Linking Life History Traits to the Threat Level of European Freshwater Fish
Abstract
1. Introduction
2. Materials and Methods
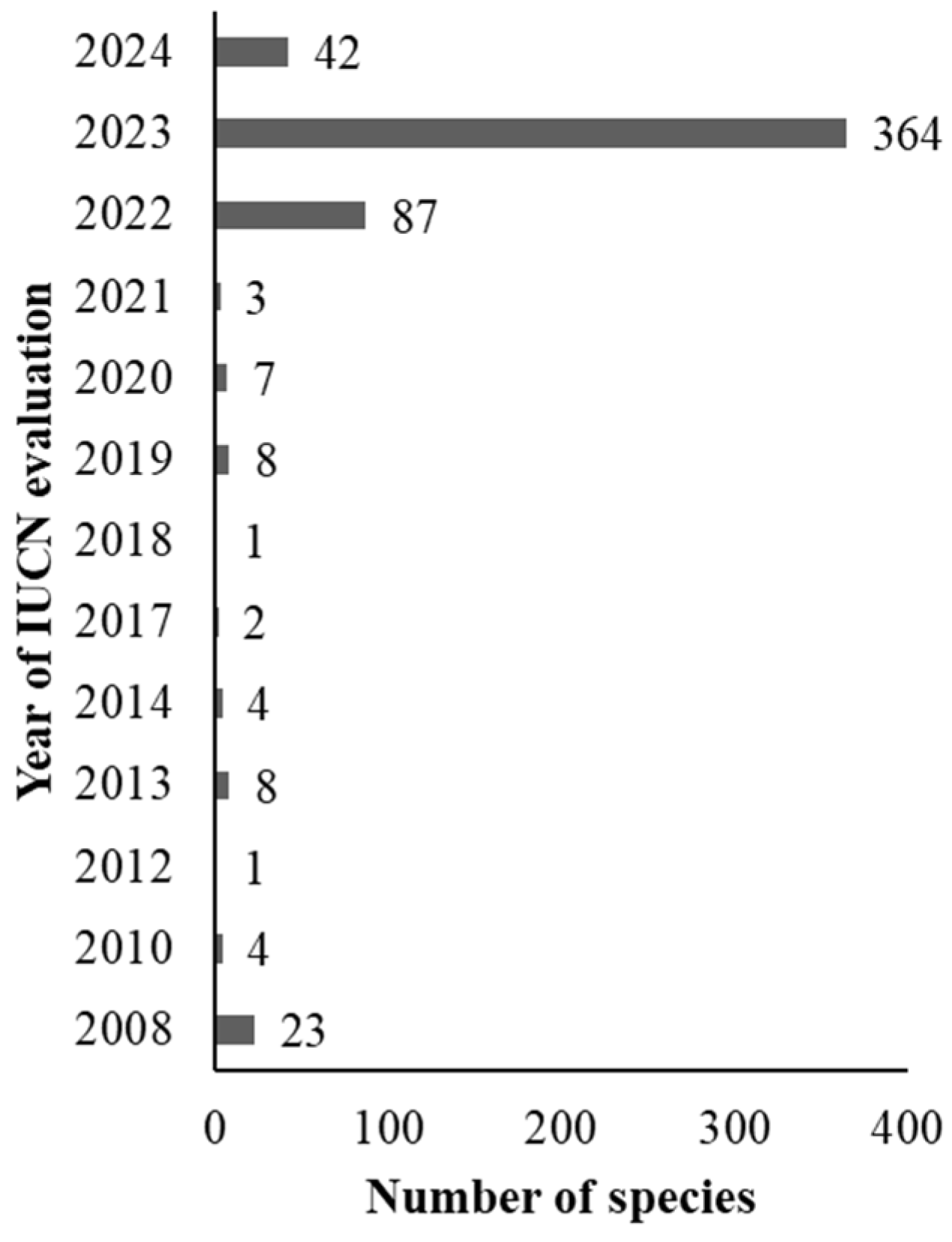
3. Results
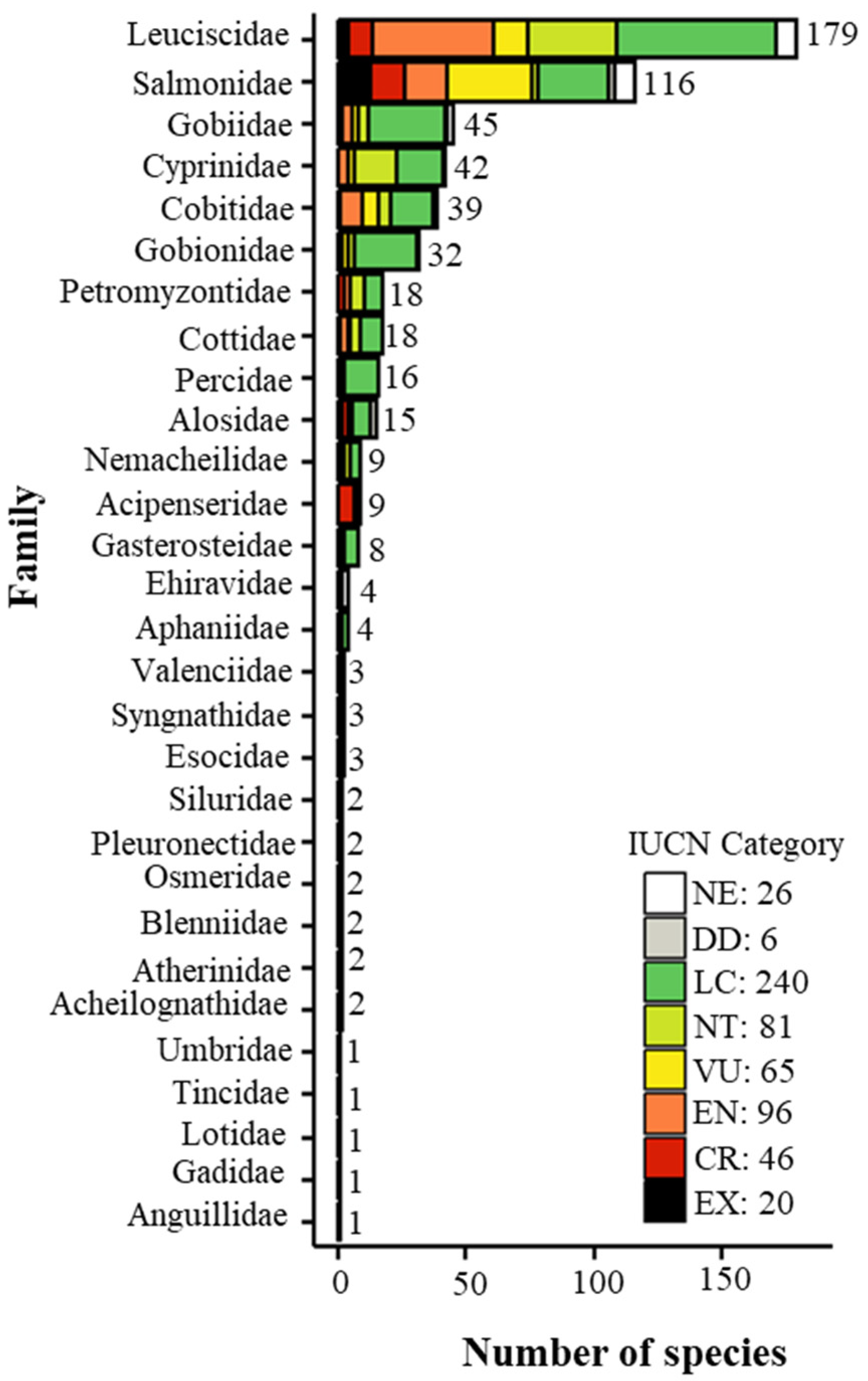
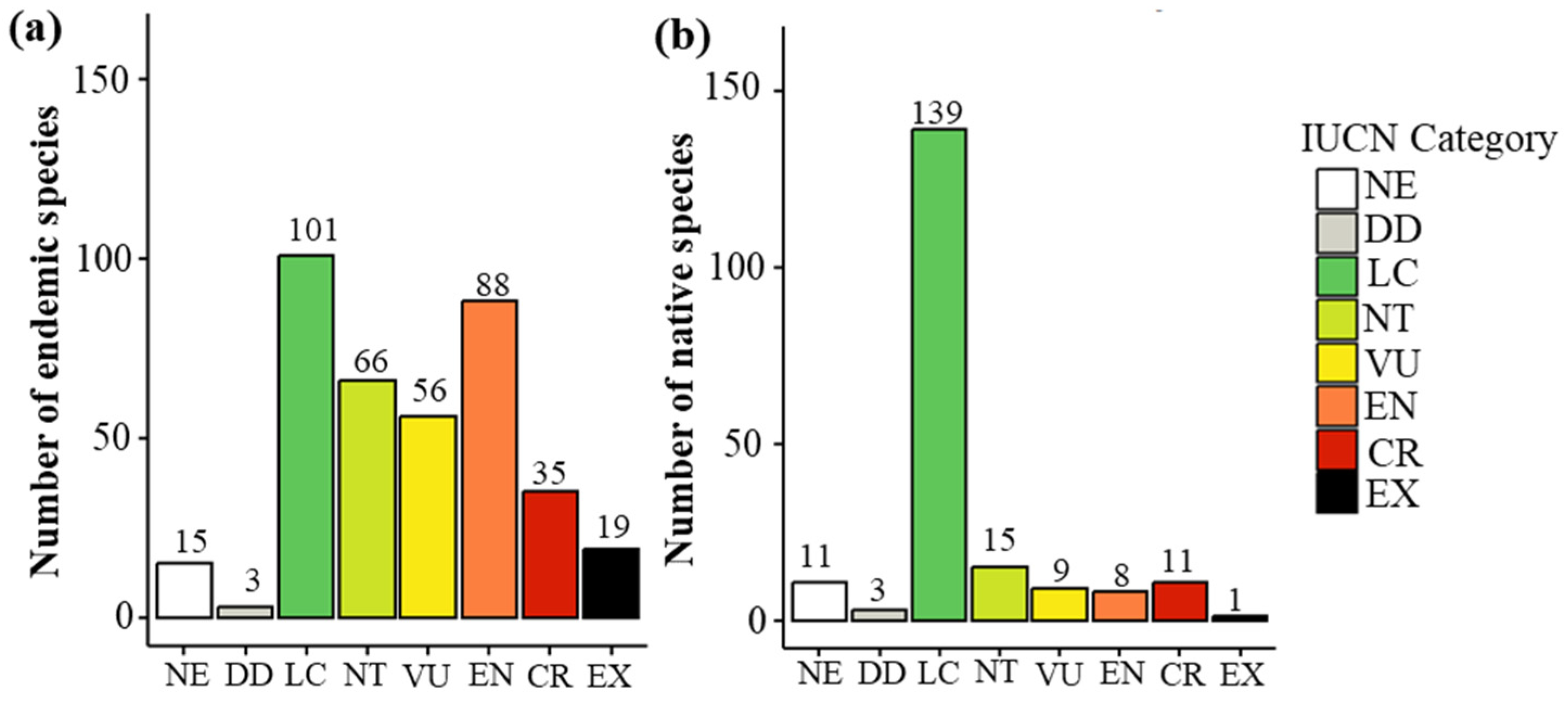
3.1. Threat Classification Across Families and Species Origin
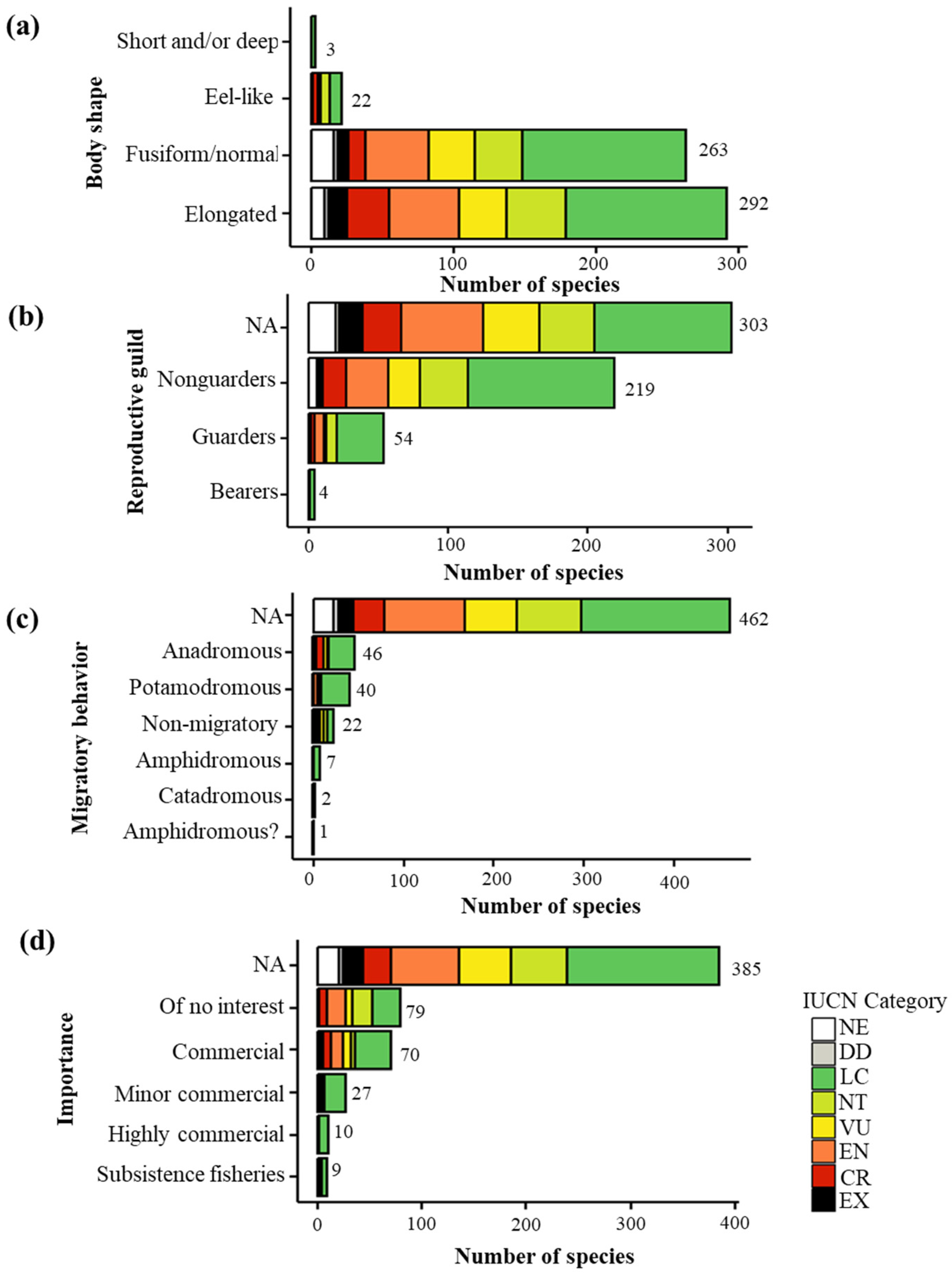
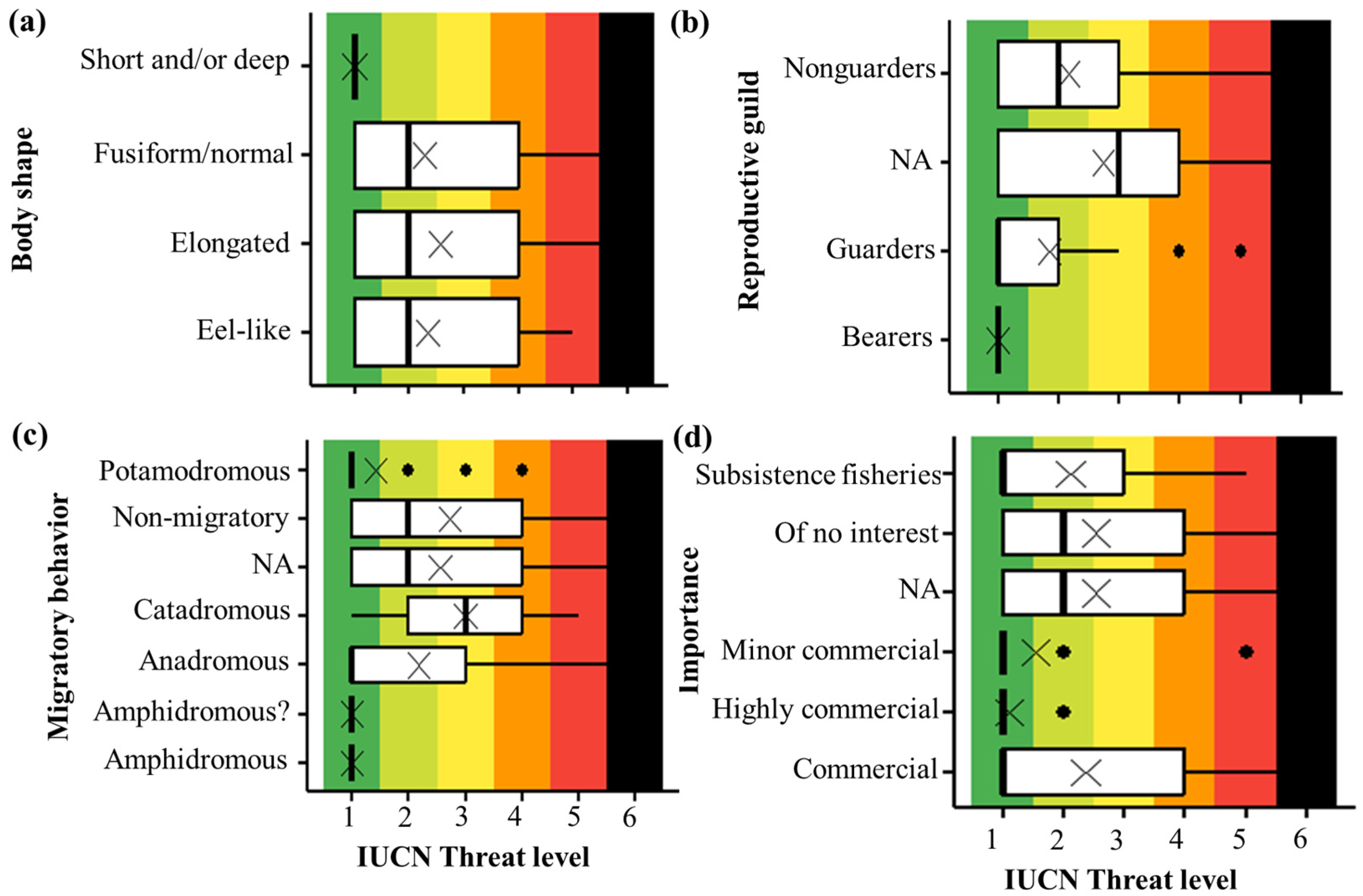
3.2. Categorical Traits and Threat Level
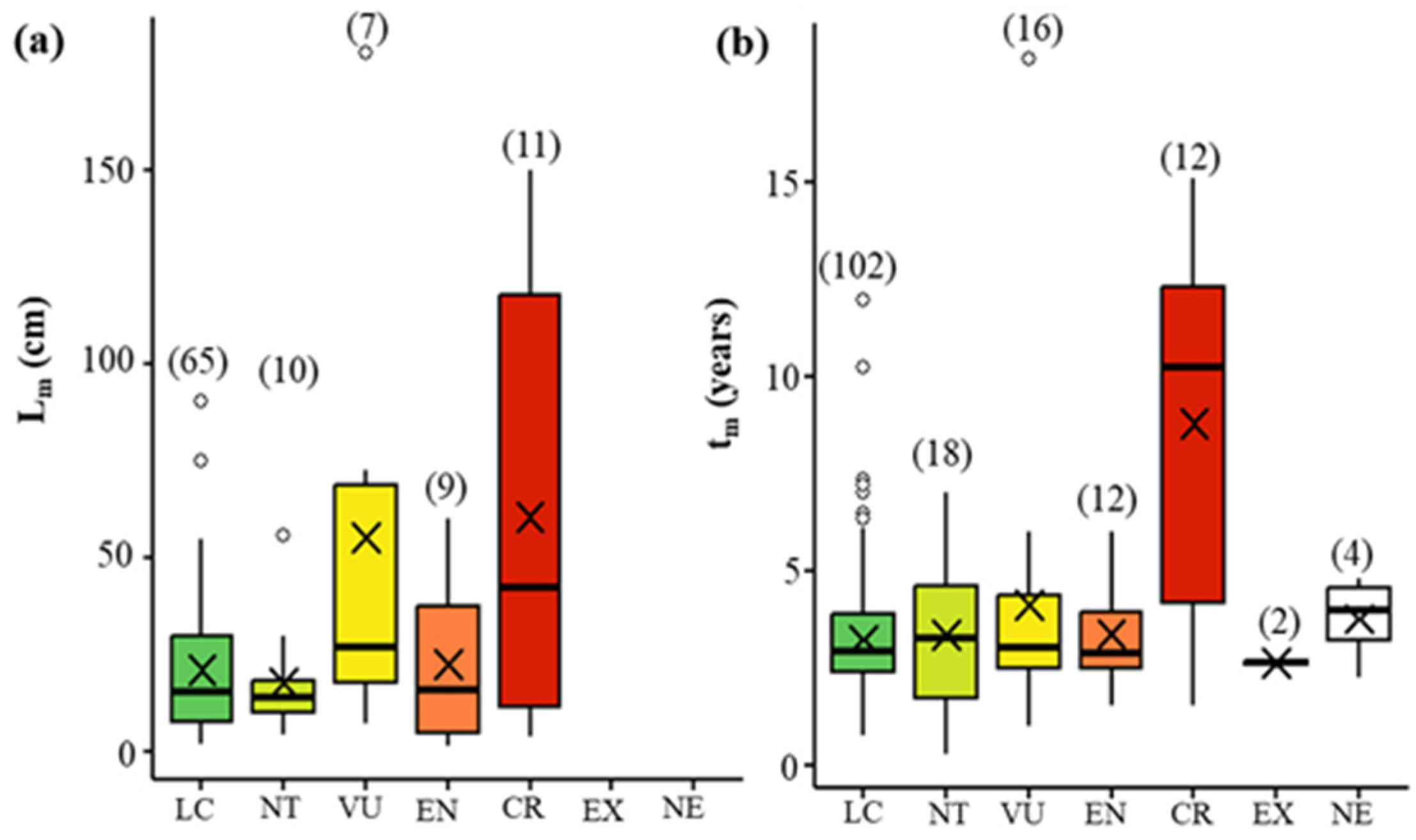
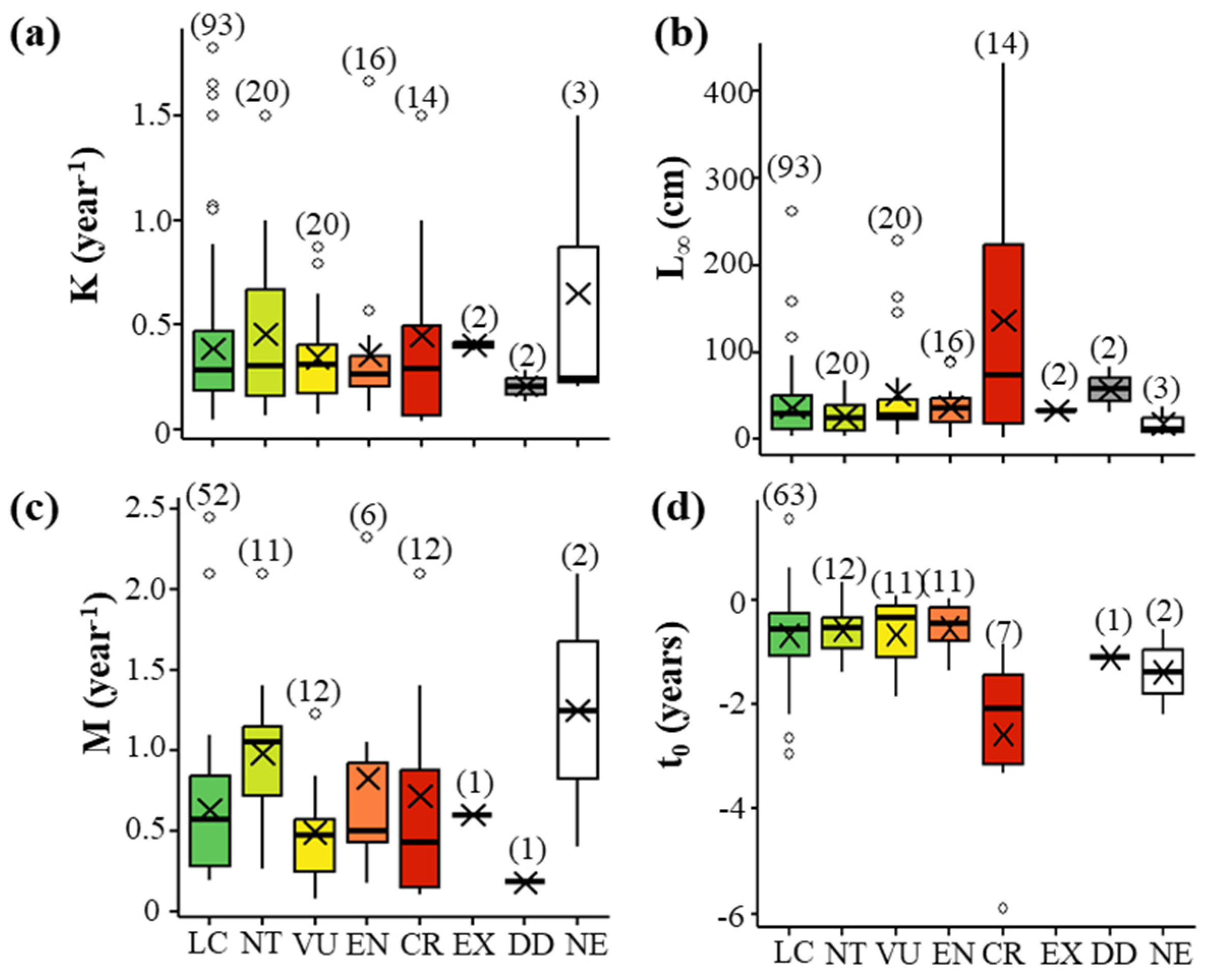
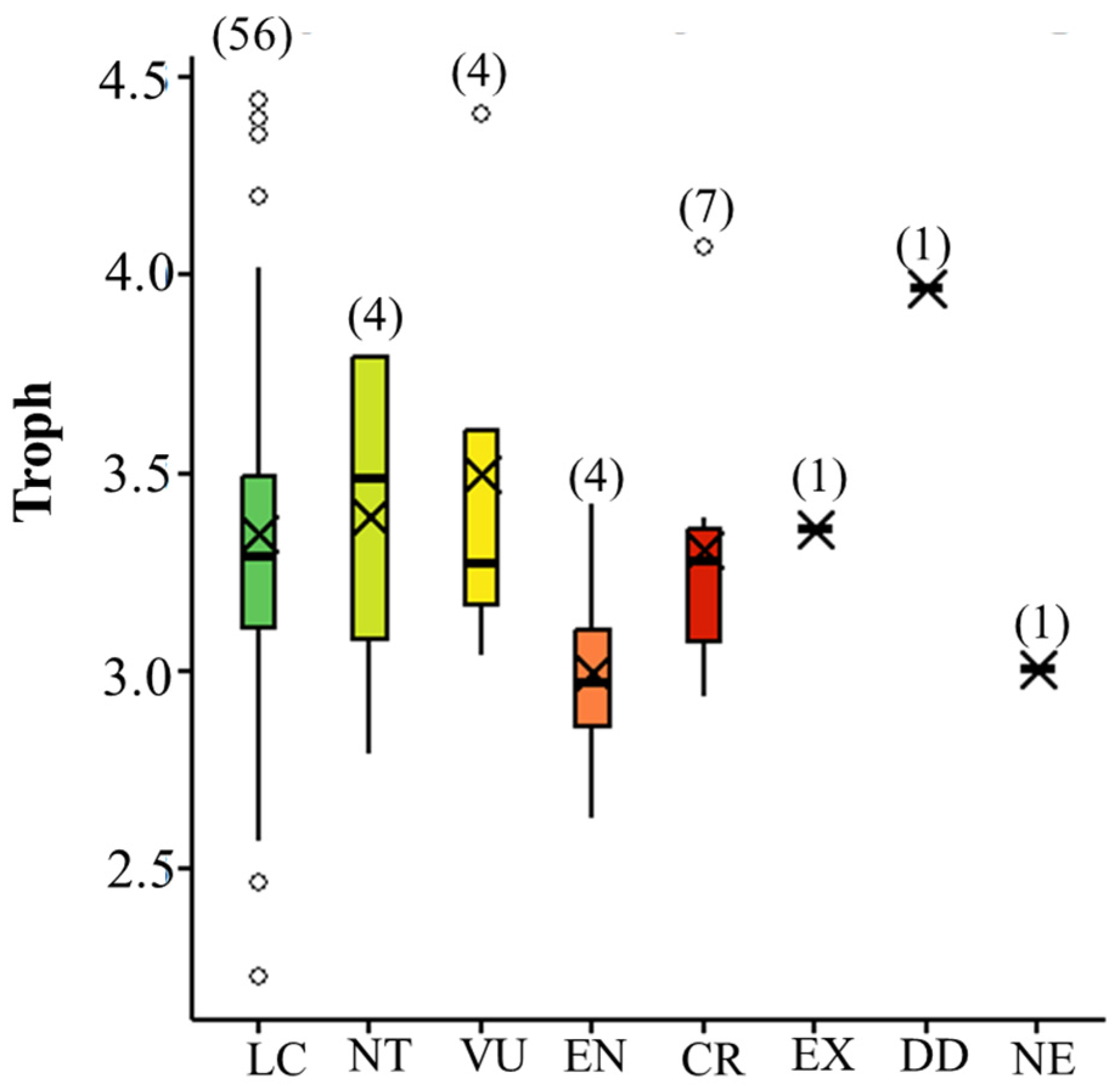
3.3. Numerical Life History Traits and Threat Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Strayer, D.L.; Dudgeon, D. Freshwater biodiversity conservation: Recent progress and future challenges. J. N. Am. Benthol. Soc. 2010, 29, 344–358. [Google Scholar] [CrossRef]
- Pelayo-Villamil, P.; Guisande, C.; Vari, R.P.; Manjarrés-Hernández, A.; García-Roselló, E.; González-Dacosta, J.; Heine, J.; Vilas, L.G.; Patti, B.; Quinci, E.M. Global diversity patterns of freshwater fishes—Potential victims of their own success. Divers. Distrib. 2015, 21, 345–356. [Google Scholar] [CrossRef]
- Tedesco, P.A.; Beauchard, O.; Bigorne, R.; Blanchet, S.; Buisson, L.; Conti, L.; Cornu, J.-F.; Dias, M.S.; Grenouillet, G.; Hugueny, B. A global database on freshwater fish species occurrence in drainage basins. Sci. Data 2017, 4, 170141. [Google Scholar] [CrossRef] [PubMed]
- Gozlan, R.E.; Karimov, B.K.; Zadereev, E.; Kuznetsova, D.; Brucet, S. Status, trends, and future dynamics of freshwater ecosystems in Europe and Central Asia. Inland Waters 2019, 9, 78–94. [Google Scholar] [CrossRef]
- Markovic, D.; Carrizo, S.F.; Kärcher, O.; Walz, A.; David, J.N. Vulnerability of European freshwater catchments to climate change. Glob. Change Biol. 2017, 23, 3567–3580. [Google Scholar] [CrossRef]
- Costa, M.J.; Duarte, G.; Segurado, P.; Branco, P. Major threats to European freshwater fish species. Sci. Total Environ. 2021, 797, 149105. [Google Scholar] [CrossRef] [PubMed]
- Xenopoulos, M.A.; Lodge, D.M.; Alcamo, J.; Märker, M.; Schulze, K.; Van Vuuren, D.P. Scenarios of freshwater fish extinctions from climate change and water withdrawal. Glob. Change Biol. 2005, 11, 1557–1564. [Google Scholar] [CrossRef]
- Jarić, I.; Lennox, R.J.; Kalinkat, G.; Cvijanović, G.; Radinger, J. Susceptibility of European freshwater fish to climate change: Species profiling based on life-history and environmental characteristics. Glob. Change Biol. 2019, 25, 448–458. [Google Scholar] [CrossRef]
- Comte, L.; Olden, J.D. Evolutionary and environmental determinants of freshwater fish thermal tolerance and plasticity. Glob. Change Biol. 2017, 23, 728–736. [Google Scholar] [CrossRef]
- Bender, M.G.; Floeter, S.R.; Mayer, F.P.; Vila-Nova, D.A.; Longo, G.O.; Hanazaki, N.; Carvalho-Filho, A.; Ferreira, C.E.L. Biological attributes and major threats as predictors of the vulnerability of species: A case study with Brazilian reef fishes. Oryx 2013, 47, 259–265. [Google Scholar] [CrossRef]
- MacLean, S.A.; Beissinger, S.R. Species’ traits as predictors of range shifts under contemporary climate change: A review and meta-analysis. Glob. Change Biol. 2017, 23, 4094–4105. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2025-1. Available online: https://www.iucnredlist.org (accessed on 10 January 2025).
- Froese, R.; Pauly, D. (Eds.) FishBase, version (02/2025); World Wide Web Electronic Publication. Available online: https://www.fishbase.org (accessed on 26 July 2025).
- Myers, G.S. Usage of anadromous, catadromous and allied terms for migratory fishes. Copeia 1949, 1949, 89–97. [Google Scholar] [CrossRef]
- McDowall, R.M. On amphidromy, a distinct form of diadromy in aquatic organisms. Fish Fish. 2007, 8, 1–13. [Google Scholar] [CrossRef]
- Northcote, T.G. Potamodromy in Salmonidae—Living and Moving in the Fast Lane. N. Am. J. Fish. Manag. 1997, 17, 1029–1045. [Google Scholar] [CrossRef]
- Boettiger, C.; Lang, D.T.; Wainwright, P.C. rfishbase: Exploring, manipulating and visualizing FishBase data from R. J. Fish Biol. 2012, 81, 2030–2039. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. dplyr: A Grammar of Data Manipulation. R Package Version 1.1.4. Available online: https://dplyr.tidyverse.org (accessed on 26 July 2025).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 26 July 2025).
- Kassambara, A. R Package, version 0.1.4.1; ggcorrplot: Visualization of a Correlation Matrix Using ‘ggplot2’. Available online: https://CRAN.R-project.org/package=ggcorrplot (accessed on 26 July 2025).
- Kassambara, A. rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R Package Version 0.7.2. Available online: https://cran.r-project.org/web/packages/rstatix/index.html (accessed on 26 July 2025).
- Liermann, C.R.; Nilsson, C.; Robertson, J.; Ng, R.Y. Implications of dam obstruction for global freshwater fish diversity. BioScience 2012, 62, 539–548. [Google Scholar] [CrossRef]
- Clavero, M.; Hermoso, V.; Levin, N.; Kark, S. Biodiversity research: Geographical linkages between threats and imperilment in freshwater fish in the Mediterranean Basin. Divers. Distrib. 2010, 16, 744–754. [Google Scholar] [CrossRef]
- Jonsson, B.; Waples, R.S.; Friedland, K.D. Extinction considerations for diadromous fishes. ICES J. Mar. Sci. 1999, 56, 405–409. [Google Scholar] [CrossRef]
- Navodaru, I.; Staras, M.; Cernisencu, I. The challenge of sustainable use of the Danube Delta Fisheries, Romania. Fish. Manag. Ecol. 2001, 8, 323–332. [Google Scholar] [CrossRef]
- Cowx, I.G.; Gerdeaux, D. The effects of fisheries management practises on freshwater ecosystems. Fish. Manag. Ecol. 2004, 11, 145–151. [Google Scholar] [CrossRef]
- Bennett, P.M.; Owens, I.P.F. Variation in extinction risk among birds: Chance or evolutionary predisposition? Proc. R. Soc. B Biol. Sci. 1997, 264, 401–408. [Google Scholar] [CrossRef]
- Jennings, S.; Reynolds, J.D.; Polunin, N.V.C. Predicting the vulnerability of tropical reef fishes to exploitation with phylogenies and life histories. Conserv. Biol. 1999, 13, 1466–1475. [Google Scholar] [CrossRef]
- Reynolds, J.D.; Jennings, S.; Dulvy, N.K. Life histories of fishes and population responses to exploitation. In Conservation of Exploited Species; Reynolds, J.D., Mace, G.M., Redford, K.H., Robinson, J.G., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 147–168. [Google Scholar]
- Cardillo, M.; Mace, G.M.; Gittleman, J.L.; Purvis, A. Latent extinction risk and the future battlegrounds of mammal conservation. Proc. Natl. Acad. Sci. USA 2006, 103, 4157–4161. [Google Scholar] [CrossRef] [PubMed]
- Dulvy, N.K.; Ellis, J.R.; Goodwin, N.B.; Grant, A.; Reynolds, J.D.; Jennings, S. Methods of assessing extinction risk in marine fishes. Fish Fish. 2004, 5, 255–276. [Google Scholar] [CrossRef]
- Stevens, J.D.; Bonfil, R.; Dulvy, N.K.; Walker, P.A. The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES J. Mar. Sci. 2000, 57, 476–494. [Google Scholar] [CrossRef]
- Musick, J.A. Criteria to define extinction risk in marine fishes: The American fisheries society initiative. Fisheries 1999, 24, 6–14. [Google Scholar] [CrossRef]
- Garcia, S.M.; Rice, J.; Charles, A. Balanced harvesting in fisheries: A preliminary analysis of management implications. ICES J. Mar. Sci. 2016, 73, 1668–1678. [Google Scholar] [CrossRef]
- Pinsky, M.L.; Jensen, O.P.; Ricard, D.; Palumbi, S.R. Unexpected patterns of fisheries collapse in the world’s oceans. Proc. Natl. Acad. Sci. USA 2011, 108, 8317–8322. [Google Scholar] [CrossRef]
- Graham, N.A.; Chabanet, P.; Evans, R.D.; Jennings, S.; Letourneur, Y.; MacNeil, M.A.; McClanahan, T.R.; Öhman, M.C.; Polunin, N.V.C.; Wilson, S.K. Extinction vulnerability of coral reef fishes. Ecol. Lett. 2011, 14, 341–348. [Google Scholar] [CrossRef]
- Myers, R.A.; Worm, B. Extinction, survival or recovery of large predatory fishes. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 13–20. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petriki, O.; Bobori, D.C. Linking Life History Traits to the Threat Level of European Freshwater Fish. Water 2025, 17, 2254. https://doi.org/10.3390/w17152254
Petriki O, Bobori DC. Linking Life History Traits to the Threat Level of European Freshwater Fish. Water. 2025; 17(15):2254. https://doi.org/10.3390/w17152254
Chicago/Turabian StylePetriki, Olga, and Dimitra C. Bobori. 2025. "Linking Life History Traits to the Threat Level of European Freshwater Fish" Water 17, no. 15: 2254. https://doi.org/10.3390/w17152254
APA StylePetriki, O., & Bobori, D. C. (2025). Linking Life History Traits to the Threat Level of European Freshwater Fish. Water, 17(15), 2254. https://doi.org/10.3390/w17152254






