Abstract
The combined effect of temperature-adapted inocula and anaerobic fermentation (AF) settings (pH 5.1 and 50 °C) were assessed to produce short-chain carboxylates (SCCs). In this study, the AF of carrot pulp was investigated using inocula adapted at different temperatures (25, 35, and 55 °C) with the aim of shifting the microbiome activity from biogas to SCC production. The highest SCC content (17.2 g COD L−1), and bioconversion (26.1%) and acidification efficiency (56.3%) were achieved with 35 °C-adapted inoculum. Lactic acid production prevailed in all reactors, demonstrating a high selectivity in SCC production. Both the microbial richness and diversity sharply diminished in the 35 °C and 55 °C operated reactors, with Firmicutes phylum identified as key players of the lactic acid production in AF. The results demonstrated that the operating temperature played a key role in shaping the microbial structure of inocula, leading to different process performances and highlighting thermophilic AF as a feasible process to produce lactic acid.
1. Introduction
Food production and consumption have increased due to the exponential growth in the world’s population, which has resulted in a notable rise in the production of organic waste. In 2022, the world wasted 1.05 billion tons of food [1]. The amount of food waste should not be disregarded when considering the Sustainable Objectives Goals pursued worldwide. Because of that, organic wastes should be recovered and upcycled for societal uses.
There are many different types of food waste, including agroindustrial waste, household food waste, and fruit/vegetable wastes [2]. More importantly, each kind of food waste exhibits unique compositions and properties. For instance, carrot pulp, a by-product of the juice and food processing industries, can be utilized as an additive in food product development [3,4] or as animal feed [5]. However, bio-based industry, which is always searching for cheap and abundant waste to be used as substrates to produce valuable products, envisages the use of this type of complex organic matter to replace expensive and/or non-sustainable carbon sources. Additionally, this approach helps in reducing the quantity of residual matter that enters the environment or needs further treatment before disposal. In this line, anaerobic digestion (AD) is a relatively straightforward biochemical conversion process that operates well with wastes that are rich in organic matter, have a high moisture content, and are highly biodegradable. Four interdependent processes—hydrolysis, acidogenesis, acetogenesis, and methanogenesis—are involved in AD, and they are all mediated by a wide variety of microorganisms. For example, acetogenesis and methanogenesis, performed by obligatory anaerobes, have an ideal pH range between 7 and 8, and a doubling time of several days. In contrast, hydrolytic and acidogenic microorganisms, performed by facultative anaerobes, have an optimal pH range between 5.5 and 6.5, and a replication time of 30 min [6]. Traditionally biogas is the main product of AD; nevertheless, intermediate products from AD have higher inherent value than biogas. These intermediate products, volatile fatty acids (VFAs), have from two (acetic acid) to six (caproic acid) carbon atoms and are generated during the acidogenesis phase of AD [7]. Yet, when lactic acid is also present in the cultivation media, instead of VFAs, the intermediate pool is referred to as short chain carboxylates (SCCs)
The market value of SCCs is USD 150 per ton, which is significantly higher than that of methane (USD 31 per ton) [8]. SCCs have been considered as essential building blocks in the chemical industry for the production of alcohols, alkanes, ketones, esters, and aldehydes, as well as polyhydroxyalkanoates [9]. In the environmental context, it is also remarkable that SCCs are normally petroderivates, while the attainment of SCCs via biological processes would entail a friendlier production route. Because of that, in the last decade, research has focused on the optimization of the biochemical steps that are involved in the production of SCCs (hydrolysis and acidogenesis) and their accumulation. This occurs when the final step of AD, methanogenesis, ceases to occur, whereby the process is referred to as anaerobic fermentation (AF).
Up to now, some of the optimal conditions for optimizing SCC production, for example, are in a pH range of 5.5 to 6.0 and a temperature of 25 °C [9]. As a result, variations in the temperature and pH throughout the AF had an impact on the profile of bacterial populations as well as the production of SCCs.
A successful AF process and the produced SCCs (composition and yield) depend on several variables, including the inoculum source, feedstock type, temperature regime, and operating conditions. In fact, pH has been evidenced to be crucial when targeting at compounds ranging from C2 to C6 (i.e., acetic to caproic acids), as pHs lower than 5.5–5.0 might end up accumulating primary metabolites (lactic acid and ethanol) rather than C2-C6 carboxylates [9]. Likewise, temperature plays a crucial role in the AF process because it affects the diversity and abundance of microorganisms, which can either promote hydrolysis or acidification. While some research showed that mesophilic and psychrophilic conditions produced the most SCCs [10], other studies indicated that increasing the temperature can significantly speed up the SCC concentration [11]. Yet, not only acidogenesis should be the focus of research when targeting SCCs, as hydrolysis is frequently the limiting stage. To overcome this limitation, one possible option is to promote enzymatic hydrolysis by using thermophilic conditions in the reactors [12]. The various feedstock properties used in those studies may be connected to these inconsistencies in how temperature affects the production of SCCs. Furthermore, these studies addressed the effects of temperature on the AF performance while neglecting the microbiome adaptation to operational conditions. To address this gap, this investigation was designed to evaluate the impact of different inocula previously acclimated at various temperatures (namely, 25, 35, and 55 °C) on the thermophilic AF performance when using a carbohydrate-rich substrate. A correlation between the inoculum and the evolved microbiome, the bioconversion and acidification efficiency, and the compositions of the SCCs was investigated.
2. Materials and Methods
2.1. Feedstock and Inoculum Used for SCC Production
To evaluate how different temperature-acclimatized inocula affect AF in terms of SCC production, three types of inocula were used in this investigation. Specifically, inocula included a conventional sludge from a mesophilic anaerobic digester (35 °C), located at the Wastewater Treatment Plant (Valladolid, Spain), a thermophilic sludge from an anaerobic digester operating at 55 °C at the Institute of Sustainable Processes of the University of Valladolid (Spain), and sludge collected from an AF process for carboxylate production carried out at 25 °C.
The carrot waste was collected at an agricultural company located in Valladolid (Spain). The selection of this residual stream was based on their potential for the production of short-chain carboxylic acids (SCCAs), due to their high carbohydrate content (Table 1), which is readily biodegradable. To prepare the substrate, 2.5 kg of carrots were ground and then liquefied in 4 L of water until a carrot pulp was obtained. The chemical characterization of the inocula and substrate can be found in Table 1 and Table 2, respectively, in terms of total and soluble chemical oxygen demand (TCOD and SCOD, respectively), total solids (TS) and volatile solids (VS), total nitrogen, pH, and the content of carbohydrates, proteins, and lipids.

Table 1.
Characterization of inocula used in the AFs.

Table 2.
Characterization of the carrot pulp used as a substrate in the AFs.
2.2. Experimental Setup
To carry out the AF, three stirred-tank reactors were operated in semi-continuous mode. The reactors had a working volume of 1.0 L and a headspace of 0.5 L. The reactors were maintained at a temperature of 50 °C using a Fisherbrand™ Isotemp™ model 4100H7 (USA) thermostatic bath. Thermophilic conditions were set aiming at favoring the hydrolysis stage. An organic loading rate (OLR) of 3.3 g COD L−1·d−1, a hydraulic retention time (HRT) of 19.5 days, and a pH of 5.1 were also established for the operation of reactors. These conditions have been selected in accordance with Greses et al. [9] so to promote carboxylate production when using agroindustrial waste. The reactors were operated for at least three HRTs, and stable concentrations were determined in the effluents. Table 3 shows the experimental setup.

Table 3.
Experimental setup description.
The process was evaluated based on organic matter bioconversion, acidification, and hydrolysis efficiencies, and nitrogen mineralization, which were calculated using the following equations:
where COD(SCC)Effluent is the sum of the concentrations of acetic (HAc), propionic (HPro), isobutyric (isoHBu), butyric (HBu), isovaleric (isoHVal), valeric (HVal), caproic (HCa), and lactic (HLac) acids at the system outlet, expressed in terms of COD (g COD/L). The stoichiometric equivalence for these acids were 1.067 for HAc, 1.513 for HPro, 1.82 for isoHBu and HBu, 2.039 for isoHVal and HVal, 2.207 for HCa, and 1.067 for HLac. TCODInfluent represents the total organic matter content at the system inlet (g COD L−1), SCODEffluent represents the soluble organic matter concentration at the system outlet (g COD L−1), VSInfluent represents the volatile solids concentration at the system inlet (g VS L−1), and VSEffluent represents the volatile solids concentration at the system outlet (g VS L−1). NH4+-N effluent and NH4+-N influent were the ammonium determined in the feedstock and the reactor effluents, respectively (g N L−1), and total nitrogen was the total Kjeldahl nitrogen (TKN) measured in the feedstock (g N L−1).
2.3. Process Monitoring
For process monitoring and substrate characterization, TCOD, SCOD, TS, VS, carbohydrates, TKN, ammonium nitrogen (NH4+-N), and pH assays were analyzed. Samples were taken twice a week during the experimental trial to monitor the process and characterize the process. Commercial kits (Merck, DE) were used for the analysis of TCOD, SCOD, and NH4+-N, respectively. For the analysis of SCOD and NH4+-N, the sample was centrifuged beforehand, and the supernatant was filtered using a 0.45 µm filter. The pH measurements and TKN, TS, VS, and ash analyses were performed according to the methodology described in APHA [13]. The pH was adjusted to 5.1 by adding sodium hydroxide (4 M NaOH). Carbohydrate content was determined using the phenol-sulfuric method [14], while protein content was obtained by multiplying the NKT value by a conversion factor of 6.25 [15]. Lipid content (as a percentage) was obtained by calculating the difference between 100 and the percentages of protein, carbohydrates, and ash. The analysis of SCC was performed using high-performance liquid chromatography (HPLC), equipped with a refractive index detector, UV–Vis detectors (Shimadzu LC-2050), and a column H+ HyperREZ XP. The column was maintained at a constant temperature of 55 °C. The mobile phase consisted of 5 mM H2SO4 and was maintained at a flow rate of 0.6 mL/min. The sample injection volume was set at 20 µL. For the analysis of SCC, the sample was previously centrifuged, and the supernatant was subsequently filtered using a 0.22 µm filter.
The gas phase composition was analyzed using a gas chromatograph equipped with a thermal conductivity detector (GC-TCD, Agilent 8860, USA). Helium was used as carrier gas, with a flow rate of 4 mL min−1. The injector, oven, and detector temperatures were 150, 60, and 200 °C, respectively. The sample volume to be injected was 500 μL.
2.4. Microbial Community Analysis
To analyze the microbial community, samples were collected from the inoculum and the steady state of each reactor, and preserved at −80 °C prior to DNA extraction. DNA was extracted using the FastDNA SPIN Kit for Soil (MP Biomedicals, LLC). Once obtained, the DNA concentration and quality were measured using a nanodrop spectrophotometer (SPECTROstar Omega—BMG Labtech, DE). For the identification of bacterial and archaeal communities, the V3 and V4 hypervariable regions of the 16S rRNA gene were amplified using the primers 341F (F—CCTACGGGNGGCWGCAG) and 805R (R—GACTACHVGGGTATC TAATCC). Amplicons were sequenced on a MiSeq Sequencer (Illumina) by FISABIO (Valencia, Spain). Once the sequences were obtained, bioinformatic analysis was performed following the procedures described by Greses et al. [16].
3. Results and Discussion
3.1. Process Performance
Three different reactors were operated under similar conditions but different inocula (more specifically, inocula adapted to 25, 35, and 55 °C). The choice of inoculum can significantly impact the AF performance, as different microorganisms may have varying degradation capabilities. Table 4 shows the SCC concentrations attained in the three reactors during the steady state of AF, including 12.5 for R1, 17.2 for R2, and 13.9 g COD L−1 for R3, resulting in bioconversion efficiencies of 18.9%, 26.1%, and 22.8%, respectively. Thereby, the reactor operated with inocula adapted at 35 °C exhibited the highest SCC concentration. This was not anticipated, as the rationale would have been that microorganisms adapted to thrive at 55 °C (the inoculum used for R3) would provide the highest production, such as the cases reported for conventional AD. It should be highlighted that not only temperature but also pH plays a key role in the AF performance. Thermophilic AD (inoculum) is characterized by a slightly basic pH due to the ammonium released from protein hydrolysis [17]. However, the thermophilic AF was performed at an acid pH (5.1), which might be detrimental for thermophilic microorganisms adapted to alkaline conditions. Moreover, the presence of methanogenic microorganisms could also support the lower bioconversion, since the SCCs were transformed into biogas, which was also reinforced by the COD removal calculated for those two reactors, ranging to 20% (Table 4).

Table 4.
Composition of reactor effluents during the steady state of the AF in terms of mean ± standard deviation.
On the other hand, the lowest SCC level was produced by R1 (12.5 g L−1). Since this inoculum was previously adapted to 25 °C, it could have promoted the washout of those microorganisms able to thrive at thermophilic conditions. Thereby, the lower bioconversion attained in R1 when subjected to the 55 °C operation can be related with a less active microbial population at this temperature. In fact, Greses et al. [12] reported that a large number of bacteria are unable to adapt to other ranges of temperature, which limits the biodiversity of metabolic pathways involved in AF. Therefore, the results showed that, although all of the reactors were operated using the same substrate, pH, OLR, and HRT, the use of an inoculum adapted to a 35 °C temperature has a significant influence in the process performance in terms of bioconversion efficiencies, since the microbiome growth at 35 °C is able to tolerate a pH oscillation between 6 and 7.5, which suggests a higher resistance to a low pH than the thermophilic ones. The literature on inocula acclimatization to a particular temperature prior to AF to produce SCCs is actually lacking, and thereby the literature is controversial when claiming which temperature is the optimal one. Some researchers have reported that mesophilic (35 °C) and thermophilic (55 °C) temperatures are the most suitable for SCC production [17,18]. Yet, the opposite has also been reported in the last five years, evidencing that psychrophilic temperatures might also help in hampering methanogens (acetate consumers) and favoring the production of SCCs [19]. As a matter of fact, the production of SCCs in a semi-continuously operated reactor at 55 °C is really limited, reducing the information that can be used to optimize the AF. However, in contrast to previous studies performing the AF of carbohydrate-rich substrates in batch mode at similar temperature (55 °C), the bioconversion efficiency in this study demonstrated a high performance. For example, after fermenting a carbohydrate-rich substrate in batch mode at 55 °C, Garcia-Aguirre et al. [19] found a 17.3% bioconversion. In the case of semi-continuously fed reactors, Greses et al. [12] achieved a 41.5% bioconversion efficiency when they fermented carbohydrate-rich food waste at 55 °C. This obvious difference could be ascribed to the different substrate composition (mainly, the macromolecular distribution of wastes).
The acidification efficiency was also higher in R2 and R3 than R1. As shown in Table 4, there were no differences in the acidification efficiency, whereby both reactors had an efficiency of 56%. Again, R1 supported the lowest acidification efficiency, indicating the limited suitability of the inoculum adapted at 25 °C for SCC production at thermophilic conditions. When compared to the literature, the acidification efficiency was comparably low. Most of the time when targeting carboxylate production during AF, a more than 90% acidogenic efficiency has been reported [20]. This can be also attributed to the differences in pH [9]. High acidogenic efficiencies are commonly linked to pHs ranging from 5.5 to 6.5, while the pH imposed in this investigation (5.1) could be detrimental for the C2-C6 SCCs.
As for organic carbon, nitrogen degradation was also followed by calculating the nitrogen mineralization. NH4+-N was released during the acidification and hydrolysis of proteins. This parameter might be utilized to evaluate how much protein degradation took place [10]. These results indicated that the conversion of protein was again very limited in R1 (Table 4). The breakdown of proteinaceous materials may have contributed to the rise in the bioconversion percentage in R2 and R3. In particular, compared to R1, ammonium concentrations in R2 and R3 were approximately two-fold higher than in R1, suggesting that more protein was hydrolyzed in the former reactors. The low nitrogen mineralization in R1 may be explained by the absence of protease activity in the inoculum adapted to 25 °C, since less energy-demanding metabolisms prevail when the microbiome is subjected to harsh conditions (25 °C and pH 5.1), such as carbohydrate degradation pathways.
The VS removal was utilized to evaluate the hydrolytic efficiency. As shown in Table 4, the VS removal for R1, R2, and R3 was 43.5, 42.5, and 40.1%, respectively. No noticeable variations were found among the reactors. Those values were similar to previous investigations, where carbohydrate-rich residues were subjected to AF regardless of the process temperature. Thus, Greses et al. [12] reached 45.2% when performing AF at 55 °C and a pH of 5.8. Similarly, 45% of the hydrolytic efficiency was attained when the AF of carbohydrate-rich food waste was performed at 37 °C and a pH of 5.5 [21]. Those results evidenced that the low bioconversion efficiency was likely related to an acidogenesis inhibition rather than a hydrolysis limitation, since almost 50% of the soluble organic matter was not converted into SCCs. This was of particular relevance, as the reason to set up the operation at 55 °C was indeed to promote the hydrolysis phase of AF. It can thus be concluded that all three inocula had the proper microbiome to perform the hydrolysis and this was the opposite to what was determined by the acidification stage. Subjecting different inoculum sources to a thermophilic operating temperature had a greater impact on acidogenesis than in the hydrolytic phase.
3.2. SCC Profile Distribution
The differences in acidogenesis efficiencies were also supported by the different SCC profile distributions. As can be seen in Figure 1, HAc, HPro, and HBu made up the majority of the SCCs that were generated during the experiments.
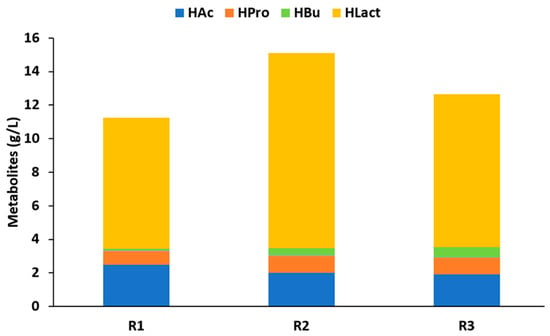
Figure 1.
SCC concentration and distribution reached during the steady state of each reactor performing AF of carrot pulp at 55 °C.
These carboxylic acids have been reported as the predominant products of the AF of carbohydrate-rich feedstock [22]. However, their relative amounts differ between previous investigations due to their dependence on the substrate, inoculum, and operational conditions [23]. HAc was the most prevalent VFA in all reactors regardless of the inoculum source. Given that carbohydrates represented most of the organic matter in the substrate (Table 2), the prevalence of HAc production was expected. When carbohydrates are hydrolyzed, glucose is released as the primary monomer, which is broken down into pyruvate through glycolysis. Pyruvate is the primary precursor for a variety of enzymatic reactions to be converted into acetyl-CoA, and subsequently HAc [23]. R1 showed the highest concentration of HAc (2.5 g VFAs L−1). In turn, the concentration of HBu was higher for R2 and R3. These findings were in line with previous research that found that different substrate types may have an impact on the pathways and enzymes involved in the production of SCCs. For example, protein-rich substrates promoted the production of HVal and isoHVal, whereas carbohydrate-rich substrates are usually converted into the shortest chain of VFAs [20,24].
It is important noting that the production of HAc-HCa carboxylates may be limited by the promotion of alternative pathways. This was the case attained herein, as important concentrations of HLact were determined in all reactors, suggesting that lactate-type fermentation took place. The content of HLact exceeded 70% of the total sCOD concentration (Figure 1), indicating the presence of a particularly selective production process. This high selectivity was mediated by the low pH that directed the AF towards a selective production of HLact. The concentrations of HLact were 7.8, 11.6, and 9.2 g L−1 for R1, R2, and R3, respectively. During acidogenesis, the lactate-dehydrogenase enzyme converts pyruvate into lactate [25]. HLact generated during acidogenesis may serve as an intermediate metabolite for the generation of HAc [26] and HPro [27,28], according to the following equations:
C3H6O3 → 0.5C4H7O2− + 0.5H+ + CO2 + H2 (ΔG0r = −32.9 kJ mol−1)
C3H6O3 → 0.67C3H5O2− + 0.33C2H3O2− + H+ + 0.33CO2 + 0.33H2O (ΔG0r = −56.6 kJ mol−1)
Consequently, the HLact accumulated in all reactors suggested a significant inhibition of the acidogenesis process (as also supported by the low acidogenesis efficiency calculated; Table 4). Also relevant was the fact that HLact productivity was higher in R2 (0.58 g L−1d−1) than R3 (0.47 g L−1d−1), evidencing that the operational temperature was not responsible for the differences in the kinetic production, but rather the microbiome used as the inoculum. This conclusion was also supported by the productivity attained in R1 (0.39 g L−1d−1) that, despite being operated at 55 °C (as was the case in R2 and R3), the differences in microbiomes did not only affect the production but also the productivity. These results gain high relevance, since HLact has been recognized as a valuable building block for the industry, opening a new niche of study to produce this metabolite following a low-cost and sustainable process in the absence of pretreatment or axenic conditions.
3.3. Microbial Community Assessment
The biodiversity in terms of richness (observed OTU) and richness and evenness (Shannon and Simpson indices) were assessed to elucidate the changes in the overall microbial structure in the inocula acclimatized to operate at different temperatures and to determine the microbial population shift when subjected to AF at 55 °C. In contrast to the R2- and R3-inocula, the OTU and Shannon and Simpson diversity indices showed lower values regarding the R1-inoculum (Table 5). This is because the R1-inoculum originated from an anaerobic fermentation process, which led to a microbial community specifically adapted to produce volatile fatty acids (VFAs) [29].

Table 5.
Biodiversity indices resulting from the analysis of the inocula and reactors.
More diversified functional groups (fermentative and hydrolytic bacteria) were probably present in the inocula collected at mesophilic and thermophilic temperatures. When the R1-inoculum was run at 55 °C, no noticeable alterations in biodiversity were observed, but the biodiversities in the R2- and R3-inocula were significantly reduced when compared with the corresponding inocula (Table 5). A similar trend was observed with the observed OTU. Microbial diversity was significantly impacted by the switch from the operational conditions of conventional AD to the operational conditions of AF (55 °C and a pH of 5.1).
The microbial profile attained at the steady state of each reactor and inocula (adapted at different temperatures) were analyzed to further understand the SCC production. Firmicutes (40%) and Proteobacteria (60%) accounted for nearly all of the total readings in the R1-inoculum (Figure 2a), being the most prevalent bacterial phyla. When R1 was run at 55 °C, the relative abundance of these phyla stayed constant, implying that the change in the operating temperature did not significantly affect the microbial population distribution. The use of an inoculum adapted to harsh AF conditions (aligned with a low temperature of 25 °C) supported the wash out of certain phyla, mediating a highly specific microbiome composed by two phyla that exhibited a high robustness, since these bacteria remained constant despite of the temperature change from 25 °C to 55 °C. These results evidenced that an inoculum adaptation to harsh conditions (acidic pH and 25 °C) is a promising strategy to develop a highly stable process, which can ensure the economic profitability for further scaling up. Regarding the R2-inoculum, the relative abundance of these phyla, Firmicutes and Proteobacteria, was lower (30% and 10%, respectively) than in the R1-inoculum. In the R2-inoculum, the other most abundant phyla were Actinobacteriota (18%) and Chloroflexi (10%). This microbial profile is typical of anaerobic digestion processes devoted to biogas production [29]. This inoculum presented a remarkably higher biodiversity than R1-inoculum (Table 5), but the microbial change was more pronounced when the AF was subjected to 55 °C. Similarly, the R3-inoculum also showed higher biodiversity than the R1-inoculum (Table 5) due to the presence of Firmicutes (15%), Proteobacteria (10%), Actinobacteriota (18%), Coprothermobacterota (15%), and Euyarchacota (10%). It should be noted that Coprothermobacterota, known for its proteolytic activity, is also commonly found in thermophilic anaerobic digestion processes [29]. When R3 was operated at 55 °C, the process was dominated by Firmicutes.
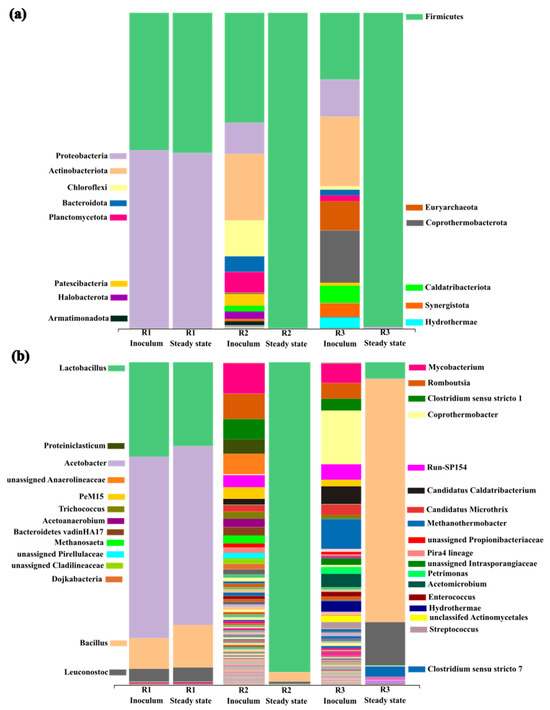
Figure 2.
Relative abundance of bacteria and archaea identified in the inocula and the reactors at (a) phylum and (b) genus level. Only microorganisms with relative abundance higher than 1% have been included in the plot legend.
The prevalence of Firmicutes and Actinobacteria in AF is a common feature, since these phyla have bacteria with hydrolytic and acidogenic capabilities to transform carbohydrates and proteins into SCCs [12,30,31]. Nevertheless, Firmicutes commonly thrive together with Actinobacteria or Bacteriodetes [24]. The boost in the Firmicutes overpopulation was attributed to the harsh operational conditions (pH 5.1) that also resulted in an important selectivity of products in the effluents. Moreover, the remarkable high relative abundance of Firmicutes in the CSTRs was probably associated to the high content of carbohydrates in the feedstocks [9].
Lactobacillus, Acetobacter, Bacillus, and Leuconostoc are among the major genera identified in R1. Nearly 95% of all bacterial genera identified in R2 was Lactobacillus (Figure 2b), which was related to the high concentration of HLact measured in R2 when compared to R1 and R3. By contrast, the dominating genera in R3 were Bacillus, Leuconostoc, Clostridium sensu sctricto 7, and Lactobacillus.
Strains of Lactobacillus have a wide range of metabolic activities and are typically associated, during the hydrolysis and acidogenesis stages, with the degradation of carbohydrates and proteins to produce lactic acid (HLa) and acetic acid (HAc) [9]. The relative abundance of Acetobacter is associated with the degradation of insoluble substrates and the accumulation of acetic acid [32], which explained the higher concentration of acetic acid observed in R1.
Both Bacillus and Clostridium sensu stricto 7 have been shown to have a large population in thermophilic AD reactors, and to be involved in the degradation of carbohydrate-rich substrate [33]. Leuconostoc is a member of the lactic acid bacteria (LAB) genus, which is characterized by the production HLact via the fermentation of carbohydrates. In this case, it should be highlighted that, despite of the fact that Leuconostoc and Bacillus are also HLact producers, their ability for conversion and productivity were lower than that of Lactobacillus. All of them supported a high selectivity towards HLact production, but when it comes to further investigation, the productivity should also be considered.
4. Conclusions
The inoculum source had an impact on the production yields of SCCs. Even though all microbial inocula behaved similarly in terms of hydrolysis when conducted at 55 °C AF, the acidogenesis efficiency was marked by the microbial diversity of the inocula. The highest biodiversity of the inoculum adapted to work at 35 °C (R2) enhanced the acidification and bioconversion efficiencies, while the same conclusion cannot be drawn with the inoculum coming from the 55 °C digester. When switching to AF conditions with mesophilic (35 °C) and thermophilic (55 °C) inocula, the diversity and richness of bacteria were sharply reduced. Firmicutes became the only phylum to dominate the new niche in reactors operated at 55 °C, but inoculated with microbiomes adapted to work at 35 and 55 °C. This was of particular relevance in terms of HLact production. Selective HLact production was mediated by Firmicutes prevalence, while the different genera developed had a direct impact on the concentration and productivity. The prevalence of Lactobacillus coming from the inoculum adapted to work at 35 °C was key in the AF conducted at 55 °C in terms of product selectivity within the carboxylates pool.
Author Contributions
Conceptualization, S.G. and C.G.-F.; methodology, C.C.-R.; formal analysis, C.C.-R., R.A.T., A.M., S.G. and C.G.-F.; investigation, C.C.-R., S.G. and C.G.-F.; data curation, C.C.-R., S.G. and C.G.-F.; writing—original draft preparation, C.C.-R., R.A.T. and A.M.; writing—review and editing, C.C.-R., R.A.T., A.M., S.G. and C.G.-F.; supervision, S.G. and C.G.-F.; funding acquisition, C.C.-R., S.G. and C.G.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the State Research Agency, the European Union—Next Generation EU, the Recovery, Transformation and Resilience Plan, and the Ministry of Science and Innovation (MICINN) through the project OILI (CNS2022-135848), by Banco Santander through the personal fellow CONTPR-2022-418 and the grant RyC2023-045083-I funded by MICIU/AEI/10.13039/501100011033 and by ESF+.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AF | Anaerobic fermentation |
| SCCs | Short-chain carboxylates |
| COD | Chemical oxygen demand |
| AD | Anaerobic digestion |
| TSs | Total solids |
| VSs | Volatile solids |
| OLR | Organic loading rate |
| HRT | Hydraulic retention time |
| HAc | Acetic acid |
| HPro | Propionic acid |
| HBu | Butyric acid |
| isoHBu | Isobutyric acid |
| HVal | Valeric acid |
| isoHVal | Isovaleric acid |
| HCa | Caproic acid |
| HLact | Lactic acid |
| NKT | Total Kjeldahl nitrogen |
References
- Food Waste Index Report 2024. Think Eat Save: Tracking Progress to Halve Global Food Waste|Knowledge for Policy. Available online: https://knowledge4policy.ec.europa.eu/publication/food-waste-index-report-2024-think-eat-save-tracking-progress-halve-global-food-waste_en?utm_source.com (accessed on 16 July 2025).
- Qin, W.; Han, S.; Meng, F.; Chen, K.; Gao, Y.; Li, J.; Lin, L.; Hu, E.; Jiang, J. Impacts of seasonal variation on volatile fatty acids production of food waste anaerobic fermentation. Sci. Total Environ. 2024, 912, 168764. [Google Scholar] [CrossRef] [PubMed]
- Akhter, M.J.; Kabir, M.I.; Sohany, M.; Islam, M.H.; Khatun, A.A.; Hosen, A.; Kabir, M.F. Effect of carrot pulp on the physicochemical, microbiological and sensory attributes of kulfi. Food Res. 2024, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, I.; Ibrahim, A. Functional jam production from blends of carrot and sweet potato pulp. Int. J. Food Sci. Nutr. 2021, 6, 10–15. [Google Scholar]
- Khan, S.R. Utilization Carrot Pulp as Corn Replacement in the Broiler Diet. J. Agric. Vet. Sci. 2019, 12, 72–74. [Google Scholar] [CrossRef]
- Akcakaya, M.; Tuncay, S.; Icgen, B. Two-stage anaerobic digestion of ozonated sewage sludge predominantly took over by acetotrophic methanogens with increased biogas and methane production. Fuel 2022, 317, 123434. [Google Scholar] [CrossRef]
- Strazzera, G.; Battista, F.; Herrero-Garcia, N.; Frison, N.; Bolzonella, D. Volatile fatty acids production from food wastes for biorefinery platforms: A review. J. Environ. Manag. 2018, 226, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ren, X.; Xuan, Y.; Liu, M.; Bai, G.; Jiang, F. Enhancement of volatile fatty acids to extremely high content in fermentation of food waste: Optimization of conditions, microbial functional genes, and mechanisms. Bioresour. Technol. 2025, 416, 131735. [Google Scholar] [CrossRef] [PubMed]
- Greses, S.; Tomás-Pejó, E.; González-Fernández, C. Assessing the relevance of acidic pH on primary intermediate compounds when targeting at carboxylate accumulation. Biomass Convers. Biorefinery 2022, 12, 4519–4529. [Google Scholar] [CrossRef]
- Shen, D.; Yin, J.; Yu, X.; Wang, M.; Long, Y.; Shentu, J.; Chen, T. Acidogenic fermentation characteristics of different types of protein-rich substrates in food waste to produce volatile fatty acids. Bioresour. Technol. 2017, 227, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Wang, H. Volatile fatty acids productions by mesophilic thermophilic sludge fermentation: Biological responses to fermentation temperature. Bioresour. Technol. 2015, 175, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Greses, S.; Tomás-Pejó, E.; González-Fernández, C. Food waste valorization into bioenergy and bioproducts through a cascade combination of bioprocesses using anaerobic open mixed cultures. J. Clean. Prod. 2022, 372, 133680. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017; ISBN 978-0875532875. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Mariotti, F.; Tomé, D.; Mirand, P.P. Converting nitrogen into protein—Beyond 6.25 and Jones’ factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Greses, S.; Gaby, J.C.; Aguado, D.; Ferrer, J.; Seco, A.; Horn, S.J. Microbial community characterization during anaerobic digestion of Scenedesmus spp. under mesophilic and thermophilic conditions. Algal Res. 2017, 27, 121–130. [Google Scholar] [CrossRef]
- Fernandez-Bayo, J.D.; Simmons, C.W.; VanderGheynst, J.S. Characterization of digestate microbial community structure following thermophilic anaerobic digestion with varying levels of green and food wastes. J. Ind. Microbiol. Biotechnol. 2020, 47, 1031–1044. [Google Scholar] [CrossRef] [PubMed]
- Cavinato, C.; Da Ros, C.; Pavan, P.; Bolzonella, D. Influence of temperature and hydraulic retention on the production of volatile fatty acids during anaerobic fermentation of cow manure and maize silage. Bioresour. Technol. 2017, 223, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Aguirre, J.; Aymerich, E.; Gonzalez-Mtnez de Goni, J.; Esteban-Gutierrez, M. Selective VFA production potential from organic waste streams: Assessing temperature and pH influence. Bioresour. Technol. 2017, 244, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- de Souza Moraes, B.; Dos Santos, G.M.; Delforno, T.P.; Fuess, L.T.; da Silva, A.J. Enriched microbial consortia for dark fermentation of sugarcane vinasse towards value-added short-chain organic acids and alcohol production. J. Biosci. Bioeng. 2019, 127, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yin, J.; Shen, D.; Li, N. Anaerobic digestion of food waste for volatile fatty acids (VFAs) production with different types of inoculum: Effect of pH. Bioresour. Technol. 2014, 161, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Strazzera, G.; Battista, F.; Tonanzi, B.; Rossetti, S.; Bolzonella, D. Optimization of short chain volatile fatty acids production from household food waste for biorefinery applications. Environ. Technol. Innov. 2021, 23, 101562. [Google Scholar] [CrossRef]
- Xiao, X.; Hu, H.; Meng, X.; Huang, Z.; Yongrui Feng, Y.; Gao, Q.; Ruan, W. Volatile fatty acids production from kitchen waste slurry using anaerobic membrane bioreactor via alkaline fermentation with high salinity: Evaluation on process performance and microbial succession. Bioresour. Technol. 2024, 399, 130576. [Google Scholar] [CrossRef] [PubMed]
- Llamas, M.; Greses, S.; Tomas-Pejo, E.; Gonzalez-Fernandez, C. Carboxylic acids production via anaerobic fermentation: Microbial communities’ responses to stepwise and direct hydraulic retention time decrease. Bioresour. Technol. 2022, 344, 126282. [Google Scholar] [CrossRef] [PubMed]
- Sikora, A.; Baszczyk, M.; Jurkowski, M.; Zielenkiewicz, U. Lactic Acid Bacteria in Hydrogen-Producing Consortia: On Purpose or by Coincidence? In Lactic Acid Bacteria—R & D for Food, Health and Livestock Purposes; IntechOpen: London, UK, 2013. [Google Scholar]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Yang, Y.-H. Microbial production of volatile fatty acids: Current status and future perspectives. Rev. Environ. Sci. Biotechnol. 2017, 16, 327–345. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, B.; Wong, J.W.C.; Zhang, Y. Enhanced volatile fatty acids production from anaerobic fermentation of food waste: A mini-review focusing on acidogenic metabolic pathways. Bioresour. Technol. 2018, 248, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.J.; González-Fernández, C.; Greses, S. Long hydraulic retention time mediates stable volatile fatty acids production against slight pH oscillations. Waste Manag. 2024, 176, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Kim, S.J.; Raza, S.; Lee, S.; Lee, J.; Son, H.; Wang, J.; Kim, Y.M. Temperature is more important than solid retention time in biogas production and resistome dynamics in anaerobic digestion with recuperative thickening. Chem. Eng. J. 2025, 514, 163355. [Google Scholar] [CrossRef]
- Garcia-Peña, E.I.; Parameswaran, P.; Kang, D.W.; Canul-Chan, M.; Krajmalnik-Brown, R. Anaerobic digestion and co-digestion processes of vegetable and fruit residues: Process and microbial ecology. Bioresour. Technol. 2011, 102, 9447–9455. [Google Scholar] [CrossRef] [PubMed]
- Elferink, S.J.O.; Krooneman, J.; Gottschal, J.C.; Spoelstra, S.F.; Faber, F.; Driehuis, F. Anaerobic conversion of lactic acid to acetic acid and 1, 2-propanediol by Lactobacillus buchneri. Appl. Environ. Microb. 2001, 67, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Cetecioglu, Z.; Atasoy, M.; Cenian, A.; Sołowski, G.; Trček, J.; Ugurlu, A.; Sedlakova-Kadukova, J. Bio-Based Processes for Material and Energy Production from Waste Streams under Acidic Conditions. Fermentation 2022, 8, 115. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).