Lithological Controls on Chemical Weathering and CO2 Consumption at Small Watershed Scale: Insights from Hydrochemistry and Stable Carbon Isotope
Abstract
1. Introduction
- (1)
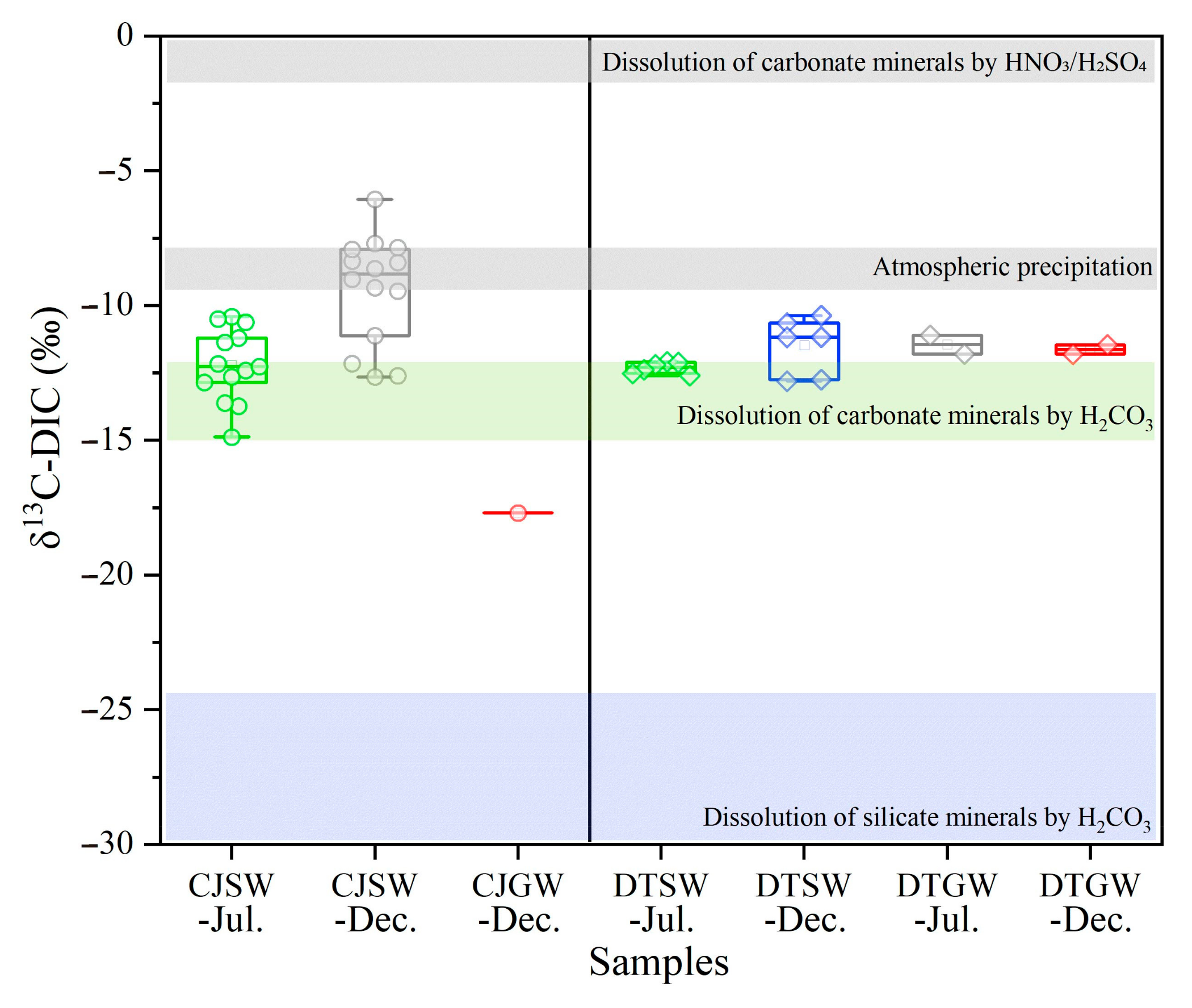
- Silicate weathering by H2CO3 produces DIC entirely derived from soil CO2 (Equations (1) and (2)), preserving the soil CO2 isotopic signature. The δ13C values of CO2 derived from both root autotrophic respiration and heterotrophic decomposition of soil organic matter closely match those of the parent plant material [7]. For instance, the predominant C3 plants in South China yields δ13C-DIC values ranging from −30.0‰ to −24.2‰.
- (2)
- Carbonate weathering by H2CO3 produces DIC, with half derived from carbonate minerals (δ13C-DIC≈0‰) and half from soil CO2 (δ13C-DIC≈−30.0‰ to −24.2‰) as shown in Equation (3), resulting in δ13C-DIC values of approximately −15.0‰ to −12.1‰.
- (3)
- Carbonate weathering by HNO3 or H2SO4 produces DIC entirely sourced from carbonate minerals (δ13C≈0‰) (Equations (4) and (5)), yielding δ13C-DIC values near 0‰.
2. Materials and Methods
2.1. Study Area
2.2. Sampling and Analysis
3. Results and Discussion
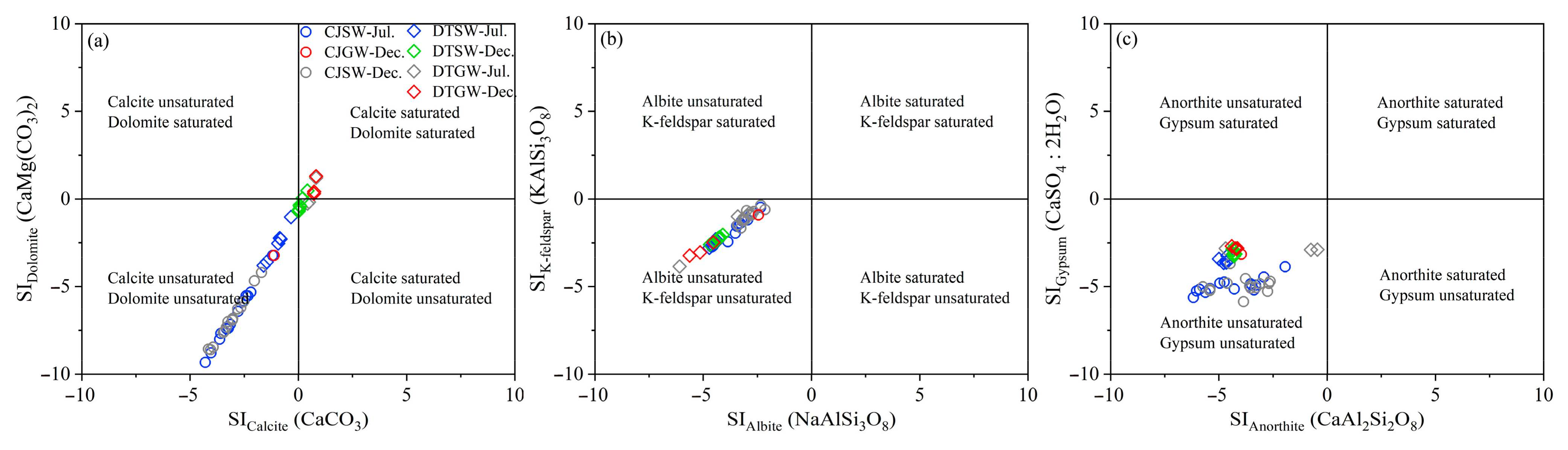
3.1. Hydrochemical Characteristics
3.2. Identification of CO2 Consumption During Chemical Weatherin Using δ13C-DIC
3.3. Quantitative Analysis of Chemical Weathering and CO2 Consumption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goudie, A.S.; Viles, H.A. Weathering and the global carbon cycle: Geomorphological perspectives. Earth Sci. Rev. 2012, 113, 59–71. [Google Scholar] [CrossRef]
- Martin, J. Carbonate minerals in the global carbon cycle. Chem. Geol. 2017, 449, 58–72. [Google Scholar] [CrossRef]
- Liu, Z.; Dreybrodt, W.; Liu, H. Atmospheric CO2 sink: Silicate weathering or carbonate weathering. Appl. Geochem. 2011, 26, S292–S294. [Google Scholar] [CrossRef]
- Tank, S.E.; Raymond, P.A.; Striegl, R.G.; McClelland, J.W.; Holmes, R.M.; Fiske, G.J.; Peterson, B.J. A land-to-ocean perspective on the magnitude, source and implication of DIC flux from major Arctic rivers to the Arctic Ocean. Global Biogeochem. Cycles 2012, 26, GB4018. [Google Scholar] [CrossRef]
- Hagedorn, B.; Cartwright, I. Climatic and lithologic controls on the temporal and spatial variability of CO2 consumption via chemical weathering: An example from the Australian Victorian Alps. Chem. Geol. 2009, 260, 234–253. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, F.; Yang, H.; Yu, S. Role of anthropogenic sulfuric and nitric acids in carbonate weathering and associated carbon sink budget in a karst catchment (Guohua), southwestern China. J. Hydrol. 2021, 599, 126287. [Google Scholar] [CrossRef]
- Liu, J.K.; Han, G.L.; Zhang, Q.; Liu, M.; Li, X.Q. Stable isotopes and Bayesian tracer mixing model reveal chemical weathering and CO2 release in the Jiulongjiang River Basin, Southeast China. Water Resour. Res. 2022, 58, e2021WR031738. [Google Scholar] [CrossRef]
- Gaillardet, J.; Dupre, B.; Louvat, P.; Allegre, C.J. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Moon, S.; Huh, Y.; Qin, J.; van Pho, N. Chemical weathering in the Hong (Red) River basin: Rates of silicate weathering and their controlling factors. Geochim. Cosmochim. Acta 2007, 71, 1411–1430. [Google Scholar] [CrossRef]
- Zhong, J.; Li, S.L.; Liu, J.; Ding, H.; Sun, X.L.; Xu, S.; Wang, T.J.; Ellam, R.M.; Liu, C.Q. Climate variability controls on CO2 consumption fluxes and carbon dynamics for monsoonal rivers: Evidence from Xijiang River, Southwest China. J. Geophys. Res. Biogeo. 2018, 123, 2553–2567. [Google Scholar] [CrossRef]
- Millot, R.; Gaillardet, J.É.; Dupré, B.; Allègre, C.J. Northern latitude chemical weathering rates: Clues from the Mackenzie River Basin, Canada. Geochim. Cosmochim. Acta 2003, 67, 1305–1329. [Google Scholar] [CrossRef]
- Qin, X.; Jiang, Z.; Zhang, L.; Huang, Q.; Liu, P. The difference of the weathering rate between carbonate rocks and silicate rocks and its effects on the atmospheric CO2 consumption in the Pearl River Basin. Geol. Bull. China 2015, 34, 1749–1757. [Google Scholar]
- Jiang, P.; Yu, G.; Zhang, Q.; Zou, Y.; Tang, Q.; Kang, Z.; Sytharith, P.; Xiao, H. Chemical weathering and CO2 consumption rates of rocks in the Bishuiyan subterranean basin of Guangxi, China. Sci. Rep. 2020, 10, 11677. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, J.; Li, S.; Lang, Y.; Zhu, X.; Chen, S. The effects of hydrological variations on chemical weathering: Evidences from temporal water chemistry, stable carbon and sulfur isotopes. CATENA 2022, 214, 106301. [Google Scholar] [CrossRef]
- Peng, H.; Liu, L.; Tao, Z.; Lv, X.; Li, S.; Deng, H.; Gao, Q. Chemical Weathering and Seasonal Variation of Chemical Compositions of Water in Humid Subtropical Basins—A Case Study for the Xizhijiang River Basin. Earth Environ. 2018, 46, 513–523. [Google Scholar]
- Sun, P.; Yu, S.; Mo, F.; He, S.; Lu, J.; Yuan, Y. Hydrochemical Characteristics and Influencing Factors in Different Geological Background: A Case Study in Darongjiang and Lingqu Basin, Guangxi, China. Environ. Sci. 2016, 37, 123–131. [Google Scholar]
- Maher, K.; Chamberlain, C.P. Hydrologic regulation of chemical weathering and the geologic carbon cycle. Science 2014, 343, 1502–1504. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S.; Wang, J.; Guo, F.; Yu, S.; Sun, P. The hydrochemistry characteristics and chemical weathering intensity of an anthropogenically involved catchment, South China. Water 2024, 16, 2444. [Google Scholar] [CrossRef]
- Amrish, V.N.; Arun, K.; Nishitha, D.S.; Balakrishna, K.; Udayashankar, H.N.; Khare, N. Major ion chemistry and silicate weathering rate of a small Western Ghats river, Sharavati, southwestern India. Appl. Geochem. 2022, 136, 105182. [Google Scholar] [CrossRef]
- Ulloa⁃Cedamanos, F.; Probst, A.; Moussa, I.; Probst, J.L. Chemical weathering and CO2 consumption in a multi-lithological karstic critical zone: Long term hydrochemical trends and isotopic survey. Chem. Geol. 2021, 585, 120567. [Google Scholar] [CrossRef]
- Xu, S.; Li, S.L.; Su, J.; Yue, F.J.; Zhong, J.; Chen, S. Oxidation of pyrite and reducing nitrogen fertilizer enhanced the carbon cycle by driving terrestrial chemical weathering. Sci. Total Environ. 2021, 768, 144343. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Dietrich, W.E.; Thurnhoffer, B.M.; Bishop, J.K.B.; Fung, I.Y. Controls on solute concentration-discharge relationships revealed by simultaneous hydrochemistry observations of hillslope runoff and stream flow: The importance of critical zone structure. Water Resour. Res. 2017, 53, 1424–1443. [Google Scholar] [CrossRef]
- Song, C.L.; Wang, G.X.; Mao, T.X.; Huang, K.W.; Sun, X.Y.; Hu, Z.Y.; Chang, R.Y.; Chen, X.P.; Raymond, P.A. Spatiotemporal variability and sources of DIC in Permafrost catchments of the Yangtze River source region: Insights from stable carbon isotope and water chemistry. Water Resour. Res. 2020, 55, e2019WR025343. [Google Scholar] [CrossRef]
- Liu, J.K.; Han, G.L. Effects of chemical weathering and CO2 outgassing on δ13CDIC signals in a karst watershed. J. Hydrol. 2020, 589, 125192. [Google Scholar] [CrossRef]
- Fu, Y.C.; Tang, C.G.; Li, J.; Zhao, Y.L.; Zhong, W.; Zeng, X.T. Sources and transport of organic carbon from the Dongjiang River to the Humen outlet of the Pearl River, southern China. J. Geogr. Sci. 2014, 24, 143–158. [Google Scholar] [CrossRef]
- Xuan, Y.X.; Cao, Y.J.; Tang, C.Y.; Li, M. Changes in dissolved inorganic carbon in river water due to urbanization revealed by hydrochemistry and carbon isotope in the Pearl River Delta, China. Environ. Sci. Pollut. 2020, 27, 24542–24557. [Google Scholar] [CrossRef]
- Levin, I.; Graul, R.; Trivett, N.B.A. Long-term observations of atmospheric CO2 and carbon isotopes at continental sites in Germany. Tellus B 1995, 47, 23–34. [Google Scholar] [CrossRef]
- Marfia, A.M.; Krishnamurthy, R.V.; Atekwana, E.A.; Panton, W.F. Isotopic and geochemical evolution of ground and surface waters in a karst dominated geological setting: A case study from Belize, Central America. Appl. Geochem. 2004, 19, 937–946. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, B.; Shen, L.J.; Kang, H.Q. Size distributions of aerosol and water-soluble ions in Nanjing during a crop residual burning event. J. Environ. Sci. 2012, 24, 1457–1465. [Google Scholar] [CrossRef]
- Trancone, G.; Spasiano, D.; Race, M.; Luongo, V.; Petrella, A.; Pirozzi, F.; Fratino, U.; Piccinni, A.F. A combined system for asbestoscement waste degradation by dark fermentation and resulting supernatant valorization in anaerobic digestion. Chemosphere 2022, 300, 134500. [Google Scholar] [CrossRef]
- El Housse, M.; Hadfi, A.; Karmal, I.; Tadoumant, S.; Ben-aazza, S.; Errami, M.; Belattar, M.; Mohareb, S.; Tounsi, A.; Driouiche, A. Study of scaling problem in the region of tata (Morocco): Analysis of the elemental composition, crystalline phases, and morphologies of scale deposition in water installations. Appl. Radiat. Isot. 2022, 188, 110388. [Google Scholar] [CrossRef] [PubMed]
- Oliva, P.; Viers, J.; Dupré, B. Chemical weathering in granitic environments. Chem. Geol. 2003, 202, 225–256. [Google Scholar] [CrossRef]
- Fan, B.; Zhao, Z.Q.; Tao, F.X.; Liu, B.J.; Tao, Z.H.; Gao, S.; Zhang, L.H. Characteristics of carbonate, evaporite and silicate weathering in Huanghe River basin: A comparison among the upstream, midstream and downstream. J. Asian Earth Sci. 2014, 96, 17–26. [Google Scholar] [CrossRef]
- Chetelat, B.; Liu, C.Q.; Zhao, Z.; Wang, Q.; Li, S.; Li, J.; Wang, B. Geochemistry of the dissolved load of the Changjiang Basin rivers: Anthropogenic impacts and chemical weathering. Geochim. Cosmochim. Acta 2008, 72, 4254–4277. [Google Scholar] [CrossRef]
- Piper, A.M. A graphic procedure in geochemical interpretation of water analyses. Eos Trans. Am. Geophys. Union 1944, 25, 914–923. [Google Scholar]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Marandi, A.; Shand, P. Groundwater chemistry and the Gibbs Diagram. Appl. Geochem. 2018, 97, 209–212. [Google Scholar] [CrossRef]
- Sun, P.; He, S.; Yuan, Y.; Yu, S.; Zhang, C. Effects of aquatic phototrophs on seasonal hydrochemical, inorganic, and organic carbon variations in a typical karst basin, Southwest China. Environ. Sci. Pollut. 2019, 26, 32836–32851. [Google Scholar] [CrossRef]
- Cao, Y.; Xuan, Y.; Tang, C.; Guan, S.; Peng, Y. Temporary and net sinks of atmospheric CO2 due to chemical weathering in subtropical catchment with mixing carbonate and silicate lithology. Biogeosciences 2020, 17, 3875–3890. [Google Scholar] [CrossRef]
- Galy, A.; France, L.C. Weathering processes in the Ganges-Brahmaputra basin and the riverine alkalinity budget. Chem. Geol. 1999, 159, 31–60. [Google Scholar] [CrossRef]
- Zhou, H.L.; Li, Z.X.; Yu, Z.L.; Wang, Y.Y.; Du, F.; Xue, J. Permafrost impacts on chemical weathering and CO2 budgets in the Tibetan Plateau: Micro-watershed perspective on a headwater catchment. CATENA 2024, 247, 108479. [Google Scholar]
- Li, L.; Liu, L.Q.; Zhou, G.Y.; Qiu, Z.J.; Zhao, H.B. Effects of canopy damage on hydrochemistry of throughfall and stemflow in evergreen broadleaved forest of Nanling Mountains. J. Soil Water Conserv. 2014, 28, 45–50. [Google Scholar]
- Hren, M.T.; Chamberlain, C.P.; Hilley, G.E.; Blisniuk, P.M.; Bookhagen, B. Major ion chemistry of the Yarlung Tsangpo–Brahmaputra river: Chemical weathering, erosion, and CO2 consumption in the southern Tibetan plateau and eastern syntaxis of the Himalaya. Geochim. Cosmoschim. Acta 2007, 71, 2907–2935. [Google Scholar] [CrossRef]
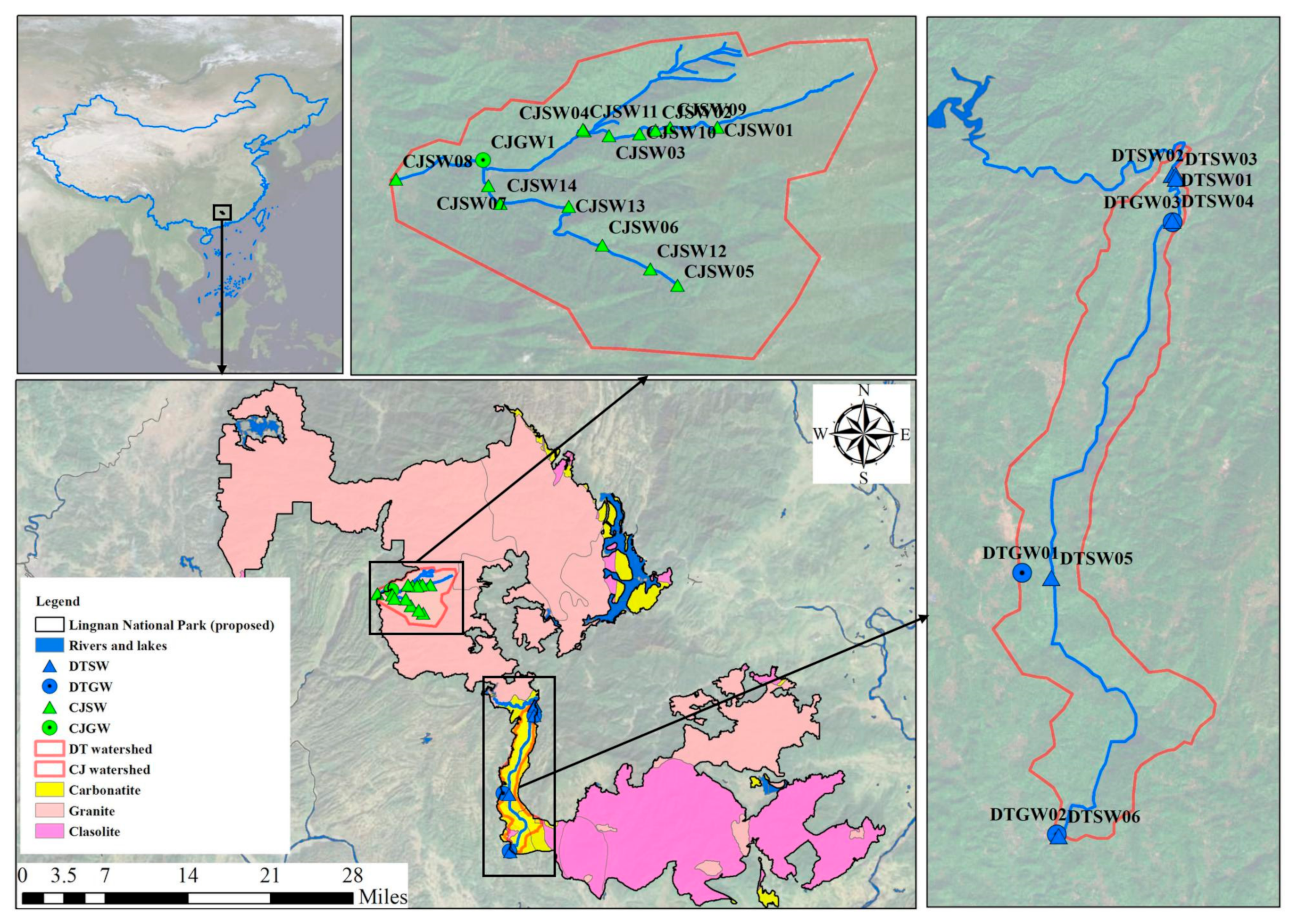
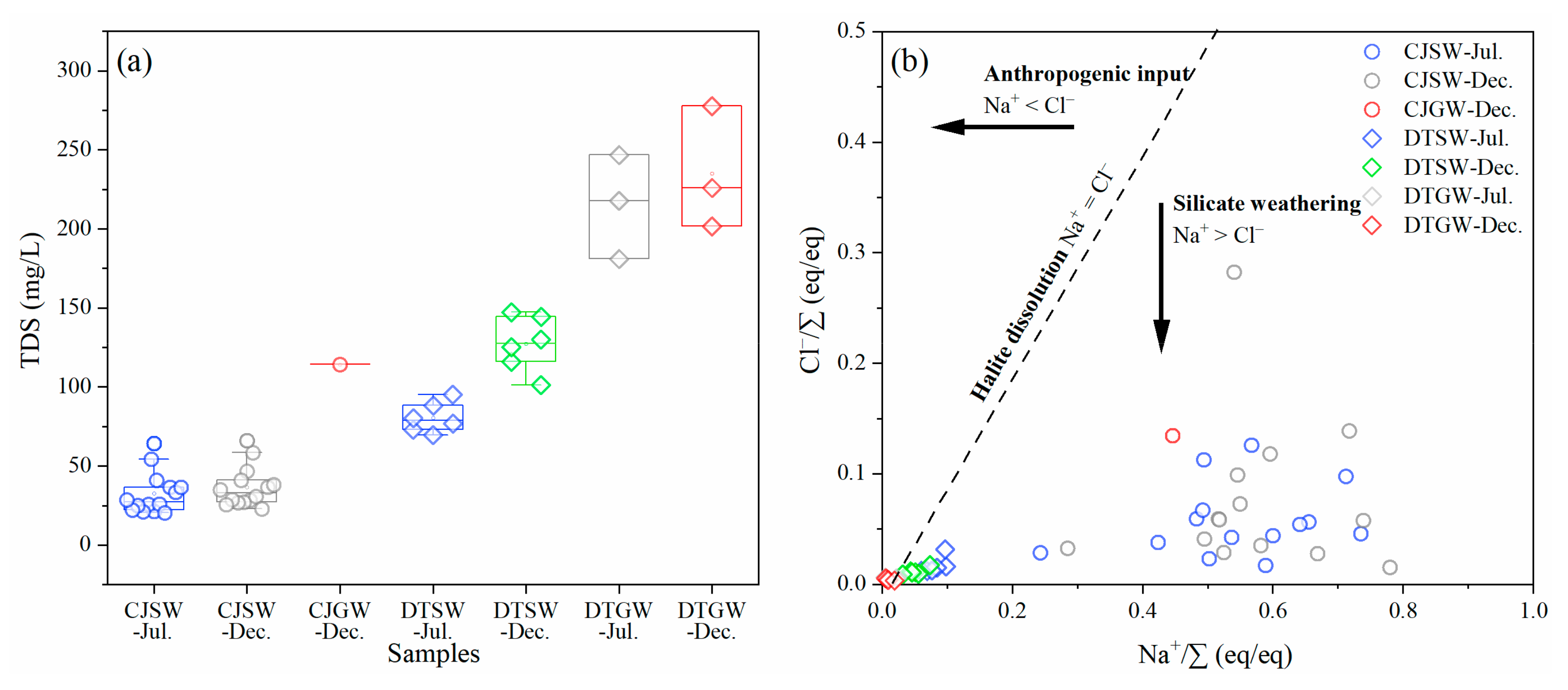
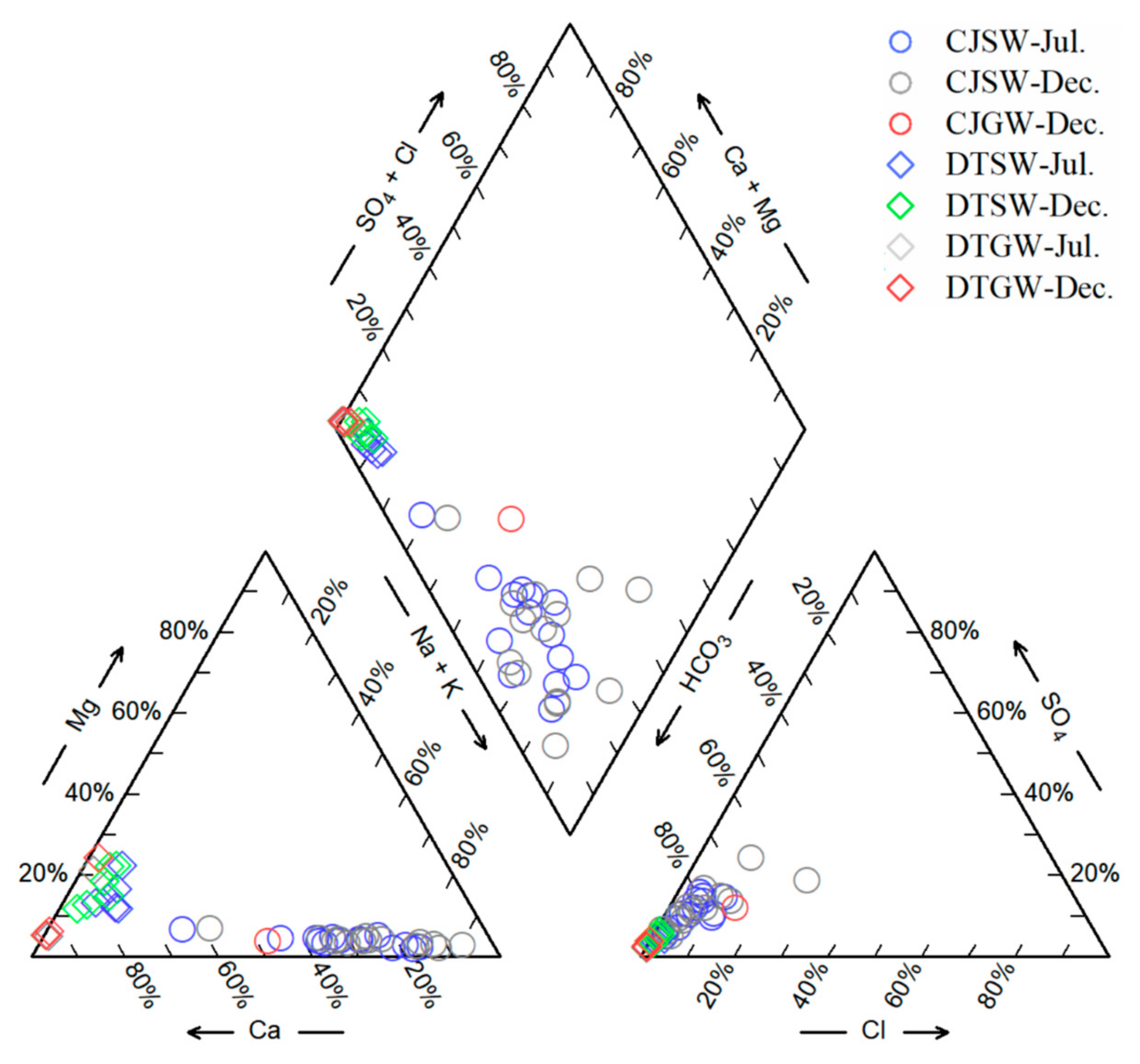
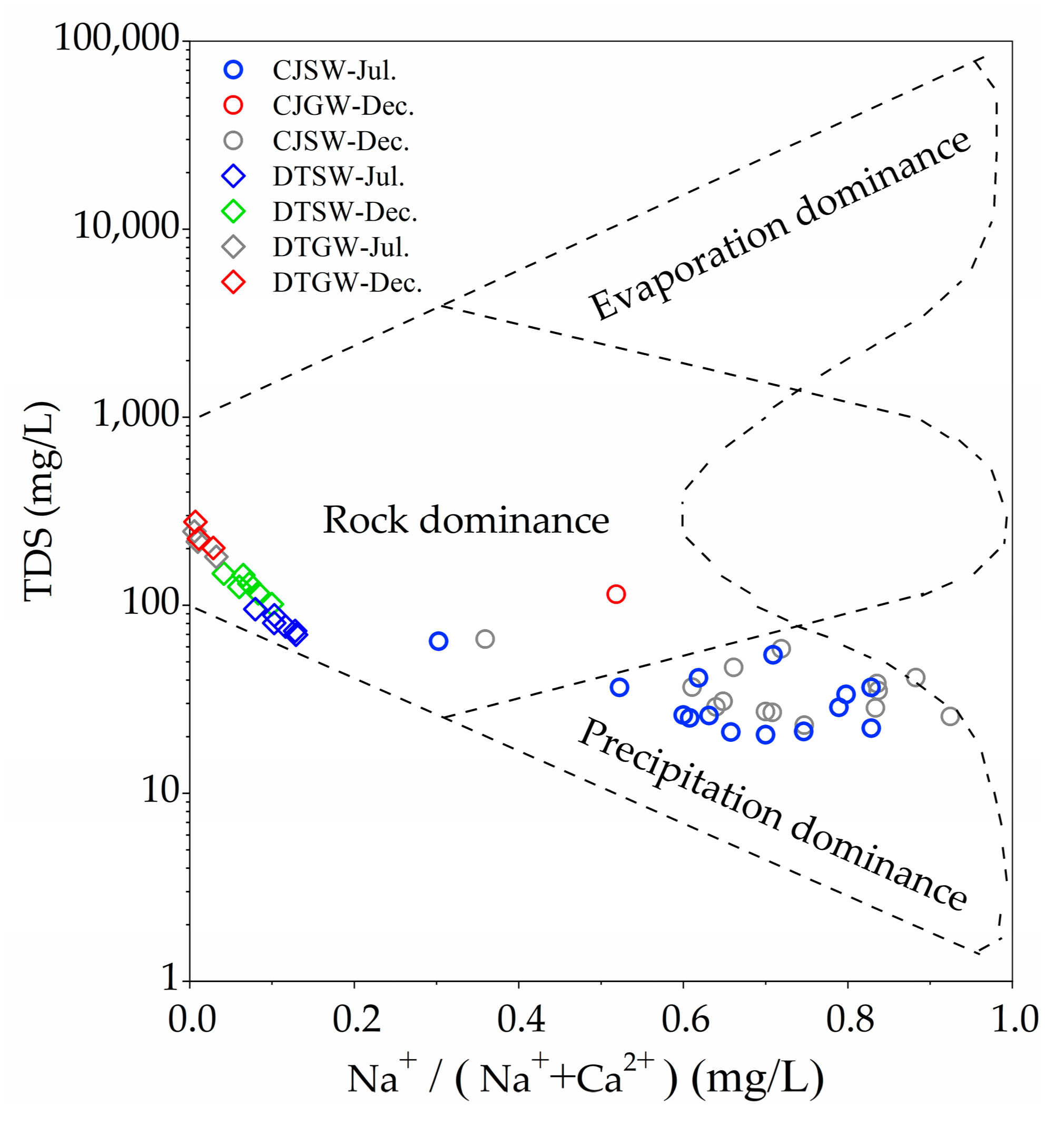

| Sample Number | TDS | pH | Ca2+ | Mg2+ | Na+ | K+ | Cl− | SO42− | HCO3− | NO3− | SiO2 | δ13C | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/L | / | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | ‰ | |||
| CJSW -July | 14 | Max | 64.4 | 7.7 | 10.5 | 0.6 | 5.7 | 1.5 | 0.8 | 1.9 | 45.0 | 2.2 | 14.5 | −10.4 |
| Min | 20.5 | 6.4 | 0.4 | 0.0 | 1.7 | 0.3 | 0.2 | 0.7 | 5.1 | 0.2 | 5.1 | −14.9 | ||
| Ave | 32.7 | 7.1 | 1.9 | 0.1 | 2.9 | 0.9 | 0.4 | 1.1 | 13.3 | 0.9 | 8.3 | −12.2 | ||
| CJSW -December | 14 | Max | 66.1 | 7.5 | 9.2 | 0.6 | 6.0 | 2.4 | 2.0 | 3.2 | 38.0 | 3.3 | 16.2 | −6.1 |
| Min | 23.0 | 6.7 | 0.2 | 0.0 | 1.9 | 0.5 | 0.2 | 0.8 | 5.4 | 0.0 | 6.1 | −12.7 | ||
| Ave | 36.7 | 7.0 | 1.7 | 0.1 | 3.4 | 1.3 | 0.6 | 1.4 | 13.6 | 0.9 | 9.5 | −9.4 | ||
| CJGW -December | 1 | Max | / | |||||||||||
| Min | / | |||||||||||||
| Ave | 114.3 | 7.2 | 14.3 | 0.7 | 15.4 | 2.4 | 7.2 | 7.8 | 62.1 | 2.6 | 15.4 | −17.7 | ||
| DTSW -July | 6 | Max | 95.3 | 7.7 | 26.1 | 4.1 | 2.5 | 1.5 | 1.3 | 3.8 | 89.0 | 2.3 | 6.1 | −12.1 |
| Min | 69.6 | 6.5 | 16.9 | 1.6 | 2.3 | 0.9 | 0.6 | 2.1 | 59.5 | 2.1 | 5.6 | −12.6 | ||
| Ave | 80.6 | 7.1 | 20.1 | 2.5 | 2.4 | 1.0 | 0.8 | 2.6 | 72.9 | 2.2 | 5.9 | −12.3 | ||
| DTSW -December | 6 | Max | 147.4 | 8.0 | 48.8 | 7.7 | 3.0 | 1.6 | 1.2 | 5.8 | 141.1 | 2.4 | 7.5 | −10.4 |
| Min | 101.3 | 7.5 | 27.3 | 3.2 | 2.1 | 1.1 | 0.8 | 3.5 | 86.8 | 1.7 | 5.4 | −12.8 | ||
| Ave | 127.4 | 7.8 | 37.5 | 5.0 | 2.7 | 1.4 | 1.0 | 4.5 | 118.3 | 2.1 | 6.6 | −11.5 | ||
| DTGW -July | 3 | Max | 246.9 | 8.2 | 92.0 | 8.5 | 1.7 | 0.9 | 1.1 | 5.7 | 277.6 | 2.4 | 6.1 | −16.6 |
| Min | 181.0 | 7.4 | 50.4 | 2.4 | 0.5 | 0.4 | 0.7 | 4.1 | 196.0 | 0.4 | 3.0 | −16.6 | ||
| Ave | 215.3 | 7.7 | 72.6 | 4.6 | 1.0 | 0.7 | 0.8 | 4.7 | 238.9 | 1.7 | 4.5 | −16.6 | ||
| DTGW -December | 3 | Max | 277.7 | 8.1 | 115.4 | 12.3 | 1.8 | 0.9 | 1.2 | 6.2 | 284.9 | 1.8 | 6.5 | −11.5 |
| Min | 201.8 | 7.6 | 61.7 | 3.5 | 0.8 | 0.5 | 0.5 | 4.3 | 205.2 | 0.3 | 3.7 | −16.5 | ||
| Ave | 235.1 | 7.8 | 88.9 | 6.5 | 1.2 | 0.8 | 0.8 | 5.1 | 239.4 | 1.3 | 5.0 | −13.2 | ||
| Contribution of Different Sources to Cations of River Water | CJSW- July | CJSW- December | DTSW- July | DTSW- December |
|---|---|---|---|---|
| Carbonate weathering (%) | / | / | 82.6 | 90.2 |
| Silicate weathering (%) | 77.9 | 81.1 | 12.9 | 7.7 |
| Atmospheric precipitation (%) | 19.6 | 14.8 | 4.5 | 2.1 |
| Anthropogenic input (%) | / | / | / | / |
| Evaporite dissolution (%) |
| Contribution to Cations of River Water | CJSW- July | CJSW- December | DTSW- July | DTSW- December |
|---|---|---|---|---|
| [Ca2+]sil (mg/L) | 1.2 | 1.1 | 0.9 | 0.9 |
| [Mg2+]sil (mg/L) | 0.1 | 0.1 | 0.1 | 0.1 |
| [K+]sil (mg/L) | 0.6 | 1.1 | 0.5 | 0.5 |
| [Na+]sil (mg/L) | 2.7 | 3.1 | 1.9 | 2.1 |
| [Ca2+]carb (mg/L) | / | / | 18.5 | 36.1 |
| [Mg2+]carb (mg/L) | / | / | 2.4 | 4.9 |
| CWRsil | CWRcarb | CWRsun | CCRsil | CCRcarb | CCRsum | |
|---|---|---|---|---|---|---|
| CJ-July | 0.22 | 0 | 0.22 | 0.26 | 0 | 0.26 |
| CJ-December | 0.26 | 0 | 0.26 | 0.30 | 0 | 0.30 |
| CJ-Ave. | 0.24 | 0 | 0.24 | 0.28 | 0 | 0.28 |
| DT-July | 0.18 | 0.65 | 0.83 | 0.21 | 1.30 | 1.51 |
| DT-December | 0.21 | 1.29 | 1.50 | 0.24 | 2.58 | 2.82 |
| DT-Ave. | 0.20 | 0.97 | 1.17 | 0.23 | 1.94 | 2.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Huang, W.; Zhuang, Z.; Hua, J.; Bai, L.; Ding, Y.; Zheng, L.; Wang, C.; Zhao, C.; Liu, Y. Lithological Controls on Chemical Weathering and CO2 Consumption at Small Watershed Scale: Insights from Hydrochemistry and Stable Carbon Isotope. Water 2025, 17, 2008. https://doi.org/10.3390/w17132008
Zhang Y, Huang W, Zhuang Z, Hua J, Bai L, Ding Y, Zheng L, Wang C, Zhao C, Liu Y. Lithological Controls on Chemical Weathering and CO2 Consumption at Small Watershed Scale: Insights from Hydrochemistry and Stable Carbon Isotope. Water. 2025; 17(13):2008. https://doi.org/10.3390/w17132008
Chicago/Turabian StyleZhang, Yuanzheng, Wenlong Huang, Zhuohan Zhuang, Jing Hua, Litong Bai, Yi Ding, Ling Zheng, Cheng Wang, Chuang Zhao, and Yunde Liu. 2025. "Lithological Controls on Chemical Weathering and CO2 Consumption at Small Watershed Scale: Insights from Hydrochemistry and Stable Carbon Isotope" Water 17, no. 13: 2008. https://doi.org/10.3390/w17132008
APA StyleZhang, Y., Huang, W., Zhuang, Z., Hua, J., Bai, L., Ding, Y., Zheng, L., Wang, C., Zhao, C., & Liu, Y. (2025). Lithological Controls on Chemical Weathering and CO2 Consumption at Small Watershed Scale: Insights from Hydrochemistry and Stable Carbon Isotope. Water, 17(13), 2008. https://doi.org/10.3390/w17132008






