Comparative Study of the Microalgae-Based Wastewater Treatment, in an Oil Refining Industry Cogeneration Concept
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgae Selection and Maintenance
2.2. Batch Growth in Photobioreactors (PBR)
2.3. Analytical Procedures
2.3.1. Biomass Analyses
2.3.2. C:H: N Elemental Analysis
2.3.3. Lipid Content
2.3.4. Inorganic N and P Nutrients Analysis
2.4. Toxicity Assessment
2.5. Growth Analysis and Estimation of Volumetric Productivity in Continuous Operation
2.6. Sustainability Assessment of the Cogeneration Production of Microalgal Biodiesel in Oil Refinery Concept
3. Results and Discussion
3.1. Nutrient Uptake and Intracellular Carbon and Nitrogen Composition
3.2. Algae Growth Dynamics and Lipid Production
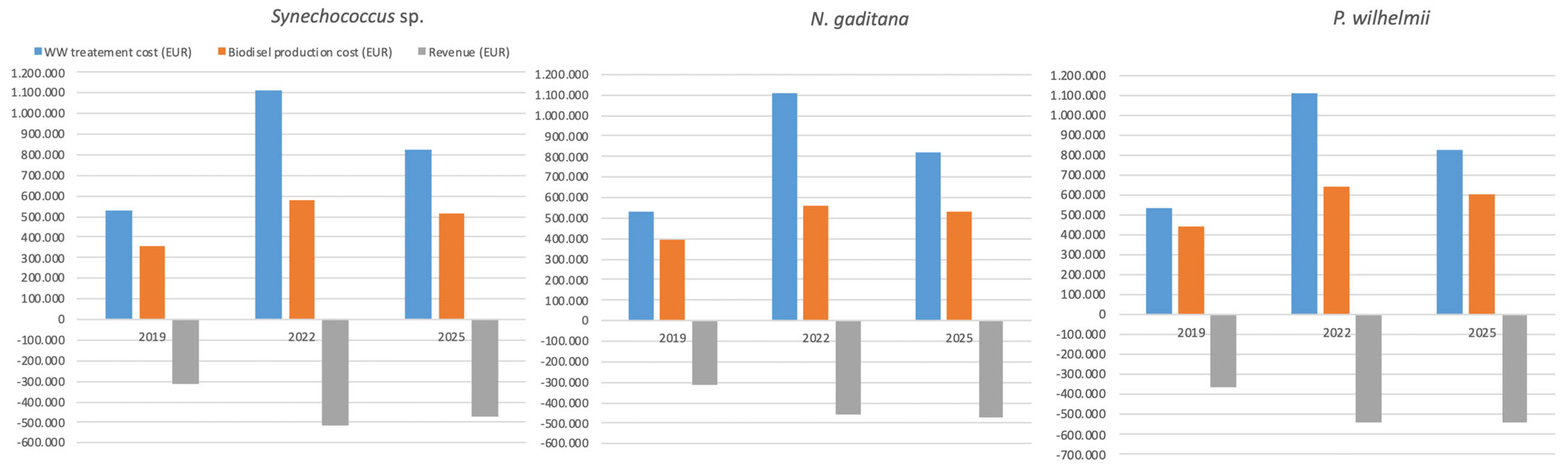
3.3. Sustainability of Upscaled Cogeneration Concept
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations and Symbols
| Parameters | Unit | Definition |
| Xo | mg/L | Initial cell concentration |
| Xm | mg/L | Maximum concentration that the system can achieve in batch |
| µ | day−1 | Maximum specific growth rate |
| Xin | mg/L | Concentration of inflow in the reactor |
| Xout | mg/L | Concentration of biomass as output from the reactor |
| dX/dt | mg/(Lh) | Change in biomass |
| V | Reactor volume | |
| D | Dilution rate | |
| P | Volumetric productivity | |
| Day | Hydraulic retention time (HRT) in the reactor | |
| Θp | HRT at maximum productivity | |
| Q | L/day | Flow rate |
| Qp | L/day | Flow rate at maximum productivity |
| Xp | mg/L | Concentration at maximum productivity |
References
- Slade, R.; Bauen, A. Micro-algae cultivation for biofuels: Cost, energy balance, environmental impacts and future prospects. Biomass Bioenergy 2013, 53, 29–38. [Google Scholar] [CrossRef]
- Serrano-Blanco, S.; Zan, R.; Harvey, A.P.; Velasquez-Orta, S.B. Intensified microalgae production and development of microbial communities on suspended carriers and municipal wastewater. JEM 2024, 370, 122717. [Google Scholar] [CrossRef]
- Clagnan, E.; D'Imporzano, G.; Dell'Orto, M.; Sanchez-Zurano, A.; Acién-Fernandez, F.G.; Pietrangeli, B.; Adani, F. Profiling microalgal cultures growing on municipal wastewater and fertilizer media in raceway photobioreactors. Biores. Technol. 2022, 360, 127619. [Google Scholar] [CrossRef]
- Moreno-Cruz, C.F.; Tzintzun-Camacho, O.; Gonzalez-Joaquin, M.C.; Aguilar-Martinez, X.E.; Martinez-Quiroz, M. Livestock wastewater as a microalgae growth medium for potential production of biodiesel in arid areas of Mexico. Algal Res. 2025, 86, 103957. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Wang, G.; Zhou, Z.; Hao, S.; Wang, L. Microalgae cultivation using unsterilized cattle farm wastewater filtered through corn stover. Bioresour. Technol. 2022, 352, 127081. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, S.; Han, Y.; Wolfe, F.A. Treatment of petrochemical wastewater and produced water from oil and gas. WER 2019, 352, 1025–1033. [Google Scholar] [CrossRef]
- Al-Jabri, H.; Das, P.; Khan, S.; Thaher, M.; Abdul, M. Treatment of Wastewaters by Microalge and the Potential Applications of the Produced Biomass—A review. Water 2021, 13, 27. [Google Scholar] [CrossRef]
- Siddique, M.N.I.; Munaim, M.S.A.; Wahid, Z.B.A. The combined effect of ultrasonic and microwave pre-treatment on bio-methane generation from codigestion of petrochemical wastewater. J. Clean. Prod. 2017, 145, 303–309. [Google Scholar] [CrossRef]
- Chaudhry, S. Integrating Microalgae Cultivation with Wastewater Treatment: A Peek into Economics. App. Biochem. Biotechn. 2021, 193, 3395–3406. [Google Scholar] [CrossRef]
- Li, K.; Liu, Q.; Fang, F.; Luo, R.; Lu, Q.; Zhou, W.; Huo, S.; Cheng, P.; Liu, J.; Addy, M.; et al. Microalgae-based wastewater treatment for nutrients recovery: A review. Bioresour. Technol. 2019, 291, 121934. [Google Scholar] [CrossRef]
- Blažina, B.; Haberle, I.; Hrustić, E.; Budiša, A.; Petrić, I.; Konjević, L.; Šilović, T.; Djakovac, T.; Geček, S. Growth aspects and biochemical composition of Synechococcus sp. MK568070 cultured in oil refinery wastewater. J. Mar. Sci. Eng. 2019, 7, 164. [Google Scholar] [CrossRef]
- Singh, P.; Borthakur, A. A review on biodegradation and photocatalytic degradation of organic pollutants: A bibliometric and comparative analysis. J. Clean. Prod. 2018, 196, 1669–1680. [Google Scholar] [CrossRef]
- Prabakar, D.; Suvetha, S.K.; Manimudi, V.T.; Mathimani, T.; Kumar, G.; Rene, E.R.; Pugazhendhi, A. Pretreatment technologies for industrial effluents: Critical Review on bioenergy production and environmental concerns. JEM 2018, 218, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Liberti, D.; Pinheriro, F.; Simōes, B.; Varela, J.; Barreira, L. Beyond Bioremediation: The Untapped Potential of Microalgae in Wastewater Treatment. Water 2024, 16, 2710. [Google Scholar] [CrossRef]
- Sudmalis, D.; DaSilva, P.; Temmink, H.; Bijmans, M.M.; Perreira, M.A. Biological treatment of produced water coupled with recovery of neutral lipids. Water Res. 2018, 147, 33–42. [Google Scholar] [CrossRef]
- Greque de Morais, E.; Fontes Sampaio, I.C.; Gonzalez-Flo, E.; Ferrer, I.; Uggetti, I.E.; García, J. Microalgae harvesting for wastewater treatment and resources recovery: A review. N. Biotechnol. 2023, 78, 84–94. [Google Scholar] [CrossRef]
- Gouveia, L.; Graça, S.; Sousa, C.; Ambrosano, L.; Ribeiro, B.; Botrel, E.P.; Neto, P.C.; Ferreira, A.F.; Silva, C.M. Microalgae biomass production using wastewater: Treatment and costs: Scale-up considerations. Algal Res. 2016, 16, 167–176. [Google Scholar] [CrossRef]
- Acién Fernández, F.G.; Gómez-Serrano, C.; Fernández-Sevilla, J.M. Recovery of nutrients from wastewaters using microalgae. Front.Sustain. Food Sys. 2018, 2, 59. [Google Scholar] [CrossRef]
- Molazadeh, M.; Ahmadzadeh, H.; Pourianfar, H.R.; Lyon, S.; Rampelotto, P.H. The use of microalgae for coupling wastewater treatment with CO2; biofixation. Front. Bioeng. Biotechnol. 2019, 7, 42. [Google Scholar] [CrossRef]
- Budiša, A.; Haberle, I.; Konjević, L.; Blažina, M.; Djakovac, T.; Lukarić-Špalj, B.; Hrustić, E. Marine microalgae Nannochloropsis gaditana and Pseudochloris wilhelmii cultivated in oil refinery wastewater—Perspective on remediation and biodiesel production. Fresenius Environ. Bull. 2019, 28, 7888–7897. [Google Scholar]
- Blažina, M.; Fafanđel, M.; Geček, S.; Haberle, I.; Klanjšček, J.; Hrustić, E.; Husinec, L.; Žilić, L.; Pritišanac, E.; Klanjšček, T. Characterization of Pseudochloris wilhelmii potential for oil refinery wastewater remediation and valuable biomass cogeneration. Front.Mar. Sci. 2022, 9, 983395. [Google Scholar] [CrossRef]
- Shoener, B.D.; Schramm, S.M.; Béline, F.; Bernard, O.; Martínez, C.; Plósz, B.G.; Snowling, S.; Steyer, J.P.; Valverde-Pérez, B.; Wágner, D.; et al. Microalgae and cyanobacteria modeling in water resource recovery facilities: A critical review. Water Res. X 2019, 2, 100024. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhao, W.; Sun, H.; Mou, J.; Liu, H.; Yu, L.; Dai, Q.; Kong, S. Yang. Phycocyanin from microalgae: A comprehensive review covering microalgal culture, phycocyanin sources and stability. Int. Food Res. 2024, 186, 114362. [Google Scholar] [CrossRef] [PubMed]
- Pancha, I.; Chokshi, K.; Mishra, S. Industrial wastewater-based microalgal biorefinery: A dual strategy to remediate waste and produce microalgal bioproducts. In Application of Microalgae in Wastewater Treatment; Kumar Gupta, S., Bux, F., Eds.; Springer: Cham, Switzerland, 2019; Volume 2, pp. 173–193. [Google Scholar] [CrossRef]
- Das, P.K.; Rani, J.; Rawat, S.; Kumar, S. Microalgal co-cultivation for biofuel production and bioremediation: Current status and benefits. Bioenergy Res. 2022, 15, 1–26. [Google Scholar] [CrossRef]
- Jacob-Lopes, E.; Manzoni, M.; Maroneze, M.; Costa Deprá, R.B.; Sartori, R.R.; Dias, L.; Zepka, Q. Bioactive food compounds from microalgae: An innovative framework on industrial biorefineries. Curr. Opin. Food Sci. 2019, 25, 1–7. [Google Scholar] [CrossRef]
- Iglewicz, B.; Hoaglin, D.C. The ASQC Basic References in Quality Control: Statistical Techniques, In How to Detect and Handle Outliers; Mykytka, E.F., Ed.; ASQC Quality Press: Milwaukee, WI, USA, 1993; Volume 16, pp. 1–87. [Google Scholar]
- APHA; AWWA; WEF. Physical and Aggregate Properties; Approved by Standard Methods Committee, 1997; APHA/AWWA/WEF: Washington, DC, USA, 1992. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Chemical and Biological Methods for Seawater. Analysis; Pergamon Press: Elmsford, NY, USA, 1984; pp. 149–153. [Google Scholar]
- Ivančić, I.; Degobbis, D. An optimal manual procedure for ammonia analysis in natural waters by the indophenol blue method. Water Res. 1989, 18, 1143–1147. [Google Scholar] [CrossRef]
- Bihari, N.; Fafanđel, M.; Piskur, V. Polycyclic aromatic hydrocarbons and ecotoxicological characterization of seawater, sediment, and mussel Mytilus galloprovincialis from the Gulf of Rijeka, the Adriatic Sea, Croatia. Arch. Environ. Contamin. Toxicol. 2007, 52, 379–387. [Google Scholar] [CrossRef]
- Ruiz, J.; Álvarez-Díaz, P.D.; Arbib, Z.; Garrido-Pérez, G.; Barragán, J.; Perales, J.A. Performance of a flat panel reactor in the continuous culture of microalgae in urban wastewater: Prediction from a batch experiment. Biores.Technol. 2013, 127, 456–463. [Google Scholar] [CrossRef]
- Verhulst, P.F. Notice on the Law That Population Will Pursue in Its Growth. Corresp. Math. Phys. 1838, 10, 113–121. [Google Scholar]
- Sompech, K.; Chisti, Y.; Srinkophakun, T. Design of raceway ponds for producing microalgae. Biofules 2012, 3, 387–397. [Google Scholar] [CrossRef]
- Available online: https://tradingeconomics.com/commodity/carbon (accessed on 10 July 2025).
- Umar, Z.; Gubareva, M.; Teplova, T. The imapact of COVID-19 on commodity markets volatility: Analyzing time-frequency relations between commodity prices and coronavirus panic levels. Resour. Policy 2021, 73, 102164. [Google Scholar] [CrossRef]
- Gharib, C.; Wali-Mefteh, S.; Serret, V.; Jabeur, S.B. Impact of COVID-19 pandemic on crude oil prices: Evidence from Economphysics approach. Resour. Policy 2021, 4, 102392. [Google Scholar] [CrossRef] [PubMed]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- European Commission. EU Emissions Trading System. Climate Action; European Commission: Brussels, Belgium.
- Harris, S.; Tsalidis, G.; Corbera, B.J.; Gallart, J.J.E.; Tegstedt, F. Application of LCA and LCC in the early stages of wastewater treatment design: A multiple case study of brine effluents. J. Clean. Prod. 2021, 307, 127298. [Google Scholar] [CrossRef]
- Vásquez-Romero, B.; Perales, J.A.; Pereira, H.; Barbosa, M.; Ruiz, J. Techno-economic assessment of microalgae production, harvesting and drying for food, feed, cosmetics, and agriculture. Sci. Total. Environ. 2022, 837, 155742. [Google Scholar] [CrossRef]
- Takeshita, T. Competitiveness, role, and impact of microalgal biodiesel in the global energy future. Appl. Energy 2011, 88, 3481–3491. [Google Scholar] [CrossRef]
- Omta, A.W.; Talmy, D.; Sher, D.; Finkel, Z.V.; Irwin, A.J.; Follows, M.J. Extracting phytoplankton physiological traits from batch and chemostat culture data. Limnol. Oceanogr. Methods 2017, 15, 453–466. [Google Scholar] [CrossRef]
- Inomura, K.; Omta, A.W.; Talmy, D.; Bragg, J.; Deutsch, C.J.; Follows, M.J. A mechanistic model of macromolecular allocation, elemental stoichiometry, and growth rate in phytoplankton. Front. Microbiol. 2020, 11, 86. [Google Scholar] [CrossRef]
- Droop, M.R. Heterotrophy of carbon. In Algal Physiology and Biochemistry; Stewart, W.D.P., Ed.; University of California Press: Berkley, CA, USA, 1974; pp. 530–559. [Google Scholar]
- Pang, M.; Liu, K.; Liu, H. Evidence for mixotrophy in Picochlorophytes from a new Pseudochloris (Trebouxiophyceae) strain. J. Phycol. 2022, 58, 80–91. [Google Scholar] [CrossRef]
- Goswami, R.K.; Mehariya, S.; Karthikeyan, O.P.; Verma, P. Influence of Carbon Sources on Biomass and Biomolecule Accumulation in Pseudochloris sp. Cultured under the Mixotrophic Condition. Int. J. Environ. Res. Public Health 2022, 19, 3674. [Google Scholar] [CrossRef]
- Hasan, R.; Kasera, N.; Beck, A.E.; Hall, S.G. Potential of Synechococcus elongatus UTEX 2973 as a feedstock for sugar production during mixed aquaculture and swine wastewater bioremediation. Heliyon 2024, 10, e24646. [Google Scholar] [CrossRef] [PubMed]
- Haberle, I.; Hrustić, E.; Petrić, I.; Pritišanac, E.; Šilović, T.; Magić, L.; Geček, S.; Budiša, A.; Blažina, M. Adriatic cyanobacteria potential for cogeneration biofuel production with oil refinery wastewater remediation. Algal Res. 2020, 50, 101978. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Madamwar, D.; Incharoesakdi, A. Bloom Dynamics of Cyanobacteria and Their Toxins: Environmental Health Impacts and Mitigation Strategies. Front. Microbiol. 2015, 6, 1245. [Google Scholar] [CrossRef] [PubMed]
- Arbib, Z.; Alvarez, J.P.; Garrido, C.; Barragan, J.; Perales, J.A. Chlorella stigmatophora for urban wastewater nutrient removal and CO2 abatement. Int. J. Phytoremediation. 2012, 14, 714–725. [Google Scholar] [CrossRef]
- Oostlander, P.C.; van Houcke, J.; Wijffels, R.H.; Barbosa, M.J. Microalge production cost in aquaculture hatcheries. Aquaculture 2020, 525, 735310. [Google Scholar] [CrossRef]
- Eurostat. Electricity Price Statistics; EU: Brussels, Belgium.
- Bora, A.; Rajan, A.S.T.; Ponnuchamy, K.; Muthusamy, G.; Alagarsamy, A. Microalgae to bioenergy production: Recent advances, influencing parameters, utilization of wastewater—A critical review. Sci.Total Environ. 2024, 946, 174230. [Google Scholar] [CrossRef]
- Bagchi, S.K.; Patnaik, R.; Prasad, R. Feasibilty of Utilizing Wastwters for Large-Scale Microalgal Cultivation and Biofuel Productions Using Hydrothermal Liquefaction Technique: A Comprehensive Review. Front. Bioeng. Biotechnol. 2021, 9, 651138. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell. Fact. 2018, 17, 36. [Google Scholar] [CrossRef]
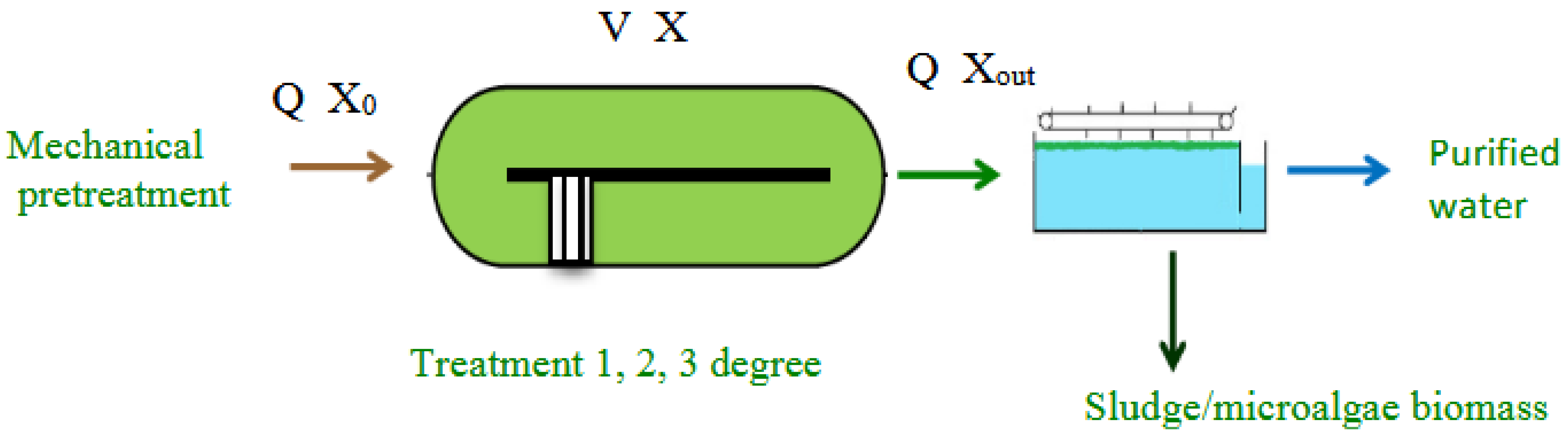

| Parameter | Unit | Range |
|---|---|---|
| pH | - | 7.48–10.62 |
| Hydrocarbon | mg/L | 5.4–152 |
| Mineral oil | mg/L | 3.3–81.3 |
| COD | mg/L O2 | 1–658 |
| NH4+ | mg/L | 0.5–124 |
| NO3− | mg/L | 15–61 |
| PO43− | mg/L | 0–1 |
| S2− | mg/L | 0–122 |
| Mercaptan | mg/kg | 0–64 |
| µ day−1 | Maximum Specific DIN Uptake Rate mmol/(gday) | Maximum Volumetric Productivity mg/(Lday) | HRT (day) | Final Lipid Content % d.w. | Lipid Productivity mg/(Lday) | Toxicity Reduction % | |
|---|---|---|---|---|---|---|---|
| P. wilhelmii | 0.432 | 0.895 | 93.9 | 2.28 | 28 | 26.30 | 76.5 |
| Synechococcus sp. | 0.336 | 0.531 | 71.0 | 3.02 | 21 | 14.91 | 12.4 |
| N. gaditana | 0.576 | 0.698 | 79.6 | 1.72 | 37 | 29.45 | 51.0 |
| Synechococcus sp. | Nannochloropsis gaditana | Pseudochloris wilhelmii | ||
|---|---|---|---|---|
| Volume of ORP | m3 | 30.241 | 17.161 | 22.785 |
| Surface of ORP | Ha | 10.08 | 5.72 | 7.59 |
| Biomass production | t/year | 129.62 | 145.23 | 171.38 |
| Θp | Day | 6.05 | 3.43 | 4.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pritišanac, E.; Fafanđel, M.; Haberle, I.; Geček, S.; Markić, M.; Bolf, N.; Vukadin, J.; Crnković, G.; Klanjšček, T.; Žilić, L.; et al. Comparative Study of the Microalgae-Based Wastewater Treatment, in an Oil Refining Industry Cogeneration Concept. Water 2025, 17, 2217. https://doi.org/10.3390/w17152217
Pritišanac E, Fafanđel M, Haberle I, Geček S, Markić M, Bolf N, Vukadin J, Crnković G, Klanjšček T, Žilić L, et al. Comparative Study of the Microalgae-Based Wastewater Treatment, in an Oil Refining Industry Cogeneration Concept. Water. 2025; 17(15):2217. https://doi.org/10.3390/w17152217
Chicago/Turabian StylePritišanac, Ena, Maja Fafanđel, Ines Haberle, Sunčana Geček, Marinko Markić, Nenad Bolf, Jela Vukadin, Goranka Crnković, Tin Klanjšček, Luka Žilić, and et al. 2025. "Comparative Study of the Microalgae-Based Wastewater Treatment, in an Oil Refining Industry Cogeneration Concept" Water 17, no. 15: 2217. https://doi.org/10.3390/w17152217
APA StylePritišanac, E., Fafanđel, M., Haberle, I., Geček, S., Markić, M., Bolf, N., Vukadin, J., Crnković, G., Klanjšček, T., Žilić, L., & Blažina, M. (2025). Comparative Study of the Microalgae-Based Wastewater Treatment, in an Oil Refining Industry Cogeneration Concept. Water, 17(15), 2217. https://doi.org/10.3390/w17152217







