Combating Environmental Antimicrobial Resistance Using Bacteriophage Cocktails Targeting β-Lactam-Resistant High-Risk Clones of Klebsiella pneumoniae and Escherichia coli in Wastewater: A Strategy for Treatment and Reuse
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Characterization of Bacteriophages
2.2. Preparation of the Bacteriophage Cocktails
2.3. Selection of Bacteria for Biocontrol Assays
2.4. Preparation of Synthetic Wastewater
2.5. Bacterial Viability in Synthetic Wastewater
2.6. Stability of Bacteriophages in Synthetic Wastewater
2.7. Biocontrol Assays Using Bacteriophages in Synthetic Wastewater
2.8. Biocontrol Assay Using Individual Bacteriophages
2.9. Biocontrol Assay Using Bacteriophage Cocktails
2.10. Biocontrol Assays Using Multiple Doses of the Cocktail
2.11. Biocontrol Assays Using Individual and Sequential Administration of the Bacteriophages Composing the Cocktail
2.12. Evaluation of Bacteriophage Resistance
2.13. Statistical Analysis
3. Results
3.1. Bacteriophages Selected After Isolation and Characterization
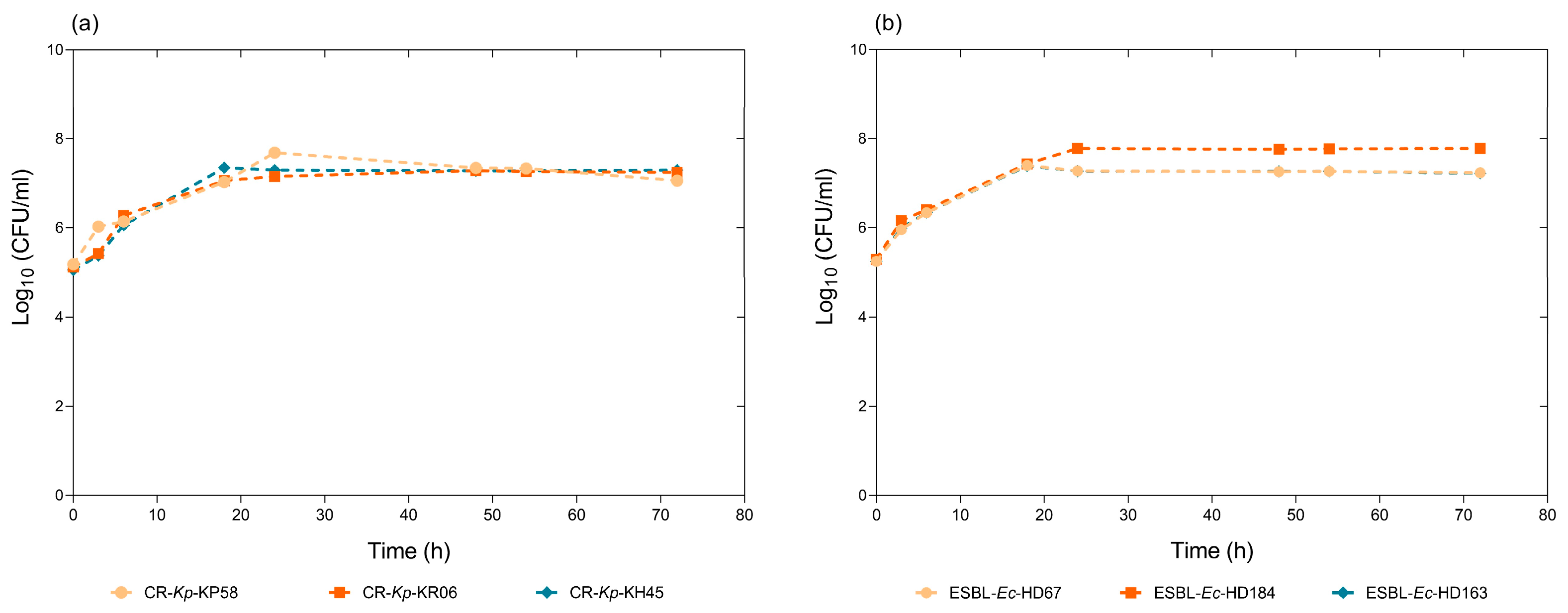
3.2. Bacterial Viability in Synthetic Wastewater
3.3. Bacteriophage Stability in Synthetic Wastewater
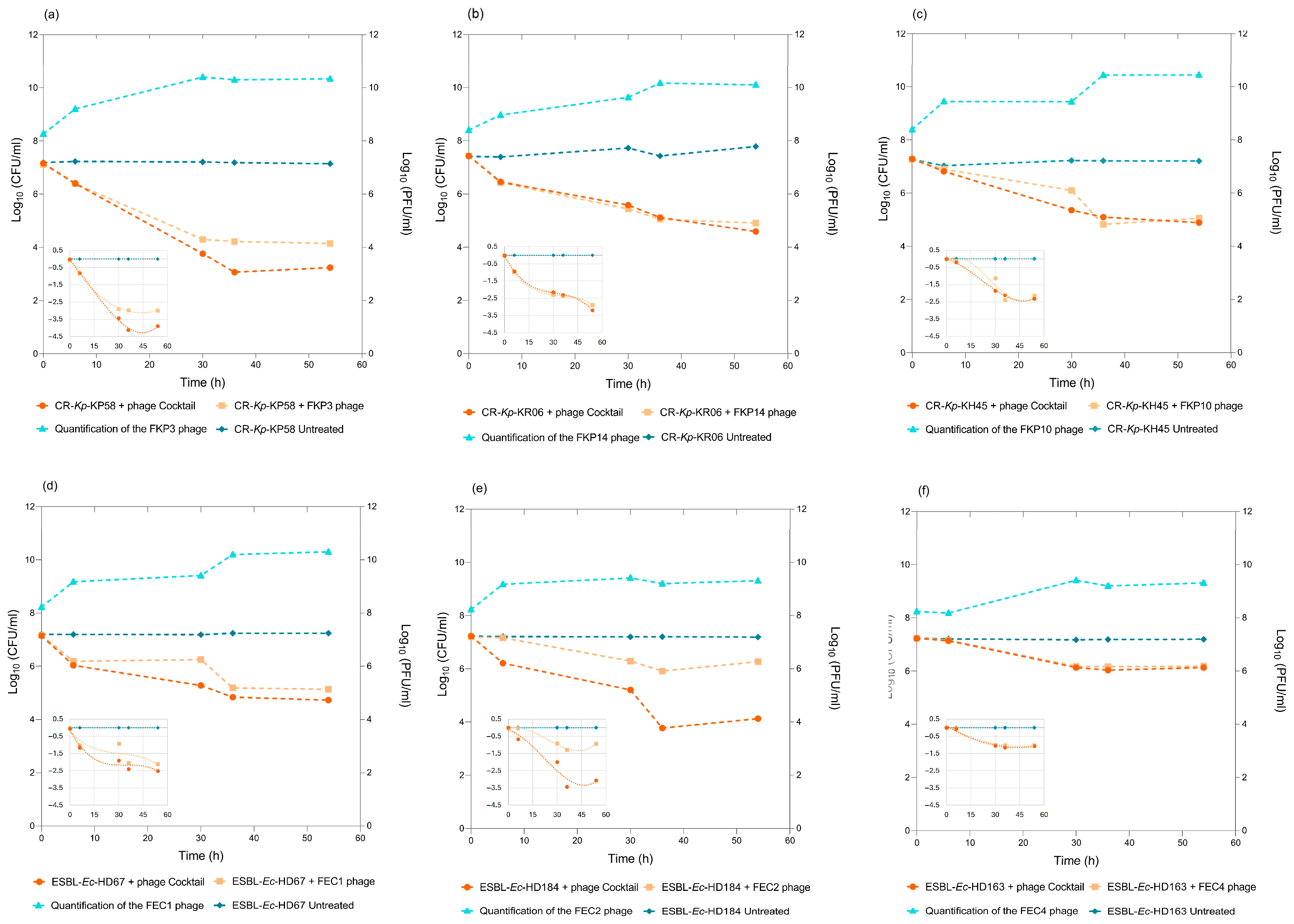
3.4. Biocontrol Assay Using Individual Bacteriophages
3.5. Biocontrol Assay Using Bacteriophage Cocktails
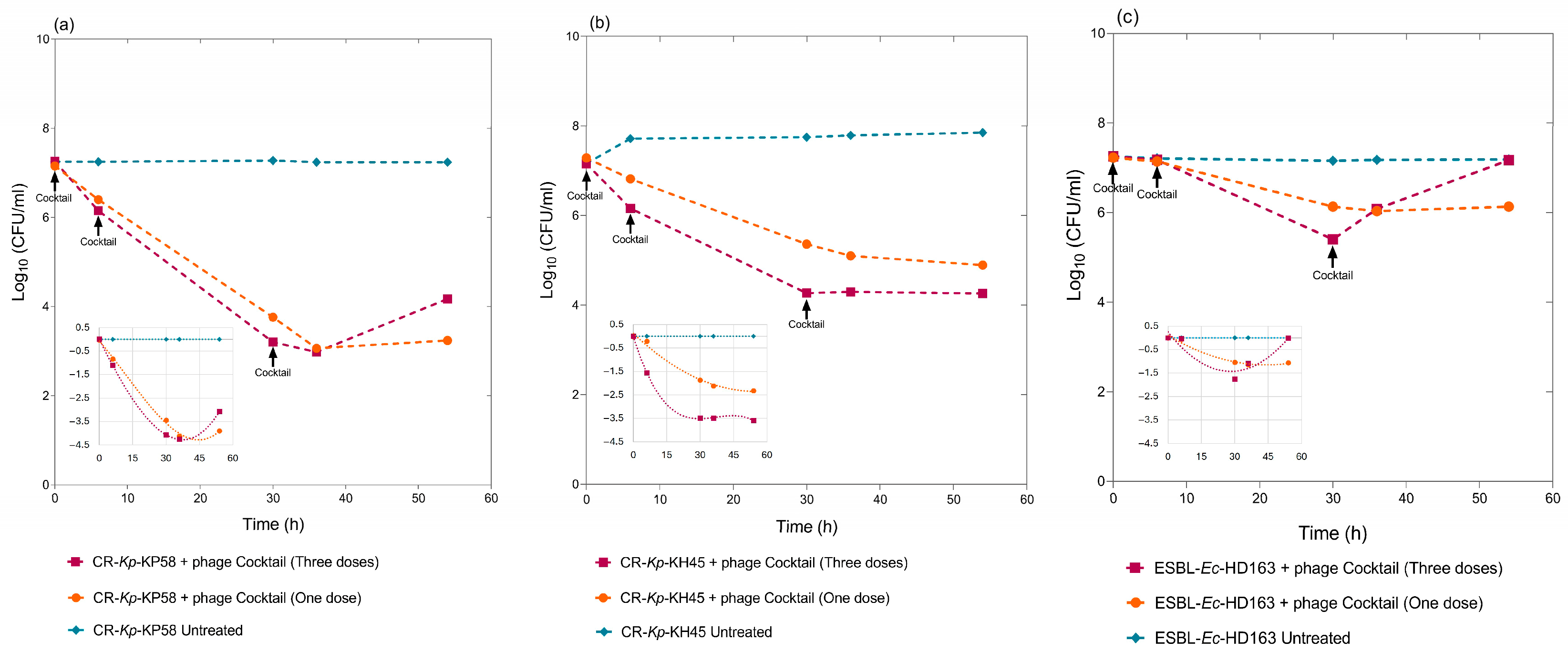
3.6. Biocontrol Assays Using Multiple Doses of the Cocktail
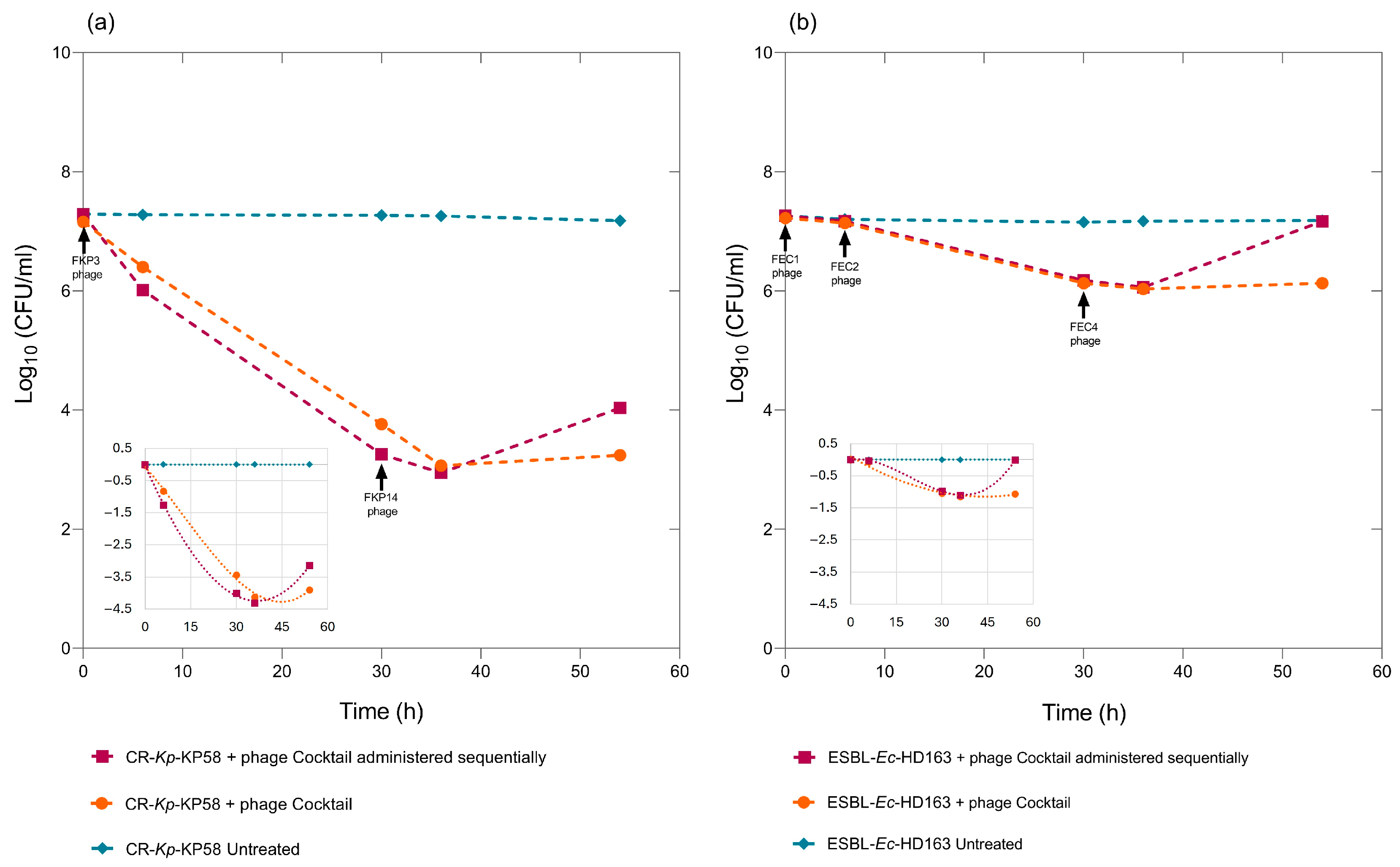
3.7. Biocontrol Assays Using Individual and Sequential Administration of the Bacteriophages Composing the Cocktail
3.8. Assessment of Bacterial Resistance to Bacteriophages
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARB | Antimicrobial-resistant bacteria |
| ARGs | Antibiotic resistance genes |
| CR-Kp | Carbapenem-resistant Klebsiella pneumoniae |
| ESBL-Ec | E. coli producers of extended spectrum β-lactamases |
| KPC | Klebsiella pneumoniae carbapenemase |
| MLST | Multi-Locus Sequence Typing |
| WHO | World Health Organization |
| WWTPs | Wastewater treatment plants |
References
- Mutuku, C.; Gazdag, Z.; Melegh, S. Occurrence of Antibiotics and Bacterial Resistance Genes in Wastewater: Resistance Mechanisms and Antimicrobial Resistance Control Approaches. World J. Microbiol. Biotechnol. 2022, 38, 152. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mozaz, S.; Chamorro, S.; Marti, E.; Huerta, B.; Gros, M.; Sànchez-Melsió, A.; Borrego, C.M.; Barceló, D.; Balcázar, J.L. Occurrence of Antibiotics and Antibiotic Resistance Genes in Hospital and Urban Wastewaters and Their Impact on the Receiving River. Water Res. 2015, 69, 234–242. [Google Scholar] [CrossRef] [PubMed]
- WHO Bacterial Priority Pathogens List 2024: Bacterial Pathogens of Public Health Importance, to Guide Research, Development, and Strategies to Prevent and Control Antimicrobial Resistance, 1st ed.; World Health Organization: Geneva, Switzerland, 2024.
- Seguni, N.Z.; Kimera, Z.I.; Msafiri, F.; Mgaya, F.X.; Joachim, A.; Mwingwa, A.; Matee, M.I. Multidrug-Resistant Escherichia Coli and Klebsiella Pneumoniae Isolated from Hospital Sewage Flowing through Community Sewage System and Discharging into the Indian Ocean. Bull. Natl. Res. Cent. 2023, 47, 66. [Google Scholar] [CrossRef]
- Uluseker, C.; Kaster, K.M.; Thorsen, K.; Basiry, D.; Shobana, S.; Jain, M.; Kumar, G.; Kommedal, R.; Pala-Ozkok, I. A Review on Occurrence and Spread of Antibiotic Resistance in Wastewaters and in Wastewater Treatment Plants: Mechanisms and Perspectives. Front. Microbiol. 2021, 12, 717809. [Google Scholar] [CrossRef] [PubMed]
- Ballesté, E.; Blanch, A.R.; Muniesa, M.; García-Aljaro, C.; Rodríguez-Rubio, L.; Martín-Díaz, J.; Pascual-Benito, M.; Jofre, J. Bacteriophages in Sewage: Abundance, Roles, and Applications. FEMS Microbes 2022, 3, xtac009. [Google Scholar] [CrossRef] [PubMed]
- Shivaram, K.B.; Bhatt, P.; Applegate, B.; Simsek, H. Bacteriophage-Based Biocontrol Technology to Enhance the Efficiency of Wastewater Treatment and Reduce Targeted Bacterial Biofilms. Sci. Total Environ. 2023, 862, 160723. [Google Scholar] [CrossRef] [PubMed]
- Tellez-Carrasquilla, S.; Salazar-Ospina, L.; Jiménez, J.N. High Activity and Specificity of Bacteriophage Cocktails against Carbapenem-Resistant Klebsiella Pneumoniae Belonging to the High-Risk Clones CG258 and ST307. Front. Microbiol. 2024, 15, 1502593. [Google Scholar] [CrossRef] [PubMed]
- Aristizabal-Hoyos, A.M.; Rodríguez, E.A.; Torres-Palma, R.A.; Jiménez, J.N. Concern Levels of β-Lactamase-Producing Gram-Negative Bacilli in Hospital Wastewater: Hotspot of Antimicrobial Resistance in Latin-America. Diagn. Microbiol. Infect. Dis. 2023, 105, 115819. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Rodríguez, D.M.; Ávila-Torres, Y.; Serna-Galvis, E.A.; Torres-Palma, R.A. Data on Treatment of Nafcillin and Ampicillin Antibiotics in Water by Sonochemistry. Data Brief 2020, 29, 105361. [Google Scholar] [CrossRef] [PubMed]
- Ocampo, A.M.; Chen, L.; Cienfuegos, A.V.; Roncancio, G.; Chavda, K.D.; Kreiswirth, B.N.; Jiménez, J.N. A Two-Year Surveillance in Five Colombian Tertiary Care Hospitals Reveals High Frequency of Non-CG258 Clones of Carbapenem-Resistant Klebsiella Pneumoniae with Distinct Clinical Characteristics. Antimicrob. Agents Chemother. 2016, 60, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Diancourt, L.; Passet, V.; Verhoef, J.; Grimont, P.A.D.; Brisse, S. Multilocus Sequence Typing of Klebsiella Pneumoniae Nosocomial Isolates. J. Clin. Microbiol. 2005, 43, 4178–4182. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Walsh, T.R.; Cuvillier, V.; Nordmann, P. Multiplex PCR for Detection of Acquired Carbapenemase Genes. Diagn. Microbiol. Infect. Dis. 2011, 70, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Serna-Galvis, E.A.; Salazar-Ospina, L.; Jiménez, J.N.; Pino, N.J.; Torres-Palma, R.A. Elimination of Carbapenem Resistant Klebsiella Pneumoniae in Water by UV-C, UV-C/Persulfate and UV-C/H2O2. Evaluation of Response to Antibiotic, Residual Effect of the Processes and Removal of Resistance Gene. J. Environ. Chem. Eng. 2020, 8, 102196. [Google Scholar] [CrossRef]
- Kauppinen, A.; Siponen, S.; Pitkänen, T.; Holmfeldt, K.; Pursiainen, A.; Torvinen, E.; Miettinen, I.T. Phage Biocontrol of Pseudomonas Aeruginosa in Water. Viruses 2021, 13, 928. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, B.; López, M.M.; Biosca, E.G. Biocontrol of the Major Plant Pathogen Ralstonia Solanacearum in Irrigation Water and Host Plants by Novel Waterborne Lytic Bacteriophages. Front. Microbiol. 2019, 10, 2813. [Google Scholar] [CrossRef] [PubMed]
- Reyneke, B.; Khan, S.; Fernández-Ibáñez, P.; Khan, W. Podoviridae Bacteriophage for the Biocontrol of Pseudomonas Aeruginosa in Rainwater. Environ. Sci. Water Res. Technol. 2020, 6, 87–102. [Google Scholar] [CrossRef]
- Swanstrom, M.; Adams, M.H. Agar Layer Method for Production of High Titer Phage Stocks. Exp. Biol. Med. 1951, 78, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, L.A.B.; Dos Santos, L.M.C.; Oliveira, F.O.; Rodrigues, L.D.A.P.; Neves, P.R.F.; Moreira, G.A.F.; Santos, A.A.B.; Lobato, G.M.; Nascimento, C.; Gerhardt, M.; et al. Evaluation of the Microbial Reduction Efficacy and Perception of Use of an Ozonized Water Spray Disinfection Technology. Sci. Rep. 2022, 12, 13019. [Google Scholar] [CrossRef] [PubMed]
- Manohar, P.; Tamhankar, A.J.; Lundborg, C.S.; Nachimuthu, R. Therapeutic Characterization and Efficacy of Bacteriophage Cocktails Infecting Escherichia Coli, Klebsiella Pneumoniae, and Enterobacter Species. Front. Microbiol. 2019, 10, 574. [Google Scholar] [CrossRef] [PubMed]
- Aristizábal-Hoyos, A.M.; Rodríguez, E.A.; Arias, L.; Jiménez, J.N. High Clonal Diversity of Multidrug-Resistant and Extended Spectrum Β-Lactamase-Producing Escherichia Coli in a Wastewater Treatment Plant. J. Environ. Manag. 2019, 245, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Pei, M.; Zhang, B.; He, Y.; Su, J.; Gin, K.; Lev, O.; Shen, G.; Hu, S. State of the Art of Tertiary Treatment Technologies for Controlling Antibiotic Resistance in Wastewater Treatment Plants. Environ. Int. 2019, 131, 105026. [Google Scholar] [CrossRef] [PubMed]
- Elbahnasawy, M.A.; ElSayed, E.E.; Azzam, M.I. Newly Isolated Coliphages for Bio-Controlling Multidrug-Resistant Escherichia Coli Strains. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100542. [Google Scholar] [CrossRef]
- Soliman, R.M.; Othman, B.A.; Shoman, S.A.; Azzam, M.I.; Gado, M.M. Biocontrol of Multi-Drug Resistant Pathogenic Bacteria in Drainage Water by Locally Isolated Bacteriophage. BMC Microbiol. 2023, 23, 118. [Google Scholar] [CrossRef] [PubMed]
- Turki, Y.; Ouzari, H.; Mehri, I.; Ammar, A.B.; Hassen, A. Evaluation of a Cocktail of Three Bacteriophages for the Biocontrol of Salmonella of Wastewater. Food Res. Int. 2012, 45, 1099–1105. [Google Scholar] [CrossRef]
- Sadeqi, S.; Hashemi Shahraki, A.; Nikkhahi, F.; Javadi, A.; Marashi, S.M.A. Application of Bacteriophage Cocktails for Reducing the Bacterial Load of Nosocomial Pathogens in Hospital Wastewater. Iran. J. Microbiol. 2022, 14, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Xing, B.; Yang, M.; Han, R.; Pan, H.; Guo, H.; Liu, Z.; Huang, T.; Du, K.; Jiang, S.; et al. Characterization of a Novel Genus of Jumbo Phages and Their Application in Wastewater Treatment. iScience 2023, 26, 106947. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Mathieu, J.; Lu, G.W.; Gabiatti, N.; Alvarez, P.J. Control of Antibiotic-Resistant Bacteria in Activated Sludge Using Polyvalent Phages in Conjunction with a Production Host. Environ. Sci. Technol. Lett. 2017, 4, 137–142. [Google Scholar] [CrossRef]
- Hall, A.R.; De Vos, D.; Friman, V.-P.; Pirnay, J.-P.; Buckling, A. Effects of Sequential and Simultaneous Applications of Bacteriophages on Populations of Pseudomonas Aeruginosa In Vitro and in Wax Moth Larvae. Appl. Environ. Microbiol. 2012, 78, 5646–5652. [Google Scholar] [CrossRef] [PubMed]
- Kotay, S.M.; Datta, T.; Choi, J.; Goel, R. Biocontrol of Biomass Bulking Caused by Haliscomenobacter Hydrossis Using a Newly Isolated Lytic Bacteriophage. Water Res. 2011, 45, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Salim, A.; Madhavan, A.; Subhash, S.; Prasad, M.; Nair, B.G.; Pal, S. Escherichia Coli ST155 as a Production-Host of Three Different Polyvalent Phages and Their Characterisation with a Prospect for Wastewater Disinfection. Sci. Rep. 2022, 12, 19406. [Google Scholar] [CrossRef] [PubMed]
- Abedon, S.T.; Danis-Wlodarczyk, K.M.; Wozniak, D.J. Phage Cocktail Development for Bacteriophage Therapy: Toward Improving Spectrum of Activity Breadth and Depth. Pharmaceuticals 2021, 14, 1019. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Pallavali, R.R.; Shin, D. Phage-Based Biocontrol of Antibiotic-Resistant Bacteria Isolated from Livestock Wastewater Treatment Plant. SSRN 2023, 15, 1616. [Google Scholar] [CrossRef]
- Beheshti Maal, K.; Soleimani Delfan, A.; Salmanizadeh, S. Isolation and Identification of Two Novel Escherichia Coli Bacteriophages and Their Application in Wastewater Treatment and Coliform’s Phage Therapy. Jundishapur J. Microbiol. 2015, 8, e14945. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ondon, B.S.; Ho, S.-H.; Jiang, J.; Li, F. Antibiotic Resistant Bacteria and Genes in Wastewater Treatment Plants: From Occurrence to Treatment Strategies. Sci. Total Environ. 2022, 838, 156544. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.-W.; Wu, Y.-H.; Yu, T.; Wang, Y.-H.; Chen, G.-Q.; Tong, X.; Bai, Y.; Xu, C.; Wang, H.-B.; Ikuno, N.; et al. Evaluating Method and Potential Risks of Chlorine-Resistant Bacteria (CRB): A Review. Water Res. 2021, 188, 116474. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Orman, M.A. UV-Induced DNA Repair Mechanisms and Their Effects on Mutagenesis and Culturability in Escherichia coli. Microbiology 2024. [Google Scholar] [CrossRef]
- Collivignarelli, M.; Abbà, A.; Benigna, I.; Sorlini, S.; Torretta, V. Overview of the Main Disinfection Processes for Wastewater and Drinking Water Treatment Plants. Sustainability 2017, 10, 86. [Google Scholar] [CrossRef]


| Bacterial Genus | Strain Code | Source | β-Lactamases (Carbapenemases or ESBL) | MLST Typing/Clermont Typing * | Antimicrobial Resistant Profile |
|---|---|---|---|---|---|
| Carbapenem-resistant K. pneumoniae | KP58 | Hospitalized patients infections | KPC3 | CG258-ST512 | Resistant to ampicillin/sulbactam, piperacillin/tazobactam, cefoxitin, ceftazidime, ceftriaxone, cefepime, ertapenem, imipenem, meropenem, amikacin, ciprofloxacin, tigecycline, and colistin. It also has intermediate resistance to gentamicin. |
| KR06 | KPC2 | CG258-ST258 | Resistant to ampicillin/sulbactam, piperacillin/tazobactam, cefoxitin, ceftazidime, ceftriaxone, cefepime, ertapenem, imipenem, meropenem, doripenem, amikacin, gentamicin, and ciprofloxacin. | ||
| KH45 | KPC2 | ST307 | Resistant to piperacillin/tazobactam, ceftazidime, cefepime, ertapenem, imipenem, meropenem, and ciprofloxacin. It also has intermediate resistance to cefoxitin, and is susceptible to amikacin and gentamicin. | ||
| E. coli resistant to third-generation cephalosporins (ESBL) | HD67 | Colonized patients | CTX-M-G9 | Non-typing B2-phylogroup | Positive ESBL test, resistant to ceftazidime, ceftriaxone, cefepime, and ciprofloxacin. Intermediate resistance to colistin, and susceptible to ampicillin/ sulbactam, piperacillin/tazobactam, cefoxitin, ertapenem, imipenem, meropenem, doripenem, amikacin, gentamicin, and tigecycline. |
| HD184 | CTX-M-G9 | ST131 E-phylogroup | |||
| HD163 | CTX-M-G1 | ST131 B2-phylogroup | Positive ESBL test, resistant to ceftazidime, ceftriaxone, and cefepime. Intermediate resistance to colistin, and susceptible to ampicillin/sulbactam, piperacillin/tazobactam, cefoxitin, ertapenem, imipenem, meropenem, doripenem, amikacin, gentamicin, ciprofloxacin, and tigecycline. |
| Component | Final Concentration on the Simulated Wastewater | |
|---|---|---|
| (mg/L) | (M) | |
| Sodium chloride (NaCl) | 7 | 119 |
| Potassium chloride (KCl) | 4 | 54 |
| Calcium chloride dihydrate (CaCl2· 2H2O) | 4 | 27 |
| Sodium bicarbonate (NaHCO3) | 96 | 1.1 |
| Calcium sulfate dihydrate (CaCO4·2H2O) | 60 | 348 |
| Magnesium sulfate heptahydrate (MgSO4· 7H2O) | 125 | 507 |
| Dibasic potassium phosphate (K2HPO4) | 28 | 161 |
| Urea (CH4N2O) | 6 | 99.9 |
| Meat Extract (Carbon source) | 32 | - |
| Peptone (Nitrogen source) | 22 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zapata-Montoya, M.D.; Salazar-Ospina, L.; Jiménez, J.N. Combating Environmental Antimicrobial Resistance Using Bacteriophage Cocktails Targeting β-Lactam-Resistant High-Risk Clones of Klebsiella pneumoniae and Escherichia coli in Wastewater: A Strategy for Treatment and Reuse. Water 2025, 17, 2236. https://doi.org/10.3390/w17152236
Zapata-Montoya MD, Salazar-Ospina L, Jiménez JN. Combating Environmental Antimicrobial Resistance Using Bacteriophage Cocktails Targeting β-Lactam-Resistant High-Risk Clones of Klebsiella pneumoniae and Escherichia coli in Wastewater: A Strategy for Treatment and Reuse. Water. 2025; 17(15):2236. https://doi.org/10.3390/w17152236
Chicago/Turabian StyleZapata-Montoya, María D., Lorena Salazar-Ospina, and Judy Natalia Jiménez. 2025. "Combating Environmental Antimicrobial Resistance Using Bacteriophage Cocktails Targeting β-Lactam-Resistant High-Risk Clones of Klebsiella pneumoniae and Escherichia coli in Wastewater: A Strategy for Treatment and Reuse" Water 17, no. 15: 2236. https://doi.org/10.3390/w17152236
APA StyleZapata-Montoya, M. D., Salazar-Ospina, L., & Jiménez, J. N. (2025). Combating Environmental Antimicrobial Resistance Using Bacteriophage Cocktails Targeting β-Lactam-Resistant High-Risk Clones of Klebsiella pneumoniae and Escherichia coli in Wastewater: A Strategy for Treatment and Reuse. Water, 17(15), 2236. https://doi.org/10.3390/w17152236







