Exploration of Phosphorus Release Characteristics in Sediments from the Plains River Network: Vertical Distribution and the Response of Phosphorus and Microorganisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Sample Collection
2.2. Experimental Methods
2.3. Methods of Analysis
2.4. Microbial Sequencing
2.5. Data and Statistical Analysis
3. Results and Discussion
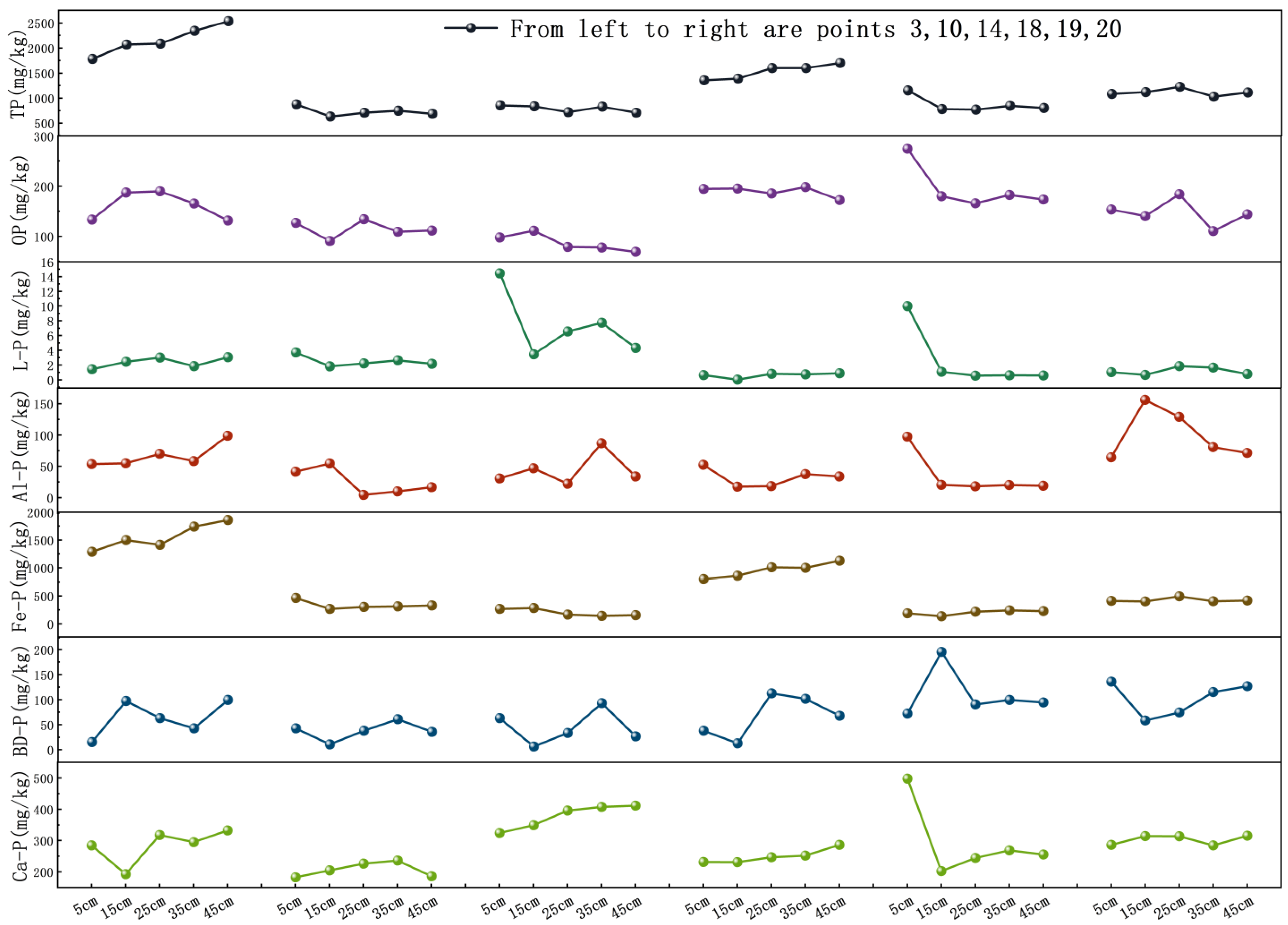
3.1. The Vertical Distribution Characteristics of Various Forms of Phosphorus in Sediments
3.1.1. Sediment Phosphorus Vertical Distribution
3.1.2. The Vertical Distribution Characteristics of Mobile Phosphorus in Sediments
3.2. Sediment Release Characteristics
3.2.1. Parameters of Phosphorus Adsorption in Sediments
3.2.2. Sediment “Source” and “Sink” Role Determination
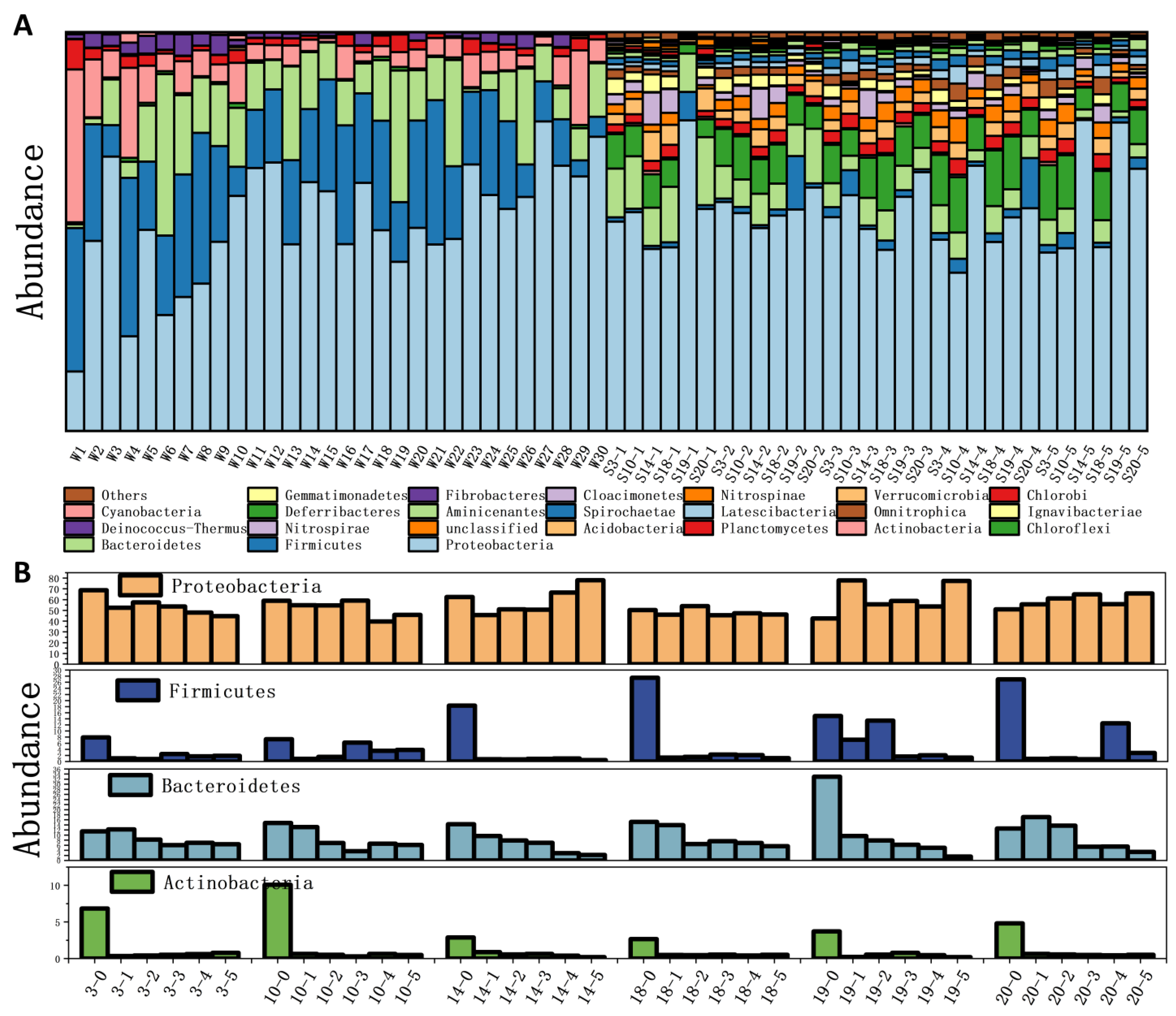
3.3. Vertical Distribution of Sediment Microorganisms
3.4. Response Between Sediment Microbes and Phosphorus
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lürling, M.; Oosterhout, F.V. Controlling eutrophication by combined bloom precipitation and sediment phosphorus inactivation. Water Res. 2013, 47, 6527–6537. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Chakraborty, S.; Das, G. A mechanistic insight into enhanced and selective phosphate adsorption on a coated carboxylated surface. J. Colloid Interface Sci. 2009, 331, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wang, S.; Wang, H.; Hu, X.; Yang, F.; Tang, M.; Zhang, M.; Zhong, J. Internal nitrogen and phosphorus loading in a seasonally stratified reservoir: Implications for eutrophication management of deep-water ecosystems. J. Environ. Manag. 2022, 319, 10. [Google Scholar] [CrossRef] [PubMed]
- Rydin, E. Potentially mobile phosphorus in Lake Erken sediment. Water Res. 2000, 34, 2037–2042. [Google Scholar] [CrossRef]
- Wang, S.; Hu, Z.; Zhang, J.; Wu, H.; Xie, H.; Liang, S.; Hu, H.; Jin, F. Formation of phosphine and its effect on phosphorus retention in constructed wetlands: Characteristic and mechanism. Environ. Technol. Innov. 2022, 28, 102653. [Google Scholar] [CrossRef]
- Liu, Z.-P.; Shao, M.-A.; Wang, Y.-Q. Spatial patterns of soil total nitrogen and soil total phosphorus across the entire Loess Plateau region of China. Geoderma 2013, 197–198, 67–78. [Google Scholar] [CrossRef]
- Shao, W.; Zhu, J.; Teng, Z.; Zhang, K.; Liu, S.; Li, M. Distribution of inorganic phosphorus and its response to the physicochemical characteristics of soil in Yeyahu Wetland, China. Process Saf. Environ. Prot. 2019, 125, 1–8. [Google Scholar] [CrossRef]
- Tu, C.; Jin, Z.; Che, F.; Cao, X.; Song, X.; Lu, C.; Huang, W. Characterization of phosphorus sorption and microbial community in lake sediments during overwinter and recruitment periods of cyanobacteria. Chemosphere 2022, 307, 11. [Google Scholar] [CrossRef]
- Enebe, M.C.; Babalola, O.O. The Influence of Soil Fertilization on the Distribution and Diversity of Phosphorus Cycling Genes and Microbes Community of Maize Rhizosphere Using Shotgun Metagenomics. Genes 2021, 12, 1022. [Google Scholar] [CrossRef]
- Pastore, G.; Kernchen, S.; Spohn, M. Microbial solubilization of silicon and phosphorus from bedrock in relation to abundance of phosphorus-solubilizing bacteria in temperate forest soils. Soil Biol. Biochem. 2020, 151, 108050. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Bergkemper, F.; Schöler, A.; Engel, M.; Lang, F.; Krüger, J.; Schloter, M.; Schulz, S. Phosphorus depletion in forest soils shapes bacterial communities towards phosphorus recycling systems (vol 18, pg 1988, 2016). Environ. Microbiol. 2016, 18, 2767. [Google Scholar] [CrossRef] [PubMed]
- Santalahti, M.; Sun, H.; Jumpponen, A.; Pennanen, T.; Heinonsalo, J.; Baldrian, P. Vertical and seasonal dynamics of fungal communities in boreal Scots pine forest soil. FEMS Microbiol. Ecol. 2016, 92, 12. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhou, X.; Lu, X.; Xu, Y.; Wei, Z.; Ruan, A. Grain size distribution drives microbial communities vertically assemble in nascent lake sediments. Environ. Res. 2023, 227, 10. [Google Scholar] [CrossRef]
- He, L.; Sun, X.; Li, S.; Zhou, W.; Chen, Z.; Bai, X. The vertical distribution and control factor of microbial biomass and bacterial community at macroecological scales. Sci. Total Environ. 2023, 869, 10. [Google Scholar] [CrossRef]
- Sun, T.; Wang, Y.; Lucas-Borja, M.E.; Jing, X.; Feng, W. Divergent vertical distributions of microbial biomass with soil depth among groups and land uses. J. Environ. Manag. 2021, 292, 9. [Google Scholar] [CrossRef]
- Pardo, P.; Rauret, G.; López-Sánchez, J.F. Shortened screening method for phosphorus fractionation in sediments. Anal. Chim. Acta 2004, 508, 201–206. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. Citation-Classic-A Modified Single Solution Method for The Determination of Phosphate in Natural-Waters. Curr. Contents Agric. Biol. Environ. Sci. 1986, 12, 16. [Google Scholar]
- Sundaram, C.S.; Viswanathan, N.; Meenakshi, S. Uptake of fluoride by nano-hydroxyapatite/chitosan, a bioinorganic composite. Bioresour. Technol. 2008, 99, 8226–8230. [Google Scholar] [CrossRef]
- Zhou, A.M.; Tang, H.X.; Wang, D.S. Phosphorus adsorption on natural sediments: Modeling and effects of pH and sediment composition. Water Res. 2005, 39, 1245–1254. [Google Scholar] [CrossRef]
- Ockenden, M.C.; Deasy, C.; Quinton, J.N.; Surridge, B.; Stoate, C. Keeping agricultural soil out of rivers: Evidence of sediment and nutrient accumulation within field wetlands in the UK. J. Environ. Manag. 2014, 135, 54–62. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, R.; Li, J. Study on influential factors of surface water quality using geographic detector- A case of Lvliang City, Shanxi Province. Acta Sci. Circumstantiae 2023, 43, 212–222. [Google Scholar]
- Li, D.-P.; Huang, Y.; Yuan, Y.; Fan, C.-X. Mechanism of phosphorus adsorption and immobility by sediments in innercity heavily polluted canal. Huan Jing Ke Xue Huanjing Kexue 2011, 32, 96–101. [Google Scholar]
- Ni, Z.; Li, Y.; Wang, S. Cognizing and characterizing the organic phosphorus in lake sediments: Advances and challenges. Water Res. 2022, 220, 118663. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Li, G.; Xia, R.; Li, C.; Zhu, Q.; Li, X.; Li, J.; Zhao, C.; Tian, Z.; Zhang, L. New insights into cyanobacterial blooms and the response of associated microbial communities in freshwater ecosystems. Environ. Pollut. 2022, 309, 11. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhang, M.; Yin, P.; Li, J. Characterization of internal phosphorus loading in the sediment of a large eutrophic lake (Lake Taihu, China). Water Res. 2022, 225, 119125. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhu, G.; Li, W.; Zhang, Y.; Zhao, L.; Gu, Z. Estimation of the algal-available phosphorus pool in sediments of a large, shallow eutrophic lake (Taihu, China) using profiled SMT fractional analysis. Environ. Pollut. 2013, 173, 216–223. [Google Scholar] [CrossRef]
- Wang, Y.T.; Zhang, T.Q.; Zhao, Y.C.; Ciborowski, J.J.; Zhao, Y.M.; O’Halloran, I.P.; Qi, Z.M.; Tan, C.S. Characterization of sedimentary phosphorus in Lake Erie and on-site quantification of internal phosphorus loading. Water Res. 2021, 188, 116525. [Google Scholar] [CrossRef]
- Hupfer, M.; Jordan, S.; Herzog, C.; Ebeling, C.; Ladwig, R.; Rothe, M.; Lewandowski, J. Chironomid larvae enhance phosphorus burial in lake sediments: Insights from long-term and short-term experiments. Sci. Total Environ. 2019, 663, 254–264. [Google Scholar] [CrossRef]
- Li, L.; Liu, L.; Wang, S.; Liu, W.; Jiao, L.; Yang, Y.; Yang, R. Spatial distribution of phosphorus fractions in sediment and the potential mobility of phosphorus in Dianchi Lake. Environ. Earth Sci. 2015, 74, 3721–3731. [Google Scholar] [CrossRef]
- Yang, C.; Li, J.; Yin, H. Phosphorus internal loading and sediment diagenesis in a large eutrophic lake (Lake Chaohu, China). Environ. Pollut. 2022, 292, 118471. [Google Scholar] [CrossRef]
- Wang, X.; Wei, J.; Bai, N.; Cha, H.; Cao, C.; Zheng, K.; Liu, Y. The phosphorus fractions and adsorption-desorption characteristics in the Wuliangsuhai Lake, China. Environ. Sci. Pollut. Res. 2018, 25, 20648–20661. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Dong, X.; Tu, C.; Yang, H.; Chang, Y.; Yang, X.; Chen, H.; Che, F. Response mechanism of sediment endogenous phosphorus release to functional microorganisms and its cyanobacterial growth and disappearance effects. Sci. Total Environ. 2024, 906, 167676. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Yao, Q.; Yu, Z. Particulate phosphorus speciation and phosphate adsorption characteristics associated with sediment grain size. Ecol. Eng. 2014, 70, 140–145. [Google Scholar] [CrossRef]
- Tian, J.R.; Zhou, P.J. Phosphorus fractions of floodplain sediments and phosphorus exchange on the sediment-water interface in the lower reaches of the Han River in China. Ecol. Eng. 2007, 30, 264–270. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Huang, X.L. Effect of Temperature and Salinity on Phosphate Sorption on Marine Sediments. Environ. Sci. Technol. 2011, 45, 6831. [Google Scholar] [CrossRef]
- Milowska, K.; Szwed, A.; Zablocka, M.; Caminade, A.M.; Majoral, J.P.; Mignani, S.; Gabryelak, T.; Bryszewska, M. In vitro PAMAM, phosphorus and viologen-phosphorus dendrimers prevent rotenone-induced cell damage. Int. J. Pharm. 2014, 474, 42–49. [Google Scholar] [CrossRef]
- Chaudhry, V.; Rehman, A.; Mishra, A.; Chauhan, P.S.; Nautiyal, C.S. Changes in Bacterial Community Structure of Agricultural Land Due to Long-Term Organic and Chemical Amendments. Microb. Ecol. 2012, 64, 450–460. [Google Scholar] [CrossRef]
- Zhou, J.; Li, D.; Zhao, Z.; Huang, Y. Phosphorus bioavailability and the diversity of microbial community in sediment in response to modified calcium peroxide ceramsite capping. Environ. Res. 2021, 195, 9. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an Ecological Classification of Soil Bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Hamdali, H.; Hafidi, M.; Virolle, M.J.; Ouhdouch, Y. Rock phosphate-solubilizing Actinomycetes: Screening for plant growth-promoting activities. World J. Microbiol. Biotechnol. 2008, 24, 2565–2575. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Gao, Q.; Dong, S.; Shi, S. Deep-sea cage culture altered microbial community composition in the sediments of the Yellow Sea Cold Water Mass. Mar. Pollut. Bull. 2022, 183, 10. [Google Scholar] [CrossRef] [PubMed]
- Flieder, M.; Buongiorno, J.; Herbold, C.W.; Hausmann, B.; Rattei, T.; Lloyd, K.G.; Loy, A.; Wasmund, K. Novel taxa of Acidobacteriota implicated in seafloor sulfur cycling. ISME J. 2021, 15, 3159–3180. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, G.; Robinson, D.; Yang, Z.; Guo, J.; Xie, J.; Fu, S.; Zhou, L.; Yang, Y. Large amounts of easily decomposable carbon stored in subtropical forest subsoil are associated with r-strategy-dominated soil microbes. Soil Biol. Biochem. 2016, 95, 233–242. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Kaur, T.; Yadav, N.; Yadav, A.N.; Kumar, M.; Kumar, V.; Dhaliwal, H.S.; Saxena, A.K. Biodiversity, current developments and potential biotechnological applications of phosphorus-solubilizing and -mobilizing microbes: A review. Pedosphere 2021, 31, 43–75. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, W.; Liu, X.; Liu, H.; Tang, G.; Yang, C. Combined impacts of algae-induced variations in water soluble organic matter and heavy metals on bacterial community structure in sediment from Chaohu Lake, a eutrophic shallow lake. Sci. Total Environ. 2023, 874, 162481. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, B.; Huang, T.; Shi, Y. Nitrate reduction by the aerobic denitrifying actinomycete Streptomyces sp. XD-11-6-2: Performance, metabolic activity, and micro-polluted water treatment. Bioresour. Technol. 2021, 326, 9. [Google Scholar] [CrossRef]
- Tammeorg, O.; Nürnberg, G.K.; Nõges, P.; Niemistö, J. The role of humic substances in sediment phosphorus release in northern lakes. Sci. Total Environ. 2022, 833, 10. [Google Scholar] [CrossRef]
- Xiao, W.J.; Song, C.L.; Cao, X.Y.; Zhou, Y.Y. Effects of Air-Drying on Phosphorus Sorption in Shallow Lake Sediment, China. Fresenius Environ. Bull. 2012, 21, 672–678. [Google Scholar]
- Jensen, H.S.; Mortensen, P.B.; Andersen, F.Ø.; Rasmussen, E.; Jensen, A. PHOSPHORUS CYCLING IN A COASTAL MARINE SEDIMENT, AARHUS BAY, DENMARK. Limnol. Oceanogr. 1995, 40, 908–917. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Zhang, J.; Xu, W.; Mou, Z. Characteristics of Inorganic Phosphate-Solubilizing Bacteria from the Sediments of a Eutrophic Lake. Int. J. Environ. Res. Public Health 2019, 16, 15. [Google Scholar] [CrossRef]
- Andrade, G.R.P.; de Azevedo, A.C.; Cuadros, J.; Souza, V.S.; Furquim, S.A.C.; Kiyohara, P.K.; Vidal-Torrado, P. Transformation of Kaolinite into Smectite and Iron-Illite in Brazilian Mangrove Soils. Soil Sci. Soc. Am. J. 2014, 78, 655–672. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Chen, H.; Chang, Y.; Yang, X.; Yang, H.; Huang, W. Exploration of Phosphorus Release Characteristics in Sediments from the Plains River Network: Vertical Distribution and the Response of Phosphorus and Microorganisms. Water 2025, 17, 2196. https://doi.org/10.3390/w17152196
Dong X, Chen H, Chang Y, Yang X, Yang H, Huang W. Exploration of Phosphorus Release Characteristics in Sediments from the Plains River Network: Vertical Distribution and the Response of Phosphorus and Microorganisms. Water. 2025; 17(15):2196. https://doi.org/10.3390/w17152196
Chicago/Turabian StyleDong, Xiaoshuang, Haojie Chen, Yongsheng Chang, Xixi Yang, Haoran Yang, and Wei Huang. 2025. "Exploration of Phosphorus Release Characteristics in Sediments from the Plains River Network: Vertical Distribution and the Response of Phosphorus and Microorganisms" Water 17, no. 15: 2196. https://doi.org/10.3390/w17152196
APA StyleDong, X., Chen, H., Chang, Y., Yang, X., Yang, H., & Huang, W. (2025). Exploration of Phosphorus Release Characteristics in Sediments from the Plains River Network: Vertical Distribution and the Response of Phosphorus and Microorganisms. Water, 17(15), 2196. https://doi.org/10.3390/w17152196





