Effects of Lanthanum-Modified Bentonite on Antibiotic Resistance Genes and Bacterial Communities in Tetracycline-Contaminated Water Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Lake Water Samples, and Sediment Samples
2.2. Culture Experiment
2.3. Gene Sampling, Extraction, and Quantitative Analysis
2.4. Bacterial Community Structure Analysis
2.5. Data Analysis and Statistical Analysis
3. Results
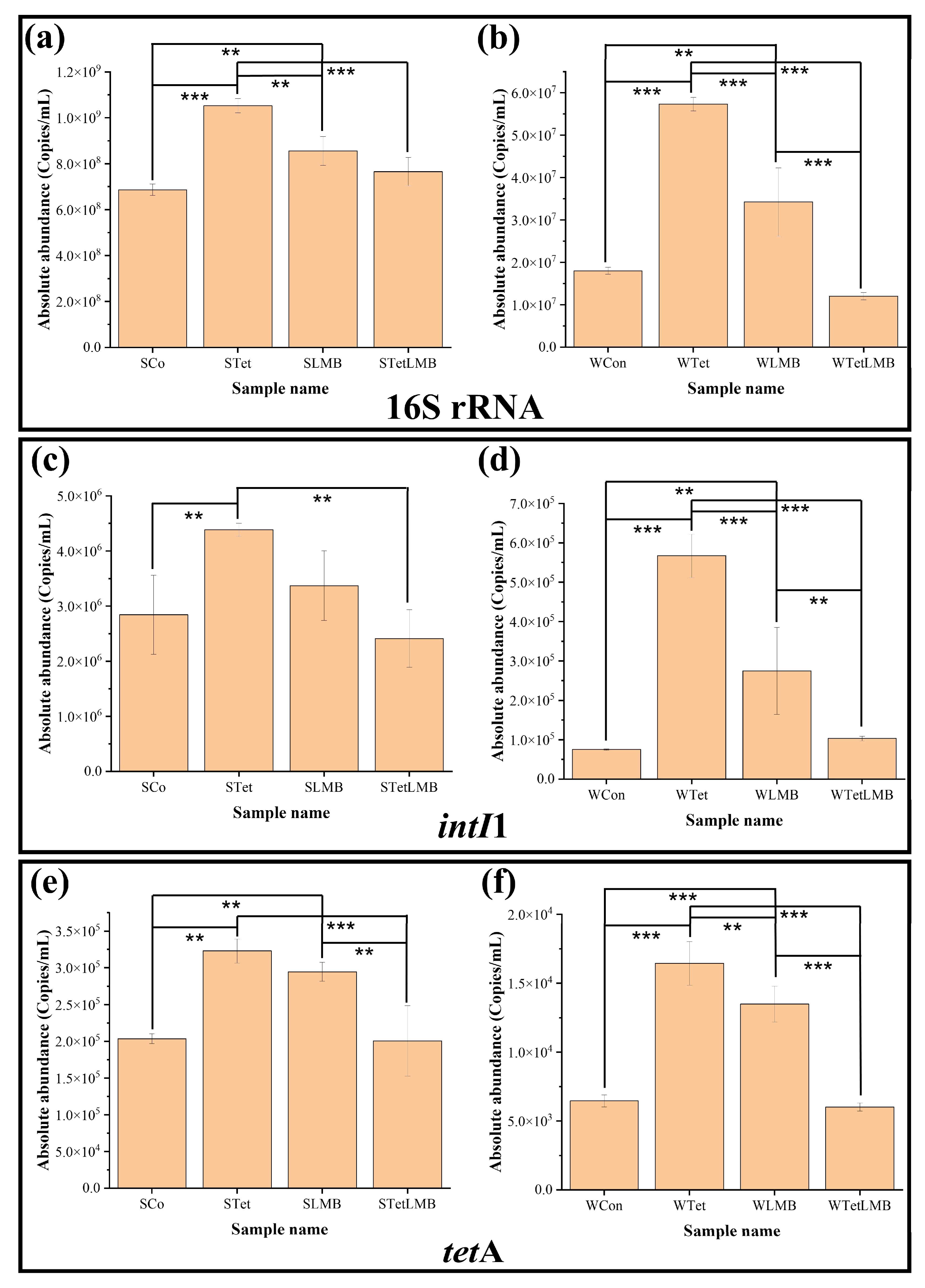
3.1. Changes in 16S rRNA, intI1, and tetA Genes After Exogenous Input of Tet and LMB
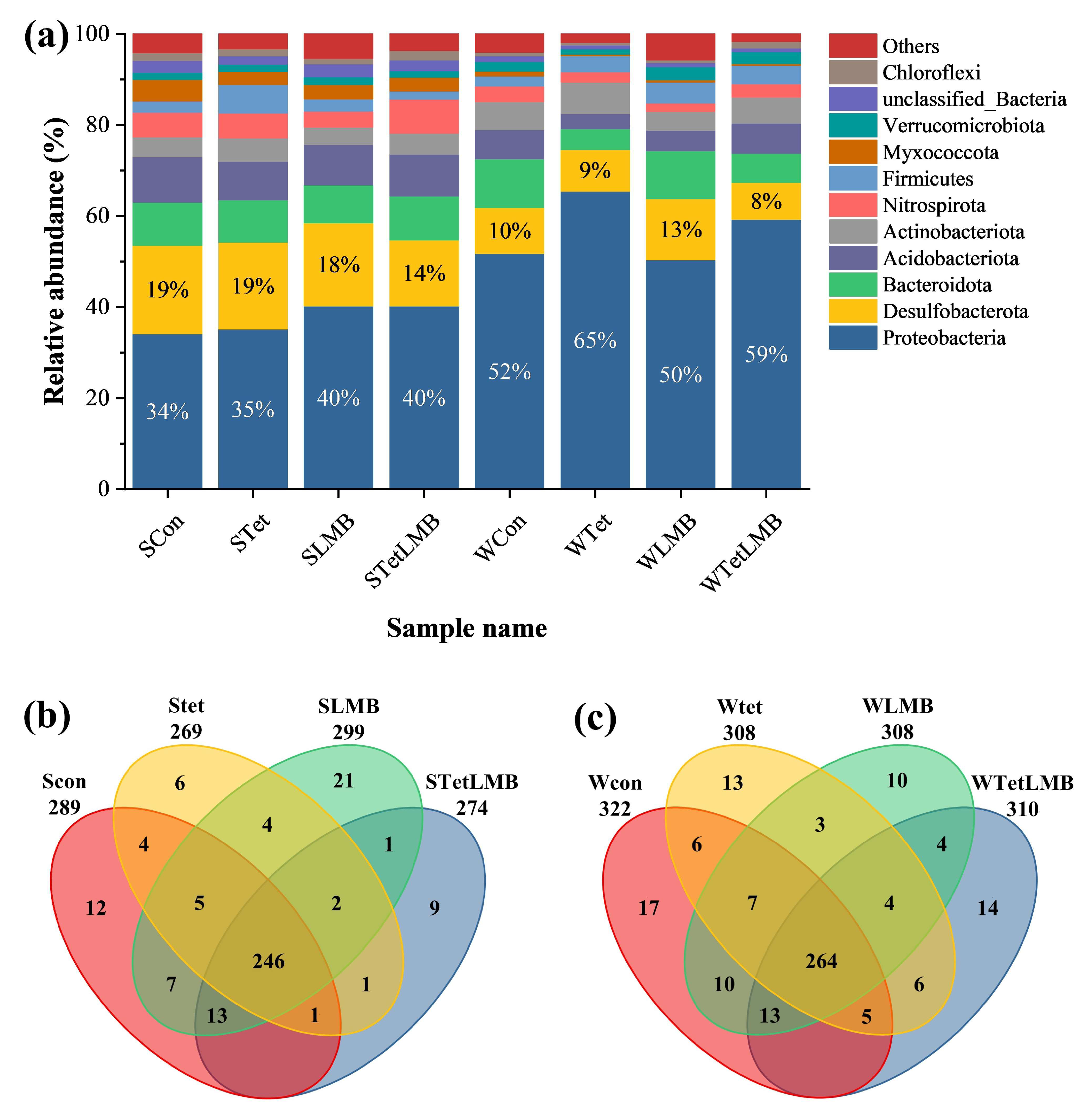
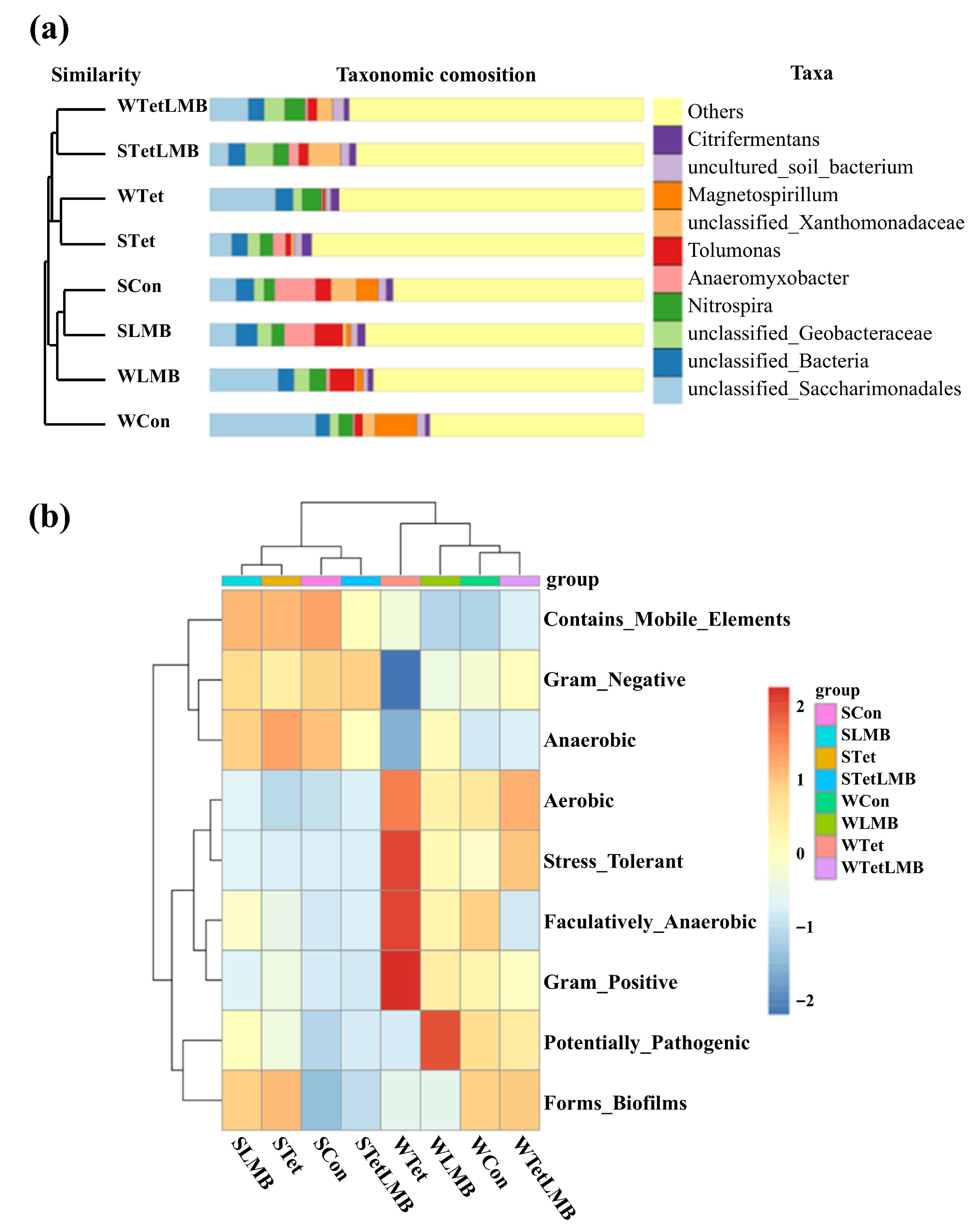
3.2. Changes in the Bacterial Community and Bacterial Functions After the Addition of Exogenous Tet or LMB
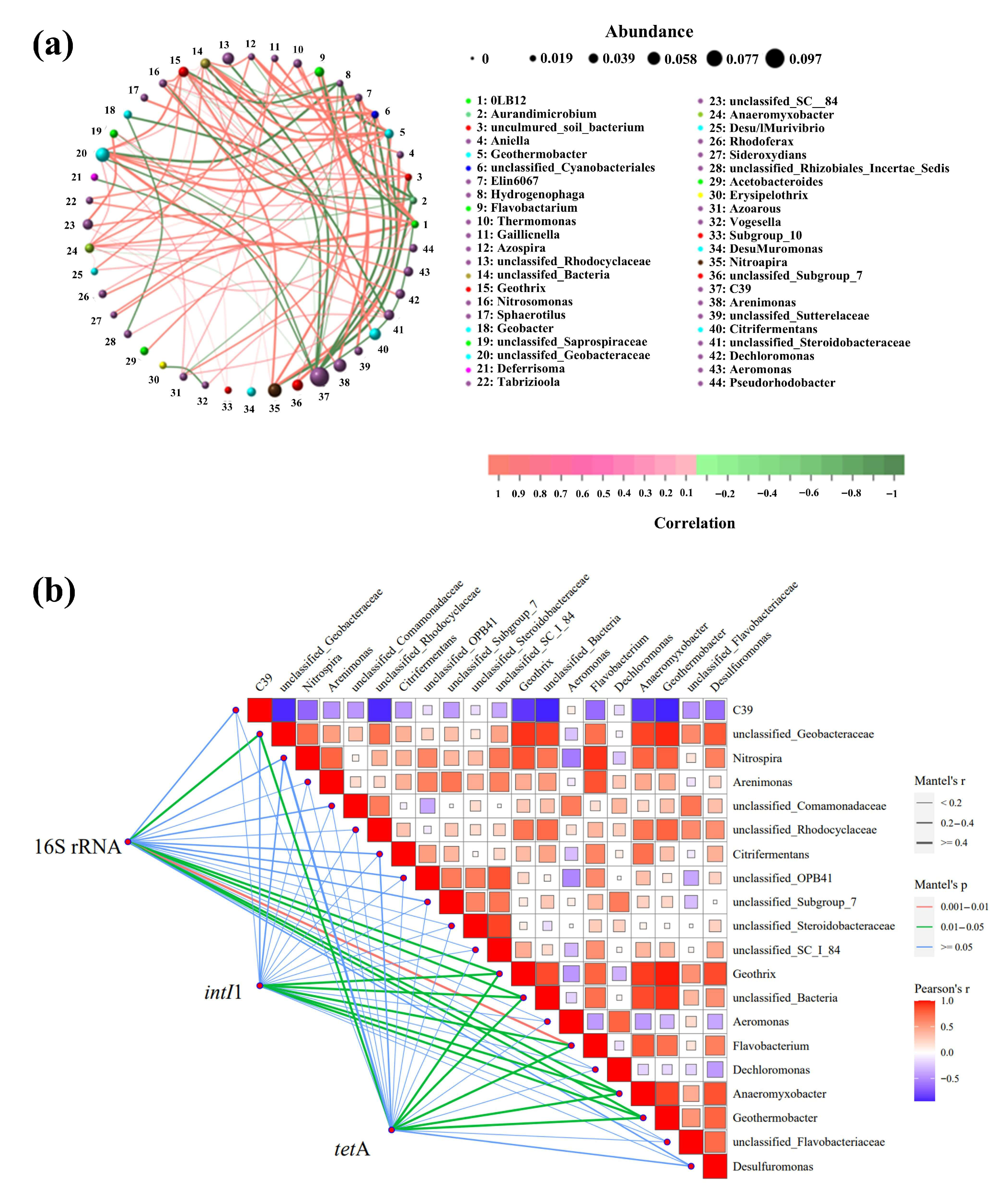
3.3. Correlations Between ARGs and the Bacterial Community
4. Discussion
4.1. Effect of Tet and/or LMB on 16S rRNA, intI1, and tetA Genes
4.2. Effect of Tet and/or LMB on Bacterial Community and Bacterial Functions
4.3. Potential Hosts of ARGs
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demain, A.L.; Sanchez, S. Microbial drug discovery: 80 years of progress. J. Antibiot. 2009, 62, 5–16. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Eckert, E.M.; Quero, G.M.; Di Cesare, A.; Manfredini, G.; Mapelli, F.; Borin, S.; Fontaneto, D.; Luna, G.M.; Corno, G. Antibiotic disturbance affects aquatic microbial community composition and food web interactions but not community resilience. Mol. Ecol. 2019, 28, 1170–1182. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Mao, Y.; Zhang, L.; Wei, H.; Wang, Z. Insight into environmental adaptability of antibiotic resistome from surface water to deep sediments in anthropogenic lakes by metagenomics. Water Res. 2024, 256, 121583. [Google Scholar] [CrossRef] [PubMed]
- Akhter, S.; Bhat, M.A.; Ahmed, S.; Siddiqui, W.A. Antibiotic residue contamination in the aquatic environment, sources and associated potential health risks. Environ. Geochem. Health 2024, 46, 387. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Zhang, D.; Xing, C.; Liang, J.; Wang, C.; Yang, X.; Liu, Z.; Zhao, Z. Effects of nitrogen-driven eutrophication on the horizontal transfer of extracellular antibiotic resistance genes in water-sediment environments. Environ. Res. 2025, 274, 121317. [Google Scholar] [CrossRef]
- Ding, C.; He, J. Effect of antibiotics in the environment on microbial populations. Appl. Microbiol. Biotechnol. 2010, 87, 925–941. [Google Scholar] [CrossRef]
- Liu, S.; Xu, Q.; Lou, S.; Tu, J.; Yin, W.; Li, X.; Jin, Y.; Radnaeva, L.D.; Nikitina, E.; Makhinov, A.N.; et al. Spatiotemporal distributions of sulfonamide and tetracycline resistance genes and microbial communities in the coastal areas of the Yangtze River Estuary. Ecotoxicol. Environ. Saf. 2023, 259, 115025. [Google Scholar] [CrossRef]
- Ahmad, F.; Zhu, D.; Sun, J. Environmental fate of tetracycline antibiotics: Degradation pathway mechanisms, challenges, and perspectives. Environ. Sci. Eur. 2021, 33, 64. [Google Scholar] [CrossRef]
- Singh, R.; Singh, A.P.; Kumar, S.; Giri, B.S.; Kim, K.H. Antibiotic resistance in major rivers in the world: A systematic review on occurrence, emergence, and management strategies. J. Clean. Prod. 2019, 234, 1484–1505. [Google Scholar] [CrossRef]
- Jiang, L.; Hu, X.; Xu, T.; Zhang, H.; Sheng, D.; Yin, D. Prevalence of antibiotic resistance genes and their relationship with antibiotics in the Huangpu River and the drinking water sources, Shanghai, China. Sci. Total Environ. 2013, 458, 267–272. [Google Scholar] [CrossRef]
- Tao, R.; Ying, G.G.; Su, H.C.; Zhou, H.W.; Sidhu, J.P.S. Detection of antibiotic resistance and tetracycline resistance genes in Enterobacteriaceae isolated from the Pearl rivers in South China. Environ. Pollut. 2010, 158, 2101–2109. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Guo, C.; Luo, Y.; Lv, J.; Zhang, Y.; Lin, H.; Wang, L.; Xu, J. Occurrence and distribution of antibiotics, antibiotic resistance genes in the urban rivers in Beijing, China. Environ. Pollut. 2016, 213, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liang, X.; Huang, X.; Zhang, T.; Li, X. Differentiating anthropogenic impacts on ARGs in the Pearl River Estuary by using suitable gene indicators. Water Res. 2013, 47, 2811–2820. [Google Scholar] [CrossRef]
- Wei, G.; Gao, H.; Li, S.; Liu, M.; Li, R.; Zhang, Y.; Shu, Q.; Wang, W.; Zhi, L.; Zeng, Y.; et al. The occurrence and abundance of antibiotic resistance genes in rivers of tropical islands: A case of Hainan Island, China. Environ. Sci. Pollut. Res. 2023, 30, 88936–88948. [Google Scholar] [CrossRef]
- Yin, H.; Yang, P.; Kong, M.; Li, W. Use of lanthanum/aluminum co-modified granulated attapulgite clay as a novel phosphorus (p) sorbent to immobilize p and stabilize surface sediment in shallow eutrophic lakes. Chem. Eng. J. 2020, 385, 123395. [Google Scholar] [CrossRef]
- Luo, D.; Tian, X.; Qin, R. Adsorption of arsenic (v) by biologically reductive lanthanum-loaded bentonite: Transportation and injection removal experiments. Environ. Technol. Innov. 2025, 37, 103922. [Google Scholar] [CrossRef]
- Copetti, D.; Finsterle, K.; Marziali, L.; Stefani, F.; Tartari, G.; Douglas, G.; Reitzel, K.; Spears, B.M.; Winfield, I.J.; Crosa, G.; et al. Eutrophication management in surface waters using lanthanum modified bentonite: A review. Water Res. 2016, 97, 162–174. [Google Scholar] [CrossRef]
- Haghseresht, F.; Wang, S.; Do, D.D. A novel lanthanum-modified bentonite, Phoslock, for phosphate removal from wastewaters. Appl. Clay Sci. 2009, 46, 369–375. [Google Scholar] [CrossRef]
- Reitzel, K.; Andersen, F.O.; Egemose, S.; Jensen, H.S. Phosphate adsorption by lanthanum modified bentonite clay in fresh and brackish water. Water Res. 2013, 47, 2787–2796. [Google Scholar] [CrossRef]
- Meis, S.; Spears, B.M.; Maberly, S.C.; O’Malley, M.B.; Perkins, R.G. Sediment amendment with Phoslock ® in Clatto Reservoir (Dundee, UK): Investigating changes in sediment elemental composition and phosphorus fractionation. J. Environ. Manag. 2012, 93, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Yang, P.; Kong, M. Effects of different chemical agents on changes in sediment phosphorus composition and the response of sediment microbial community. J. Environ. Manag. 2023, 342, 118321. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Yang, C.; Yang, P.; Kaksonen, A.H.; Douglas, G.B. Contrasting effects and mode of dredging and In Situ adsorbent amendment for the control of sediment internal phosphorus loading in eutrophic lakes. Water Res. 2021, 189, 116644. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Huang, D.L.; Chen, S.; Wang, G.F.; Chen, Y.S.; Tao, J.X.; Chen, H.J.; Gao, L. Removing antibiotic resistance genes under heavy metal stress with carbon-based materials and clay minerals: By sorption alone? Chem. Eng. J. 2022, 446, 137121. [Google Scholar] [CrossRef]
- Chowdhury, N.N.; Wiesner, M.R. Persistence and environmental relevance of extracellular antibiotic resistance genes: Regulation by nanoparticle association. Environ. Eng. Sci. 2021, 38, 1129–1139. [Google Scholar] [CrossRef]
- Deng, C.; Liu, X.; Li, L.; Shi, J.; Guo, W.; Xue, J. Temporal dynamics of antibiotic resistant genes and their association with the bacterial community in a water-sediment mesocosm under selection by 14 antibiotics. Environ. Int. 2020, 137, 105554. [Google Scholar] [CrossRef]
- Gillings, M.R.; Gaze, W.H.; Pruden, A.; Smalla, K.; Tiedje, J.M.; Zhu, Y.-G. Using the class 1 integron-integrase gene as a proxy for anthropogenic pollution. ISME J. 2014, 9, 1269–1279. [Google Scholar] [CrossRef]
- Cheng, D.; Liu, Y.; Shehata, E.; Feng, Y.; Lin, H.; Xue, J.; Li, Z. In-feed antibiotic use changed the behaviors of oxytetracycline, sulfamerazine, and ciprofloxacin and related antibiotic resistance genes during swine manure composting. J. Hazard. Mater. 2021, 402, 123710. [Google Scholar] [CrossRef]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Bates, S.T.; Berg-Lyons, D.; Caporaso, J.G.; Walters, W.A.; Knight, R.; Fierer, N. Examining the global distribution of dominant archaeal populations in soil. ISME J. 2011, 5, 908–917. [Google Scholar] [CrossRef]
- Sun, R.; He, L.; Li, T.; Dai, Z.; Sun, S.; Ren, L.; Liang, Y.-Q.; Zhang, Y.; Li, C. Impact of the surrounding environment on antibiotic resistance genes carried by microplastics in mangroves. Sci. Total Environ. 2022, 837, 155771. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Song, J.; Liu, X.; Chen, B.; Zhang, C.; Zhang, S. Tea polyphenols and catechins postpone evolution of antibiotic resistance genes and alter microbial community under stress of tetracycline. Ecotoxicol. Environ. Saf. 2023, 253, 114675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, Y.; Wang, Y.; Liu, X.; Li, M.; Fang, H.; Kong, M. Effect of antibiotics, antibiotic-resistant bacteria, and extracellular antibiotic resistance genes on the fate of ARGs in marine sediments. Sci. Total Environ. 2023, 891, 164305. [Google Scholar] [CrossRef] [PubMed]
- Mwanamoki, P.M.; Devarajan, N.; Thevenon, F.; Atibu, E.K.; Tshibanda, J.B.; Ngelinkoto, P.; Mpiana, P.T.; Prabakar, K.; Mubedi, J.I.; Kabele, C.G.; et al. Assessment of pathogenic bacteria in water and sediment from a water reservoir under tropical conditions (Lake Ma Vallée), Kinshasa Democratic Republic of Congo. Environ. Monit. Assess. 2014, 186, 6821–6830. [Google Scholar] [CrossRef]
- Devarajan, N.; Laffite, A.; Graham, N.D.; Meijer, M.; Prabakar, K.; Mubedi, J.I.; Elongo, V.; Mpiana, P.T.; Ibelings, B.W.; Wildi, W.; et al. Accumulation of clinically relevant antibiotic-resistance genes, bacterial load, and metals in freshwater lake sediments in central europe. Environ. Sci. Technol. 2015, 49, 6528–6537. [Google Scholar] [CrossRef]
- Anand, U.; Reddy, B.; Singh, V.K.; Singh, A.K.; Kesari, K.K.; Tripathi, P.; Kumar, P.; Tripathi, V.; Simal-Gandara, J. Potential Environmental and human health risks caused by antibiotic-resistant bacteria (ARB), antibiotic resistance genes (ARGs) and emerging contaminants (ECs) from municipal solid waste (MSW) landfill. Antibiotics 2021, 10, 374. [Google Scholar] [CrossRef]
- Li, Z.; Yang, F.; Han, B.; Zhao, R.; Yang, M.; Zhang, K. Vermicomposting significantly reduced antibiotic resistance genes in cow manure even under high tetracycline concentrations. Bioresour. Technol. 2025, 419, 132002. [Google Scholar] [CrossRef]
- Qiu, X.; Feng, M.; Zhou, G.; Wang, H. Effects of mineral additives on antibiotic resistance genes and related mechanisms during chicken manure composting. Bioresour. Technol. 2022, 346, 126631. [Google Scholar] [CrossRef]
- Jang, H.M.; Kim, Y.B.; Choi, S.; Lee, Y.; Shin, S.G.; Unno, T.; Kim, Y.M. Prevalence of antibiotic resistance genes from effluent of coastal aquaculture, South Korea. Environ. Pollut. 2018, 233, 1049–1057. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, Y.; Yu, B.; Yang, M. Changes of resistome, mobilome and potential hosts of antibiotic resistance genes during the transformation of anaerobic digestion from mesophilic to thermophilic. Water Res. 2016, 98, 261–269. [Google Scholar] [CrossRef]
- Dong, P.; Cui, Q.; Fang, T.; Huang, Y.; Wang, H. Occurrence of antibiotic resistance genes and bacterial pathogens in water and sediment in urban recreational water. J. Environ. Sci. 2019, 77, 65–74. [Google Scholar] [CrossRef]
- Low, A.; Ng, C.; He, J. Identification of antibiotic resistant bacteria community and a GeoChip based study of resistome in urban watersheds. Water Res. 2016, 106, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Heijnen, C.E.; Vanveen, J.A. A determination of protective microhabitats for bacteria introduced into soil. FEMS Microbiol. Ecol. 1991, 85, 73–80. [Google Scholar] [CrossRef]
- Khalaf, S.M.; Al-Mahmoud, S.M. Adsorption of tetracycline antibiotic from aqueous solutions using natural iraqi bentonite. Egypt. J. Chem. 2021, 64, 5511–5519. [Google Scholar] [CrossRef]
- Paula Fagundes, A.; Felipe Viana da Silva, A.; Bueno de Morais, B.; Lusitâneo Pier Macuvele, D.; Nones, J.; Gracher Riella, H.; Padoin, N.; Soares, C. A novel application of bentonite modified with copper ions in the tetracycline adsorption: An experimental design study. Mater. Lett. 2021, 291, 129552. [Google Scholar] [CrossRef]
- Zhang, K.; Li, K.; Xin, R.; Han, Y.; Guo, Z.; Zou, W.; Wei, W.; Cui, X.; Zhang, Z.; Zhang, Y. Antibiotic resistomes in water supply reservoirs sediments of central China: Main biotic drivers and distribution pattern. Environ. Sci. Pollut. Res. 2022, 29, 37712–37721. [Google Scholar] [CrossRef]
- Lin, L.; Lai, Z.; Yang, H.; Zhang, J.; Qi, W.; Xie, F.; Mao, S. Genome-centric investigation of bile acid metabolizing microbiota of dairy cows and associated diet-induced functional implications. ISME J. 2023, 17, 172–184. [Google Scholar] [CrossRef]
- Waajen, G.; Pauwels, M.; Lurling, M. Effects of combined flocculant—Lanthanum modified bentonite treatment on aquatic macroinvertebrate fauna. Water Res. 2017, 122, 183–193. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; Zhao, H. Propagation of antibiotic resistance genes in an industrial recirculating aquaculture system located at northern China. Environ. Pollut. 2020, 261, 114155. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, Y.; Kong, L.; Bai, G.; Luo, F.; Liu, Z.; Wang, C.; Ding, Z.; He, F.; Wu, Z.; et al. Effects of bentonite on the growth process of submerged macrophytes and sediment microenvironment. J. Environ. Manag. 2021, 287, 112308. [Google Scholar] [CrossRef]
- Nandi, S.; Chakrabarty, S.; Bandopadhyay, P.; Mandal, D.; Azaharuddin, M.; Das, A.; Pal, A.; Ghosh, S.; Nandy, S.; Sett, U.; et al. Molecular mechanism of action of tetracycline-loaded calcium phosphate nanoparticle to kill multi-drug resistant bacteria. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2025, 1869, 130733. [Google Scholar] [CrossRef]
- Ding, J.; Yang, W.; Liu, X.; Zhao, J.; Fu, X.; Zhang, F.; Liu, H. Hydraulic conditions control the abundance of antibiotic resistance genes and their potential host microorganisms in a frequently regulated river–lake system. Sci. Total Environ. 2024, 946, 174143. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, L.; Li, Z.-H.; Zhang, M.-Q.; Shao, W.; Sheng, G.-P. Antibiotic resistance and microbiota in the gut of Chinese four major freshwater carp from retail markets. Environ. Pollut. 2019, 255, 113327. [Google Scholar] [CrossRef]
- Xu, M.; Huang, X.-H.; Shen, X.-X.; Chen, H.-Q.; Li, C.; Jin, G.-Q.; Cao, J.-S.; Xue, Z.-X. Metagenomic insights into the spatiotemporal responses of antibiotic resistance genes and microbial communities in aquaculture sediments. Chemosphere 2022, 307, 135596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, X.; Liu, H.; Gao, L.; Wang, Q. Free ammonia pretreatment enhances the removal of antibiotic resistance genes in anaerobic sludge digestion. Chemosphere 2021, 279, 130910. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Xu, Y.; Zhong, Z.; Xu, Y.; Lin, X.; Cao, Z.; Feng, G. Insights into antibiotic resistance-related changes in microbial communities, resistome and mobilome in paddy irrigated with reclaimed wastewater. Sci. Total Environ. 2023, 900, 165672. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Singh, A.; Chowdhary, P.; Pandey, A.; Gupta, P. Occurrence of emerging sulfonamide resistance (sul1 and sul2) associated with mobile integrons-integrase (intI1 and intI2) in riverine systems. Sci. Total Environ. 2021, 751, 142217. [Google Scholar] [CrossRef]
- Zhai, W.; Yang, F.; Mao, D.; Luo, Y. Fate and removal of various antibiotic resistance genes in typical pharmaceutical wastewater treatment systems. Environ. Sci. Pollut. Res. 2016, 23, 12030–12038. [Google Scholar] [CrossRef]
- Chen, X.; Ke, Y.; Zhu, Y.; Xu, M.; Chen, C.; Xie, S. Enrichment of tetracycline-degrading bacterial consortia: Microbial community succession and degradation characteristics and mechanism. J. Hazard. Mater. 2023, 448, 130984. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Liang, S.; Zhang, S.; Wei, D.; Xu, X.; Zhang, P. Effects of Lanthanum-Modified Bentonite on Antibiotic Resistance Genes and Bacterial Communities in Tetracycline-Contaminated Water Environments. Water 2025, 17, 2188. https://doi.org/10.3390/w17152188
Wang W, Liang S, Zhang S, Wei D, Xu X, Zhang P. Effects of Lanthanum-Modified Bentonite on Antibiotic Resistance Genes and Bacterial Communities in Tetracycline-Contaminated Water Environments. Water. 2025; 17(15):2188. https://doi.org/10.3390/w17152188
Chicago/Turabian StyleWang, Wanzhong, Sijia Liang, Shuai Zhang, Daming Wei, Xueting Xu, and Peng Zhang. 2025. "Effects of Lanthanum-Modified Bentonite on Antibiotic Resistance Genes and Bacterial Communities in Tetracycline-Contaminated Water Environments" Water 17, no. 15: 2188. https://doi.org/10.3390/w17152188
APA StyleWang, W., Liang, S., Zhang, S., Wei, D., Xu, X., & Zhang, P. (2025). Effects of Lanthanum-Modified Bentonite on Antibiotic Resistance Genes and Bacterial Communities in Tetracycline-Contaminated Water Environments. Water, 17(15), 2188. https://doi.org/10.3390/w17152188





