Dual-Functioned Magnesium-Enriched Biochar Hydrogels for Phosphate Recovery and Slow-Release Nutrient Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Mg-Modified Biochar
2.3. Preparation of Mg-Modified Biochar Hydrogel
2.4. Characterization of Mg-Modified Biochar and Mg-Modified Biochar Hydrogel
2.5. Adsorption Experiments (Aqueous Solution Condition)
2.6. Release of Phosphate into Soil
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Adsorbent Materials
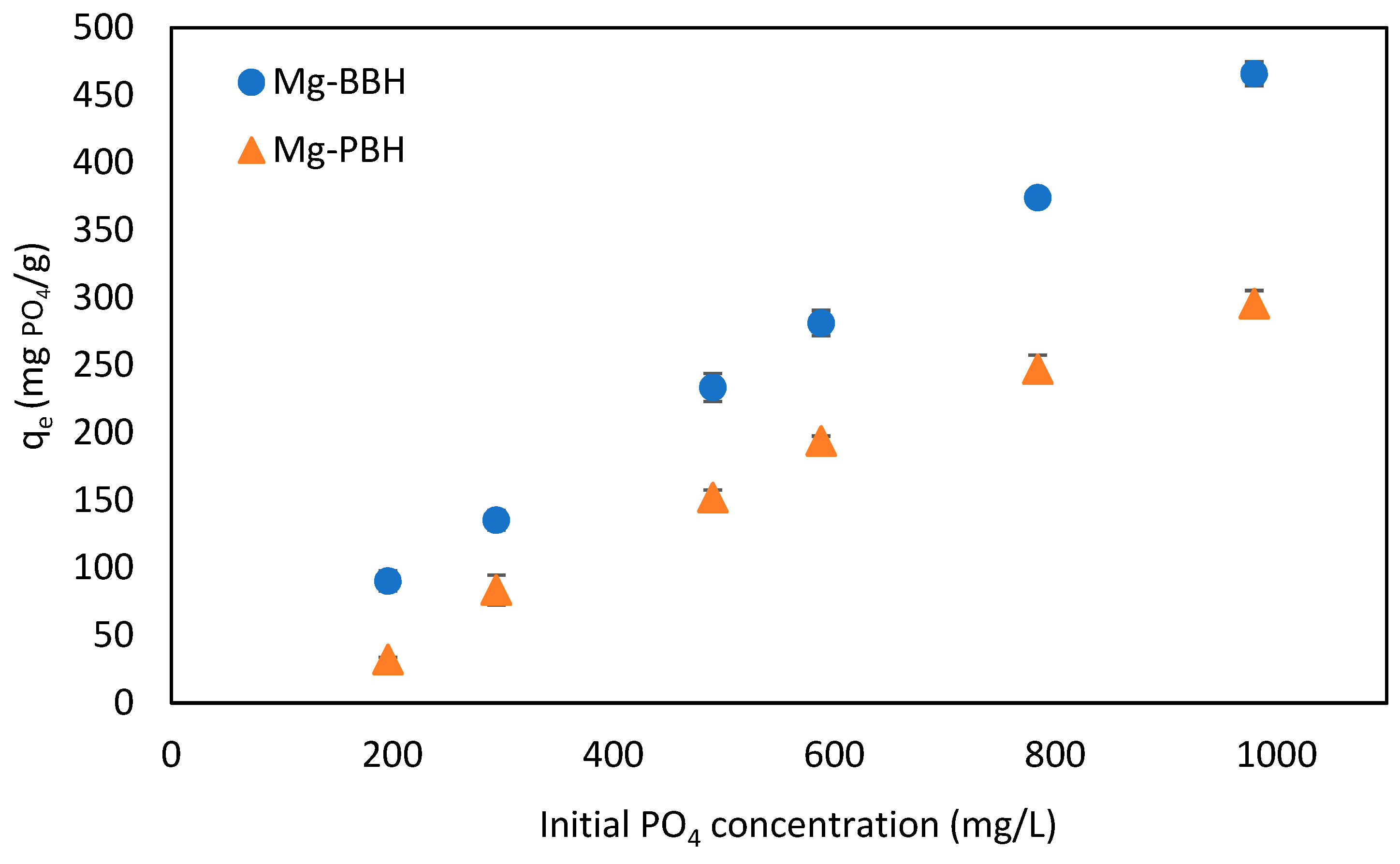
3.2. Adsorption of PO4 (Influence of Initial PO4 Concentration)
3.3. Adsorption of PO4 (Influence of Contact Time and Temperature)
3.4. Adsorption Isotherm Studies
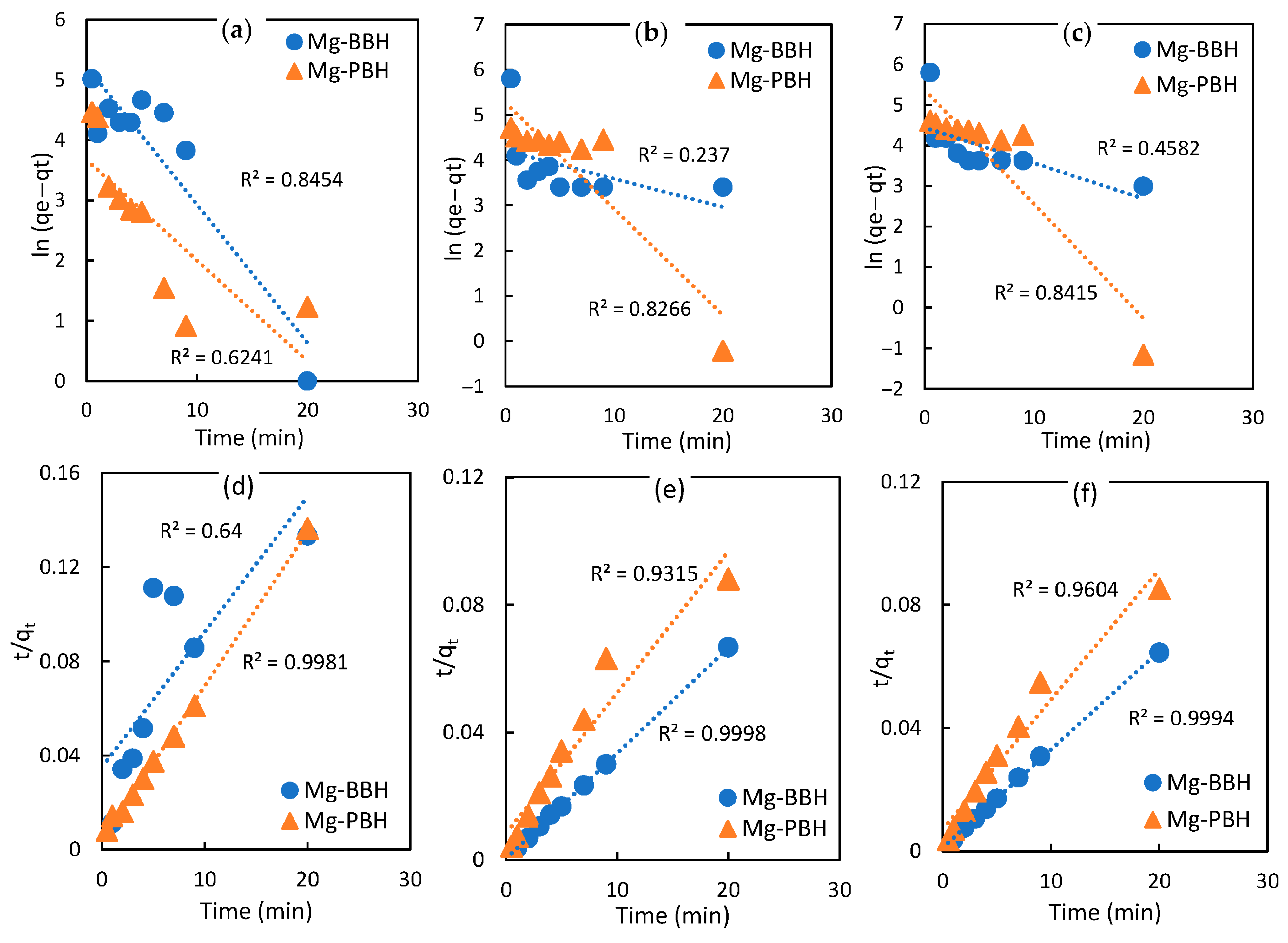
3.5. Kinetic Studies
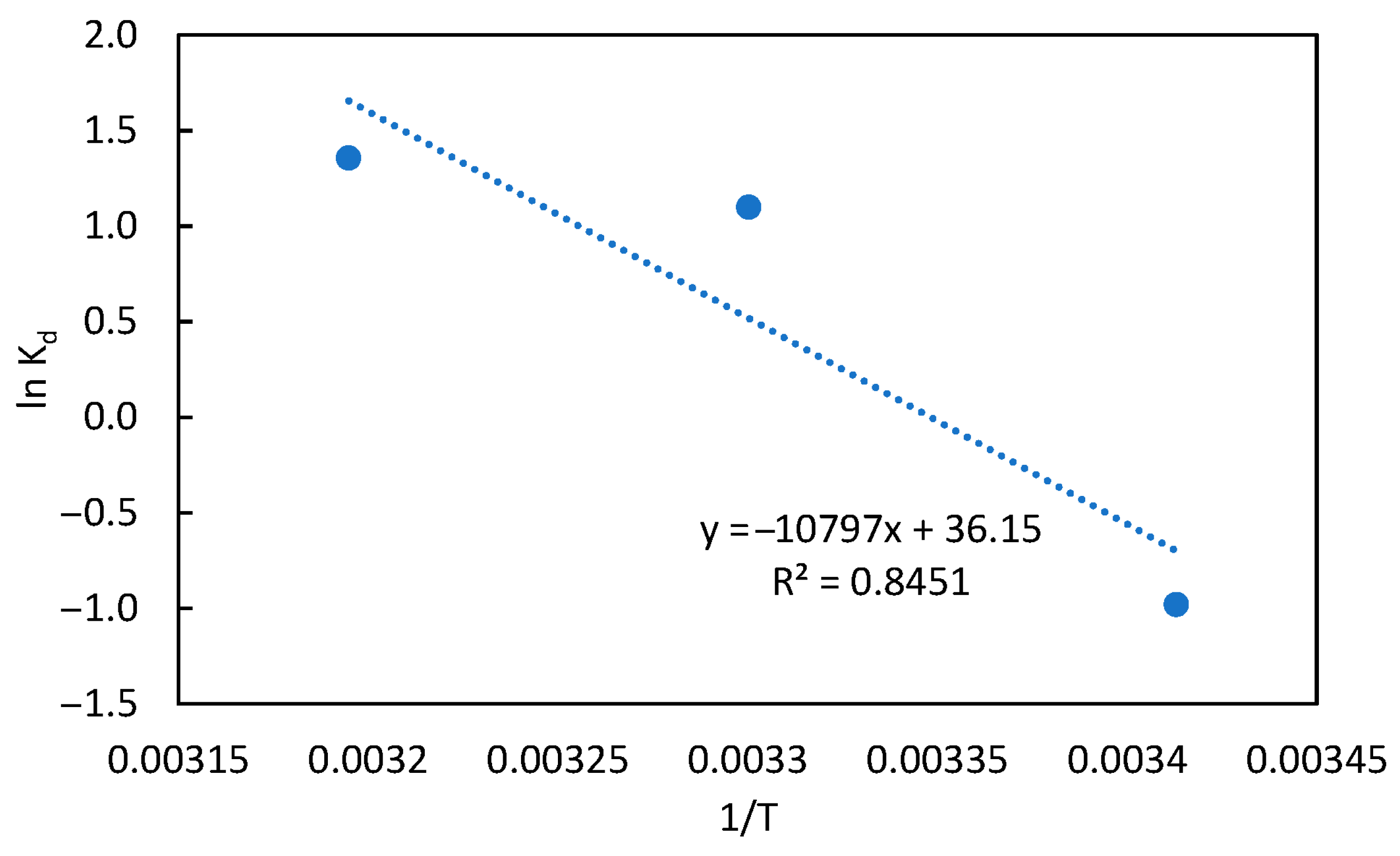
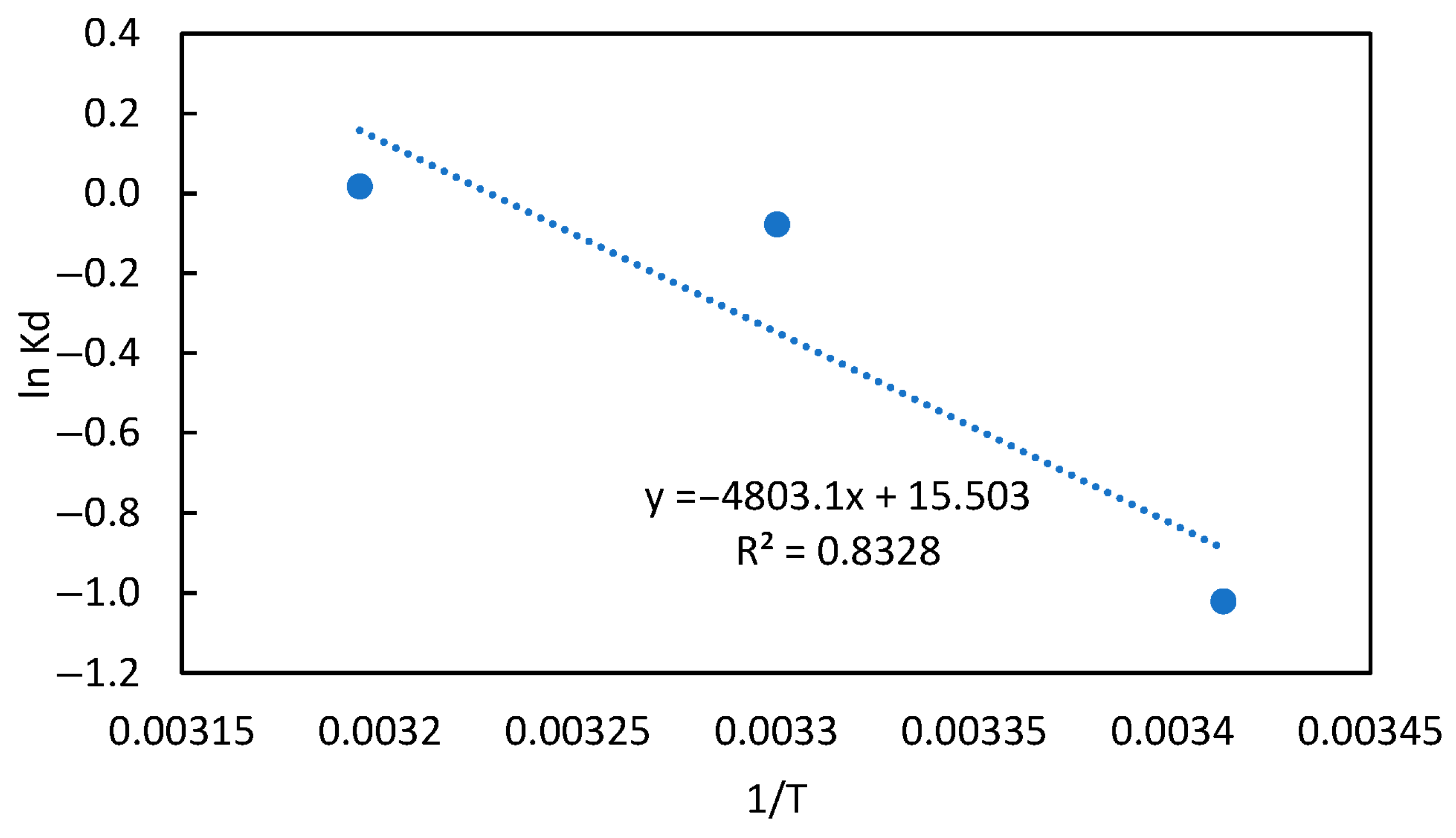
3.6. Thermodynamic Studies
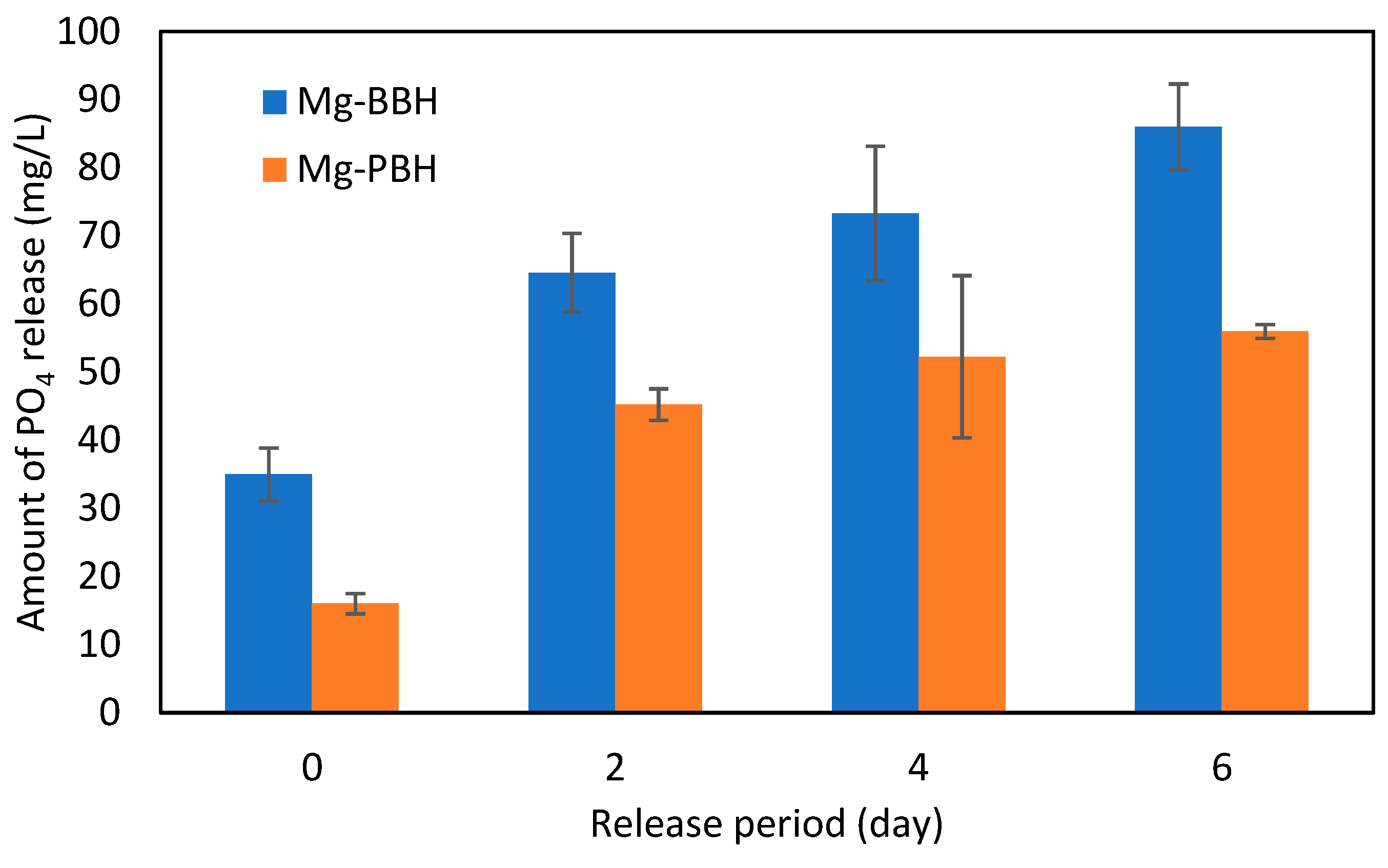
3.7. Release of Phosphate in Soil
3.8. Estimation of Cost Analysis of Adsorbents
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Richardson, K.; Steffen, W.; Lucht, W.; Bendtsen, J.; Cornell, S.E.; Donges, J.F.; Drüke, M.; Fetzer, I.; Bala, G.; von Bloh, W.; et al. Earth beyond six of nine planetary boundaries. Sci. Adv. 2023, 9, eadh2458. [Google Scholar] [CrossRef]
- Akinnawo, S.O. Eutrophication: Causes, consequences, physical, chemical and biological techniques for mitigation strategies. Environ. Chall. 2023, 12, 100733. [Google Scholar] [CrossRef]
- Hoque, T.S.; Sarkar, D.; Datta, R.; Kibria, M.G.; Ullah, R.; Ahmed, N.; Hossain, M.A.; Masood, A.; Anjum, N.A.; Hoque, T.S.; et al. Sustainable Management of Phosphorus in Agriculture for Environmental Conservation. In Phosphorus in Soils and Plants; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Iizuka, A.; Ho, H.J.; Sugimoto, T.; Adachi, K.; Shibata, E. Simultaneous Separation and Recovery of Phosphorus from Aqueous Solution by Bipolar Membrane Electrodialysis. ISIJ Int. 2023, 63, 1172–1177. [Google Scholar] [CrossRef]
- Jin, W.; Hao, D.; Tabatabai, S.A.A.; Kennedy, M.; Schippers, J.C. Theoretical and experimental investigation of phosphate removal from seawater by multi-stage coagulation. Water Supply 2021, 21, 3725–3734. [Google Scholar] [CrossRef]
- Tran, L.B.; Nguyen, T.T.; Padungthon, S.; Le, T.T.; Nguyen Thi, Q.A.; Nguyen, N.H. Advanced natural hydrated iron-alum oxides cation exchange resin for simultaneous phosphate and hardness removal. npj Clean Water 2022, 5, 43. [Google Scholar] [CrossRef]
- Akram, M.; Xu, X.; Gao, B.; Wang, S.; Khan, R.; Yue, Q.; Duan, P.; Dan, H.; Pan, J. Highly efficient removal of phosphate from aqueous media by pomegranate peel co-doping with ferric chloride and lanthanum hydroxide nanoparticles. J. Clean. Prod. 2021, 292, 125311. [Google Scholar] [CrossRef]
- Sarker, P.; Liu, X.; Hata, N.; Takeshita, H.; Miyamura, H.; Maruo, M. Thermally modified bamboo-eggshell adsorbent for phosphate recovery and its sustainable application as fertilizer. Environ. Res. 2023, 231 Pt 1, 115992. [Google Scholar] [CrossRef]
- Shao, Y.; Li, J.; Fang, X.; Yang, Z.; Qu, Y.; Yang, M.; Tan, W.; Li, G.; Wang, H. Chemical modification of bamboo activated carbon surface and its adsorption property of simultaneous removal of phosphate and nitrate. Chemosphere 2022, 287, 132118. [Google Scholar] [CrossRef]
- Odega, C.A.; Ayodele, O.O.; Ogutuga, S.O.; Anguruwa, G.T.; Adekunle, A.E.; Fakorede, C.O. Potential application and regeneration of bamboo biochar for wastewater treatment: A review. Adv. Bamboo Sci. 2023, 2, 100012. [Google Scholar] [CrossRef]
- Yadav, S.; Pipil, H.; Haritash, A.K.; Reddy, K.R. Fe(III)-modified bamboo biochar for the removal of phosphate from synthetic and field stormwater runoff. Sustain. Water Resour. Manag. 2024, 10, 140. [Google Scholar] [CrossRef]
- Zeng, S.; Lan, X.; Liu, P.; Zhang, Z.; Cheng, X.; Xu, N.; Yin, H. Removal of Phosphate from Water by Iron/Calcium Oxide-Modified Biochar: Removal Mechanisms and Adsorption Modeling. Water 2024, 16, 3245. [Google Scholar] [CrossRef]
- Biswas, B.; Adhikari, S.; Jahromi, H.; Ammar, M.; Baltrusaitis, J.; Torbert, A.; Linhoss, J.; Lamba, J. Magnesium doped biochar for simultaneous adsorption of phosphate and nitrogen ions from aqueous solution. Chemosphere 2024, 358, 142130. [Google Scholar] [CrossRef]
- Humayro, A.; Harada, H.; Naito, K.; Humayro, A.; Harada, H.; Naito, K. Adsorption of Phosphate and Nitrate Using Modified Spent Coffee Ground and Its Application as an Alternative Nutrient Source for Plant Growth. J. Agric. Chem. Environ. 2020, 10, 80–90. [Google Scholar] [CrossRef]
- Muratova, S.A.; Papikhin, R.V.; Khoroshkova, Y.V. The effect of caffeine in a nutrient medium on rhizogenesis of the Rubus genus plants. BIO Web Conf. 2020, 23, 03013. [Google Scholar] [CrossRef]
- Cabral, F.; Ribeiro, H.M.; Hilário, L.; Machado, L.; Vasconcelos, E. Use of pulp mill inorganic wastes as alternative liming materials. Bioresour. Technol. 2008, 99, 8294–8298. [Google Scholar] [CrossRef][Green Version]
- Rasmus, J.; Adesanya, E.; Silva Santos, H.; Kilpimaa, K.; Illikainen, M. Effects of thermal treatment on the characteristics of pulp mill residue. J. Environ. Manag. 2024, 351, 119793. [Google Scholar] [CrossRef]
- JIS Z 8801; Japansese Industrial Standard—Test Sieves. Japanese Standard Association: Tokyo, Japan, 2020.
- ASTM E11-24; Standard Specification for Woven Wire Test Sieve Cloth and Test Sieves. ASTM International: West Conshohocken, PA, USA, 2024.
- Hidayat, E.; Sarbani, N.M.M.; Samitsu, S.; Nugroho, F.A.A.; Lahiri, S.K.; Aoyagi, M.; Yonemura, S.; Harada, H. Evaluation of slow-release fertilizers derived from hydrogel beads: Sodium alginate-poly (acrylic acid) and humic acid-encapsulated struvite for soil salinity amelioration. Arab. J. Chem. 2024, 17, 105877. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Standard operating procedure for soil available phosphorus-Olsen method. In Food and Agriculture Organization of the United Nations; Food and Agriculture Organization (FAO): Rome, Italy, 2021. [Google Scholar]
- Yin, Q.; Wang, R.; Zhao, Z. Application of Mg–Al-modified biochar for simultaneous removal of ammonium, nitrate, and phosphate from eutrophic water. J. Clean. Prod. 2018, 176, 230–240. [Google Scholar] [CrossRef]
- Li, A.; Ge, W.; Liu, L.; Qiu, G. Preparation, adsorption performance and mechanism of MgO-loaded biochar in wastewater treatment: A review. Environ. Res. 2022, 212, 113341. [Google Scholar] [CrossRef]
- Rahman, S.; Navarathna, C.M.; Krishna Das, N.; Alchouron, J.; Reneau, P.; Stokes, S.; Thirumalai, V.K.G.R.; Perez, F.; Barbary Hassan, E.; Mohan, D.; et al. High capacity aqueous phosphate reclamation using Fe/Mg-layered double hydroxide (LDH) dispersed on biochar. J. Colloid Interface Sci. 2021, 597, 182–195. [Google Scholar] [CrossRef]
- Sadiq, A.; Choubey, A.; Bajpai, A.K. Biosorption of chromium ions by calcium alginate nanoparticles. J. Chil. Chem. Soc. 2018, 63, 4077–4081. [Google Scholar] [CrossRef]
- Faried, M.; Shameli, K.; Miyake, M.; Zakaria, Z.; Hara, H.; Khairudin, N.A.; Etemadi, M. Ultrasound-assisted in the synthesis of silver nanoparticles using sodium alginate mediated by green method. Dig. J. Nanomater. Biostruct. 2016, 11, 547–552. [Google Scholar]
- Behnami, A.; Croué, J.-P.; Aghayani, E.; Pourakbar, M. A catalytic ozonation process using MgO/persulfate for degradation of cyanide in industrial wastewater: Mechanistic interpretation, kinetics and by-products. Rsc Adv. 2021, 11, 36965–36977. [Google Scholar] [CrossRef]
- Tu, P.; Zhang, G.; Cen, Y.; Huang, B.; Li, J.; Li, Y.; Deng, L.; Yuan, H. Enhanced phosphate adsorption and desorption characteristics of MgO-modified biochars prepared via direct co-pyrolysis of MgO and raw materials. Bioresour. Bioprocess. 2023, 10, 49. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, B.; Feng, Q.; Chen, M.; Zhang, X.; Zhao, R. Recovery of nitrogen and phosphorus in wastewater by red mud-modified biochar and its potential application. Sci. Total Environ. 2023, 860, 160289. [Google Scholar] [CrossRef]
- Deng, Y.; Li, M.; Zhang, Z.; Liu, Q.; Jiang, K.; Tian, J.; Zhang, Y.; Ni, F. Comparative study on characteristics and mechanism of phosphate adsorption on Mg/Al modified biochar. J. Environ. Chem. Eng. 2021, 9, 105079. [Google Scholar] [CrossRef]
- Bezza, F.A.; Brink, H.G.; Chirwa, E.M.N. Selective and efficient removal of phosphate from aqueous solution using activated carbon-supported Mg–Fe layered double oxide nanocomposites. Can. J. Chem. Eng. 2024, 103, 524–542. [Google Scholar] [CrossRef]
- Zhu, D.; Chen, Y.; Yang, H.; Wang, S.; Wang, X.; Zhang, S.; Chen, H. Synthesis and characterization of magnesium oxide nanoparticle-containing biochar composites for efficient phosphorus removal from aqueous solution. Chemosphere 2020, 247, 125847. [Google Scholar] [CrossRef]
- Wan, C.; Ding, S.; Zhang, C.; Tan, X.; Zou, W.; Liu, X.; Yang, X. Simultaneous recovery of nitrogen and phosphorus from sludge fermentation liquid by zeolite adsorption: Mechanism and application. Sep. Purif. Technol. 2017, 180, 1–12. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zhou, H.D.; Wang, Q.; Xu, B.X.; Zhu, G. Efficiency and mechanism of phosphate adsorption and desorption of a novel Mg-loaded biochar material. Environ. Sci. Pollut. Res. Int. 2024, 31, 4425–4438. [Google Scholar] [CrossRef]
- Civan Çavuşoğlu, F.; Özçelik, G.; Bayazit, Ş.S. Comparative Investigation of Phosphate Adsorption Efficiencies of MOF-76 (Ce) and Metal Oxides Derived from MOF-76 (Ce). Langmuir 2024, 40, 4255–4266. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Li, A.Y.; Deng, H.; Ye, C.H.; Wu, Y.Q.; Linmu, Y.D.; Hang, H.L. Characteristics of nitrogen and phosphorus adsorption by Mg-loaded biochar from different feedstocks. Bioresour. Technol. 2019, 276, 183–189. [Google Scholar] [CrossRef]
- Alagha, O.; Manzar, M.S.; Zubair, M.; Anil, I.; Mu’azu, N.D.; Qureshi, A. Comparative Adsorptive Removal of Phosphate and Nitrate from Wastewater Using Biochar-MgAl LDH Nanocomposites: Coexisting Anions Effect and Mechanistic Studies. Nanomaterials 2020, 10, 336. [Google Scholar] [CrossRef]
- Khalil, A.K.A.; Dweiri, F.; Almanassra, I.W.; Chatla, A.; Atieh, M.A. Mg-Al Layered Double Hydroxide Doped Activated Carbon Composites for Phosphate Removal from Synthetic Water: Adsorption and Thermodynamics Studies. Sustainability 2022, 14, 6991. [Google Scholar] [CrossRef]
- Sepúlveda-Cadavid, C.; Romero, J.H.; Torres, M.; Becerra-Agudelo, E.; López, J.E. Evaluation of a biochar-based slow-release P fertilizer to improve Spinacia oleracea P use, yield, and nutritional quality. J. Soil Sci. Plant Nutr. 2021, 21, 2980–2992. [Google Scholar] [CrossRef]
- Luo, W.; Qian, L.; Liu, W.; Zhang, X.; Wang, Q.; Jiang, H.; Cheng, B.; Ma, H.; Wu, Z. A potential Mg-enriched biochar fertilizer: Excellent slow-release performance and release mechanism of nutrients. Sci. Total Environ. 2021, 768, 144454. [Google Scholar] [CrossRef]

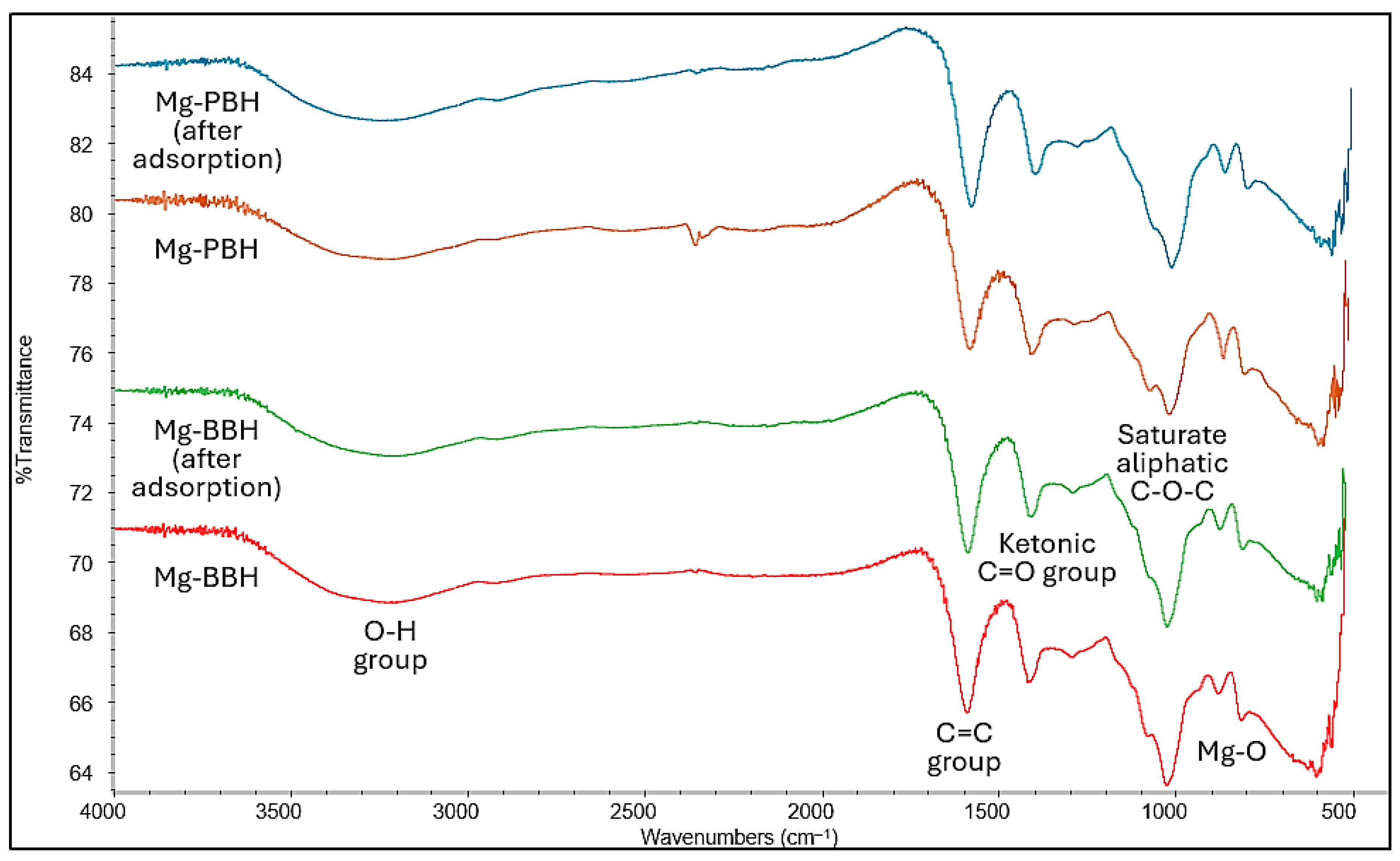



| Elements | Mg-Modified Bamboo Biochar (mg/100 g) | Mg-Containing Pulp Biochar (mg/100 g) |
|---|---|---|
| Nitrogen | 0.4 ± 0.06 | 0.1 ± 0.08 |
| Phosphoric acid | 378.9 ± 8.3 | <0.1 |
| Potassium | 168.2 ± 6.6 | 90.1 ± 7.2 |
| Calcium | 20.4 ± 2.9 | 152.7 ± 4.9 |
| Magnesium | >140.0 | 82.8 ± 8.9 |
| Isotherm Models | Parameters | Mg-BBH | Mg-PBH |
|---|---|---|---|
| Langmuir | 563.1725 | 193.703 | |
| 0.0103 | 0.0018 | ||
| 0.4921 | 0.8470 | ||
| R2 | 0.2918 | 0.2014 | |
| Freundlich | 1.9935 | 0.026 | |
| 1/ | 1.4438 | 1.6094 | |
| R2 | 0.8528 | 0.7344 | |
| Temkin | 341.52 | 218.1893 | |
| 0.0812 | 0.0106 | ||
| R2 | 0.9131 | 0.937 |
| Kinetic Models | Parameter | 20 °C (293 K) | 30 °C (303 K) | 40 °C (313 K) | |||
|---|---|---|---|---|---|---|---|
| Mg-BBH | Mg-PBH | Mg-BBH | Mg-PBH | Mg-BBH | Mg-PBH | ||
| Pseudo-first-order | 185.6987 | 39.3709 | 67.2336 | 193.4196 | 84.8002 | 215.8872 | |
| 0.2295 | 0.1672 | 0.0623 | 0.2345 | 0.0877 | 0.2819 | ||
| R2 | 0.8454 | 0.6241 | 0.237 | 0.8266 | 0.4582 | 0.8415 | |
| Pseudo-second- order | 173.9835 | 152.9544 | 302.0623 | 226.2074 | 313.1222 | 236.8047 | |
| 0.0009 | 0.0101 | 0.0302 | 0.0024 | 0.0091 | 0.0025 | ||
| R2 | 0.64 | 0.9981 | 0.9998 | 0.9315 | 0.9994 | 0.9604 | |
| Adsorbents | Temperature (K) | ΔG° (kJ mol−1) | ΔH° (kJ/mol) | ΔS° (J/mol K) | |
|---|---|---|---|---|---|
| 293 | 0.3750 | 2.3893 | 89.7663 | 300.5511 | |
| Mg-BBH | 303 | 3.000 | −2.7676 | ||
| 313 | 3.8750 | −3.5249 | |||
| 293 | 0.3602 | 2.4873 | 39.9330 | 128.8919 | |
| Mg-PBH | 303 | 0.9249 | 0.1966 | ||
| 313 | 1.0176 | −0.0454 |
| Items | Unit Cost (JPY) | Mg-BBH | Mg-PBH | ||
|---|---|---|---|---|---|
| Amount Used | Cost (JPY) | Amount | Cost (JPY) | ||
| Bamboo | 0 | 100 g | 0 | – | – |
| Pulp residue | 0 | – | – | 100 g | 0 |
| MgCl26H2O | 1900/500 g | 102 g | 387.6 | – | – |
| CaCl2 | 3300/500 g | 45 g | 297.0 | 45 g | 297.0 |
| Sodium alginate | 2900/300 g | 50 g | 483.3 | 50 g | 483.3 |
| Heating cost | 18.84 JPY/kWh | 6.0 kWh (1 kW, 6 h) | 113.04 | 6.0 kWh (1 kW, 6 h) | 113.04 |
| Drying cost | 18.84 JPY/kWh | 12 kWh (1 kW, 12 h) | 226.08 | 12 kWh (1 kW, 12 h) | 226.08 |
| Total cost (JPY) | – | – | 1507.02 | – | 1119.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamad Sarbani, N.M.; Harada, H.; Aoyagi, M.; Hidayat, E. Dual-Functioned Magnesium-Enriched Biochar Hydrogels for Phosphate Recovery and Slow-Release Nutrient Delivery. Water 2025, 17, 2235. https://doi.org/10.3390/w17152235
Mohamad Sarbani NM, Harada H, Aoyagi M, Hidayat E. Dual-Functioned Magnesium-Enriched Biochar Hydrogels for Phosphate Recovery and Slow-Release Nutrient Delivery. Water. 2025; 17(15):2235. https://doi.org/10.3390/w17152235
Chicago/Turabian StyleMohamad Sarbani, Nur Maisarah, Hiroyuki Harada, Mitsuru Aoyagi, and Endar Hidayat. 2025. "Dual-Functioned Magnesium-Enriched Biochar Hydrogels for Phosphate Recovery and Slow-Release Nutrient Delivery" Water 17, no. 15: 2235. https://doi.org/10.3390/w17152235
APA StyleMohamad Sarbani, N. M., Harada, H., Aoyagi, M., & Hidayat, E. (2025). Dual-Functioned Magnesium-Enriched Biochar Hydrogels for Phosphate Recovery and Slow-Release Nutrient Delivery. Water, 17(15), 2235. https://doi.org/10.3390/w17152235







