers
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parameter | Lake Ińsko | Lake Wisola | ||||||
|---|---|---|---|---|---|---|---|---|
| Surface Water | Bottom Water | Inflows | Outflows | Surface Water | Bottom Water | Inflows | Outflows | |
| Visibility (m)/sampling depth (m) * | 5.1 ± 0.6 (4.0–6.0) | 9.7 ± 2.2 (7.3–14.0) | - | - | 2.6 ± 1.2 (1.5–5.5) | 9.8 ± 2.5 6.0–14.0 | - | - |
| temperature (°C) | 12.1 ± 7.1 (3.6–21.0) | 8.8 ± 5.2 (3.4–18.6) | 11.8 ± 7.4 (3.2–20.7 | 10.9 ± 6.1 3.7–18.0 | 12.5 ± 7.3 (3.7–21.0) | 7.9 ± 3.7 (4.2–15.3) | 11.8 ± 6.5 (1.6–19.4) | 13.0 ± 6.9 (3.5–19.9) |
| pH | 8.0 ± 0.3 (7.3–8.8) | 7.9 ± 0.5 (7.1–9.)2 | 7.2 ± 0.2 (6.9–7.6) | 8.1 ± 0.1 8.0–8.2 | 8.3 ± 0.6 (7.4–9.4) | 7.6± 0.5 (7.1–8.)9 | 7.5 ± 0.4 (6.8–7.9) | 8.2 ± 0.8 (6.4–9.2) |
| Oxygen concentration (mg∙L−1) | 9.0 ± 1.7 (6.5–12.6) | 7.4 ± 1.9 (3.6–10.0) | 7.4 ± 2.0 (5.1–10.0) | 8.8 * | 9.6 ± 3.9 (7.6–12.5) | 3.8 ± 2.8 (1.0–10.6) | 7.1 ± 2.1 (2.0–10.4) | 15.5 ± 10.1 (2.8) |
| Dry Weight (%, Mean ± SD; Range) | ||||||
|---|---|---|---|---|---|---|
| Organ/ | PIKE | BREAM | Roach | |||
| Tissue | Lake Ińsko | Lake Wisola | Lake Ińsko | Lake Wisola | Lake Ińsko | Lake Wisola |
| gonads | 23.2 ± 7.1 (14.4–36.3) | 24.1 ± 7.7 (15.5–36.4) | 23.2 ± 6.3 (13.4–35.8) | 22.2 ± 5.2 (10.0–36.7) | 24.7 ± 8.6 (18.3–33.4) | 24.9 ± 5.6 (14.8–35.5) |
| kidney | 18.7 ± 1.1 (13.7–21.0) | 18.6 ± 1.5 (16.0–20.7) | 19.3 ± 2.2 (17.1–23.6) | 20.0 ± 1.4 (15.0–24.2) | 21.5 ± 2.4 (16.0–23.1) | 19.9 ± 2.1 (19.2–27.3) |
| liver | 27.5 ± 5.3 (18.9–34.7) | 28.2 ± 4.2 (18.0–39.2) | 22.5 ± 2.6 (17.4–27.3) | 22.6 ± 1.8 (18.2–29.1) | 23.0 ± 1.9 (19.528.9) | 23.1 ± 2.8 (19.8–25.7) |
| spleen | 24.5 3.7 (21.5–26.7) | 23.7 1.5 (20.5–41.7) | 20.3 1.0 (18.1–23.9) | 20.4 1.3 (17.9–22.5) | 23.6 1.0 (20.5–25.6) | 22.7 1.9 (22.6–25.0) |
| stomach * | 22.8 ± 4.0 (18.8–25.8) | 21.8 ± 1.7 (17.0–43.5) | 23.0 ± 1.4 (20.4–26.6) | 23.5 ± 1.4 (19.8–27.2) | - | - |
| intestine | 24.6 ± 3.3 (21.2–27.6) | (18.7–40.0) | 23.5 ± 2.3 (18.8–28.8) | 23.7 ± 1.7 (18.8–30.4) | 21.3 ± 2.7 (18.4–24.4) | 21.6 ± 1.6 (15.6–23.9) |
| muscle | 21.0 ± 2.1 (18.8–24.0) | 20.7 ± 1.0 (18.7–35.6) | 21.6 ± 2.5 (17.2–38.3) | 21.5 ± 2.9 (17.8–37.1) | 22.9 ± 5.3 (18.3–21.6) | 20.7 ± 0.7 (20.4–39.6) |
| skin | 34.3 ± 2.7 (29.6–38.0) | 34.6 ± 2.1 (28.3–40.8) | 37.3 ± 3.1 (31.8–42.4) | 37.4 ± 2.5 (29.6–43.2) | 45.1 ± 5.0 (38.3–49.5) | 42.2 ± 2.4 (37.1–50.6) |
| gills | 20.9 ± 2.8 (19.2–29.6) | 22.5 ± 1.7 (15.2–25.5) | 23.0 ± 1.8 (20.3–26.5) | 23.6 ± 1.4 (17.4–26.1) | 24.4 ± 3.5 (24.1–26.4) | 25.2 ± 0.8 (17.4–27.5) |
| Fish Species | Bioconcentration Factors (BAFws), Mean (Range) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gonads | Kidneys | Liver | Spleen | Stomach * | Intestine * | Muscle | Skin | Gills | Total | |
| Lake Ińsko | ||||||||||
| Pike | 511 (nd-31,11) | 533 (nd-3,556) | 667 (nd-7,778) | 622 (nd-3,556) | 444.4 (nd-2,000) | 577.8 (nd-2,888) | 444 (nd-2,444) | 382 (nd-2,444) | 467 (nd-1,778) | 517 (nd-7,778) |
| Bream | 66.7 (nd-1,556) | 407 (nd-2,444) | 156 (nd-1,333) | 111 (nd-1,111) | 156 (nd-2889) | 178 (nd-2,222) | 44 (nd-889) | 222 (nd-1,111) | 133 (nd-1,778) | 164 (nd-2,889) |
| Roach | 667 (nd-1,778) | 444 (nd-2,222) | 444 (nd-1,111) | 889 (nd-1,333) | - | 444 (nd-1,556) | 222 (nd-889) | 667 (nd-5,556) | 889 (nd-4,889) | 583 (nd-5,556) |
| Lake Wisola | ||||||||||
| Pike | 88 (nd-305) | 92 (nd-203) | 108 (nd-271) | 119 (nd-542) | 75 (nd-305) | 92 (nd-305) | 75 (nd-203) | 68 (nd-305) | 81 (nd-237) | 88 (nd-542) |
| Bream | 169 (nd-1.254) | 136 (nd-576) | 68 (nd-339) | 102 (nd-441) | 68 (nd-305) | 102 (nd-271) | 68 (nd-237) | 102 (nd-508) | 169 (nd-1.458) | 109 (nd-1,254) |
| Roach | 78 (nd-271) | 77 (nd-237) | 108 (nd-359) | 84 (nd-203) | - | 68 (nd-237) | 34 (nd-136) | 59 (nd-271) | 34 (nd-1,153) | 68 (nd-359) |
| Fish Species | Bioconcentration Factors (BAFws), Mean (Range) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gonads | Kidneys | Liver | Spleen | Stomach * | Intestine * | Muscle | Skin | Gills | Total | |
| Lake Ińsko | ||||||||||
| Pike | 958 (nd-5,833 | 1,000 (nd-6,667) | 1,250 (nd-14,583) | 1,167 (nd-6,667) | 833 (nd-3,750) | 1,083 (nd-5,417) | 833 (nd-4,583) | 717 (nd-4,583) | 875 (nd-3,333) | 969 (nd-14,583) |
| Bream | 125 (nd-2,917) | 763 (nd-4,583) | 292 (nd-2,500) | 208 (nd-2,083) | 292 (nd-5,417) | 333 (nd-4,167) | 83 (nd-1,667) | 417 (nd-2,083) | 250 (nd-3,333) | 307 (nd-5,417) |
| Roach | 1250 (nd-3,333) | 833 (nd-4,167) | 833 (nd-2,083) | 1667 (nd-2,500) | - | 833 (nd-2,917) | 417 (nd-1,667) | 1250 (nd-1,0417) | 1667 (nd-9,167) | 1094 (nd-10,417) |
| Lake Wisola | ||||||||||
| Pike | 311 (<nd-1,078) | 323 (<nd-719) | 381 (<nd-958) | 419 (<nd-1,916) | 263 (<nd-1,078) | 323 (<nd-1,078) | 263 (<nd-719) | 240 (<nd-1.078) | 287 (<nd-838) | 312 (<nd-1,916) |
| Bream | 599 (<nd-4,431) | 479 (<nd-2,036) | 240 (<120-1,198) | 359 (<nd-1,557) | 240 (<nd-1,078) | 359 (<nd-958) | 240 (<nd-838) | 359 (<nd-1,796) | 599 (<nd-5,150) | 386 (<nd-5,150) |
| Roach | 275 (<nd-958) | 273 (<nd-838) | 383 (<nd-1,269) | 296 (<nd-719) | 240 (<nd-838) | 120 (<nd-479) | 207 (<nd-958) | 120 (<nd-4072) | 239 (<nd-1,269) | |
References
- Rahman, M.S.; Molla, A.H.; Saha, N.; Rahman, A. Study on heavy metals levels and its risk assessment in some edible fishes from Bangshi River, Savar, Dhaka, Bangladesh. Food Chem. 2012, 134, 847–854. [Google Scholar] [CrossRef]
- Moiseenko, T.I.; Gashkina, N.A. Distribution and bioaccumulation of heavy metals (Hg, Cd and Pb) in fish: Influence of the aquatic environment and climate. Environ. Res. Lett. 2020, 15, 115013. [Google Scholar] [CrossRef]
- Al-Rudainy, A.J.; Al-Samawi, S.M. Determination of lead concentration in water and in different organs of Carrasobarbus luteus and Cyprinus carpio Tigris River. Iraqi J. Vet. Med. 2017, 41, 43–48. [Google Scholar] [CrossRef]
- Badawi, A.; Magdy, S. Evaluation of the pollution extent of heavy metals in the sediment of the Nile Delta, Mediterranean Coast, Egypt. Egypt. J. Aquat. Res. 2023, 49, 221–228. [Google Scholar] [CrossRef]
- Protasowicki, M.; Niedźwiecki, E. Ecological Threats in the Countries of the Oder Estuary Due to the Use of Metals (Zagrożenia Ekologiczne w Strefie Ujścia Odry ze Szczególnym Uwzględnieniem Metali Ciężkich). In Materiały Sympozjum „Substancje toksyczne w środowisku”; Drabent, Z., Smoczyński, L., Eds.; ART, Katedra Chemii: Olsztyn, Poland, 1991; pp. 3–6. (In Polish) [Google Scholar]
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef] [PubMed]
- Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy (OJ L 327, 22.12.2000, p. 1), Amended by Commission Directive 2014/101/EU of 30 October 2014 (OJ L 311, 31.10.2014). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02000L0060-20141120 (accessed on 12 April 2025).
- Zhang, Y.; Xu, X.; Sun, D.; Cao, J.; Zhang, Y.; Huo, X. Alteration of the number and percentage of innate immune cells in preschool children from an e-waste recycling area. Ecotoxicol. Environ. Saf. 2017, 145, 615–622. [Google Scholar] [CrossRef]
- Satarug, S.; Garrett, S.H.; Sens, M.A.; Sens, D.A. Cadmium, environmental exposure, and health outcomes. Environ. Health Perspect. 2010, 118, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Mielcarek, K.; Nowakowski, P.; Puścion-Jakubik, A.; Gromkowska-Kępka, K.J.; Soroczyńska, J.; Markiewicz-Zukowska, R.; Naliwajko, S.K.; Grabia, M.; Bielecka, J.; Żmudzińska, A.; et al. Arsenic, cadmium, lead and mercury content and health risk assessment of consuming freshwater fish with elements of chemometric analysis. Food Chem. 2022, 379, 132167. [Google Scholar] [CrossRef]
- Environmental Fact Sheet: EPA Releases RCRA Waste Minimization PBT Chemical List; National Service Center for Environmental Publications (NSCEP): Cincinnati, OH, USA, 1998.
- Calmano, W.; Förstner, U. Chemical extraction of heavy metals in polluted river sediments in Central Europe. Sci. Total Environ. 1982, 28, 77–90. [Google Scholar] [CrossRef]
- Calmano, W.; Ahlf, W.; Förstner, U. Exchange of Heavy Metals Between Sediment Component and Water. In Metal Speciation in the Environment; NATO ASI G23; Springer: Berlin/Heidelberg, Germany, 1990; pp. 503–520. [Google Scholar]
- Wardas, M.; Budek, L.; Helios Rybicka, E. Variability of heavy metals in bottom sediments of the Wilga River, a tributory of the Vistula River (Kraków area, Poland). Appl. Geochem. 1996, 11, 197–202. [Google Scholar] [CrossRef]
- Girolametti, F.; Fanelli, M.; Ajdini, B.; Truzzi, C.; Illuminati, S.; Susmel, S.; Celussi, M.; Šangulin, J.; Annibaldi, A. Dissolved Potentially Toxic Elements (PTEs) in Relation to Depuration Plant Outflows in Adriatic Coastal Waters: A Two Year Monitoring Survey. Water 2022, 14, 569. [Google Scholar] [CrossRef]
- Ricart, M.; Guasch, H.; Barceló, D.; Brix, R.; Conceicao, M.H.; Geiszinger, A.; López de Alda, M.J.; López-Doval, J.C.; Munoz, I.; Postigo, C.; et al. Primary and complex stressors in polluted Mediterranean rivers: Pesticide effects on biological communities. J. Hydrol. 2010, 383, 52–61. [Google Scholar] [CrossRef]
- Maceda-Veiga, A.; Monroy, M.; Navarro, E.; Viscor, E.; de Sostoa, A. Metal concentrations and pathological responses of wild native fish exposed to sewage discharge in a Mediterranean river. Sci. Total Environ. 2013, 449, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.I.; Kiyani, A.; Santiago-Herrera, M.; Ibáñez, J.; Yousaf, S.; Iqbal, M.; Martel-Martín, S.; Barros, R. Sustainability of phytoremediation: Post-harvest stratagems and economic opportunities for the produced metals contaminated biomass. J. Environ. Manag. 2023, 326, 116700. [Google Scholar] [CrossRef] [PubMed]
- Garai, P.; Banerjee, P.; Mondal, P.; Saha, N.C. Effect of Heavy Metals on Fishes: Toxicity and Bioaccumulation. J. Clin. Toxicol. 2021, S18, 001. [Google Scholar]
- Hama Aziz, K.H.; Mustafa, F.S.; Omer, K.M.; Hama, S.; Hamarawf, R.F.; Rahman, K.O. Heavy metal pollution in the aquatic environment: Efficient and low-cost removal approaches to eliminate their toxicity: A review. RSC Adv. 2023, 13, 17595. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Kalamdhad, A.S. Effects of Heavy Metals on Soil, Plants, Human Health and Aquatic Life. Int. J. Res. Chem. Environ. 2011, 1, 15–21. [Google Scholar]
- Bąkowski, M.; Winiarska-Mieczan, A.; Kwiecień, M.; Krusiński, R.; Jachimowicz-Rogowska, K.; Twarużek, M.; Grobelska, D. Effect of cadmium and lead on living organisms—Transport, accumulation, metabolism. J. Elem. 2024, 29, 311–328. [Google Scholar] [CrossRef]
- Perera, P.; Kodithu, P.S.; Sundara, V.T.; Edirisingh, U. Bioaccumulation of Cadmium in Freshwater Fish: An Environmental Perspective. Insight Ecol. 2015, 4, 1–12. [Google Scholar] [CrossRef]
- Prica, M.; Andrejić, G.; Šinžar-Sekulić, J.; Rakić, T.; Dželetović, Ž. Bioaccumulation of heavy metals in common reed (Phragmites australis) growing spontaneously on highly contaminated mine tailing ponds in Serbia and potential use of this species in phytoremediation. Bot. Serbica 2019, 43, 85–95. [Google Scholar] [CrossRef]
- Niemi, N.; Hansen, J.P.; Eklöf, J.S.; Eriksson, B.K.; Andersson, H.C.; Bergström, U.; Östman, Ö. Influence of reed beds (Phragmites australis) and submerged vegetation on pike (Esox Lucius). Fish. Res. 2023, 261, 106621. [Google Scholar] [CrossRef]
- Bonanno, G. Comparative performance of trace element bioaccumulation and biomonitoring in the plant species Typha domingensis, Phragmites australis and Arundo donax. Ecotoxicol. Environ. Saf. 2013, 97, 124–130. [Google Scholar] [CrossRef]
- Marek, J. Heavy Metals in the Aquatic Environment of the Barycz Valley—Assessment of Threats to Fisheries Management (Metale ciężkie w środowisku wodnym Doliny Baryczy ocena zagrożeń gospodarki rybackiej); 1991/wPol/1/4; Wyd; Akademii Rolniczej we Wrocławiu: Wrocław, Poland, 1991. (In Polish) [Google Scholar]
- Ali, H.; Khan, E. Trophic Transfer, Bioaccumulation, and Biomagnification of Non-Essential Hazardous Heavy Metals and Metalloids in Food Chains/Webs—Concepts and Implications for Wildlife and Human Health. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 1353–1376. [Google Scholar] [CrossRef]
- Jamil Emon, F.; Rohani, M.F.; Sumaiya, N.; Tuj Jannat, M.F.; Akter, Y.; Shahjahan, M.; Abdul Kari, Z.; Tahiluddin, A.B.; Goh, K.W. Bioaccumulation and Bioremediation of Heavy Metals in Fishes-A Review. Toxics. 2023, 11, 510. [Google Scholar] [CrossRef] [PubMed]
- Ab Latif Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef]
- Witkowska, D.; Słowik, J.; Chilicka, K. Heavy metals and human health: Possible exposure pathways and the competition for protein binding sites. Molecules 2024, 26, 6060. [Google Scholar] [CrossRef]
- Linnik, P.M.; Zubenko, I.B. Role of bottom sediments in the secondary pollution of aquatic environments by heavy-metal compounds. Lakes Reserv. Res. Manag. 2000, 5, 11–21. [Google Scholar] [CrossRef]
- Filipiak, J.; Raczyński, M. West Pomeranian Lakes (Outline of Factography); Akademia Rolnicza w Szczecinie: Szczecin, Poland, 2000. (In Polish) [Google Scholar]
- Trojanowski, J.; Parzych, A.; Trojanowska, C.; Bruski, J. The trophic condition of Lake Ińsko. Arch. Environ. Prot. 2004, 30, 85–100. (In Polish) [Google Scholar]
- Kubiak, J.; Machula, S.; Czerniejewski, P.; Brysiewicz, A.; Wawrzyniak, W. Long term changes in the quality and water trophy of Lake Ińsko—The effect of the re-oligotrophication? J. Water Land Dev. 2021, 51, 30–37. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Method 950.46; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- ASTM D3174-12(2018); Standard Test Method for Ash in the Analysis Sample of Coal and Coke from Coal. ASTM: West Conshohocken, PA, USA, 2020. Available online: https://store.astm.org/d3174-12r18e01.html (accessed on 12 April 2025).
- Chakravarty, M.M.; Patgiri, A.D. Metal pollution assessment in sediments of the Dikrong River, NE India. J. Hum. Ecol. 2009, 27, 63–67. [Google Scholar] [CrossRef]
- Seshan, B.R.R.; Natesan, U.; Deepthi, K. Geochemical and statistical approach for evaluation of heavy metal pollution in core sediments in southeast coast of India. Int. J. Environ. Sci. Technol. 2010, 7, 291–306. [Google Scholar] [CrossRef]
- Bojakowska, I.; Sokołowska, G. Geochemiczne klasy czystości osadów wodnych. Geochem. Purity Cl. Aqueous Sediments Przegląd Geol. 1998, 46, 49–54. (In Polish) [Google Scholar]
- US EPA. Risk-Based Concentration Table; United States Environmental Protection Agency: Washington, DC, USA, 2000. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables (accessed on 10 May 2025).
- Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing REGULATION (EC) No 1881/2006. Off. J. Eur. Union 2023, 119, 103–157.
- US EPA. Quantitative Risk Assessment Calculations; Environmental Protection Agency: Washington, DC, USA, 2015. Available online: https://www.epa.gov/sites/default/files/2015-05/documents/13.pdf (accessed on 10 May 2025).
- European Food Safety Authority (EFSA). Lead dietary exposure in the European population. EFSA J. 2012, 10, 2831. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Cadmium dietary exposure in the European population. EFSA J. 2012, 10, 2551. [Google Scholar] [CrossRef]
- Rozporządzenie Ministra Infrastruktury z Dnia 25 Czerwca 2021 r. w Sprawie Klasyfikacji Stanu Ekologicznego, Potencjału Ekologicznego i Stanu Chemicznego Oraz Sposobu Klasyfikacji Stanu Jednolitych Części Wód Powierzchniowych, a Także Środowiskowych Norm Jakości dla Substancji Priorytetowych. Dz.U. 2021 poz. 1475. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20210001475 (accessed on 10 May 2025).
- Gwoździński, K.; Mazur, J.; Pieniążek, A. Concentrations of Metals in Water of Unmonitored Lakes Near a Landscape Park. Pol. J. Environ. Stud. 2014, 23, 1317–1321. [Google Scholar]
- CCME. Summary of Existing Canadian Environmental Quality Guidelines. 2002. Available online: https://ccme.ca/en/current-activities/canadian-environmental-quality-guidelines (accessed on 12 April 2025).
- Zerbe, J.; Sobczyński, T.; Elbanowska, H.; Siepak, J. Speciation of Heavy Metals in Bottom Sediments of Lakes. Pol. J. Environ. Stud. 1999, 8, 331–339. [Google Scholar]
- Kwapulinski, J.; Wiechula, D. Forms of Selected Heavy Metals in the Bottom Sediments of Goczalkowice Reservoir; Chemistry and Environmental Protection; Polytechnic University of Lublin: Lublin, Poland, 1993; Volume 142. [Google Scholar]
- Environmental Protection Agency (EPA). Emissions of Arsenic Compounds. In Technology Transfer Network, National Air Toxics Assessment 2005, Pollutant-Specific Data Tables, US; EPA: Washington, DC, USA, 2005. [Google Scholar]
- Rahman, S.U.; Qin, A.; Zain, M.; Mushtaq, Z.; Mehmood, F.; Riaz, L.; Naveed, S.; Ansari, M.J.; Saeed, M.; Ahmad, I.; et al. Pb uptake, accumulation, and translocation in plants: Plant physiological, biochemical, and molecular response: A review. Heliyon 2024, 10, e27724. [Google Scholar] [CrossRef] [PubMed]
- Allaway, W.H. Agronomic Controls Over the Environmental Cycling of Trace Elements. In Advances in Agronomy; Norman, A.G., Ed.; Academic Press: Cambridge, MA, USA, 1968; Volume 20, pp. 235–274. [Google Scholar]
- Szarek-Gwiazda, E.; Amirowicz, A. Bioaccumulation of trace elements in roach, silver bream, rudd, and perch living in an inundated opencast sulphur mine. Aquat. Ecol. 2006, 40, 221–236. [Google Scholar] [CrossRef]
- Chałabis-Mazurek, A.; Rechulicz, J.; Pyz-Łukasik, R.A. Food-Safety Risk Assessment of Mercury, Lead and Cadmium in Fish Recreationally Caught from Three Lakes in Poland. Animals 2021, 11, 3507. [Google Scholar] [CrossRef]
- Burkhard, L.P. Evaluation of published bioconcentration factor (BCF) and bioaccumulation factor (BAF) Data for per- and polyfluoroalkyl substances across aquatic species. Environ. Toxicol. Chem. 2021, 40, 1530–1543. [Google Scholar] [CrossRef] [PubMed]
- Has-Schön, E.; Bogut, I.; Strelec, I. Heavy metal profile in five fish species included in human diet, domiciled in the end flow of river Neretva (Croatia). Arch. Environ. Contam. Toxicol. 2006, 50, 545–551. [Google Scholar] [CrossRef]
- Evaluation of Certain Food Additives and Contaminants: Seventy-Third Report of the Joint FAO/WHO Expert Committee on Food Additives; FAO/WHO Technical Report Series 960—JECFA 73; WHO: Geneva, Switzerland, 2011.


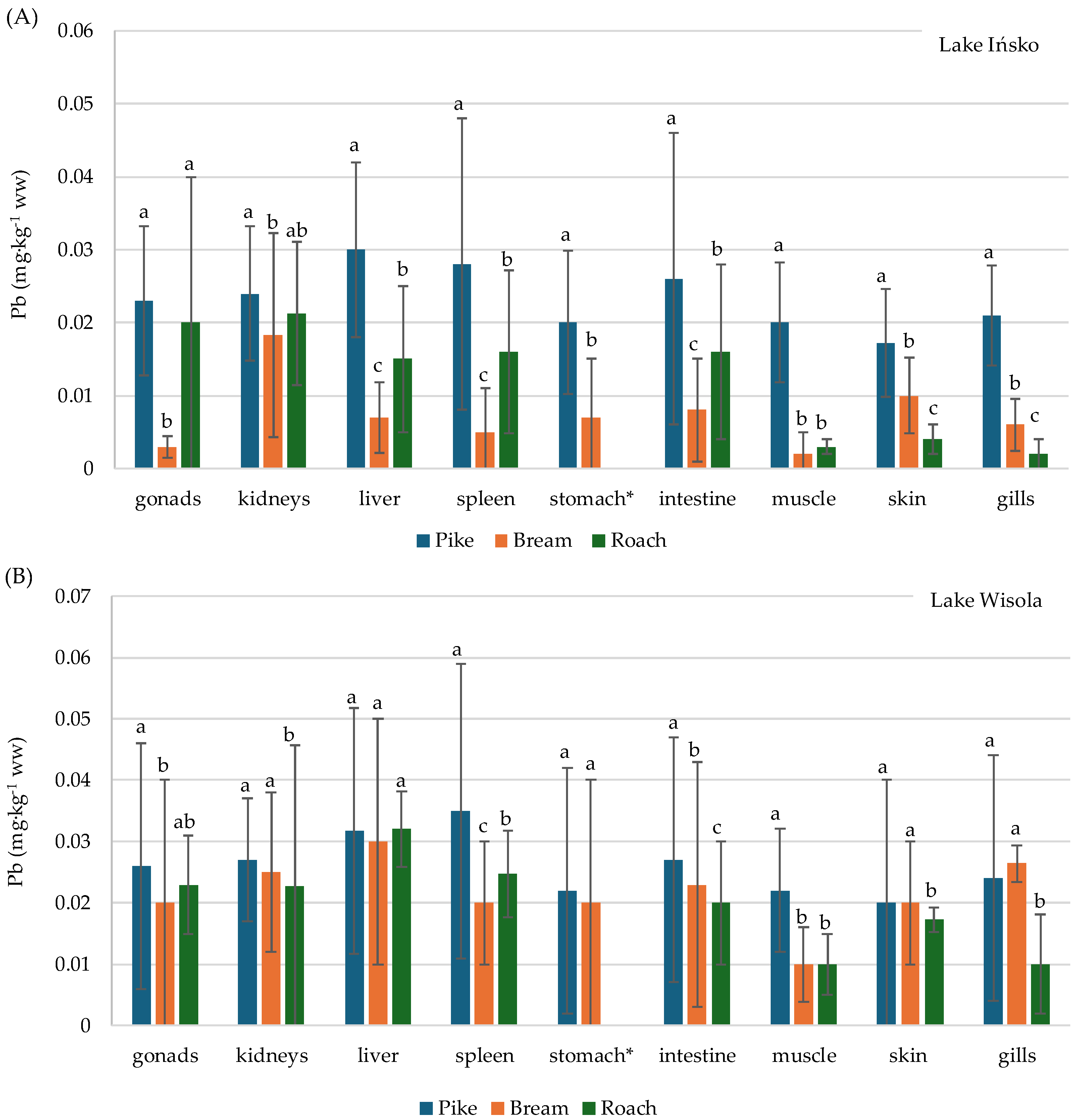
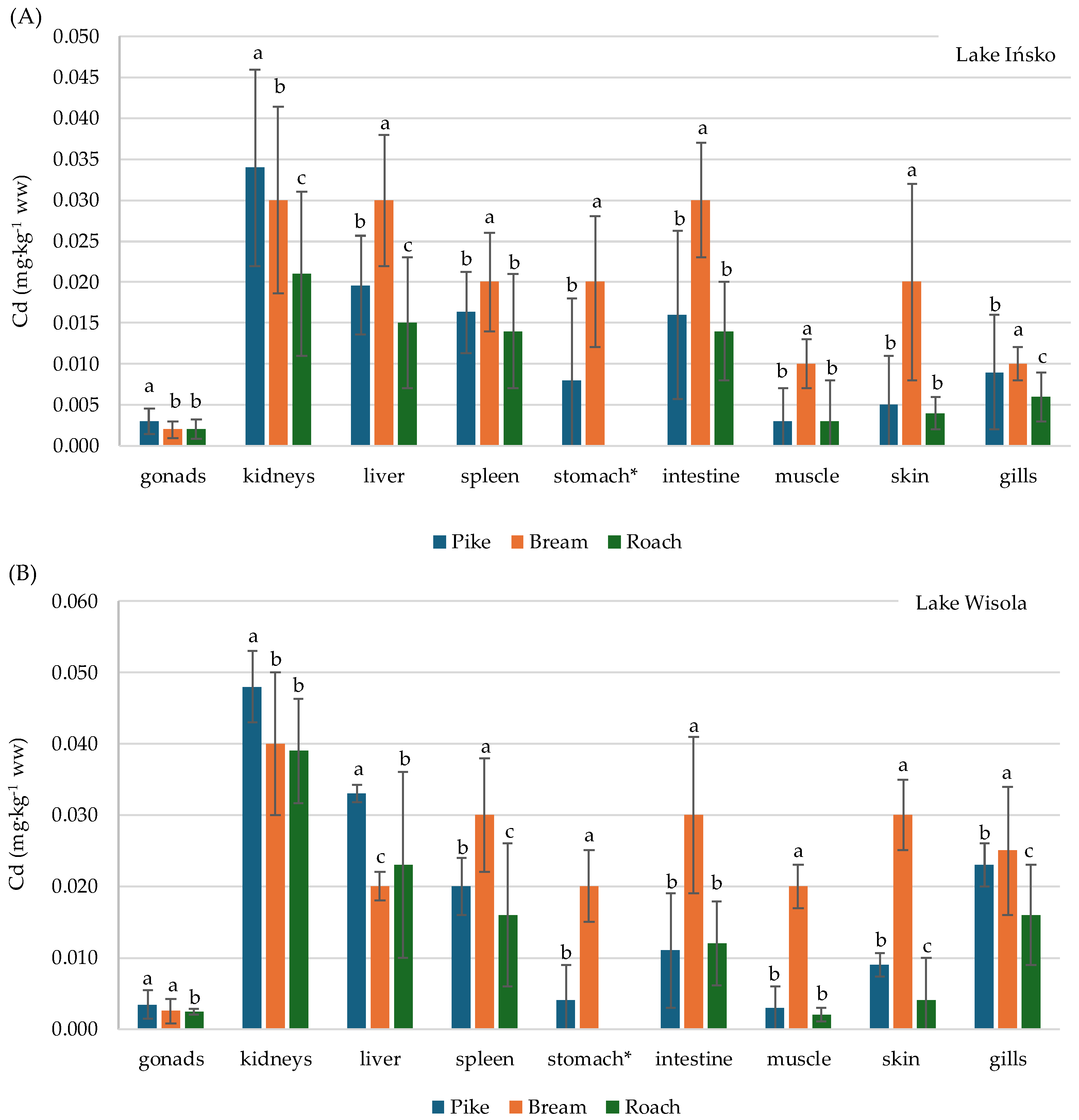
| Lake | Fish Species | No. of Fish | Body Weight (g) | Total Length (cm) | ||
|---|---|---|---|---|---|---|
| Mean ± SD | (Range) | Mean ± SD | (Range) | |||
| Ińsko | Pike | 80 | 683 ± 134.6 | 487.4–943.5 | 46.8 ± 3.2 | 42.1–53.1 |
| Bream | 80 | 707.9 ± 160.3 | 409.9–931.9 | 39.4 ±2.7 | 35.5–43.1 | |
| Roach | 80 | 120.6 ± 21.5 | 89.4–154.5 | 25.7 ± 11.7 | 19.8–56.3 | |
| Wisola | Pike | 80 | 665.1 ± 224.0 | 450.5–1180.1 | 46.5 ± 4.5 | 41.1–55.9 |
| Bream | 80 | 599.3 ± 136.5 | 407.4–820.9 | 37.7 ± 2.7 | 33.7–232.9 | |
| Roach | 80 | 101.5 ± 22.1 | 76.4–141.4 | 21.2 ± 1.7 | 19.0–24.2 | |
| Material | Amount (g) | Reagents * | Parameters of Digestion Process (Step Number) ** |
|---|---|---|---|
| Bottom sediments | 0.2 ± 0.001 (dw) | 3 mL | (I) 90 PSI, 20 min; (II) 100 PSI, 30 min; (III) 150 PSI, 35 min; (IV) 160 PSI, 10 min |
| Common reed | 0.3 ± 0.001 g dw | 6 mL HNO3; 2 mL H2O2 * | (I) 65 PSI, 20 min; (II) 70 PSI, 20 min |
| Fish | 1 ± 0.001 g ww | 5 mL HNO3 | (1) 20 PSI, 5 min; (2) 40 PSI, 10 min; (3) 85 PSI, 10 min; (4) 135 PSI, 10 min; (5) 175 PSI, 5 min |
| Certificate MATERIAL | Pb (mg·kg−1 ± SD) | Cd (mg·kg−1 ± SD) | ||||
|---|---|---|---|---|---|---|
| Certified Value | Obtained Value | Recovery (%) | Certified Value | Obtained Value | Recovery (%) | |
| MESS-3 | 21.1 ± 1.0 | 20.0 ± 1.2 | 94.8 | 0.24 ± 0.01 | 0.23 ± 0.02 | 95.8 |
| INCT-MPH-2 | 2.16 ± 0.23 | 2.08 ± 0.10 | 96.3 | 0.199 ± 0.015 | 0.203 ± 0.030 | 102.0 |
| DOLT-3 | 0.32 ± 0.05 | 0.31 ± 0.06 | 97.4 | 19.4 ± 0.6 | 18.5 * ± 0.10 | 95.4 |
| Lake | Water | n | Pb (μg·L−1) Mean ± SD (Range) | Cd (μg·L−1) Mean ± SD (Range) |
|---|---|---|---|---|
| Ińsko | surface | 120 | 0.04 aA ± 0.03 (<0.02–0.16) | 0.020 aA ± 0.018 (0.001–0.071) |
| bottom | 48 | 0.05 aA ± 0.04 (<0.02–0.25) | 0.028 acA ± 0.023 (0.006–0.089) | |
| tributaries | 18 | 0.10 bA ± 0.05 (0.04–0.19) | 0.030 cA ± 0.030 (0.004–0.128) | |
| outflow | 9 | 0.06 aA ± 0.05 (<0.02–0.17) | 0.014 dA ± 0.009 (0.007–0.031) | |
| Wisola | surface | 72 | 0.17 aB ± 0.11 (<0.02–0.54) | 0.059 aB ± 0.043 (0.012–0.253) |
| bottom | 66 | 0.42 bB ± 0.36 (0.05–1.81) | 0.108 bB ± 0.112 (0.018–0.543) | |
| tributaries | 39 | 0.44 bB ± 0.23 (0.14–1.03) | 0.257 cB ± 0.201 (0.027–0.709) | |
| outflow | 39 | 0.26 cB ± 0.24 (<0.02–1.20) | 0.122 bB ± 0.101 (0.015–0.545) |
| Bottom Sediments | Organic Matter (%) | |||
|---|---|---|---|---|
| Lake Ińsko | Lake Wisola | |||
| n | Mean ± SD (Range) | n | Mean ± SD (Range) | |
| organic (muddy) 1 | 66 | 19.4 aA ± 3.8 (14.9–28.1) | 48 | 36.4 aB ± 8.2 (12.5–47.9) |
| mineral (sandy) 2 | 66 | 1.2 bA ± 0.9 (0.3–3.6) | 114 | 1.6 bA ± 1.3 (0.2–5.1) |
| Metal | Bottom Sediments | Lake Ińsko | Lake Wisola |
|---|---|---|---|
| Mean ± SD (Range) | Mean ± SD (Range) | ||
| Pb | organic (muddy) * | 47.6 aA ± 13.3 (13.3–78.5) | 59.4 aB ± 24.4 (12.1–108.0) |
| mineral (sandy) ** | 3.5 b ± 1.5 (1.1–6.5) | 4.4 b ± 3.1 (0.5–11.4) | |
| Cd | organic (muddy) * | 0.97 aA ± 0.42 (0.06–1.90) | 1.12 aB ± 0.86 (0.03–3.42) |
| mineral (sandy) ** | 0.05 b ± 0.03 (0.02–0.14) | 0.10 b ± 0.19 (0.01–1.30) |
| Metal/ | Bottom | Lake Ińsko | Lake Wisola | ||
|---|---|---|---|---|---|
| PLI | Sediments | AF | CF | AF | CF |
| Pb | organic | 1,057,777 | 4.8 | 201,356 | 5.9 |
| (muddy) | (29,555–1,744,444) | (41,017–366,102) | |||
| Cd | organic | 40,417 | 1.9 | 40,417 | 2.2 |
| (muddy) | (2500–179,167) | (2500–179,167) | |||
| PLI | 9.2 | 13.3 | |||
| Pb | mineral | 77,777 | 0.4 | 14,915 | 0.4 |
| (sandy) | (24,444–144,444) | (1695–38,644) | |||
| Cd | mineral | 2083 | 0.1 | 119 | 0.2 |
| (sandy) | (833–5833) | (119–15,476) | |||
| PLI | 0.04 | 0.09 | |||
| Plant Organ | Lake Ińsko, n = 72 | Lake Wisola, n = 75 | ||
|---|---|---|---|---|
| Pb | Cd | Pb | Cd | |
| Leaves | 0.17 aA ± 0.11 | 0.015 aA ± 0.010 | 0.18 aA ± 0.13 | 0.021 aB ± 0.005 |
| (0.06–0.55) | (0.001–0.042) | (0.01–0.86) | (0.002–0.029) | |
| Stems | 0.20 bA ± 0.20 | 0.015 abA ± 0.015 | 0.24 bB ± 0.13 | 0.019 bB ± 0.013 |
| (0.01–0.94) | (0.002–0.087) | (0.03–0.60) | (0.002–0.069) | |
| Rhizomes | 0.16 aA ± 0.06 | 0.016 bA ± 0.019 | 0.18 aA ± 0.12 | 0.057 cB ± 0.022 |
| (0.03–0.34) | (0.001–0.090) | (0.04–0.58) | (0.004–0.041) | |
| Roots | 4.41 cA ± 1.72 | 0.494 cA ± 0.340 | 4.65 cA ± 1.64 | 0.455 dA ± 0.361 |
| (1.54–9.56) | (0.087–1.569) | (1.64–7.51) | (0.045–1.658) | |
| Lake | Plant Organs | Pb, Mean (Range) | Cd, Mean (Range) | ||
|---|---|---|---|---|---|
| BAFw | BAFs | BAFw | BAFs | ||
| Ińsko | Leaves | 3777 (1333–12,222) | 0.05 (0.02–0.16) | 625 (42–1750) | 0.3 (0.02–0.84) |
| Stems | 4444 (222–20,888) | 0.06 (<0.01–0.27) | 625 (83–3625) | 0.30 (0.04–1.74) | |
| Rhizomes | 3555 (667–7556) | 0.05 (0.01–0.10) | 667 (42–3750) | 0.32 (0.02–1.8) | |
| Roots | 98,000 (34,222–212,444) | 1.26 (0.44–2.7) | 20,583 (3625–65,375) | 9.88 (1.74–31.38) | |
| Wisola | Leaves | 610 (31–2915) | 0.04 (0.01–0.13) | 875 (83–1208) | 0.19 (0.02–0.69) |
| Stems | 814 (102–2034) | 0.05 (<0.01–0.14) | 792 (83–2875) | 0.1 (0.02–0.21) | |
| Rhizomes | 610 (136–1966) | 0.041 (<0.01–0.13) | 2375 (167–1708) | 0.57 (0.04–0.41) | |
| Roots | 15,763 (5559–25,458) | 1.05 (0.37–1.71) | 18,958 (1875–69,083) | 4.55 (0.45–16.58) | |
| Lake | Fish Species | EDI (µg∙kg−1 bw) | EWI (µg∙kg−1 bw) | Percentage of BMDL01 | Percentage of BMDL10 | THQ |
|---|---|---|---|---|---|---|
| Ińsko | Pike | 0.012 (0.006–0.067) | 0.052 (0.026–0.289) | 0.82 (0.41–4.49) | 1.94 (0.97–10.69) | 0.003 (0.002–0.019) |
| Bream | 0.006 (<0.001–0.024) | 0.026 (<0.001–0.105) | 0.41 (<0.01–1.63) | 0.97 (<0.01–3.89) | 0.002 (<0.001–0.007) | |
| Roach | 0.006 (0.006–0.024) | 0.026 (0.026–0.105) | 0.41 (0.41–1.63) | 0.97 (0.97–3.89) | 0.002 (0.002–0.007) | |
| Wisola | Pike | 0.012 (0.006–0.037) | 0.052 (0.026–0.157) | 0.82 (0.41–2.45) | 1.94 (0.97–5.83) | 0.003 (0.002–0.010) |
| Bream | 0.012 (<0.001–0.043) | 0.052 (<0.001–0.184) | 0.82 (<0.01–2.86) | 1.94 (<0.01–6.80) | 0.003 (<0.001–0.012) | |
| Roach | 0.012 (0.006–0.024) | 0.052 (0.026–0.105) | 0.82 (0.41–1.63) | 1.94 (0.97–3.89) | 0.003 (0.002–0.007) |
| Lake | Fish Species | EDI (µg∙kg−1 bw) | EWI (µg∙kg−1 bw) | Percentage of TWI | THQ |
|---|---|---|---|---|---|
| Ińsko | Pike | 0.002 (0.001–0.013) | 0.008 (0.003–0.055) | 0.31 (0.10–2.20) | 0.002 (0.001–0.013) |
| Bream | 0.001 (<0.001–0.010) | 0.005 (<0.001–0.045) | 0.21 (<0.01–1.78) | 0.001 (<0.001–0.010) | |
| Roach | 0.002 (0.001–0.013) | 0.008 (0.003–0.055) | 0.31 (0.10–2.20) | 0.002 (0.001–0.013) | |
| Wisola | Pike | 0.002 (0.001–0.010) | 0.008 (0.003–0.045) | 0.31 (0.10–1.78) | 0.002 (0.001–0.010) |
| Bream | 0.001 (<0.001–0.013) | 0.005 (<0.001–0.058) | 0.21 (<0.01–2.31) | 0.001 (<0.010–0.013) | |
| Roach | 0.001 (0.001–0.004) | 0.005 (0.003–0.016) | 0.21 (0.10–0.63) | 0.001 (0.001–0.004) |
| Lake Ińsko | Lake Wisola | |||||
|---|---|---|---|---|---|---|
| Pike | Bream | Roach | Pike | Bream | Roach | |
| HI | 0.005 | 0.003 | 0.004 | 0.005 | 0.005 | 0.005 |
| (0.002–0.032) | (<0.001–0.017) | (0.002–0.020) | (0.002–0.021) | (<0.001–0.025) | (0.002–0.010) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajkowska-Myśliwiec, M.; Protasowicki, M.; Witczak, A. The Mobility and Distribution of Lead and Cadmium in the Ecosystems of Two Lakes in Poland and Their Effect on Humans and the Environment. Water 2025, 17, 2255. https://doi.org/10.3390/w17152255
Rajkowska-Myśliwiec M, Protasowicki M, Witczak A. The Mobility and Distribution of Lead and Cadmium in the Ecosystems of Two Lakes in Poland and Their Effect on Humans and the Environment. Water. 2025; 17(15):2255. https://doi.org/10.3390/w17152255
Chicago/Turabian StyleRajkowska-Myśliwiec, Monika, Mikołaj Protasowicki, and Agata Witczak. 2025. "The Mobility and Distribution of Lead and Cadmium in the Ecosystems of Two Lakes in Poland and Their Effect on Humans and the Environment" Water 17, no. 15: 2255. https://doi.org/10.3390/w17152255
APA StyleRajkowska-Myśliwiec, M., Protasowicki, M., & Witczak, A. (2025). The Mobility and Distribution of Lead and Cadmium in the Ecosystems of Two Lakes in Poland and Their Effect on Humans and the Environment. Water, 17(15), 2255. https://doi.org/10.3390/w17152255







