Enhancing Photocatalytic Activity with the Substantial Optical Absorption of Bi2S3-SiO2-TiO2/TiO2 Nanotube Arrays for Azo Dye Wastewater Treatment
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of TNAs
2.3. Fabrication of Bi2S3-TiO2-SiO2/TNA
2.4. Instruments
2.5. Photocatalytic Performance Tests
2.6. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Characterization of Prepared Films
3.1.1. Structural Properties
3.1.2. FT-IR Spectra
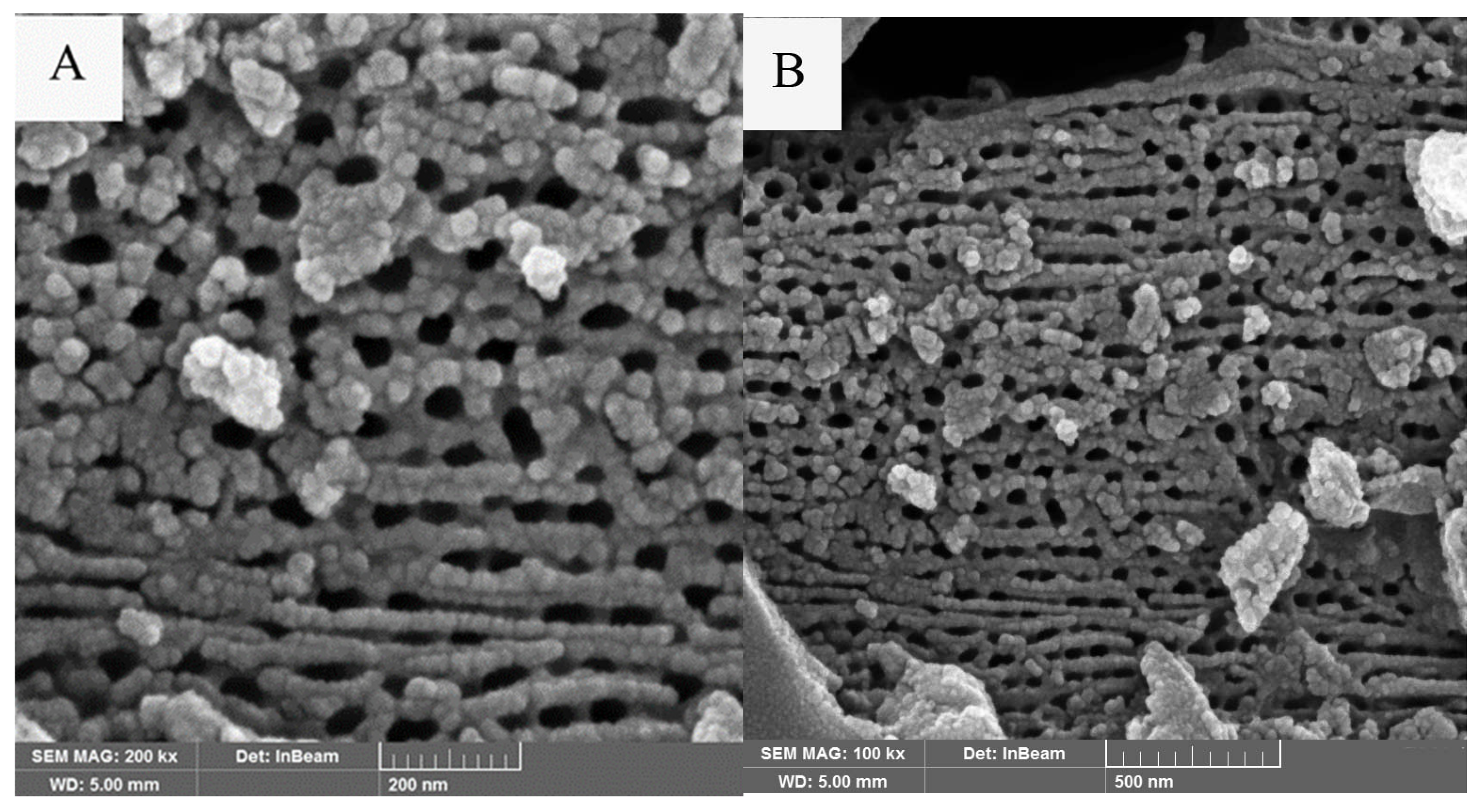
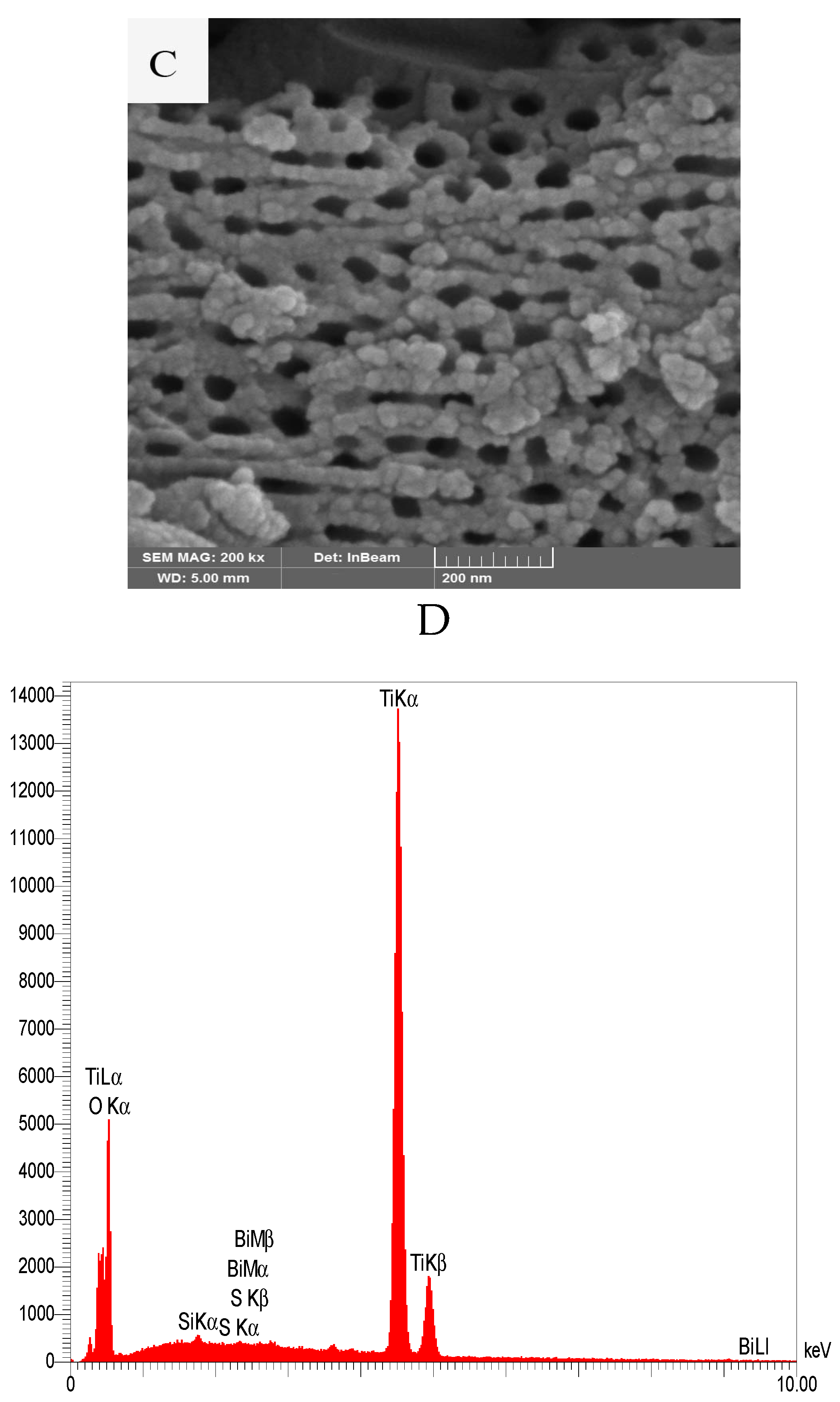
3.1.3. SEM-EDX Studies
3.1.4. Band Gap Energy and UV–Vis/DRS Analysis
3.1.5. Electrochemical Analysis
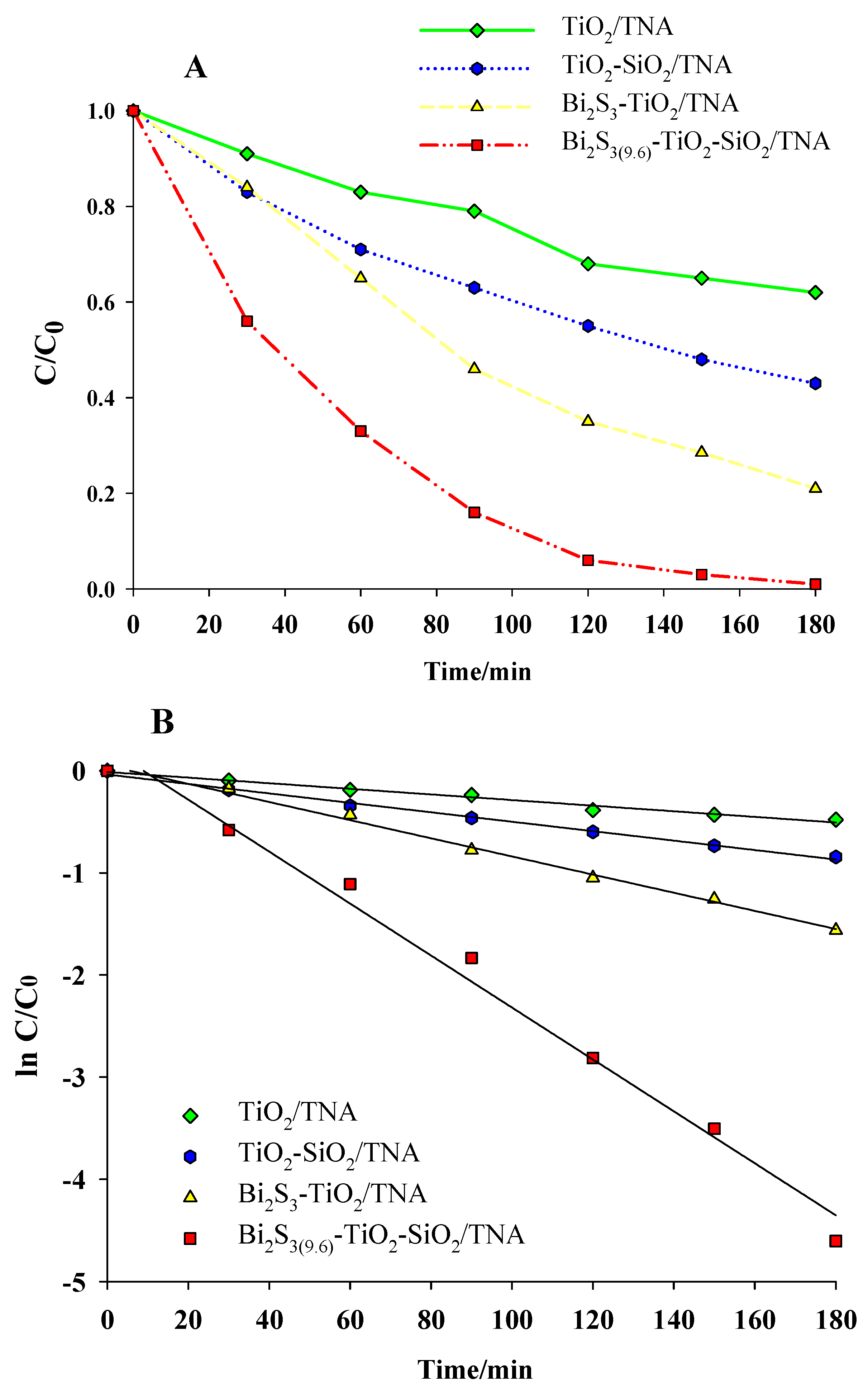
3.2. Photocatalytic Performance of Photocatalysts
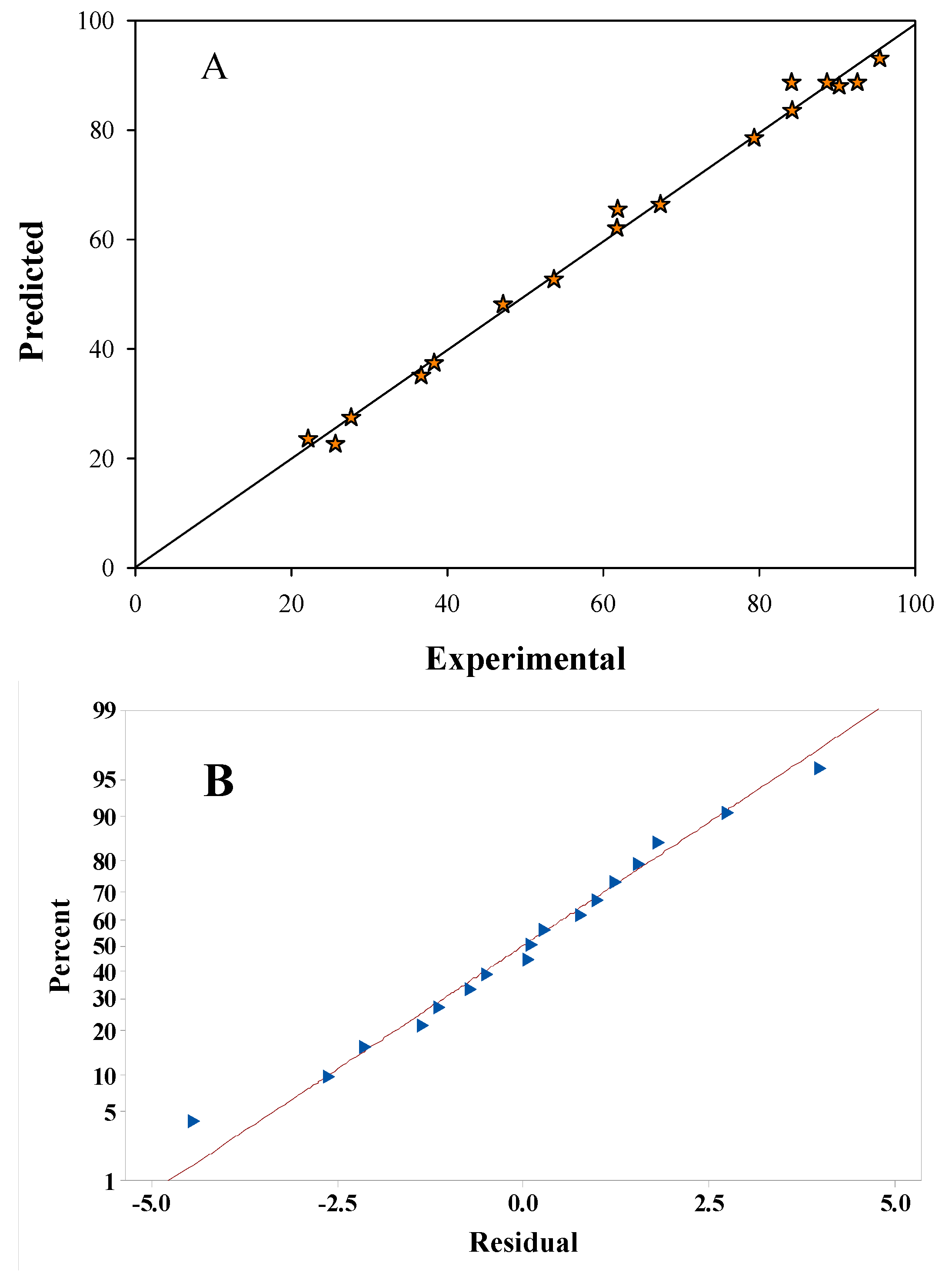
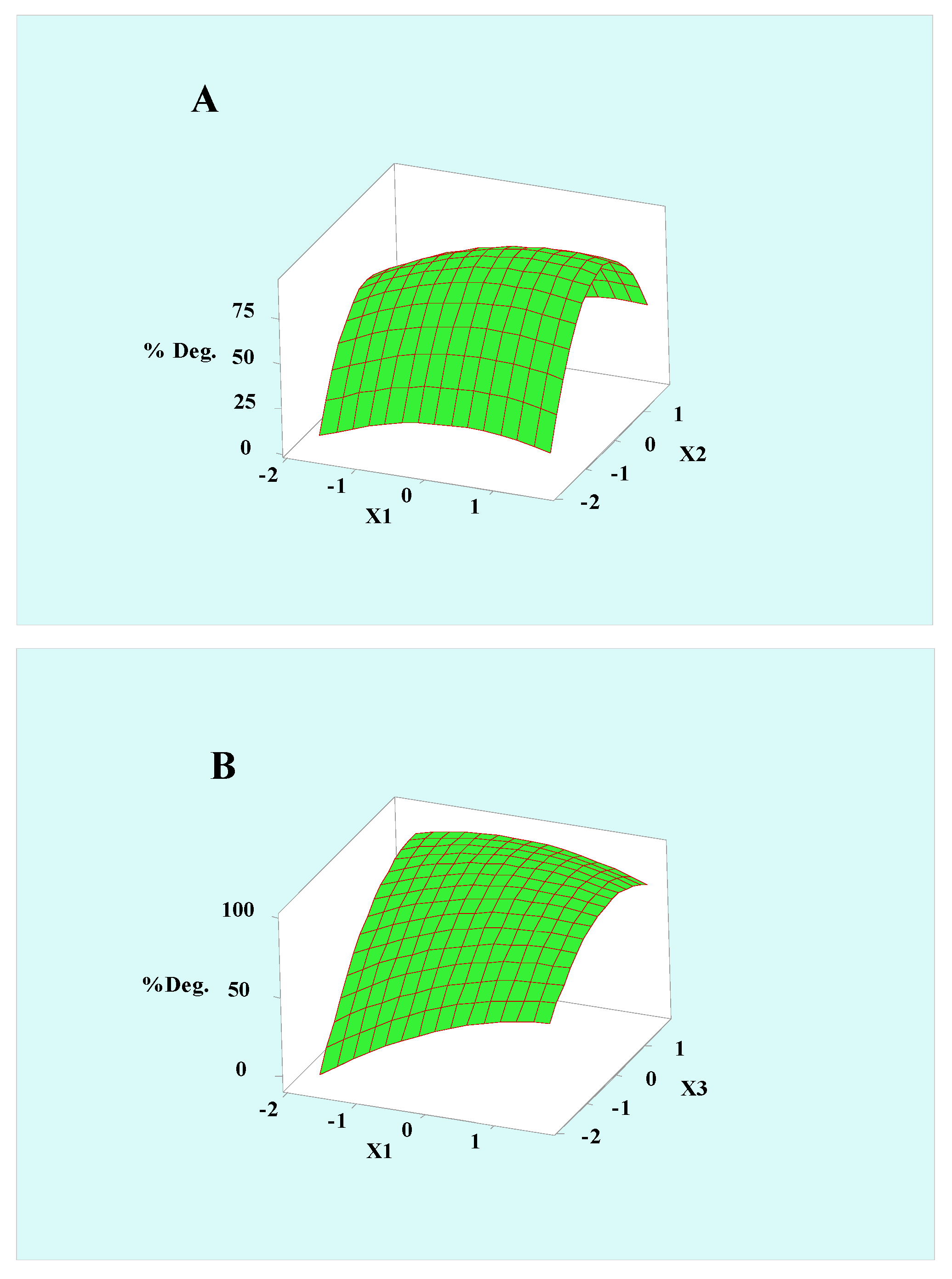
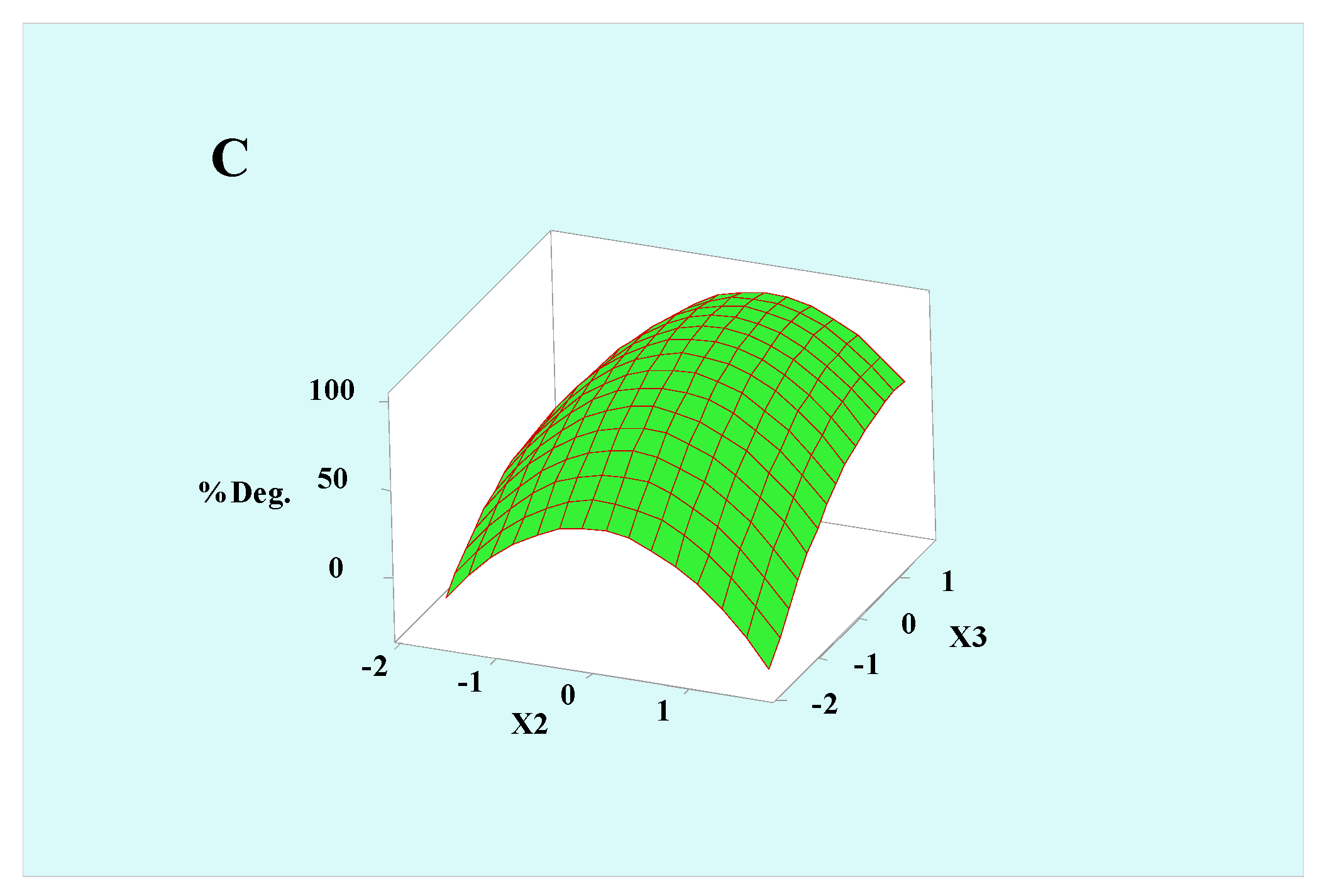
3.3. Degradation Parameter Optimization Using RSM
3.4. Key Parameter Optimization Using RSM
3.5. Energy Level Structure and the Photocatalytic Degradation Mechanism
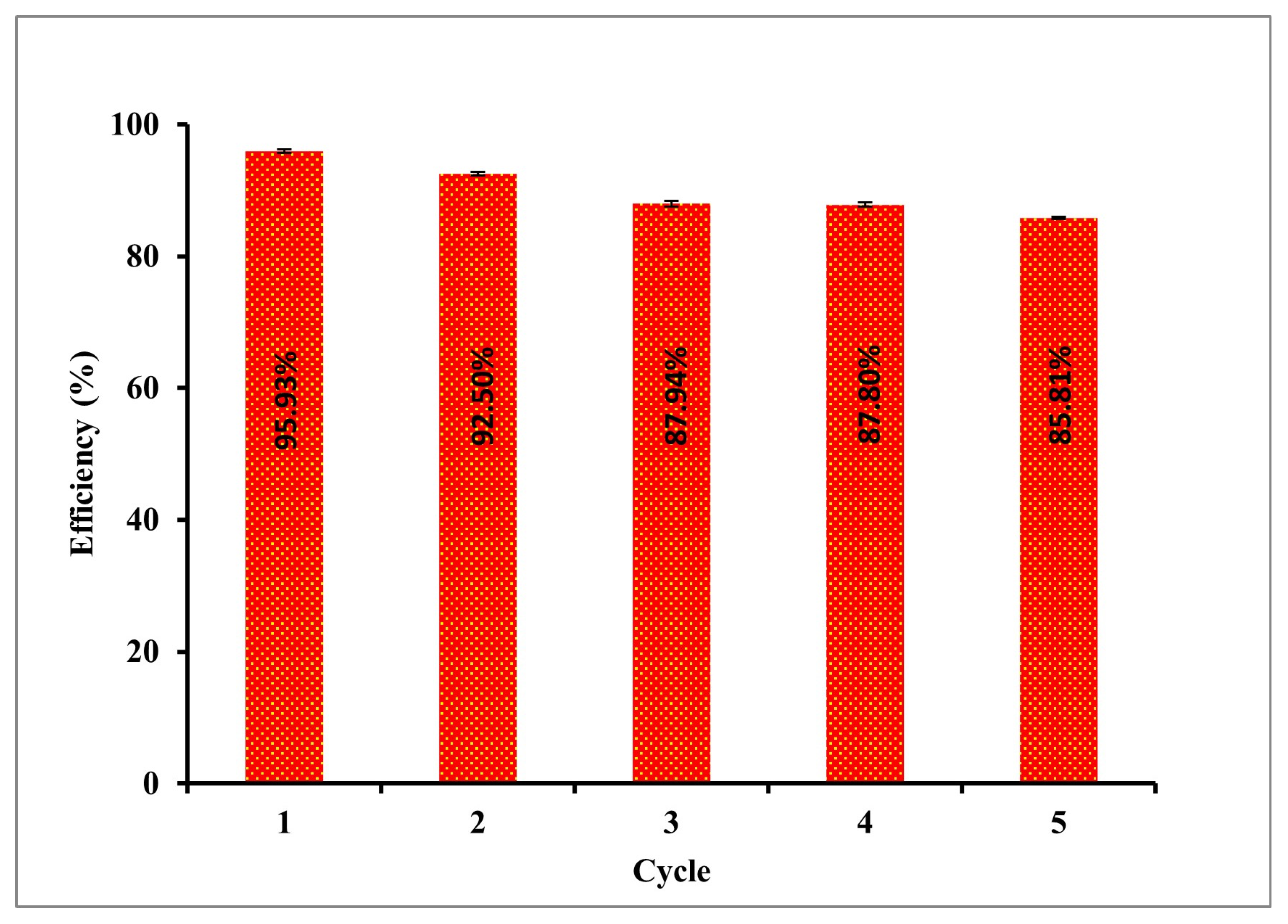
3.6. Reusability of Bi2S3(9.6)-TiO2-SiO2/TNA
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Wan, Y.; He, Q.; Zhou, X.; Song, Q.; Chen, Z.; Wang, P.; Wang, J.; Wang, T.; Chen, P.; et al. Enhanced photoelectrocatalytic performance of ZnO/Co3O4 nanoflowers modified TiO2 nanotube array under visible light. J. Environ. Chem. Eng. 2023, 11, 111604. [Google Scholar] [CrossRef]
- Liu, X.; Liu, W.; Xin, S.; Gao, S.; Huo, S.; Fu, W.; Gao, M.; Xie, H. Photoelectrocatalytic degradation of tetracycline through C3N5/TiO2 nanotube arrays photoanode under visible light: Performance, DFT calculation and mechanism. J. Environ. Chem. Eng. 2023, 11, 110576. [Google Scholar] [CrossRef]
- Belal, R.M.; Zayed, M.A.; El-Sherif, R.M.; Ghany, N.A.A. Advanced electrochemical degradation of basic yellow 28 textile dye using IrO2/Ti meshed electrode in different supporting electrolytes. J. Electroanal. Chem. 2021, 882, 114979. [Google Scholar] [CrossRef]
- Shi, J.; Liao, R.; Jia, R.; Liu, Y.; Wu, D.; Chang, S.; Zhang, N.; Gao, G.; Wang, X.; Hu, D.; et al. A novel combustion drying synthesis route of 3D WO3–SiO2 composite aerogels for enhanced adsorption and visible light photocatalytic activity. J. Non-Cryst. Solids 2023, 609, 122259. [Google Scholar] [CrossRef]
- Wu, Y.; Guan, M.; Chang, X.; Wang, J.; Xu, S. Homogeneous double-layer TiO2-ZrO2-SiO2 photocatalyst with multi-heterojunction structure for enhanced visible light-responsive photocatalytic activity. J. Mol. Liq. 2023, 369, 120959. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, S.; Zhao, Y.; Deng, Y.; Yang, W.; Ye, Y.; Wang, K. Construction of Z-scheme Bi2O3/CeO2 heterojunction for enhanced photocatalytic capacity of TiO2 NTs. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 304, 123405. [Google Scholar] [CrossRef]
- Fakhravar, S.; Farhadian, M.; Tangestaninejad, S. Excellent performance of a novel dual Z-scheme Cu2S/Ag2S/BiVO4 heterostructure in metronidazole degradation in batch and continuous systems: Immobilization of catalytic particles on α-Al2O3 fiber. Appl. Surf. Sci. 2020, 505, 144599. [Google Scholar] [CrossRef]
- Geioushy, R.; El-Sheikh, S.; Azzam, A.B.; Salah, B.A.; El-Dars, F.M. One-pot fabrication of BiPO4/Bi2S3 hybrid structures for visible-light driven reduction of hazardous Cr (VI). J. Hazard. Mater. 2020, 381, 120955. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, C.; Zhao, Y.; Fang, F.; Yin, Y.; Ye, Y.; Yang, K.; Yang, Q.; Wang, K. Photocatalytic pollutant elimination and hydrogen production over TiO2 NTs/Bi2S3-MoS2 with Z-scheme configuration: Kinetics and mechanism. Mater. Res. Bull. 2023, 167, 112430. [Google Scholar] [CrossRef]
- Hou, J.; Chen, Y.; Yimiti, M.; Xue, Y.; Zhang, H.; Wang, Q. One-pot solvothermal synthesis of Bi2S3-CdS sensitized TiO2 NT films for improved photocatalytic performance. Mol. Catal. 2024, 569, 114560. [Google Scholar] [CrossRef]
- Hmamouchi, S.; El Yacoubi, A.; El Hezzat, M.; Sallek, B.; El Idrissi, B.C. Optimization of photocatalytic parameters for MB degradation by g-C3N4 nanoparticles using Response Surface Methodology (RSM). Diam. Relat. Mater. 2023, 136, 109986. [Google Scholar] [CrossRef]
- Eskandari, P.; Amarloo, E.; Zangeneh, H.; Rezakazemi, M.; Aminabhavi, T.M. Photocatalytic degradation of metronidazole and oxytetracycline by novel l-Arginine (C, N codoped)-TiO2/g-C3N4: RSM optimization, photodegradation mechanism, biodegradability evaluation. Chemosphere 2023, 337, 139282. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.; Chainy, Y.; Sardar, P.; Samanta, A.N. Construction of a novel Bi2S3/MIL-101 (Fe) Z-scheme heterojunction for the effective visible-light assisted photocatalytic degradation of Tetracycline Hydrochloride: Performance, mechanism, and degradation pathway. Surf. Interfaces 2025, 60, 106011. [Google Scholar] [CrossRef]
- Ali, H.; Yasir, M.; Ngwabebhoh, F.A.; Sopik, T.; Zandraa, O.; Sevcik, J.; Masar, M.; Machovsky, M.; Kuritka, I. Boosting photocatalytic degradation of estrone hormone by silica-supported g-C3N4/WO3 using response surface methodology coupled with Box-Behnken design. J. Photochem. Photobiol. A Chem. 2023, 441, 114733. [Google Scholar] [CrossRef]
- Karimi-Shamsabadi, M.; Behpour, M. Comparing photocatalytic activity consisting of Sb2S3 and Ag2S on the TiO2–SiO2/TiO2 nanotube arrays-support for improved visible-light-induced photocatalytic degradation of a binary mixture of basic blue 41 and basic red 46 dyes. Int. J. Hydrogen. Energy 2021, 46, 26989–27013. [Google Scholar] [CrossRef]
- Ansari, F.; Sheibani, S.; Fernández-García, M. A response surface methodology optimization for efficient photocatalytic degradation over reusable CuxO/TiO2 nanocomposite on copper wire. Mater. Res. Bull. 2023, 166, 112342. [Google Scholar] [CrossRef]
- Koutavarapu, R.; Babu, B.; Reddy, C.V.; Reddy, I.N.; Reddy, K.R.; Rao, M.; Aminabhavi, T.M.; Cho, M.; Kim, D.; Shim, J. ZnO nanosheets-decorated Bi2WO6 nanolayers as efficient photocatalysts for the removal of toxic environmental pollutants and photoelectrochemical solar water oxidation. J. Environ. Manag. 2020, 265, 110504. [Google Scholar] [CrossRef]
- Bai, H. Synthesis of Bi2S3-TiO2 nanocomposite and its electrochemical and enhanced photocatalytic properties for phenol degradation. Int. J. Electrochem. Sci. 2023, 18, 100071. [Google Scholar]
- Asadpoor, M.; Arjmand, M.; Farhadian, M.; Omidkhah, M.; Zinatizadeh, A. Optimization and modeling of the photocatalytic activities of a novel visible driven CNT/TiO2/BiOBr/Bi2S3 nanocomposite. Desalination Water Treat. 2021, 209, 219–229. [Google Scholar] [CrossRef]
- Wu, Z.; Yuan, D.; Lin, S.; Guo, W.; Zhan, D.; Sun, L.; Lin, C. Enhanced photoelectrocatalytic activity of Bi2S3–TiO2 nanotube arrays hetero-structure under visible light irradiation. Int. J. Hydrogen. Energy 2020, 45, 32012–32021. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, K.; Yu, H.; Zhang, M.; Sun, Z. In-situ preparation of double Z-scheme Bi2S3/BiVO4/TiO2 ternary photocatalysts for enhanced photoelectrochemical and photocatalytic performance. Appl. Surf. Sci. 2021, 545, 148986. [Google Scholar] [CrossRef]
- Drmosh, Q.; Hezam, A.; Hendi, A.; Qamar, M.; Yamani, Z.; Byrappa, K. Ternary Bi2S3/MoS2/TiO2 with double Z-scheme configuration as high performance photocatalyst. Appl. Surf. Sci. 2020, 499, 143938. [Google Scholar] [CrossRef]
- Ghattavi, S.; Nezamzadeh-Ejhieh, A. A visible light driven AgBr/g-C3N4 photocatalyst composite in methyl orange photodegradation: Focus on photoluminescence, mole ratio, synthesis method of g-C3N4 and scavengers. Compos. Part B Eng. 2020, 183, 107712. [Google Scholar] [CrossRef]
- Nasseh, N.; Pour, A.K.; Azqandi, M.; Kassim, W.M.; Barikbin, B.; Al-Musawi, T.J. Integrated Fenton-like and photocatalytic system for metronidazole degradation in water using FeNi3/SiO2/CuS magnetic nanocomposite. Desalination Water Treat. 2023, 303, 160–170. [Google Scholar] [CrossRef]
- Luo, X.-L.; Yang, S.-Y.; Wang, Z.-L.; Xu, Y.-H. Synthesis of Z–scheme Bi2S3/RGO/BiVO4 photocatalysts with superior visible light photocatalytic effectiveness for pollutant degradation. Sep. Purif. Technol. 2023, 318, 123966. [Google Scholar] [CrossRef]
- Muniyandi, G.R.; Ubagaram, J.; Srinivasan, A.; James, D.R.; Pugazhenthiran, N.; Govindasamy, C.; Joseph, J.A.; Bosco, A.J.; Mahalingam, S.; Kim, J. Advanced Z-scheme Hg-C3N4/Bi2S3 nanocomposites: Boosting photocatalytic degradation of antibiotics under visible light exposure. J. Ind. Eng. Chem. 2024, 140, 647–657. [Google Scholar] [CrossRef]
- Chawla, A.; Sudhaik, A.; Sonu; Kumar, R.; Raizada, P.; Ahamad, T.; Khan, A.A.P.; Van Le, Q.; Nguyen, V.-H.; Thakur, S.; et al. Bi2S3-based photocatalysts: Properties, synthesis, modification strategies, and mechanistic insights towards environmental sustainability and green energy technologies. Coord. Chem. Rev. 2025, 529, 216443. [Google Scholar] [CrossRef]
- Lan, M.; Dong, X.; Zheng, N.; Zhang, Y. One-step construction of sulfur-vacancy-rich SnS/Bi2S3 Z-scheme heterostructure photocatalyst for significant removal of hazardous Cr (VI). Chem. Eng. Sci. 2025, 311, 121626. [Google Scholar] [CrossRef]
- Verma, M.K.; Sharma, M.; Dudi, M.; Singla, L.; Kumar, R. Curcumin stabilized Bi2S3@Ce2S3 nanoflowers: An efficient photocatalyst against harmful bacteria and toxic cationic dyes in aqueous medium. Next Mater. 2025, 8, 100694. [Google Scholar] [CrossRef]
- Mirzaeinia, S.; Pazhang, M.; Imani, M.; Chaparzadeh, N.; Amani-Ghadim, A.R. Improving the stability of uricase from Aspergillus flavus by osmolytes: Use of response surface methodology for optimization of the enzyme stability. Process Biochem. 2020, 94, 86–98. [Google Scholar] [CrossRef]
- Niknam, H.; Sadeghzadeh-Attar, A. Mg-doped TiO2 nanorods-SrTiO3 heterojunction composites for efficient visible-light photocatalytic degradation of basic yellow 28. Opt. Mater. 2023, 136, 113395. [Google Scholar] [CrossRef]
- Chahkandi, M.; Zargazi, M.; Ghiasabadi, K.B.; Chung, J.S. Core-shell structured Ag-HAp@Bi2S3 as effective S-scheme heterojunction photocatalyst: Induced with internal polarization electric field effect and co-catalyst fashions. J. Mol. Liq. 2024, 399, 124423. [Google Scholar] [CrossRef]
- Rao, V.N.; Reddy, N.L.; Kumari, M.M.; Ravi, P.; Sathish, M.; Kuruvilla, K.; Preethi, V.; Reddy, K.R.; Shetti, N.P.; Aminabhavi, T.M.; et al. Photocatalytic recovery of H2 from H2S containing wastewater: Surface and interface control of photo-excitons in Cu2S@TiO2 core-shell nanostructures. Appl. Catal. B Environ. 2019, 254, 174–185. [Google Scholar]
- Reddy, C.V.; Reddy, I.N.; Harish, V.; Reddy, K.R.; Shetti, N.P.; Shim, J.; Aminabhavi, T.M. Efficient removal of toxic organic dyes and photoelectrochemical properties of iron-doped zirconia nanoparticles. Chemosphere 2020, 239, 124766. [Google Scholar] [CrossRef]
- Reddy, N.R.; Bharagav, U.; Kumari, M.M.; Cheralathan, K.; Shankar, M.; Reddy, K.R.; Saleh, T.A.; Aminabhavi, T.M. Highly efficient solar light-driven photocatalytic hydrogen production over Cu/FCNTs-titania quantum dots-based heterostructures. J. Environ. Manag. 2020, 254, 109747. [Google Scholar] [CrossRef]
- Jiang, B.; Brandt, S.A. A fractal perspective on scale in geography. ISPRS Int. J. Geo-Inf. 2016, 5, 95. [Google Scholar] [CrossRef]
- Saravanan, S.; Dubey, R.S.; Subbarao, P.S. Optical investigation of sol–gel-synthesized and spin-coated SiO2/TiO2 multilayers. Nanomater. Energy 2023, 12, 44–48. [Google Scholar] [CrossRef]
- Shamsabadi, M.K.; Behpour, M. Fabricated CuO–ZnO/nanozeolite X heterostructure with enhanced photocatalytic performance: Mechanism investigation and degradation pathway. Mater. Sci. Eng. B 2021, 269, 115170. [Google Scholar] [CrossRef]
- Wang, J.; Ran, Q.; Xu, X.; Zhu, B.; Zhang, W. Preparation and optical properties of TiO2-SiO2 thin films by sol-gel dipping method. IOP Conf. Ser. Earth Environ. Sci. 2019, 310, 042029. [Google Scholar] [CrossRef]
- Babyszko, A.; Wanag, A.; Kusiak-Nejman, E.; Morawski, A.W. Effect of calcination temperature of SiO2/TiO2 photocatalysts on UV-VIS and VIS removal efficiency of color contaminants. Catalysts 2023, 13, 186. [Google Scholar] [CrossRef]
- Stelo, F.; Kublik, N.; Ullah, S.; Wender, H. Recent advances in Bi2MoO6 based Z-scheme heterojunctions for photocatalytic degradation of pollutants. J. Alloys Compd. 2020, 829, 154591. [Google Scholar] [CrossRef]
- Sundaram, P.S.; Sangeetha, T.; Rajakarthihan, S.; Vijayalaksmi, R.; Elangovan, A.; Arivazhagan, G. XRD structural studies on cobalt doped zinc oxide nanoparticles synthesized by coprecipitation method: Williamson-Hall and size-strain plot approaches. Phys. B Condens. Matter 2020, 595, 412342. [Google Scholar] [CrossRef]
- Uma, K.; Chen, S.-W.; KrishnaKumar, B.; Jeyaprabha, C.; Yang, T.C.-K.; Lin, J.-H. Enhanced photocatalytic activity of CdS nanostar decorated SiO2/TiO2 composite spheres and the simulation effect using FDTD model. Ionics 2021, 27, 397–406. [Google Scholar] [CrossRef]
- Huerta-Flores, A.M.; García-Gómez, N.A.; de la Parra, S.M.; Sánchez, E.M. Comparative study of Sb2S3, Bi2S3 and In2S3 thin film deposition on TiO2 by successive ionic layer adsorption and reaction (SILAR) method. Mater. Sci. Semicond. Process. 2015, 37, 235–240. [Google Scholar] [CrossRef]
- Belghiti, M.; El Mersly, L.; Tanji, K.; Belkodia, K.; Lamsayety, I.; Ouzaouit, K.; Faqir, H.; Benzakour, I.; Rafqah, S.; Outzourhit, A. Sol-gel combined mechano-thermal synthesis of Y2O3, CeO2, and PdO partially coated ZnO for sulfamethazine and basic yellow 28 photodegradation under UV and visible light. Opt. Mater. 2023, 136, 113458. [Google Scholar] [CrossRef]
- Niknam, H.; Sadeghzadeh-Attar, A. Constructing trinary heterostructure of TiO2/CoCr2O4/SrTiO3 to enhance photocatalytic activity toward degradation of yellow 28 dye. Mater. Chem. Phys. 2023, 299, 127489. [Google Scholar] [CrossRef]
- Behpour, M.; Mehrzad, M.; Hosseinpour, M.S. TiO2 thin film: Preparation, characterization, and its photocatalytic degradation of basic yellow 28 dye. J. Nanostruct. 2015, 5, 183–187. [Google Scholar]
- Foulady-Dehaghi, R.; Behpour, M. Visible and solar photodegradation of textile wastewater by multiple doped TiO2/Zn nanostructured thin films in fixed bed photoreactor mode. Inorg. Chem. Commun. 2020, 117, 107946. [Google Scholar] [CrossRef]
- Behpour, M.; Foulady-Dehaghi, R.; Mir, N. Considering photocatalytic activity of N/F/S-doped TiO2 thin films in degradation of textile waste under visible and sunlight irradiation. Sol. Energy 2017, 158, 636–643. [Google Scholar] [CrossRef]
- Dehaghi, R.F.; Behpour, M.; Mir, N. Purification of textile wastewater by using coated Sr/S/N doped TiO2 nanolayers on glass orbs. Korean J. Chem. Eng. 2018, 35, 1441–1449. [Google Scholar] [CrossRef]
- Wang, D.; Hou, P.; Yang, P.; Cheng, X. BiOBr@ SiO2 flower-like nanospheres chemically-bonded on cement-based materials for photocatalysis. Appl. Surf. Sci. 2018, 430, 539–548. [Google Scholar] [CrossRef]
- Wang, Z.; Su, B.; Xu, J.; Hou, Y.; Ding, Z. Direct Z-scheme ZnIn2S4/LaNiO3 nanohybrid with enhanced photocatalytic performance for H2 evolution. Int. J. Hydrogen. Energy 2020, 45, 4113–4121. [Google Scholar] [CrossRef]
- Helal, A.; Harraz, F.A.; Ismail, A.A.; Sami, T.M.; Ibrahim, I. Hydrothermal synthesis of novel heterostructured Fe2O3/Bi2S3 nanorods with enhanced photocatalytic activity under visible light. Appl. Catal. B Environ. 2017, 213, 18–27. [Google Scholar] [CrossRef]
- Reddy, C.V.; Koutavarapu, R.; Reddy, K.R.; Shetti, N.P.; Aminabhavi, T.M.; Shim, J. Z-scheme binary 1D ZnWO4 nanorods decorated 2D NiFe2O4 nanoplates as photocatalysts for high efficiency photocatalytic degradation of toxic organic pollutants from wastewater. J. Environ. Manag. 2020, 268, 110677. [Google Scholar] [CrossRef]
- Cao, J.; Chen, X.; Chen, C.; Chen, A.; Zheng, W. High efficiency Bi2S3/Bi2MoO6/TiO2 photoanode for photoelectrochemical hydrogen generation. J. Alloys Compd. 2023, 965, 171139. [Google Scholar] [CrossRef]
- Zabihi, M.; Motavalizadehkakhky, A. PbS/ZIF-67 nanocomposite: Novel material for photocatalytic degradation of basic yellow 28 and direct blue 199 dyes. J. Taiwan Inst. Chem. Eng. 2022, 140, 104572. [Google Scholar] [CrossRef]
- Zhou, X.; Mu, Y.; Zhang, S.; Gao, L.; Chen, H.; Mu, J.; Zhang, X.; Zhang, M.; Liu, W. CdS nanoparticles sensitized high energy facets exposed SnO2 elongated octahedral nanoparticles film for photocatalytic application. Mater. Res. Bull. 2019, 111, 118–125. [Google Scholar] [CrossRef]
- Li, X.; Wang, K.; Mao, Z.; Kumar, J.M.; Han, C.; Li, L.; Yu, R.; Gu, Y.; Zhang, Y. Facile synthesis of hierarchical structure Bi2S3/TiO2 heterojunction and enhancing light-harvesting performance. Vacuum 2023, 217, 112579. [Google Scholar] [CrossRef]
- Zare, M.H.; Mehrabani-Zeinabad, A. Photocatalytic activity of ZrO2/TiO2/Fe3O4 ternary nanocomposite for the degradation of naproxen: Characterization and optimization using response surface methodology. Sci. Rep. 2022, 12, 10388. [Google Scholar] [CrossRef]








| Factors | Levels | ||
|---|---|---|---|
| Low | Central (0) | High | |
| −1 | 0 | +1 | |
| X1: [Bi3+] (M) | 0.30 | 0.40 | 0.50 |
| X2: [BY 28] (mg/L) | 10 | 20 | 30 |
| X3: pH | 4 | 6 | 8 |
| Photocatalysts | Sources of Irradiation | Time (min) | Degradation (%) | Ref. |
|---|---|---|---|---|
| 1.5 Mg–TiO2/SrTiO3 | Visible-light irradiation | 150 | 97% | [31] |
| Y2O3, CeO2, and ZnO/PdO | Visible-light irradiation | 120 | 94% | [45] |
| TiO2/CoCr2O4/SrTiO3 | Visible-light irradiation | 120 | 98% | [46] |
| N-S doped TiO2 | Visible-light irradiation | 180 | 98% | [47] |
| Zn/Tu-TiO2 | Visible-light irradiation | 240 | 95% | [48] |
| N/F/S-doped TiO2 | Visible-light irradiation | 360 | 94% | [49] |
| Sr/S/N doped TiO2 nanolayers on glass orbs | Visible-light irradiation | 480 | 91% | [50] |
| Bi2S3-TiO2-SiO2/TNA | Visible-light irradiation | 180 | 95.45% | This work |
| Item | Degrees of Freedom | Sum of Squares | F-Value | p-Value |
|---|---|---|---|---|
| Model | 9 | 10,396.8 | 120.05 | 0.000 |
| Linear | 3 | 4996.3 | 173.08 | 0.000 |
| X1 | 1 | 651.4 | 67.69 | 0.000 |
| X2 | 1 | 283.5 | 29.47 | 0.001 |
| X3 | 1 | 4061.4 | 422.07 | 0.000 |
| Square | 3 | 4907.7 | 170.01 | 0.000 |
| X1*X1 | 1 | 176.6 | 18.36 | 0.004 |
| X2*X2 | 1 | 4744.7 | 493.08 | 0.000 |
| X3*X3 | 1 | 808.0 | 83.97 | 0.000 |
| 2-Way Interaction | 3 | 492.7 | 17.07 | 0.001 |
| X1*X2 | 1 | 36.1 | 3.75 | 0.094 |
| X1*X3 | 1 | 267.2 | 27.76 | 0.001 |
| X2*X3 | 1 | 189.4 | 19.69 | 0.003 |
| Error | 7 | 67.4 | ||
| Lack of Fit | 5 | 31.8 | 0.36 | 0.847 |
| Pure Error | 2 | 35.6 | ||
| Total | 16 | 10,464.1 |
| Catalysts | C (M) | Mass Loading (mg/cm2) | |||||
|---|---|---|---|---|---|---|---|
| Bi3+ | Bi2S3 | ||||||
| Bi2S3(6.1)-TiO2-SiO2/TNA | 0.13 | 6.1 | |||||
| Bi2S3(9.6)-TiO2-SiO2/TNA | 0.20 | 9.6 | |||||
| Bi2S3(11.4)-TiO2-SiO2/TNA | 0.30 | 11.4 | |||||
| Bi2S3(13.7)-TiO2-SiO2/TNA | 0.40 | 13.7 | |||||
| Bi2S3(15.8)-TiO2-SiO2/TNA | 0.46 | 15.8 | |||||
| The Bi2S3-TiO2-SiO2/TNA photocatalytic process under optimal conditions in degradation of BY 28. | |||||||
| Dyes | X1 | X2 | X3 | Deg. (%) | |||
| Coded | Actual (M) | Coded | Actual (mg/L) | Coded | Actual | ||
| BY 28 | 0.18 | 0.30 | 0.25 | 22.5 | 1.03 | 8.34 | 95.45 |
| Run | Independent Variables | % Degradation | |||
|---|---|---|---|---|---|
| X1: [Bi3+] (M) | X2: Dye Concentration (mg/L) | X3: pH | Experimental Value | Predicted Value | |
| 1 | 1 | 1 | 1 | 84.21 | 83.58 |
| 2 | 1 | 1 | −1 | 53.66 | 52.73 |
| 3 | −1.68 | 0 | 0 | 67.33 | 66.41 |
| 4 | 1 | −1 | −1 | 47.13 | 48.16 |
| 5 | 0 | 0 | 0 | 92.57 | 88.68 |
| 6 | −1 | 1 | 1 | 79.35 | 78.56 |
| 7 | −1 | 1 | −1 | 22.14 | 23.56 |
| 8 | 1.68 | 0 | 0 | 90.25 | 88.06 |
| 9 | 0 | 0 | 0 | 88.69 | 88.68 |
| 10 | 1 | −1 | 1 | 61.76 | 62.09 |
| 11 | 0 | 0 | 1.68 | 95.45 | 93.09 |
| 12 | −1 | −1 | −1 | 27.65 | 27.43 |
| 13 | −1 | −1 | 1 | 61.85 | 65.52 |
| 14 | 0 | 1.68 | 0 | 38.28 | 37.42 |
| 15 | 0 | −1.68 | 0 | 25.64 | 22.61 |
| 16 | 0 | 0 | 0 | 84.14 | 88.68 |
| 17 | 0 | 0 | −1.68 | 36.63 | 35.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulrahman, A.; Algarni, Z.; Ghazouani, N.; Sammen, S.S.; Amari, A.; Scholz, M. Enhancing Photocatalytic Activity with the Substantial Optical Absorption of Bi2S3-SiO2-TiO2/TiO2 Nanotube Arrays for Azo Dye Wastewater Treatment. Water 2025, 17, 1875. https://doi.org/10.3390/w17131875
Abdulrahman A, Algarni Z, Ghazouani N, Sammen SS, Amari A, Scholz M. Enhancing Photocatalytic Activity with the Substantial Optical Absorption of Bi2S3-SiO2-TiO2/TiO2 Nanotube Arrays for Azo Dye Wastewater Treatment. Water. 2025; 17(13):1875. https://doi.org/10.3390/w17131875
Chicago/Turabian StyleAbdulrahman, Amal, Zaina Algarni, Nejib Ghazouani, Saad Sh. Sammen, Abdelfattah Amari, and Miklas Scholz. 2025. "Enhancing Photocatalytic Activity with the Substantial Optical Absorption of Bi2S3-SiO2-TiO2/TiO2 Nanotube Arrays for Azo Dye Wastewater Treatment" Water 17, no. 13: 1875. https://doi.org/10.3390/w17131875
APA StyleAbdulrahman, A., Algarni, Z., Ghazouani, N., Sammen, S. S., Amari, A., & Scholz, M. (2025). Enhancing Photocatalytic Activity with the Substantial Optical Absorption of Bi2S3-SiO2-TiO2/TiO2 Nanotube Arrays for Azo Dye Wastewater Treatment. Water, 17(13), 1875. https://doi.org/10.3390/w17131875









